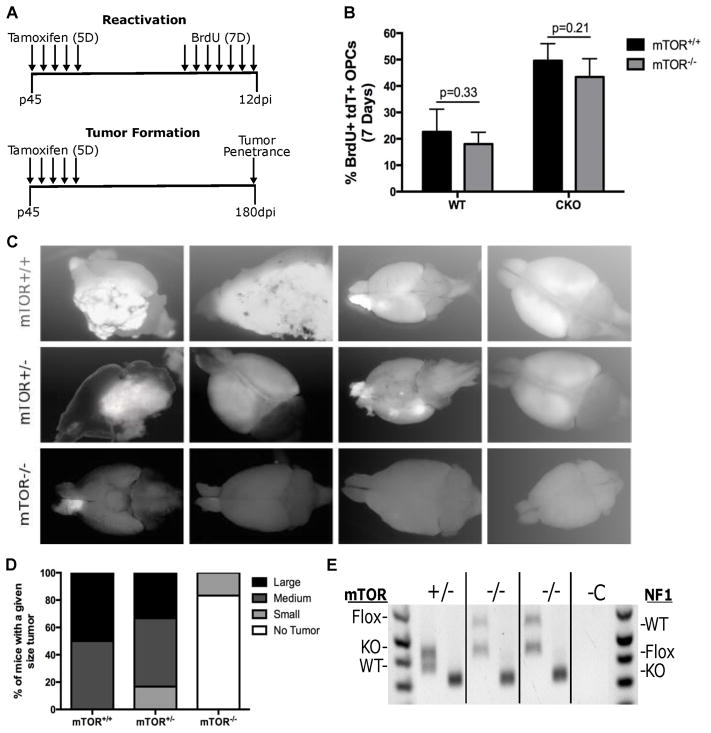
Fig. 7. mTOR deletion blocked gliomagenesis.
(A) Top schematic shows the experimental design for testing the effect of mTOR deletion on PreT-OPC reactivation. Mice were treated for 5 days with Tamoxifen to induce cre-mediated recombination and then treated for 7 days with BrdU to label dividing OPCs. Bottom schematic shows the experimental design for testing the effect of mTOR deletion on gliomagenesis. Following 5 days of Tamoxifen treatment, mice were kept for 180 days to determine the tumor penetrance.
(B) Following 7 days of BrdU injections, mice were examined for the percent of dividing OPCs. In both WT and glioma model mice, mTOR-KO led to no significant differences of OPC reactivation. (n=4 WT, n=5 CKO) Student t Test; Error Bar ± SD
(C) Glioma model mice (p53-null,NF1-null) with either mTOR-WT, mTOR-het, and mTOR-null status were dissected at P240 to determine the tumor penetrance. While mTOR-WT CKO mice always had large tumors, mTOR-null CKO mice had either none or occasionally very small tumors. The images are representative of the average tumor size in mTOR-WT, mTOR-het, and mTOR-null glioma mice. More than 10 mice were examined in each group.
(D) Semi-quantitative representation of tumor penetrance and sizes in glioma model mice (p53-null,NF1-null) with either mTOR-WT, mTOR-het, and mTOR-null status.
(E) Genotyping of tumor OPCs purified from mTOR-null brains. For each genotype (indicated at top), the left bands represent mTOR PCR and the right band represents NF1 PCR. While Cre-mediated recombination of the NF1-flox alleles was complete (only KO band was detected) in all tumor cells, the recombination of mTOR-flox alleles was incomplete in both mTOR-null tumors (evidenced by the presence of the mTOR-flox band). We are confident that the mTOR-flox allele in tumor cells came from “escaper cells” rather than contaminated non-tumor cells because of the absence of mTOR-flox band in the +/− lane and our confirmation of cell purity based on PDGFRα staining after immunopanning of tumor OPCs.