Abstract
Objective:
The treatment of hypertension requires special attention because of comorbidities and polypharmacy. In a previous study, polypharmacy has been associated with a high risk of drug-related problems (DRPs). This study aimed to analyze DRPs in Indonesian hypertensive patients focusing on drug therapy effectiveness and adverse drug reactions.
Methods:
A cross-sectional study was conducted using medical records' data, prescriptions, and nursing records to observe DRPs that occurred in outpatients with hypertension from February to April 2019. A total of 114 outpatients aged ≥23 years with a primary diagnosis of primary hypertension were included in this study. DRPs were reviewed based on literature, recent guidelines, and drug interaction software. Classification DRPs were done using Indonesian-translated Pharmaceutical Care Network Europe V6.02. The data obtained were analyzed using univariate descriptive analysis.
Findings:
Of all participants, 65 (57%) outpatients were found to have DRPs related to treatment effectiveness (54 cases) and adverse drug reactions (36 cases). The primary cause of the problems was an inappropriate drug (94.14%) and dose selection (2.86%). Potential drug interactions were found high (62.14%) in the combination of an antihypertensive agent with other drugs among patients. Overprescribing drugs without clear indications, untreated indications, and subtherapeutic dosage were also reported in this study.
Conclusion:
A significant percentage of outpatients being treated for hypertension experienced DRPs. The role of clinical pharmacists and physicians in monitoring drug therapy needs to be prioritized to prevent and resolve DRPs in outpatients with hypertension.
Keywords: Drug-related problems, hypertension, outpatient, Pharmaceutical Care Network Europe V6.02
INTRODUCTION
The American College of Cardiology 2017 defines hypertension as systolic blood pressure above 130 mmHg and diastolic blood pressure of 80 mmHg measured in ≥2 careful readings obtained on ≥2 occasions.[1] The prevalence of hypertension in Indonesians >18 years old increased by 8.3%–34.1% from 2013 to 2018.[2] Hypertension can be a complication for hemorrhagic stroke, ischemic stroke, myocardial infarction, sudden death, heart failure, peripheral arterial disease, cognitive decline, and dementia.[3] With multiple comorbidities, hypertension patients also tend to experience polypharmacy, a factor that has been associated with the high risk of drug-related problems (DRPs).[4,5]
The Pharmaceutical Care Network Europe (PCNE) defines a DRP as an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes. DRPs are divided into four domains: treatment effectiveness, adverse drug reactions, treatment costs, and others.[6] One of the classifications that are often used by researchers and health workers in the pharmacy area is PCNE. Comparing with other DRP classification systems, PCNE can provide a more structured evaluation for optimizing medicines and improving health service.[7] It can identify DRPs from many prospective, including the problems itself, causes, interventions, and outcomes of interventions. In Indonesia, the PCNE mostly used is the PCNE V6.02 classification system since it has been validated in the local language and recommended by the Ministry of Health of the Republic of Indonesia.
DRPs in outpatients with hypertension have been previously investigated at Adama Hospital Medical College and were found to have occurred in 155 patients. A total of 93 (57.4%) patients experienced 1 DRP, 57 (36.1%) patients – 2 DRPs, and 5 (6.5%) patients – 3 DRPs. The most common drug interactions in outpatients with hypertension were of moderate (88.4%) and minor (8.9%) severity, with significant drug interactions occurring in only 2.7% of the outpatients.[8]
Study about DRPs in patient with hypertension has been reported before at four primary health centers in Medan, Indonesia. Among 66 patients who experienced DRPs, 31 (47%) did not receive drug therapy for their indication, 14 (21.2%) received ineffective drugs, 9 (13.6%) did not comply with their medication requirements, 6 (9.1%) experienced adverse drug reactions, 5 (7.6%) received drug therapy without an indication, and 1 (1.5%) received too low drug dose.[9]
We chose to investigate DRPs associated with hypertension because this disease affects the highest percentage of patients seen at Koja District Hospital, Jakarta, and there has never been a study to evaluate DRPs in outpatients with hypertension at a district hospital. The study aims to analyze DRPs described in the PCNE guidelines version V6.02 in Koja District Hospital outpatients with hypertension treated from February to April 2019.
METHODS
This research was a cross-sectional study and had been approved by the Jakarta Utara Center of research permission with number 037/16.1/31.72/-1.862.9/2019. This study was conducted using secondary data obtained from medical records, prescriptions, and nursing records of outpatient hypertension patients at Koja District Hospital, Jakarta, Indonesia, from February to April 2019. The inclusion criteria were outpatients with primary hypertension as the principal diagnosis, those who assigned the ICD X code with I10 code, those aged ≥23 years, and those who were admitted to hospital as an outpatient in period February 25–April 25, 2019. The exclusion criteria were patients with incomplete or unreadable medical and nursing records (no data on gender, age, diagnosis, and medication) and patients who had not been prescribed any antihypertensive medication by a physician. To prevent the selection bias, all of the outpatients who met the criteria were captured in this study.
Demographic and medication data were obtained from the prescriptions, patient medical records, and nursing records. The data collected were medical record number, gender, age, diagnosis, patient complaints, duration of treatment and treatment status, drugs used (name of the drug, dosage form, the strength of the drug, dosage interval, and amount of medication), blood pressure, and clinical and laboratory data.
The researchers identified the existence of a DRP using the PCNE V6.02[6] that has been translated in Indonesian language by focusing on two domains, which were treatment effectiveness (P1) and adverse drug reactions (P2). The domain of problems with the treatment effectiveness of the therapy was divided into four subdomains: therapy failure (P1.1), the nonoptimal effect of drug treatment (P1.2), the wrong effect of drug treatment (P1.3), and untreated indication (P1.4). The domain of adverse reactions (ARs) was divided into three subdomains: nonallergic (P2.1), allergic (P2.2), and toxic adverse drug events (P2.3). The DRPs were also classified into the domain of causes, which was divided into the subdomains of drug selection, drug form, dose selection, treatment duration, dispensing, drug use/administration process, logistics related, patient related, and others.
Medication review was conducted based on the literature, national, and international guidelines. The potential drug interactions were analyzed using recent updated IBM Micromedex.
Univariate descriptive analysis was performed using Microsoft Excel to determine the frequency distribution of patient characteristics according to the demographic factors and clinical characteristics of patients, such as type of hypertension, medications prescribed, patients' complaints, DRPs, and also the causes of DRPs.
RESULTS
A total of 150 outpatients with hypertension who were treated in Koja District Hospital between February and April 2019 were initially considered for inclusion in the study. After applying the inclusion and exclusion criteria, 114 patients were included in the research and 36 patients were excluded because of incomplete medical and nursing records.
Most of the participants in the study were between 53 and 62 years old (46%) and female (68%). The type of hypertension of analysis patients was varied from prehypertension, stage 1, and stage 2 hypertension. The staging of hypertension was classified using Seventh Joint National Committee (JNC 7) for the systole blood pressure (SBP) and diastole blood pressure (DBP) (prehypertension SBP: 120–139 mmHg and DBP: 80–99 mmHg; Stage 1 hypertension SBP 140–159 mmHg and DBP 90–99 mmHg; and Stage 2 hypertension SBP ≥160 mmHg and DBP ≥100 mmHg). The most frequently used hypertension medications by participants were calcium channel blockers (85.09%) and angiotensin-converting enzyme (ACE) inhibitors (42.98%), with amlodipine 5 mg or 10 mg once daily being the most prescribed. The characteristics of the study participants, including age, gender, the type of hypertension, and the medication lowering blood pressure used by participants, are shown in Table 1.
Table 1.
Demographical characteristics of studied patients
| Patients’ characteristics | Number of patients (%) (n=114) |
|---|---|
| Age (year) | |
| 23-32 | 2 (1.75) |
| 33-42 | 3 (2.63) |
| 43-52 | 22 (19.30) |
| 53-62 | 46 (40.35) |
| 63-72 | 33 (28.95) |
| >72 | 8 (7.02) |
| Gender | |
| Male | 46 (40.35) |
| Female | 68 (59.65) |
| Stage of hypertension | |
| Prehypertension | 32 (28.07) |
| Stage 1 hypertension | 36 (31.58) |
| Stage 2 hypertension | 46 (40.35) |
| Used antihypertensive drug | |
| Calcium antagonist | 97 (85.09) |
| ACE inhibitor | 49 (42.98) |
| Beta-blocker | 40 (35.09) |
| Central alpha-agonist | 10 (8.77) |
ACE: Angiotensin-converting enzyme
The study showed that DRPs were found in 65 (57%) outpatients with hypertension in Koja District Hospital. The primary DRPs found were related to treatment effectiveness (54 cases) and adverse reaction (36 cases) which were dominated by adverse drug event nonallergic (40%) and untreated indications (25.6%) as subdomain problems [Figure 1].
Figure 1.
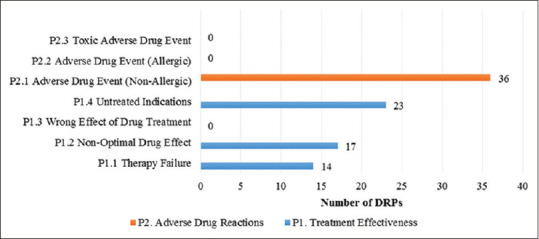
Number of drug-related problems classified using Pharmaceutical Care Network Europe V6.02
The primary cause of the DRPs was an inappropriate drug (94.14%) and dose selection (2.86%). Improper drug selection was described as no indication for drugs, inappropriate combination of drugs, indication for drug note notice, and a new indication for drug treatment presented. Potential drug interactions were found high (62.14%) in the combination of an antihypertensive agent with other symptomatic or comorbidity treatments among patients and cause the most DRPs found [Figure 2]. Antihypertensive was the most frequent contributor to the DRPs found. The list of drugs' combination that was contributed to the DRP is shown in Table 2. The study also showed that prescribers, in some situations, sometimes also gave medication for symptomatic therapy with unclear indications [Table 2].
Figure 2.
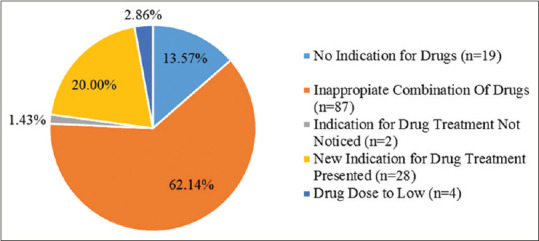
Causes of drug-related problems according to Pharmaceutical Care Network Europe V 6.02 Classification (n = 140)
Table 2.
List of drugs that mostly caused drug-related problem in outpatients with hypertension
| Drugs having potential interactions |
| Amlodipine-clopidogrel |
| Acetosal-clopidogrel |
| Amlodipine-domperidone |
| Clonidine-bisoprolol |
| Amitriptyline-meloxicam |
| Overprescribed drugs without cleared indications |
| Analgesic-anti-inflammation (potassium diclofenac and paracetamol) |
| Laxative (paraffin) |
| Antiseptic mouthwash (povidone-iodine) |
| Antiemetic (domperidone) |
| Antiulcer (lansoprazole, omeprazole, and sucralfate) |
DISCUSSION
In this current study, most of the patients with hypertension were female and above 50 years old. Factors related to a patient's gender are very influential in the occurrence of hypertension, and the risk for the development of hypertension is thought to be higher in men than in women because of lifestyles that tend to increase blood pressure. However, women >65 years old have a reduced vasodilation ability because of hormonal changes after menopause, such as reduced estrogen and reduced activation of nitric oxide and prostacyclin, which can lead to increased blood pressure.[10]
Amlodipine was the most frequently prescribed calcium antagonist in this study. Calcium antagonist drugs are used according to a therapeutic algorithm in patients with hypertension who are >60 years old with or without a combination of ACE inhibitor or angiotensin receptor blocker (ARB). The results of this study are consistent with the age of the patients; calcium antagonists were used more often in patients ≥60 years old than in younger patients and can be combined with ACE inhibitors, which were the second-most widely used drugs among the participants. When related to the number of patients based on their hypertension level, these results were also consistent because patients with second-degree hypertension were the highest (40.32%) users of calcium antagonists. According to the guideline, Stage 2 hypertension patients must be treated with a combination of two drugs at once, either a calcium or thiazide antagonist combined with an ACE inhibitor or ARB. Our results were also consistent with research related to the management of DRPs in outpatients with hypertension in four primary health centers, which found that amlodipine was the most used drug at 47.7%, followed by captopril at 22.4%.[9]
The DRPs found in this study were related to treatment effectiveness and adverse drug events. Treatment effectiveness of medicines can be influence by many factors, such as dosage, drug interaction, and drug choice. In this study, a combination of antihypertensive medication with other cardiovascular or symptomatic medicines might lead to the decreasing effect of therapy and increasing the risk of adverse effects. A study about DRP done in Malaysia also showed that the most frequent DRP was drug interactions (33.7%) with an antihypertensive and antiplatelet agent that was mostly associated with DRPs in hypertensive patients with comorbidity.[11]
Drug interactions between amlodipine and clopidogrel can cause the effectiveness of treatment not optimal. Concomitant of amlodipine and clopidogrel can reduce clopidogrel activity because amlodipine inhibits CYP3A4, which metabolizes clopidogrel to an active metabolite. This effect of amlodipine was proven by a prospective study of 900 randomized Korean patients suffering from coronary heart disease with coronary percutaneous intervention. The patients were given clopidogrel with acetosal (acetylsalicylic acid) and a calcium antagonist. This regimen can significantly reduce the antiplatelet activity of clopidogrel and increase cardiac death, nonfatal myocardial infarction, and ischemic stroke.[12] However, this effect was not detected in hypertensive outpatients in Koja District Hospital during the study. The pharmacist educates the patient to separate the drug administration, amlodipine in the morning, and clopidogrel during the day. This education reduces the risk of these interactions.
Adverse drug event nonallergic was found in the interactions between acetosal and clopidogrel that leads to bleeding and gastrointestinal (GI) damage. Low-dose acetosal causes GI mucosal and systemic effects of prostaglandin depletion through inhibition of cyclooxygenase-1. Prostaglandins play an important role in protecting the integrity of the gastric mucosa by increasing local blood flow and promoting the synthesis and secretion of mucus and bicarbonate. The acidic environment causes the acetosal to remain un-ionized, which forces it to accumulate in the gastric mucosal cells, changes cell permeability, and causes ulceration.[13] Although these two drugs have a significant drug interaction, some cardiovascular disease patients need it as dual antiplatelet therapy. In order to avoid the negative effects of this drug interaction, pharmacists in Koja District Hospital had educated patients regarding the time lag between giving acetosal in the morning and clopidogrel during the day. However, it is still necessary to conduct GI bleeding and blood monitoring of patients to ensure that side effects do not occur.
The coadministration of amlodipine with domperidone can increase the domperidone concentration in blood and prolong QT because amlodipine is a CYP3A4 inhibitor. This interaction can cause serious heart disease, such as ventricular arrhythmias and sudden cardiac death, which has been shown by cohort studies that found a link between serious ventricular arrhythmias and sudden cardiac death in the use of domperidone at dosages >30 mg/day in patients >60 years old.[14] To avoid this interaction, the lowest dosage and short duration of domperidone are recommended. If the patient experiences fainting, dizziness, palpitation, and seizures after receiving domperidone, it must be stopped.
The interaction between clonidine and bisoprolol can cause a risk of sinus bradycardia. Therefore, the heart rate must always be monitored when simultaneously taking both drugs. Discontinuation of clonidine while using bisoprolol can lead to rebound hypertension due to alpha-stimulation that is not inhibited. In patients who take clonidine together with bisoprolol but wish to stop taking clonidine, they must first stop consuming bisoprolol within a few days before slowly stopping clonidine.[15] Although the result of this interaction was not appeared clinically in the patients analyzed, monitoring and education of patients should be done, especially when deciding to stop one of the medications.
The interaction of amitriptyline and meloxicam also contributes in DRPs since it can increase the risk of bleeding, as shown by a cohort study in The Netherlands. The study demonstrated that concurrent consumption of tricyclic antidepressants with nonsteroidal anti-inflammatory drugs (NSAIDs) in patients increased the incidence of side effects in the GI tract relative to the incidence in patients who received tricyclic antidepressants alone. This finding can be explained by the fact that tricyclic antidepressants substantially inhibit CYP2C9 but not more substantially than inhibition by selective serotonin reuptake inhibitor antidepressants.[16] Physicians in Koja District Hospital sometimes prescribe a combination of amitriptyline and meloxicam once daily to relieve pain in outpatients with hypertension, although the evidence based on this practice is not established yet. Prevention of interactions has been accomplished at Koja District Hospital by administering these combinations in minimum doses of amitriptyline at 5 mg and meloxicam at 3 mg. However, complete blood count and laboratory test need to be done regularly to monitor the bleeding effects of this interaction.
Among the participants, 13.57% of the patients were prescribed by medications that were not indicated, such as paracetamol, diclofenac potassium, mucolytic syrup, laxative agent, mouthwash, domperidone, and antiulcer drugs. Analgesic-anti-inflammation commonly prescribes to treat pain or headache that is often suffered by hypertension patients. Antiulcer like proton-pump inhibitor (PPI) in cardiovascular patients usually prescribes to treat a peptic ulcer that is induced by an antiplatelet agent. While only prescribed for additional medications, these drugs should not be overused when there is no clear indication. Besides increasing the therapy cost, this medication can lead to other adverse effects such as GI disturbance for the NSAID or increasing the risk of Clostridium difficile-associated diarrhea, nutritional deficiencies, or bone fracture for the PPI.[17,18]
The problem in drug dose was also found in this study. Inappropriate dose selection occurred in subtherapeutic dosage of sucralfate and ambroxol prescribed by the physician. Although these drugs were prescribed for symptomatic treatment only, the monitoring of outcome therapy should have been paid attention by the physician or clinical pharmacist. If the therapeutic outcome does not reach, it can contribute to decreasing patient quality of life.
Another finding of this study was that some patients were not prescribed by the medication that they might be needed. Some indications that were not written in the medical record but not being treated were diarrhea, heartburn, cough, headache, and myalgia. This case might happen for some reason, such as the patients have had the symptomatic medications at home, or the physician prescribed some drugs but was not correctly recorded.
The limitations of this study were that the researchers only looked at patient complaints from patients' integrated care to note that was written by health-care providers in a medical record without interacting doctors to confirm directly. The researchers also could not directly interact with the patients to verify whether the prescribing of those medicines appropriate or not and to ensure that they were already taking a drug at home that was effectively treating the problem written in the medical record.
From this study, it can be concluded that a significant percentage of outpatients being treated for hypertension experienced DRPs. The Koja District Hospital pharmacy practice offers services to manage DRPs among outpatients with hypertension. However, the role of clinical pharmacists and physicians in drug monitoring needs to be improved to help to prevent and resolve DRPs in outpatients with hypertension.
AUTHORS' CONTRIBUTION
All authors contributed the idea of research, study design, collecting data, data analysis and manuscript preparation.
Financial support and sponsorship
The research was financially supported by Universitas Indonesia.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank the Universitas Indonesia for providing research grant PITTA B and Koja District Hospital for permitting to use the data in this study. The authors state that there is no conflict of interest in this research.
REFERENCES
- 1.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison HC, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 2.Hasil Utama Riskesdas 2018. Jakarta: Kemenkes; 2018. Kementerian Kesehatan Republik Indonesia. [Google Scholar]
- 3.European Society of Cardiology. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension The Task Force for the Management of Arterial Hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension. 2018:1–98. [Google Scholar]
- 4.Viktil KK, Blix HS, Moger TA, Reikvam A. Polypharmacy as commonly defined is an indicator of limited value in the assessment of drug-related problems. Br J Clin Pharmacol. 2007;63:187–95. doi: 10.1111/j.1365-2125.2006.02744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hailu BY, Berhe DF, Gudina EK, Gidey K, Getachew M. Drug related problems in admitted geriatric patients: The impact of clinical pharmacist interventions. BMC Geriatr. 2020;20:13. doi: 10.1186/s12877-020-1413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pharmaceutical Care Network Europe. Classification for drug related problems v6.02. Pharmaceutical Care Network Europe Foundation. 2010 [Google Scholar]
- 7.Griese-Mammen N, Hersberger KE, Messerli M, Leikola S, Horvat N, van Mil JWF, et al. PCNE definition of medication review: Reaching agreement. Int J Clin Pharm. 2018;40:1199–208. doi: 10.1007/s11096-018-0696-7. [DOI] [PubMed] [Google Scholar]
- 8.Hussein M, Lenjisa J, Woldu M, Tegegne G, Umeta G, Dins H. Assessment of drug related problems among hypertensive patients on follow up in Admaa Hospital Medical College, East Ethiopia. Clin Pharmol Biopharm. 2014;3:1–6. [Google Scholar]
- 9.Nasution A, Khairunnisa, Tanjung HR. Drug related problems in management of hypertensive outpatients admitted to four Indonesian primary health centers. Asian J Pharm Clin Res. 2016;9:87–90. [Google Scholar]
- 10.Supraptia B, Nilamsari WP, Hapsari PP, Muzayana HA, Firdausi H. Problems Related to Antihypertensive Drugs in Elderly Patients at the Geriatric Clinic Dr. Soetomo Hospital, Surabaya. Indones J Clin Pharm. 2014;1:36–41. [Google Scholar]
- 11.Redzuan AM, Ramli AR, Pheng MT. Drug related problems in hypertensive patients with multiple comorbidites. J Pharm Res. 2017;1:1–8. [Google Scholar]
- 12.Lee SP, Bae JW, Park KW, Rha SW, Bae JH, Suh JW, et al. Inhibitory interaction between calcium channel blocker and clopidogrel.-Efficacy of cilostazol to overcome it- Circ J. 2011;75:2581–9. doi: 10.1253/circj.cj-11-0113. [DOI] [PubMed] [Google Scholar]
- 13.Inayah N, Manggau MA, Amran Y. Analysis of effectiveness and side effects of using clopidogrel alone and in combination clopidogrel-aspilet in ischemic stroke patients at the RSUP Dr. Wahidin Sudirohusodo Makassar. Majalah Farmasi dan Farmakologi. 2018;22:81–4. [Google Scholar]
- 14.Puspitasari AW, Azizahwati A, Hidayat AR. Analysis of potential drugs interaction on Antihypertensions drugs prescription in community health center of Sukmajaya district in period of June-November 2015. Asian J Pharm Clin Res. 2017;10:61–5. [Google Scholar]
- 15.Bailey RR, Neale TJ. Rapid clonidine withdrawal with blood pressure overshoot exaggerated by beta-blockade. Br Med J. 1976;1:942–3. doi: 10.1136/bmj.1.6015.942-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore N, Pollack C, Butkerait P. Adverse drug reactions and drug-drug interactions with over-the-counter NSAIDs. Ther Clin Risk Manag. 2015;11:1061–75. doi: 10.2147/TCRM.S79135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton-pump inhibitors: What the clinician needs to know. Therap Adv Gastroenterol. 2012;5:219–32. doi: 10.1177/1756283X12437358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maes ML, Fixen DR, Linnebur SA. Adverse effects of proton-pump inhibitor use in older adults: A review of the evidence. Ther Adv Drug Saf. 2017;8:273–97. doi: 10.1177/2042098617715381. [DOI] [PMC free article] [PubMed] [Google Scholar]


