Abstract

The efficient selectivity of heavy metal ions from wastewater is still challenging but gains great public attention in water treatment on a world scale. In this study, the novel disulfide cross-linked poly(methacrylic acid) iron oxide (Fe3O4@S-S/PMAA) nanoparticles with selective adsorption, improved adsorption capability, and economic reusability were designed and prepared for selective adsorption of Pb(II) ions in aqueous solution. In this study, nuclear magnetic resonance, dynamic light scattering, scanning electron microscopy, X-ray diffraction, vibrating sample magnetometry, and thermogravimetric analysis were utilized to study the chemophysical properties of Fe3O4@S-S/PMAA. The effect of different factors on adsorption properties of the Fe3O4@S-S/PMAA nanoparticles for Co(II) and Pb(II) ions in aqueous solution was explored by batch adsorption experiments. For adsorption mechanism investigation, the adsorption of Fe3O4@S-S/PMAA for Co(II) and Pb(II) ions can be better fitted by a pseudo-second-order model, and the adsorption process of Fe3O4@S-S/PMAA for Co(II) and Pb(II) matches well with the Freundlich isotherm equation. Notably, in the adsorption experiments, the Fe3O4@S-S/PMAA nanoparticles were demonstrated to have a maximum adsorption capacity of 48.7 mg·g–1 on Pb(II) ions with a selective adsorption order of Pb2+ > Co2+ > Cd2+ > Ni2+ > Cu2+ > Zn2+ > K+ > Na+ > Mg2+ > Ca2+ in the selective experiments. In the regeneration experiments, the Fe3O4@S-S/PMAA nanoparticles could be easily recovered by desorbing heavy metal ions from the adsorbents with eluents and showed good adsorption capacity for Co(II) and Pb(II) after eight recycles. In brief, compared to other traditional nanoadsorbents, the as-prepared Fe3O4@S-S/PMAA with improved adsorption capability and high regeneration efficiency demonstrated remarkable affinity for adsorption of Pb(II) ions, which will provide a novel technical platform for selective removal of heavy metal ions from actual polluted water.
1. Introduction
In recent years, water contamination has caused severe environmental problems along with serious impacts to the ecosystem and human health on a worldwide scale.1,2 The water pollutants such as the heavy metal ions generated by the chemical-intensive industries are intentionally discharged and pumped into the river, which are nonbiodegradable and of high environmental toxicity in natural environments.3−5 As a representative toxic metal ion, the lead ion [Pb(II)] and its compounds existing in environmental samples are considered as a major environmental health problem. Because of its high toxicity, long-term drinking water containing Pb(II) ions will cause chronic poisoning to the human body.6−8 Additionally, Pb(II) ions are considered to be easily accumulated in the human body, which will cause severely irreversible damages to human organs such as kidneys and the nervous system.9−11 In this regard, Pb(II) ions need to be efficiently removed from the wastewater, which has emerged as an urgent issue for the environmental protection. Recently, many physicochemical technologies have been used for the treatment of heavy metal ions from polluted water, such as chemical precipitation,12 ion exchange,13 membrane filtration,14 reverse osmosis,15 and adsorption.16,17 Among these various technologies, the adsorption method is featured by cost-effectiveness, large-scale choice of materials, and ease of operation. Moreover, the adsorbents can be directly dispersed in wastewater to completely contact with pollutants, facilitating an efficient adsorption process.18−20 Although adsorption has been widely applied in the removal of Pb(II) ions in water treatment, nonselectivity, low adsorption capacity, and nonreusability are still challenging in the application of the conventional adsorbents in the adsorption process for actual wastewater treatment.
In this regard, the rational design and manufacture of novel functionalized adsorbents are therefore required for the efficiently selective adsorption of Pb(II) ions in practical wastewater treatment. In general, conventional inorganic adsorbents, such as activated carbon, clay, alumina, and their derivatives, have low selectivity toward the Pb(II) ion adsorption from the complex actual wastewater.21,22 Recently, polymeric adsorbents which can be modified with functional groups have been widely developed in pollutant adsorption and gained great interest in the selective removal of heavy metal ions.23 As reported by the previous literature, oxygen and nitrogen-enriched polymer adsorbents exhibited good affinity to Pb(II) adsorption via covalent bonds on the basis of “hard-soft-acid–base” (HSAB) theory; therefore, such kinds of functional polymer adsorbents may overcome the obstacles for efficiently selective removal of Pb(II) ions.24 Additionally, the disulfide bond also shows good selective interaction with the Pb(II) ions because of its electron-rich structure, which can form the chemical interaction bond with the Pb(II) ions.25 Therefore, grafting Lewis soft base ligands such as N–H and C=O bonds and the electron-rich S–S groups on the adsorbents may achieve the efficiently selective adsorption of Pb(II) ions in water treatment. Nevertheless, the application of these kinds of polymeric adsorbents is generally impeded by their complicated separation techniques, resulting in subsequently high costs during the adsorption process. Therefore, materials with easy separability are considered as promising candidates for both isolation and reuse of the adsorbents for efficient pollutant removal in water treatment. In this regard, iron oxide nanoparticles as a kind of representative magnetic materials have gained vast attention because of their easy magnetic separability under an external magnetic field.26,27 Thus, to design a promising adsorption material, the above-mentioned ligand-enriched functional polymer can be modified on the surface of iron oxide nanoparticles to obtain the functionalized magnetic nanocomposites, which can achieve selective adsorption, high adsorption capacity, fast separability, and economic reusability for the adsorption process. Unfortunately, to our best knowledge, few studies based on disulfide-containing and oxygen and nitrogen-enriched polymer magnetic nanoadsorbents have been reported for efficiently selective adsorption of Pb(II) so far.
Herein, novel disulfide cross-linked poly(methacrylic acid) iron oxide (Fe3O4@S-S/PMAA) nanoparticles were designed and prepared for efficiently selective adsorption of Pb(II) ions. The preparation of the Fe3O4@S-S/PMAA nanoparticles underwent a three-step process including the following: (i) the coprecipitation method was used to prepare the Fe3O4 nanoparticles, (ii) the Fe3O4 nanoparticles were modified with 3-methacryloxypropyltrimethoxysilane (MPS) to form the Fe3O4@MPS nanoparticles with a vinyl-enriched coating layer, and (iii) subsequently, poly(methacrylic acid) was assembled onto Fe3O4@MPS by using N,N-bis(acrylate) cystamine (BACy) as the cross-linker to form the Fe3O4@S-S/PMAA nanoparticles via the free-radical copolymerization. The chemophysical properties of the Fe3O4@S-S/PMAA nanoparticles were fully characterized, and Fe3O4@S-S/PMAA was then utilized to study the adsorption process on Co(II) and Pb(II) ions in aqueous solution. The adsorption kinetics and isothermal adsorption equilibrium on Co(II) and Pb(II) ion adsorption were analyzed. Notably, during the adsorption process, Fe3O4@S-S/PMAA demonstrated high selectivity toward Pb(II) ions upon other coexisting metal cations, and the mechanism for the selective adsorption is also discussed. Finally, the adsorption–desorption and reusability of the as-prepared Fe3O4@S-S/PMAA for removal of Co(II) and Pb(II) ions were performed.
2. Results and Discussion
2.1. Material Characterization
As shown in Scheme 1, the preparation procedure of the Fe3O4@S-S/PMAA nanoparticles was carried out through the coprecipitation of Fe2+ and Fe3+ forming the Fe3O4 nanoparticles and subsequent modification with MPS on the Fe3O4 nanoparticles to form the Fe3O4@MPS nanoparticles. Finally, the free-radical copolymerization was utilized to prepare the network structure outlayer of the Fe3O4@S-S/PMAA nanoparticles by using MAA and BACy as the cross-linker. Remarkably, Fe3O4@S-S/PMAA was endowed with multifunctions in water treatment because of the multilayered structure. Magnetic separation will be achieved by the superparamagnetic Fe3O4 inner core of Fe3O4@S-S/PMAA, and the disulfide bond and the oxygen and nitrogen-enriched polymer structure are supposed to demonstrate good selectivity toward Pb(II) ion adsorption. As anticipated, the as-prepared Fe3O4@S-S/PMAA nanoparticles can achieve efficiently selective removal of Pb(II) ions and improved reusability in wastewater treatment.
Scheme 1. Preparation Route of Fe3O4@S-S/PMAA Nanoparticles and Its Application for Pb(II) Adsorption.
The 1H NMR spectrum was used to analyze the synthesized BACy in dimethyl sulfoxide (DMSO) (shown in Figure S1). The signals of methylene (e,f) neighboring the S–S bond, vinyl groups (a–c), and the imino group (d) can be assigned to δ = 2.82 (f), 3.43 (e), 5.62–6.25 (a–c), and 8.32 ppm (d), respectively. The preparation of Fe3O4, Fe3O4@MPS, and Fe3O4@S-S/PMAA was also confirmed by Fourier transform infrared (FT-IR) spectra and powder X-ray diffraction (XRD) patterns (as shown in Figure 1). In Figure 1a, the stretching vibration of the Fe–O bond which appeared at 588 cm–1 was observed for the Fe3O4 nanoparticles. In the FT-IR spectrum of Fe3O4@MPS (shown in Figure 1a), the absorption peaks at 1716, 1636, and 1054 cm–1 can be assigned to the vibrations of carbonyl groups, vinyl groups, and Si–O bonds, respectively. As shown in Figure 1a, the amide I bands around 1655 cm–1 and the amide II bands at 1541 cm–1 belong to the amido bond in BACy. Additionally, the C–S bond, ether linkage, and disulfide appeared at 1266, 1102, and 454 cm–1, respectively, indicating the successful incorporation of disulfide and PMAA units into the polymer network as the outer layer of Fe3O4@S-S/PMAA. As shown in Figure 1b, the powder XRD patterns of Fe3O4, Fe3O4@MPS, and Fe3O4@S-S/PMAA were obtained. The powder diffraction peaks at 30.1, 35.4, 43.2, 53.5, 57.1, and 62.7° belong to a series of characteristic magnetite lattice planes, such as the (220), (311), (400), (422), (511), and (440) planes, respectively. The powder XRD patterns of Fe3O4@MPS and Fe3O4@S-S/PMAA nanoparticles showed the characteristic diffraction peaks of the Fe3O4 nanoparticles; thus, the same crystal structure of magnetite as Fe3O4 nanoparticles in Fe3O4@MPS and Fe3O4@S-S/PMAA after the manufacturing process is suggested.
Figure 1.

FT-IR spectra (a) and XRD patterns (b) of Fe3O4, Fe3O4@MPS, and Fe3O4@S-S/PMAA nanoparticles.
The micromorphologies of the Fe3O4, Fe3O4@MPS, and Fe3O4@S-S/PMAA nanoparticles were studied by using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The SEM microphotos of the Fe3O4, Fe3O4@MPS, and Fe3O4@S-S/PMAA nanoparticles with the average sizes of about 40, 60, and 80 nm, respectively, are shown in Figure 2a–c. The upward trend in particle size indicates the successful modification of Fe3O4 nanoparticles in the outer layers. Moreover, a microspherical structure was observed in the SEM microphotos of Fe3O4@S-S/PMAA nanoparticles with a smooth surface, whereas Fe3O4 and Fe3O4@MPS had an irregular structure and rough surface. It can be explained by the soft polymer network coated on the surface of the Fe3O4 nanoparticles. The TEM microphotos of the Fe3O4, Fe3O4@MPS, and Fe3O4@S-S/PMAA nanoparticles are also shown in Figure 2d–f. The growth trend in particle size of Fe3O4, Fe3O4@MPS, and Fe3O4@S-S/PMAA nanoparticles was observed, as shown in the SEM microphotos. Moreover, the same results were presented in the dynamic light scattering (DLS) measurements, as shown in Figure S2. Based on the particle size distribution of the nanoparticles, an increasing trend of particle size was observed, indicating the core–shell structure of the Fe3O4@S-S/PMAA nanoparticles. Therefore, based on the SEM and TEM observations of the polymer network coated on Fe3O4 nanoparticles, the successful preparation of Fe3O4@S-S/PMAA nanoparticles with a multilayer structure is suggested.
Figure 2.
(a–c) SEM microphotos of Fe3O4, Fe3O4@MPS, and Fe3O4@S-S/PMAA nanoparticles and (d–f) TEM microphotos of Fe3O4, Fe3O4@MPS, and Fe3O4@S-S/PMAA nanoparticles.
Figure 3 shows the N2 adsorption–desorption isotherms and the pore size distribution curves of Fe3O4, Fe3O4@MPS, and Fe3O4@S-S/PMAA nanoparticles. In Figure 3a, the N2 adsorption curves of all samples exhibited the conspicuous hysteresis loops. According to the N2 adsorption curves, all the samples fit well with the typical IV isotherm, which suggests the mesoporous structure of the nanoparticles. Moreover, Fe3O4, Fe3O4@MPS, and Fe3O4@S-S/PMAA nanoparticles demonstrated a H2 hysteresis loop in a p/p0 range from 0.6 to 0.9, suggesting the presence of uniform channel-like mesopores in the nanoparticles. In Figure 3b, the pore size distribution curves were also evaluated. Based on the pore size distribution curves, the pore size of the samples mainly distributes at around 10 nm, indicating the majority of the mesoporous structure; however, macropores also existed in the nanoparticles. Additionally, Table 1 shows the parameters of the porous structure of Fe3O4, Fe3O4@MPS, and Fe3O4@S-S/PMAA nanoparticles. Among the three samples, the SBET of Fe3O4 (100.7 m2·g–1) was much larger than that of Fe3O4@MPS (66.2 m2·g–1) and Fe3O4@S-S/PMAA (96.9 m2·g–1). Nevertheless, the Fe3O4@S-S/PMAA magnetic nanoparticles owned a uniform mesopore size (average 9.4 nm) with a large pore volume (0.321 cm3·g–1), which indicates a potentially efficient adsorption capacity for pollutant removal in water solutions.
Figure 3.
(a) N2 adsorption–desorption isotherms; (b) pore size distribution curves; (c) VSM analysis; (d) TGA curves of Fe3O4, Fe3O4@MPS, and Fe3O4@S-S/PMAA nanoparticles.
Table 1. Parameters of the Porous Structure of the Fe3O4, Fe3O4@MPS, and Fe3O4@S-S/PMAA Nanoparticles.
| samples | SBET (m2·g–1) | pore volume (cm3·g–1) | pore size (nm) |
|---|---|---|---|
| Fe3O4 | 100.7 | 0.406 | 14.5 |
| Fe3O4@MPS | 66.2 | 0.207 | 9.3 |
| Fe3O4@S-S/PMAA | 96.9 | 0.321 | 9.4 |
The vibrating sample magnetometry (VSM) measurement and thermogravimetric analysis (TGA) were utilized to investigate the magnetic and thermo-oxidative degradation behaviors of the Fe3O4, Fe3O4@MPS, and Fe3O4@S-S/PMAA nanoparticles. The magnetization curves of the samples as a function of the variation of magnetic field are shown in Figure 3c. Among the three samples, the saturation magnetization of Fe3O4 (57.6 emu·g–1) was larger than that of Fe3O4@MPS (44.1 emu·g–1) and Fe3O4@S-S/PMAA (11.8 emu·g–1) nanoparticles, and it is probably due to the polymer networks out of Fe3O4 that have no contribution to the magnetic performance to the nanocomposites. Notably, the Fe3O4@S-S/PMAA nanoparticles were endowed with the superparamagnetic nature as Fe3O4, according to the zero coercivity and remanence of the magnetization curve, which can be reused after the adsorption process by magnetic separation. Moreover, the TGA curves for the thermo-oxidative degradation behaviors are shown in Figure 3d. A 9.2% weight loss was observed in the TGA curve of Fe3O4 nanoparticles ranging from room temperature to 900 °C because of the escape of adsorbed water and the solvent, which was less than the weight loss of Fe3O4@MPS (34.4%) caused by the thermo-oxidative degradation of the organic substance (such as MPS) in the sample. Based on the TGA curve of Fe3O4@S-S/PMAA, a much greater weight loss (46.8%) appeared around 400 °C, while a total weight loss of 71.8% ranging from room temperature to 900 °C was observed. In conclusion, the Fe3O4@S-S/PMAA nanoparticles demonstrated superparamagnetic nature and good thermostability for separable usage in actual water treatment.
2.2. Adsorption Property Study
2.2.1. Effect of pH and Amount of the Adsorbent
The comparative adsorption capacity of Fe3O4@S-S/PMAA for Co(II) and Pb(II) ions was evaluated under different solution pH and initial concentrations. As shown in Figure 4a, the adsorption capacities of Fe3O4@S-S/PMAA for Co(II) and Pb(II) ions at different pH ranging from 2.0 to 8.0 were investigated. Fe3O4@S-S/PMAA showed a better adsorption capacity for Pb(II) ions compared to Co(II) ions and reached the maximum adsorption capacity at the pH around 7.0, whereas a decline of adsorption capacity in qe was observed at pH around 8.0. The adsorption capacity of Pb(II) does not decrease significantly, while the adsorption capacity of Co(II) obviously decreases at pH lower than 5. The decline of the adsorption capacity of Fe3O4@S-S/PMAA on Co(II) at lower pH (pH < 5) could be explained by the low affinity of Fe3O4@S-S/PMAA toward Co(II); however, Fe3O4@S-S/PMAA exhibited higher affinity on Pb(II), resulting in higher adsorption capacity at pH ranging from 2 to 8 because of the Lewis soft base ligands (such as N–H and C=O bonds and the electron-rich S–S groups) on the adsorbents. Moreover, the effect of amount of the adsorbent on the adsorption capacities and removal efficiency of Fe3O4@S-S/PMAA for Co(II) and Pb(II) ions was also investigated and is shown in Figure 4b,c, respectively. The equilibrium adsorption capacities of Fe3O4@S-S/PMAA on Co(II) and Pb(II) were 58.1 and 36.7 mg·g–1, respectively. Additionally, it was indicated that the adsorption capacities toward Co(II) and Pb(II) ions declined when increasing the amount of the adsorbent, while the removal efficiency of Fe3O4@S-S/PMAA increased when increasing the amount of the adsorbent. The experiment on the effect of the pH value on zeta potential for the solid adsorbent has been carried out. A generally negative zeta potential was observed at different pH values ranging from 3 to 8, which can be explained by the carboxyl groups of PMAA segments existing in the outer layer of the nanocomposites.
Figure 4.
(a) Effect of initial pH on the adsorption of Fe3O4@S-S/PMAA for Co(II) and Pb(II) ions. Effect of adsorbent amount on the adsorption capacities and removal efficiency of Fe3O4@S-S/PMAA for Pb(II) (b) and Co(II) ions (c). (d) Effect of the pH value on zeta potential (initial concentration 100 mg·L–1, pH 2–8, adsorbent 10–45 mg, shaking rate 120 rpm, 25 °C).
2.2.2. Effect of Contact Time and Adsorption Kinetics
In Figure 5, the effect of contact time for Co(II) and Pb(II) ion adsorption of Fe3O4@S-S/PMAA was evaluated. As shown in Figure 5a,b, the adsorption capacities of Fe3O4@S-S/PMAA nanoparticles increased rapidly in the first 150 min for Co(II) and Pb(II) ion adsorption and then reached the adsorption equilibrium until the end of the measurement. The maximum qe of the Fe3O4@S-S/PMAA nanoparticles for Pb(II) ion adsorption was 84.6 mg·g–1 at 180 min, which was higher than that for Co(II) ion adsorption of 47.9 mg·g–1 at 240 min. The differences between the adsorption capacities of Fe3O4@S-S/PMAA toward Co(II) and Pb(II) ion adsorption can be attributed to the different interaction mechanisms between the adsorbate and adsorbent. Therefore, the adsorption kinetics for the Co(II) and Pb(II) ion adsorption process was further analyzed by the pseudo-first-order and pseudo-second-order models. The following equations were used to analyze the linear expressions of the pseudo-first-order and pseudo-second-order models of Fe3O4@S-S/PMAA for Co(II) and Pb(II) ion adsorption
| 1 |
| 2 |
where qe and qt are the amounts of adsorbed metal ions at equilibrium and at time t (min), respectively; k1 (min–1) and k2 (g·mg–1·min–1) represent the kinetic rate constants for the pseudo-first-order and second-order models, respectively; and qe,cal (mg·g–1) represents the calculated equilibrium adsorption capacity of Co(II) and Pb(II) ions. The adsorption kinetic data were fitted to eqs 1 and 2, and Figure 5c,d shows the plots of the pseudo-first-order and pseudo-second-order kinetic models, respectively. Additionally, Table 2 describes the calculated kinetic parameters. The correlation coefficient (R2) value of the pseudo-second-order model is 0.9995 for Pb(II) ion adsorption, which is higher than that of the pseudo-first-order model (0.9943). For Co(II) ion adsorption, the R2 value of the pseudo-second-order model (0.9987) is higher than the R2 value of the pseudo-first-order model (0.9893). Based on the experimental data, the adsorption process matches well with the pseudo-second-order model, which suggests that the adsorption process for both Co(II) and Pb(II) ions on the adsorbent surface may be a rate-limiting step via chemical adsorption.
Figure 5.
Effect of the contact time on the adsorption of Fe3O4@S-S/PMAA for Pb(II) (a) and Co(II) (b) ion adsorption. Adsorption kinetic models: pseudo-first-order (c) and pseudo-second-order model (d) (initial concentration 100 mg·L–1, adsorbent 20 mg, shaking rate 120 rpm, 25 °C).
Table 2. Kinetic Parameters on the Adsorption of Fe3O4@S-S/PMAA Nanoparticles for Co(II) and Pb(II) in Different Models.
| pseudo-first-order |
pseudo-second-order |
||||||
|---|---|---|---|---|---|---|---|
| qe,exp (mg·g–1) | qe,cal (mg·g–1) | K1 (min–1) | R2 | qe,cal (mg·g–1) | K2 (g·mg–1·min–1) | R2 | |
| Pb(II) | 84.6 | 28.6 | 0.0163 | 0.9943 | 86.6 | 0.0018 | 0.9995 |
| Co(II) | 47.9 | 39.8 | 0.0202 | 0.9893 | 51.8 | 0.0008 | 0.9987 |
2.2.3. Adsorption Isotherms
As most commonly employed in the isothermal model study, herein, the Langmuir and Freundlich adsorption models were used to analyze the isothermal adsorption of Fe3O4@S-S/PMAA nanoparticles for Co(II) and Pb(II) ion adsorption. As shown in Figure 6a,b, the concentration of the adsorbed amounts for Co(II) and Pb(II) ion adsorption was shown as a function of the equilibrium concentrations. In addition, the adsorption isotherms of Fe3O4@S-S/PMAA nanoparticles for Co(II) and Pb(II) ion adsorption evaluated by the Langmuir and Freundlich adsorption models are shown in Figure 6c,d. Table 3 lists all parameters of the Langmuir and Freundlich constants. The following equations were used to describe the Langmuir and Freundlich adsorption models
| 3 |
| 4 |
where ce is the concentration of the adsorbate at equilibrium, qe is the concentration of the adsorbed amount in the equilibrated solution, qm (mg·g–1) is the theoretical maximum sorption capacity, KL (L·mg–1) is the Langmuir sorption equilibrium constant that represents the affinity of the adsorbate and adsorbent, KF [(mg·g–1)(L·mg–1)(1/n)] is the Freundlich constant that relates to the adsorption capacity, and n is related to the adsorption intensity.
Figure 6.
Effect of the concentration of the adsorbate at equilibrium on the adsorption of Fe3O4@S-S/PMAA for Pb(II) (a) and (b) Co(II) ions. Equilibrium isotherm of Fe3O4@S-S/PMAA nanoparticles for Co(II) and Pb(II) ion adsorption by the Langmuir (c) and Freundlich isotherm models (d) (initial concentration 50–500 mg·L–1, adsorbent 20 mg, shaking rate 120 rpm, 25 °C).
Table 3. Adsorption Parameters of the Langmuir and Freundlich Isotherm Models.
| Langmuir |
Freundlich |
|||||
|---|---|---|---|---|---|---|
| KL (L·mg–1) | qm (mg·g–1) | R2 | KF (mg·g–1)(L·mg–1)(1/n) | n | R2 | |
| Pb(II) | 0.0043 | 543.5 | 0.9733 | 5.4 | 1.36 | 0.9962 |
| Co(II) | 0.0036 | 156.3 | 0.7840 | 2.0 | 1.56 | 0.9913 |
As shown in Table 3, the R2 value for Pb(II) adsorption by the Freundlich adsorption isotherm was 0.9962 and that for Co(II) adsorption by the Freundlich adsorption isotherm was 0.9913, which were both higher than the R2 value by Langmuir adsorption. Therefore, the Freundlich adsorption isotherm fitted better with the adsorption process of Co(II) and Pb(II) ions onto Fe3O4@S-S/PMAA nanoparticles. Moreover, a higher value of KF for Pb(II) adsorption than that of Co(II) adsorption was observed. Because the value of KF is directly proportional to the adsorption capacity, it is suggested that the adsorption capacity of Fe3O4@S-S/PMAA on Pb(II) ions is higher than that on Co(II) ions. Moreover, the theoretical maximum adsorption capacities of Fe3O4@S-S/PMAA nanoparticles for Co(II) and Pb(II) ions are 543.5 and 156.3 mg·g–1, respectively. In brief, according to the investigation of the adsorption isotherms, the Fe3O4@S-S/PMAA nanoparticles demonstrated good affinity to Pb(II) ions, resulting in a higher adsorption capacity than Co(II) adsorption.
2.2.4. Adsorption Thermodynamic Analysis
For adsorption thermodynamic analysis, the adsorption capacities of Fe3O4@S-S/PMAA on Co(II) and Pb(II) ions were studied at different temperatures. The adsorption thermodynamics was studied at the temperatures of 303, 313, and 323 K. The standard Gibbs free energy (ΔGθ, kJ·mol–1), standard enthalpy change (ΔHθ, kJ·mol–1), and standard entropy change (ΔSθ, kJ·mol–1·K–1) are calculated by the following equations
| 5 |
| 6 |
| 7 |
where Kθ is the thermodynamic equilibrium constant, Cs is the amount of metal ions adsorbed per mass (mg·g–1) of Fe3O4@S-S/PMAA, Ce is the equilibrium concentration (mg·L–1), T is the temperature in kelvin, and the ideal gas constant (R) is equal to 8.314.
Table 4 presents the calculated thermodynamic parameters by the above equations. Based on the experimental data, it was suggested that the adsorption of Co(II) and Pb(II) ions onto Fe3O4@S-S/PMAA is processing spontaneously because of the negative values of ΔGθ at different temperatures. A more spontaneous adsorption process with a more negative value of ΔGθ was observed at a lower temperature because of the negative value of ΔSθ. Additionally, the negative value of ΔSθ showed a declined randomness at the solid/liquid interface during metal-ion adsorption, and the adsorption process was exothermic because of the negative values of ΔHθ.
Table 4. Thermodynamic Data for the Adsorption of Co(II) and Pb(II) Ions on Fe3O4@S-S/PMAAa.
| ΔGθ (kJ·mol–1) |
|||||
|---|---|---|---|---|---|
| ΔHθ (kJ·mol–1) | ΔSθ (kJ·mol–1·K–1) | 303 K | 313 K | 323 K | |
| Pb(II) | –42.12 | –0.13 | –2.73 | –1.46 | –0.13 |
| Co(II) | –7.89 | –0.02 | –0.76 | –0.68 | –0.29 |
Initial concentration 50 mg·L–1, adsorbent 20 mg, shaking rate 120 rpm.
2.2.5. Adsorption Selectivity
In order to study the selective adsorption of Fe3O4@S-S/PMAA nanoparticles for Pb(II) ions, the selective adsorption experiments were investigated, as shown in Figure 7. A specific selectivity was shown with the adsorption order of Pb2+ > Co2+ > Cd2+ > Ni2+ > Cu2+ > Zn2+ > K+ > Na+ > Mg2+ > Ca2+ with interfering metal-ion tests. The adsorption capacity of Fe3O4@S-S/PMAA was 48.7 mg·g–1 for Pb(II) ion adsorption in the comparative experiments with common and toxic interfering metal ions. Table 5 presents the comparison of the adsorption capacity toward toxic metal ions on Fe3O4@S-S/PMAA with those of other reported adsorbents. It was revealed that our prepared Fe3O4@S-S/PMAA is competitive and even better in the adsorption capacity compared to other various reported adsorbents.
Figure 7.

Comparative adsorption characteristics of Fe3O4@S-S/PMAA nanoparticles for adsorption with interfering metal ions (initial concentration of each metal ion, 40 mg·L–1; adsorbent 20 mg; shaking rate 120 rpm; 25 °C).
Table 5. Comparison of Heavy-Metal-Ion Adsorption Capacity of Fe3O4@S-S/PMAA with Those of Reported Adsorbents.
| adsorption
capacity (mg·g–1) |
|||||||
|---|---|---|---|---|---|---|---|
| Adsorbents | Pb2+ | Co2+ | Zn2+ | Cd2+ | Cu2+ | Ni2+ | references |
| polypyrrole-modified magnetic Fe3O4/reduced graphene oxide composites | 60.9 | 8.4 | 30.2 | 12.1 | (28) | ||
| magnetic CA nanofibers | 44.1 | (29) | |||||
| hexadentate ligand-modified magnetic nanocomposites | 11.31 | 4.86 | 13.88 | 78.67 | 7.64 | (30) | |
| magnetic polydopamine-coated reduced graphene oxide composite | 35.2 | (31) | |||||
| lead-ion-imprinted magnetic biosorbent | 69.15 | (32) | |||||
| carboxymethyl–cyclodextrin polymer-modified Fe3O4 nanoparticle | 64.5 | 27.7 | 13.2 | (33) | |||
| magnetic ion-imprinted and −SH-functionalized polymer | 32.58 | (34) | |||||
| Fe3O4@S-S/PMAA | 48.7 | 25.1 | 18.1 | 22.4 | 19.9 | 21.5 | this work |
2.3. X-ray Photoelectron Spectroscopy Analysis
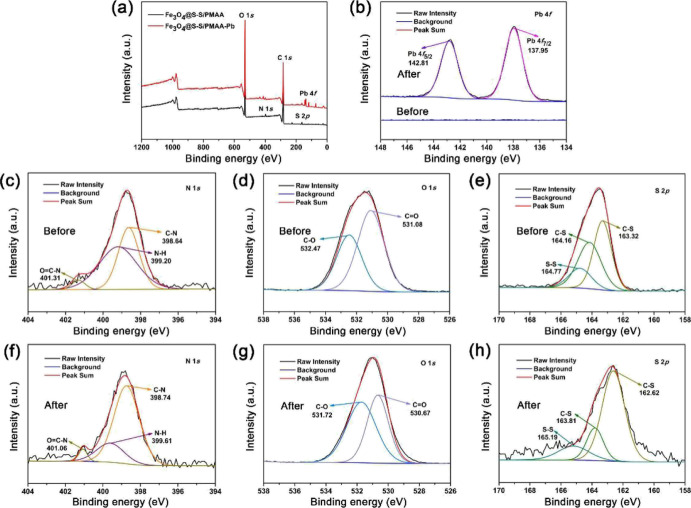
To investigate the adsorption mechanism of the as-prepared magnetic nanoadsorbent, the X-ray photoelectron spectroscopy (XPS) spectra of Fe3O4@S-S/PMAA and that with adsorbed Pb(II) ions (Fe3O4@S-S/PMAA-Pb) were recorded, as shown in Figure 8. Figure 8a shows the full-survey scan spectra of the samples, and the peaks can be assigned to C, N, O, and S atoms which exist in the polymer network outlayer of Fe3O4@S-S/PMAA and Fe3O4@S-S/PMAA-Pb. As shown in Figure 8b, after adsorption for Pb(II) ions, the bonding energies of Pb(II) ions 4f7/2 and Pb 4f5/2 shifted to the lower values of 137.95 and 142.81 eV compared to the bonding energies of 139.4 and 144.3 eV for the free Pb(II) ions, respectively.35 The existence of adsorbed Pb(II) ions on the surface of Fe3O4@S-S/PMAA is suggested. In Figure 8c, the peaks for N 1s at 398.64, 399.20, and 401.31 eV can be assigned to the C–N, N–H, and O=C–N groups in the polymer network, respectively. After adsorption for Pb(II) ions, these characteristic peaks shifted to the higher values at 398.74, 399.61, and 401.06 eV in the sample of Fe3O4@S-S/PMAA-Pb (as shown in Figure 8f). The blue shift for N 1s can be explained by the chemical bonding between the N atoms and the Pb(II) ions. Additionally, as shown in Figure 8d,g, after adsorption for Pb(II) ions, the bonding energies of O 1s at 531.08 and 532.47 eV corresponding to the C=O and C–O groups, respectively, shifted to lower values of 530.67 and 531.72 eV, respectively, suggesting the interaction between the O atoms and the Pb(II) ions. In Figure 8e,h, the peaks at 164.77 eV attributed to the S–S bond shifted to a higher value of 165.19 eV, which can be explained by the electron transmission form the disulfide bond, suggesting the chemical bond interaction between Pb(II) ions and the S–S bond. Based on the above experimental results, the Pb(II) ions are considered as the Lewis soft acid, and N, O, and S atoms in chemical groups such as C–N, N–H, O=C–N, C=O, C–O, and S–S in the as-prepared Fe3O4@S-S/PMAA can serve as the Lewis soft base, which can form chemical bonds with the Pb(II) ions on the basis of HSAB theory and facilitate the selective adsorption for Pb(II) ion removal in water solutions.
Figure 8.
(a) XPS full-survey scan spectra of Fe3O4@S-S/PMAA and ion-adsorbed composites Fe3O4@S-S/PMAA-Pb; (b) high-resolution Pb 4f spectra of Fe3O4@S-S/PMAA and Fe3O4@S-S/PMAA-Pb; (c–e) high-resolution N 1s, O 1s, and S 2p spectra of Fe3O4@S-S/PMAA, respectively. (f–h) High-resolution N 1s, O 1s, and S 2p spectra of Fe3O4@S-S/PMAA-Pb, respectively.
2.4. Adsorption–Desorption Experiments and Reusability of Fe3O4@S-S/PMAA Nanoparticles
To evaluate the reusability of the Fe3O4@S-S/PMAA nanoparticles, adsorption–desorption experiments were performed toward Co(II) and Pb(II) ions. Typically, 20 mg of the adsorbent was added into 25 mL of the Pb(II) or Co(II) solution by using a thermostat orbital shaker for 12 h. After magnetic separation, the Fe3O4@S-S/PMAA nanoparticles were then regenerated by using 0.5 M HCl as the eluent, and subsequently, several adsorption–desorption cycle experiments were carried out until there was an obvious decline in the adsorption capacity. The removal efficiency of Fe3O4@S-S/PMAA in the adsorption–desorption experiments is shown in Figure 9. At the first round, the removal efficiencies of Co(II) and Pb(II) ions were 98.3 and 81.3%, respectively; after eight cycles, high removal efficiencies of 75.1 and 58.4% for Pb(II) and Co(II) ion adsorption were respectively observed. It is suggested that Fe3O4@S-S/PMAA exhibits excellent reusability, which can be cyclically used as a potential adsorbent for heavy metal removal in actual polluted water.
Figure 9.

Effect of cycle number on the adsorption capacity of Fe3O4@S-S/PMAA nanoparticles for Co(II) and Pb(II) ions (initial concentration of each metal ion, 50 mg·L–1; adsorbent 20 mg; shaking rate 120 rpm; 25 °C).
3. Conclusions
The novel disulfide cross-linked poly(methacrylic acid) iron oxide, Fe3O4@S-S/PMAA, nanoparticles were prepared and utilized in selective adsorption of Pb(II) ions. The adsorption process matched well with the pseudo-second-order kinetic equation and the Freundlich isotherm model. Remarkably, Fe3O4@S-S/PMAA demonstrated a specific selectivity for Pb(II) ion adsorption in the selective adsorption experiments with interfering metal ions, which is competitive compared to various reported adsorbents. In eight adsorption–desorption cycles, the as-prepared Fe3O4@S-S/PMAA nanoparticles show high removal efficiency toward Pb(II) ions. In conclusion, the as-prepared Fe3O4@S-S/PMAA nanoparticles have excellent selectivity, efficient adsorption capacity, and improved reusability for potential use in the removal of heavy metal ions from wastewater.
4. Experimental Section
4.1. Chemicals and Materials
All the solid reagents are of analytical grade and used as received, including iron trichloride (98%, Chengdu Jinshan Chemical Reagent, China), ferrous chloride (98%, Chengdu Jinshan Chemical Reagent, China), 3-(trimethoxysilyl)propyl methacrylate (MPS, 99%, Best Reagent, China), citric acid (98%, Chengdu Jinshan Chemical Reagent, China), cystamine dihydrochloride (96%, J&K Chemical, China), acryloyl chloride (98%, J&K Chemical, China), methacrylic acid (MAA, 99%, Chengdu Cologne Chemicals Co.. Ltd., China), and 2,2-azobisisobutyronitrile (AIBN, 99%, Kemio Chemical Reagent Ltd., China). All the solvents are commercially available and used after purification, including ethylene acetate (99%, Chengdu Jinshan Chemical Reagent, China), dichloromethane (DCM, 99%, Chengdu Jinshan Chemical Reagent Co., Ltd., China), and acetonitrile (ACN, Tianjin Zhiyuan Chemical Reagent Co., Ltd., China).
4.1.1. Preparation of Fe3O4@MPS Nanoparticles
According to an already published procedure,36 60 mL of deionized water was used to dissolve FeCl3·6H2O (2.7 g, 15.5 mmol) and FeCl2·4H2O (1.0 g, 15.2 mmol). The synthesis procedure was under N2 protection, and ammonia solution was added into the mixture with vigorous mechanical stirring at 80 °C for half an hour. Citric acid was added into the mixture at 90 °C, and the mixture was stirred for another 90 min and then cooled to room temperature. The solid products were collected by magnetic separation following a vacuum drying procedure. A mixture of ethanol and deionized water was used to dissolve the as-prepared Fe3O4 nanoparticles by ultrasonication and then transferred into a three-neck flask. After bubbling with N2 for 30 min, the three-neck flask was connected with a condensing reflux device. 3 mL of MPS was added dropwise into the three-neck flask in an oil bath with vigorous mechanical stirring at 60 °C. After the reaction proceeded for 8 h, the Fe3O4@MPS nanoparticles were obtained by centrifugation following a vacuum drying procedure.
4.1.2. Preparation of the Fe3O4@S-S/PMAA Nanoparticles
BACy was synthesized following an already published procedure.37 Cystamine dihydrochloride was dissolved in deionized water, and acryloyl chloridein was dissolved in 4 mL of DCM. The above solutions were transferred into a three-necked flask, and NaOH solution (40 mM) was slowly added into the three-necked flask within 5 min, which was carried out in an ice–water bath with magnetic stirring for 4 h. After that, the product in the organic phase was extracted with DCM. The solid product was obtained via a recrystallization procedure following a vacuum drying procedure. For the preparation of Fe3O4@S-S/PMAA nanoparticles, 0.15 g of BACy in 30 mL of ACN, 0.20 g of the as-prepared Fe3O4@ Fe3O4@MPS nanoparticles, 3 mL of MAA, and 20 mg of AIBN were all added into a three-necked flask. The reaction then proceeded with vigorous mechanical stirring at 80 °C for 4 h under a nitrogen atmosphere. After that, Fe3O4@S-S/PMAA was obtained via centrifugation following a vacuum drying procedure. To ensure repeatability, the purity of chemical agents, mole ratio of monomers, reaction temperature, and vacuum degree should be precisely controlled. FT-IR measurements were used to monitor the chemical structure of the nanocomposites, and DLS, SEM, and TEM were utilized to investigate the nanosized morphology for ensuring the experimental repeatability.
4.2. Characterization
The 1H NMR spectrum was used to characterize the chemical structure of BACy in DMSO. The FT-IR spectra of the sample were recoreded in the solid state ranging from 400 to 4000 cm–1 with a scan speed of 4 cm–1/S at room temperature. N2 adsorption–desorption isotherms were used to analyze the Brunauer–Emmett–Teller (BET) surface areas and pore size distributions of each sample using a nitrogen adsorption apparatus (BET, Autosorb-iQ, Quantachrome, USA). Micromorphologies of the samples were observed by SEM (FEI Inspect F50, USA) and TEM (Hitachi H-600, Japan). The nanosize distributions and zeta potential of the samples were investigated by DLS (Malvern Zetasizer Nano ZS90, UK) at the concentration of 1 mg·mL–1 under ambient temperature. Powder XRD patterns were obtained on a Persee XD-6 diffractometer using Cu Kα radiation (λ = 0.154 nm) with an accelerating voltage of 36 kV. Magnetic behaviors of samples were investigated by VSM (PPMS-9, Quantum Design Company, USA). A thermogravimetric analyzer (NETZSCH STA 449 C, Germany) was used to evaluate the thermo-oxidative degradation behaviors of the samples ranging from 20 to 900 °C. The elemental chemical states on the surface of the samples were measured by XPS (ESCALAB 250Xi, USA).
4.3. Adsorption Studies
The adsorption activities of as-prepared Fe3O4@S-S/PMAA were investigated by choosing Pb(II) and Co(II) as the adsorption targets. Batch adsorption experiments were performed to investigate the adsorption behavior of the magnetic nanoparticles upon the effect of different conditions such as pH, amount of adsorbent, and temperature. Additionally, adsorption isotherm experiments were carried out by setting the Pb(II) or Co(II) solution at different concentrations, and the adsorption kinetics toward Pb(II) or Co(II) was investigated at different time intervals. All the adsorption experiments were carried out using a thermostat orbital shaker at the speed of 120 rpm. The Pb(II) and Co(II) ion concentrations were measured by the inductively coupled plasma mass spectrometry (ICP–MS) after the magnetic adsorbents were magnetically separated. Adsorption capacities (qe, mg·g–1) were calculated by the following equation
| 8 |
where C0 and Ce are the initial and equilibrium concentrations of the metal ion, respectively, V is the volume of the solution, and m represents the weight of the adsorbent.
The adsorption kinetics of Pb(II) and Co(II) adsorption was investigated by the pseudo-first-order and pseudo-second-order models, and adsorption isotherms were studied with the Langmuir and Freundlich models. Adsorption thermodynamics of Pb(II) and Co(II) for Fe3O4@S-S/PMAA nanoparticle adsorption was investigated at different temperatures. The selective adsorption tests were carried out with a mixture solution containing Pb(II), Cu(II), Zn(II), Cd(II), Co(II), Ni(II), Ca(II), Mg(II), Na(I), and K(I) ions. Each metal ion was set to 40 ppm, and 20 mg of the adsorbent was then dispersed in the mixture solution. All the adsorption experiments were carried out using a thermostat orbital shaker at the speed of 120 rpm. The equilibrium concentrations of metal ions in the solution were measured by ICP–MS. The data of all of the treatment groups are presented as mean values ± standard deviation. The equilibrium concentrations of Pb(II) and Co(II) were analyzed by ICP–MS. The preparation process and selective removal of Pb(II) ions are illustrated in Scheme 1.
4.4. Adsorption–Desorption and Recycling Experiments
For adsorption–desorption and recycling experiments, the magnetic adsorbents were obtained by magnetic separation after achieving saturated Pb(II) or Co(II) adsorption and then washed by HCl (0.5 M) to remove the adsorbed metal ions. Next, the adsorption cycle was subsequently performed by using the regenerated magnetic adsorbents. Several cycles were carried out until an obvious decline occurred in the adsorption capacity for metal ions.
Acknowledgments
This work was financially supported by the Sichuan Science and Technology Program (18YYJC0265) and the Fundamental Research Funds for the Central Universities of Southwest Minzu University (2018NZD07).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c05623.
1H NMR spectrum of BACy in DMSO and nanosized distribution of Fe3O4, Fe3O4@MPS, and Fe3O4@S-S/PMAA nanoparticles by DLS measurements (PDF)
Author Contributions
∥ R.W. and J.L. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Bojdi M. K.; Mashhadizadeh M. H.; Behbahani M.; Farahani A.; Davarani S. S. H.; Bagheri A. Synthesis, Characterization and Application of Novel Lead Imprinted Polymer Nanoparticles as a High Selective Electrochemical Sensor for Ultra-trace Determination of Lead Ions in Complex Matrixes. Electrochim. Acta 2014, 136, 59–65. 10.1016/j.electacta.2014.05.095. [DOI] [Google Scholar]
- Quan X.; Sun Z.; Meng H.; Han Y.; Wu J.; Xu J.; Xu Y.; Zhang X. Surface Functionalization of MIL-101(Cr) by Aminated Mesoporous Silica and Improved Adsorption Selectivity Toward Special Metal Ions. Dalton Trans. 2019, 48, 5384–5396. 10.1039/c9dt00501c. [DOI] [PubMed] [Google Scholar]
- Deng S.; Liu X.; Liao J.; Lin H.; Liu F. PEI Modified Multiwalled Carbon Nanotube as a Novel Additive in PAN Nanofiber Membrane for Enhanced Removal of Heavy Metal Ions. Chem. Eng. J. 2019, 375, 122086. 10.1016/j.cej.2019.122086. [DOI] [Google Scholar]
- Bao S.; Li K.; Ning P.; Peng J.; Jin X.; Tang L. Synthesis of Amino-functionalization Magnetic Multi-metal Organic Framework (Fe3O4/MIL-101(Al0.9Fe0.1)/NH2) for Efficient Removal of Methyl Orange from Aqueous Solution. J. Taiwan Inst. Chem. Eng. 2018, 87, 64–72. 10.1016/j.jtice.2018.03.009. [DOI] [Google Scholar]
- Xu G.; Wang L.; Xie Y.; Tao M.; Zhang W. Highly Selective and Efficient Adsorption of Hg2+ by a Recyclable Aminophosphonic Acid Functionalized Polyacrylonitrile Fiber. J. Hazard. Mater. 2018, 344, 679–688. 10.1016/j.jhazmat.2017.11.017. [DOI] [PubMed] [Google Scholar]
- Saravaia H.; Gupta H.; Popat P.; Sodha P.; Kulshrestha V. Single Step Synthesis of Magnesium Doped Lithium Manganese Oxide Nanosorbent and their Polymer Composite Beads for Selective Heavy Metals Removal. ACS Appl. Mater. Interfaces 2018, 10, 44059–44070. 10.1021/acsami.8b17141. [DOI] [PubMed] [Google Scholar]
- Li Z.; Xiao D.; Ge Y.; Koehler S. Surface Functionalized Porous Lignin for Fast and Efficient Lead Removal from Aqueous Solution. ACS Appl. Mater. Interfaces 2015, 7, 15000–15009. 10.1021/acsami.5b03994. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Ni S.; Wang X.; Zhang W.; Lagerquist L.; Qin M.; Willför S.; Xu C.; Fatehi P. Ultrafast Adsorption of Heavy Metal Ions onto Functionalized Lignin-based Hybrid Magnetic Nanoparticles. Chem. Eng. J. 2019, 372, 82–91. 10.1016/j.cej.2019.04.111. [DOI] [Google Scholar]
- Zou L.; Shao P.; Zhang K.; Yang L.; You D.; Shi H.; Pavlostathis S. G.; Lai W.; Liang D.; Luo X. Tannic Acid-based Adsorbent with Superior Selectivity for Lead(II) Capture: Adsorption Site and Selective Mechanism. Chem. Eng. J. 2019, 364, 160–166. 10.1016/j.cej.2019.01.160. [DOI] [Google Scholar]
- Hao L.; Song H.; Zhang L.; Wan X.; Tang Y.; Lv Y. SiO2/Graphene Composite for Highly Selective Adsorption of Pb(II) Ion. J. Colloid Interface Sci. 2012, 369, 381–387. 10.1016/j.jcis.2011.12.023. [DOI] [PubMed] [Google Scholar]
- Song Y.; Wang N.; Yang L.-Y.; Wang Y. g.; Yu D.; Ouyang X.-K. Facile Fabrication of ZIF-8/Calcium Alginate Microparticles for Highly Efficient Adsorption of Pb(II) from Aqueous Solutions. Ind. Eng. Chem. Res. 2019, 58, 6394–6401. 10.1021/acs.iecr.8b05879. [DOI] [Google Scholar]
- Uluozlu O. D.; Tuzen M.; Mendil D.; Soylak M. Coprecipitation of Trace Elements with Ni2+/2-Nitroso-1-naphthol-4-sulfonic Acid and Their Determination by Flame Atomic Absorption Spectrometry. J. Hazard. Mater. 2010, 176, 1032–1037. 10.1016/j.jhazmat.2009.11.144. [DOI] [PubMed] [Google Scholar]
- Liu C.; Jin R.-N.; Ouyang X.-k.; Wang Y.-G. Adsorption Behavior of Carboxylated Cellulose Nanocrystal-polyethyleneimine Composite for Removal of Cr(VI) Ions. Appl. Surf. Sci. 2017, 408, 77–87. 10.1016/j.apsusc.2017.02.265. [DOI] [Google Scholar]
- Seoane B.; Téllez C.; Coronas J.; Staudt C. NH2-MIL-53(Al) and NH2-MIL-101(Al) in Sulfur-containing Copolyimide Mixed Matrix Membranes for Gas Separation. Sep. Purif. Technol. 2013, 111, 72–81. 10.1016/j.seppur.2013.03.034. [DOI] [Google Scholar]
- Zhang Y.; Xiong Y.; Tang Y.; Wang Y. Degradation of Organic Pollutants by an Integrated Photo-Fenton-like Catalysis/Immersed Membrane Separation System. J. Hazard. Mater. 2013, 244–245, 758–764. 10.1016/j.jhazmat.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Shi S.; Zhu W.; Yang C.; Li S.; Liu X.; Hu N.; Huang L.; Wang R.; Suo Y.; Li Z.; Wang J. In-Situ Fixation of All-Inorganic Mo-Fe-S Clusters for Highly Selective Removal of Lead(II). ACS Appl. Mater. Interfaces 2017, 9, 32720–32726. 10.1021/acsami.7b08967. [DOI] [PubMed] [Google Scholar]
- Sun W.; Wu H.; Xu Z.; Li C.; Qian X.; Chen L. Adsorption of Heavy Metal Ions by Carbon-Nanofibers-Blended Carbon Nanotubes. ChemistrySelect 2018, 3, 12410–12414. 10.1002/slct.201800203. [DOI] [Google Scholar]
- Fu Y.; Liu X.; Chen G. Adsorption of Heavy Metal Sewage on Nano-materials such as Titanate/TiO2 Added Lignin. Results Phys. 2019, 12, 405–411. 10.1016/j.rinp.2018.11.084. [DOI] [Google Scholar]
- Liu D.; Ding C.; Chi F.; Pan N.; Wen J.; Xiong J.; Hu S. Polymer Brushes on Graphene Oxide for Efficient Adsorption of Heavy Metal Ions from Water. J. Appl. Polym. Sci. 2019, 136, 48156. 10.1002/app.48156. [DOI] [Google Scholar]
- Wang R.; Chen K.; Peng S.; Wang Q.-Y.; Huang S.-H.; Zhou Q.-H.; Wang J.; Lin J. Environmentally Friendly Magnetic Nanoparticles for Efficient Removal of Methylene Blue and Cr(VI) from Water. NANO 2020, 15, 2050126. 10.1142/s179329202050126x. [DOI] [Google Scholar]
- Puspitasari T.; Kadja G. T. M.; Radiman C. L.; Darwis D.; Mukti R. R. Two-step Preparation of Amidoxime-functionalized Natural Zeolites Hybrids for The Removal of Pb Ions in Aqueous Environment. Mater. Chem. Phys. 2018, 216, 197–205. 10.1016/j.matchemphys.2018.05.083. [DOI] [Google Scholar]
- Tang J.; Lv H.; Gong Y.; Huang Y. Preparation and Characterization of A Novel Graphene/Biochar Composite for Aqueous Phenanthrene and Mercury Removal. Bioresour. Technol. 2015, 196, 355–363. 10.1016/j.biortech.2015.07.047. [DOI] [PubMed] [Google Scholar]
- Lu M. Adsorption of Cd2+ and Zn2+ on Carboxylated Polyvinyl Alcohol. ChemistrySelect 2020, 5, 6894–6898. 10.1002/slct.202001569. [DOI] [Google Scholar]
- Bo S.; Luo J.; An Q.; Xiao Z.; Wang H.; Cai W.; Zhai S.; Li Z. Efficiently Selective Adsorption of Pb(II) with Functionalized Alginate-based Adsorbent in Batch/Column Systems: Mechanism and Application Simulation. J. Cleaner Prod. 2020, 250, 119585. 10.1016/j.jclepro.2019.119585. [DOI] [Google Scholar]
- Fu W.; Huang Z. One-pot Synthesis of Two-dimensional Porous Fe3O4/poly (C3N3S3) Network Nanocomposite for Selective Removal of Pb(II) and Hg(II) from Synthetic Wastewater. ACS Sustainable Chem. Eng. 2018, 6, 14785–14794. 10.1021/acssuschemeng.8b03320. [DOI] [Google Scholar]
- Peng S.; Wang Q. y.; Xiao X.; Wang R.; Lin J.; Zhou Q. h.; Wu L. n. Redox-responsive Polyethyleneimine-coated Magnetic Iron Oxide Nanoparticles for Controllable Gene Delivery and Magnetic Resonance Imaging. Polym. Int. 2020, 69, 206–214. 10.1002/pi.5943. [DOI] [Google Scholar]
- Wei J.; Yang Z.; Sun Y.; Wang C.; Fan J.; Kang G.; Zhang R.; Dong X.; Li Y. Nanocellulose-based Magnetic Hybrid Aerogel for Adsorption of Heavy Metal Ions from Water. J. Mater. Sci. 2019, 54, 6709–6718. 10.1007/s10853-019-03322-0. [DOI] [Google Scholar]
- Liu Z.; Gao Z.; Xu L.; Hu F. Polypyrrole Modified Magnetic Reduced Graphene Oxide Composites: Synthesis, Characterization and Application for Selective Lead Adsorption. RSC Adv. 2020, 10, 17524–17533. 10.1039/d0ra01546f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby T. I.; El-Kady M. F.; Zaki A. E. H. M.; El-Kholy S. M. Preparation and Application of Magnetite Nanoparticles Immobilized on Cellulose Acetate Nanofibers for Lead Removal from Polluted Water. Water Sci. Technol.: Water Supply 2017, 17, 176–187. 10.2166/ws.2016.124. [DOI] [Google Scholar]
- Sahoo J. K.; Kumar A.; Rout L.; Rath J.; Dash P.; Sahoo H. An Investigation of Heavy Metal Adsorption by Hexa-dentate Ligand-modified Magnetic Nanocomposites. Sep. Sci. Technol. 2018, 53, 863–876. 10.1080/01496395.2017.1406950. [DOI] [Google Scholar]
- Mehdinia A.; Heydari S.; Jabbari A. Synthesis and Characterization of Reduced Graphene Oxide-Fe3O4@polydopamine and Application for Adsorption of Lead Ions: Isotherm and Kinetic Studies. Mater. Chem. Phys. 2020, 239, 121964. 10.1016/j.matchemphys.2019.121964. [DOI] [Google Scholar]
- He Y.; Xiao W.; Li G.; Yang F.; Wu P.; Yang T.; Chen C.; Ding P. A Novel Lead-ion-imprinted Magnetic Biosorbent: Preparation, Optimization and Characterization. Environ. Technol. 2019, 40, 499–507. 10.1080/09593330.2017.1397762. [DOI] [PubMed] [Google Scholar]
- Badruddoza A. Z. M.; Shawon Z. B. Z.; Tay W. J. D.; Hidajat K.; Uddin M. S. Fe3O4/Cyclodextrin Polymer Nanocomposites for Selective Heavy Metals Removal from Industrial Wastewater. Carbohydr. Polym. 2013, 91, 322–332. 10.1016/j.carbpol.2012.08.030. [DOI] [PubMed] [Google Scholar]
- Guo B.; Deng F.; Zhao Y.; Luo X.; Luo S.; Au C. Magnetic Ion-imprinted and −SH Functionalized Polymer for Selective Removal of Pb(II) from Aqueous Samples. Appl. Surf. Sci. 2014, 292, 438–446. 10.1016/j.apsusc.2013.11.156. [DOI] [Google Scholar]
- Ma Y.; Lv L.; Guo Y.; Fu Y.; Shao Q.; Wu T.; Guo S.; Sun K.; Guo X.; Wujcik E. K.; Guo Z. Porous Lignin Based Poly (Acrylic Acid)/Organo-montmorillonite Nanocomposites: Swelling Behaviors and Rapid Removal of Pb(II) Ions. Polymer 2017, 128, 12–23. 10.1016/j.polymer.2017.09.009. [DOI] [Google Scholar]
- Qu J.; Tian Z.; Wang Q.; Peng S.; Luo J.-B.; Zhou Q.-H.; Lin J. Surface Design and Preparation of Multi-functional Magnetic Nanoparticles for Cancer Cell Targeting, Therapy, and Imaging. RSC Adv. 2018, 8, 35437–35447. 10.1039/c8ra06718j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J.; Wang R.; Peng S.; Shi M.; Yang S.-T.; Luo J.-B.; Lin J.; Zhou Q.-H. Stepwise Dual pH and Redox-responsive Crosslinked Polypeptide Nanoparticles for Enhanced Cellular Uptake and Effective Cancer Therapy. J. Mater. Chem. B 2019, 7, 7129–7140. 10.1039/c9tb01773a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.