Abstract
Context: Lewy body (LB)-related α-synucleinopathy (LBAS) is the neuropathological hallmark of several neurodegenerative diseases such as Parkinson disease (PD), but it is also found in neurologically asymptomatic subjects. An abnormal accumulation of α-synuclein has been reported also in the spinal cord, but extent and significance of the spinal cord involvement are still poorly defined.
Objective: We aimed to review the studies addressing the spinal cord involvement of LBAS in healthy subjects and in patients with PD or other neurodegenerative diseases.
Methods: A MEDLINE search was performed using following terms: “spinal cord”, “ α-synucleinopathy”, “α-synuclein”, “Lewy body”, “Parkinson’s disease”, “multiple system atrophy”, “neurodegenerative disorder”.
Results: LBAS in the spinal cord is associated with that of the medullary reticular formation and locus ceruleus in the brainstem but not with that in the olfactory bulb and amygdala. The intermediolateral columns of the thoracic and sacral cord are the most frequently and severely affected region of the spinal cord. LBAS occurs in centrally projecting spinal cord neurons integrating pain, in particular from lower body periphery. It also involves the sacral parasympathetic nucleus innervating the smooth muscles of the bladder and distal colon and the Onuf’s nucleus innervating the striated sphincters. The spinal cord lesions may thus play a crucial role in the genesis of frequent non-motor symptoms such as pain, urinary symptoms, bowel dysfunction, autonomic failure including orthostatic hypotension and sexual disturbances. Moreover, these may also contribute to the motor symptoms, since α-synuclein inclusions have been observed in the pyramidal tracts of patients with PD and multiple system atrophy.
Conclusion: Recognition of this peculiar spinal cord pathology may help in the management of the related symptoms in subjects affected by α-synucleinopathies.
Keywords: Lewy body, α-synucleinopathy, α-synuclein, Spinal cord, Parkinson's disease, Neurodegenerative disorders
Introduction
Lewy body (LB)-related α-synucleinopathy (LBAS) is the neuropathological hallmark of several neurodegenerative diseases.1 The abnormal accumulation of pathologic α-synuclein in the brain is a characteristic feature of Parkinson disease (PD),2,3 Lewy body disease (LBD),4 and dementia with Lewy bodies (DLB).5 LBAS was also found in Alzheimer disease (AD),6 multiple system atrophy (MSA),7 progressive supranuclear palsy,7 Down syndrome,8 and pure autonomic failure (PAF).9,10
LBAS appears as Lewy neurites (LNs) in the affected dendrites and neurons,11–13 and/or as particular aggregates,14,15 punctuate structures, pale bodies, and LBs in the somata of the involved neurons.16–20
LBAS was found in the brain stem nuclei of 8%–12% of neurologically asymptomatic subjects older than 60 years;21,22 this so-called “incidental LBD” (ILBD) is thought to represent the early asymptomatic phase of some of the neurodegenerative diseases mentioned above. However, an abnormal accumulation of α-synuclein has been reported also in the central and peripheral nervous systems, including the spinal cord and dorsal root ganglia (DRG).
The present narrative review focuses on spinal cord involvement of LBAS in normal subjects and in patients with PD or other neurodegenerative diseases.
Methods
We performed a comprehensive review of computerized literature databases. The MEDLINE, accessed by Pubmed (1966–February 2018) and EMBASE (1980–February 2018) electronic databases were searched using the following free terms and medical subject headings (MeSH) combined in multiple search strategies: “spinal cord”, “α-synucleinopathy”, “α-synuclein”, “Lewy body”, “Parkinson’s disease”, “multiple system atrophy”, and “neurodegenerative disorder”.
Only original studies written in English and conducted in humans were considered eligible for inclusion. Single case reports or letters were excluded. The titles and abstracts of the initially identified studies were screened to determine if they satisfied the selection criteria. Full-text articles were retrieved for the selected titles, and their reference lists were searched for additional publications. Principal investigators of included trials were contacted in the case of missing or incomplete data, and additional information was requested. Two review authors screened the titles and abstracts of the initially identified studies to determine if they satisfied the selection criteria, and independently assessed the methodological quality of each study and risk of bias, focusing on blinding. The search strategy described above yielded 36 results, one of which was excluded after reading the full paper, thereby leaving 35 studies which contributed to this review.
A flow-chart (Fig. 1) illustrates the selection/inclusion process.
Figure 1.
Flow-chart showing the selection/inclusion process.
Spinal cord involvement in subjects with LBAS
In a neuropathological study, ILBD was identified in 17 out of 98 subjects (17.3%) with a mean age of 80.7 years and no evidence of PD.23 LBAS, mainly LNs, was found in the brain stem, olfactory bulbs and autonomic nuclei of the thoracic spinal cord in all cases, in the sacral parasympathetic nuclei (15 out of 16 cases), in the myenteric plexus of the esophagus (14 out of 17 cases), in the sympathetic ganglia (14 out of 17 cases), and in the vagus nerve (12 out of 16 cases). Notably, a high number of LBAS lesions was detected in the non-autonomic spinal cord nuclei.
In another study, ILBD was reported in 13 out of 106 (12%) healthy subjects aged between 60 and 100 years.24 In nine cases (8%) mainly LNs were localized at the T2 and T9 levels of the spinal cord, most often in the intermediolateral columns of the thoracic cord (Th/IML). In all of these subjects, LBAS was found in the dorsal motor nucleus of the vagus, in the locus coeruleus, and in the central raphe nucleus. It has been hypothesized that LBAS spinal cord involvement likely occurs after the brain stem involvement, thus at stage 2 of the Braak’s scheme.
In a general population of nondemented subjects, LBAS was found in 11 out of 73 brains.25 It may be hypothesized, that the occurrence of LBAS is related to age. In these respects, an interesting study focused on the characteristics of LBAS in 23 centenarians (six of them nondemented).26 In 8 subjects (34.8%) LBAS was observed in the brain, and in 4 subjects was also found in the spinal cord, which was examined from the third cervical to the third sacral segment. This study has some limitations: the sample size was limited and the site of the lesions was rather variable. However, it is conceivable that LBAS occurs quite frequently in the spinal cord of neurologically healthy subjects older than 60 years, likely in 8%–10% of the cases; the frequency seems to increase with age and display a correlational trend with cerebral LBAS.
Another study in an elderly population assessed the distribution of LBAS in 265 consecutive subjects who underwent autopsy at the Tokio Metropolitan Geriatric Hospital.27 The presence of LBAS in specific regions of the lower brainstem (i.e. the medullary reticular formation and locus ceruleus) showed a high correlation with that in the spinal cord, while the presence of LBAS in the spinal cord was found to be independent of the presence of LBAS in the olfactory bulb and amygdala. It seems, thus, that the propagation LBAS occurs retrogradely from the dorsal horn to the dorsal root ganglia through the dorsal root and from the sympathetic ganglia to the Th-IML. Moreover, LBAS in the spinal cord accumulates mainly at the caudal level.
Nevertheless, the LB-related pathology is not simply limited to the brainstem, but is widely involves in the spinal cord (83% of ILBD cases).28 On the basis of its frequency and distribution, it has been speculated that LB-related pathology starts in the spinal cord. Most phosphorilated α-synuclein (pαSyn)-positive structures were distributed in and around the autonomic nuclei of the spinal cord. The Th/IML are likely the first affected structure, since there were the most frequently and severely affected region. LB-related pathology could progress towards the caudal vertebrae by affecting neurons in the spinal cord that are vulnerable to α-synuclein.
Spinal cord involvement in patients with neurodegenerative disorders and LBAS
Findings from animal models
Genetic animal models of parkinsonism provided interesting data. A severe glial cells pathology in the cervical spinal cord was found in α-synuclein transgenic mice only during aging. Interestingly, the spinal cord is more affected than brain regions.29 By using monoclonal antibodies two different epitopes of alpha-synuclein were recognized in normal rats.
Human studies
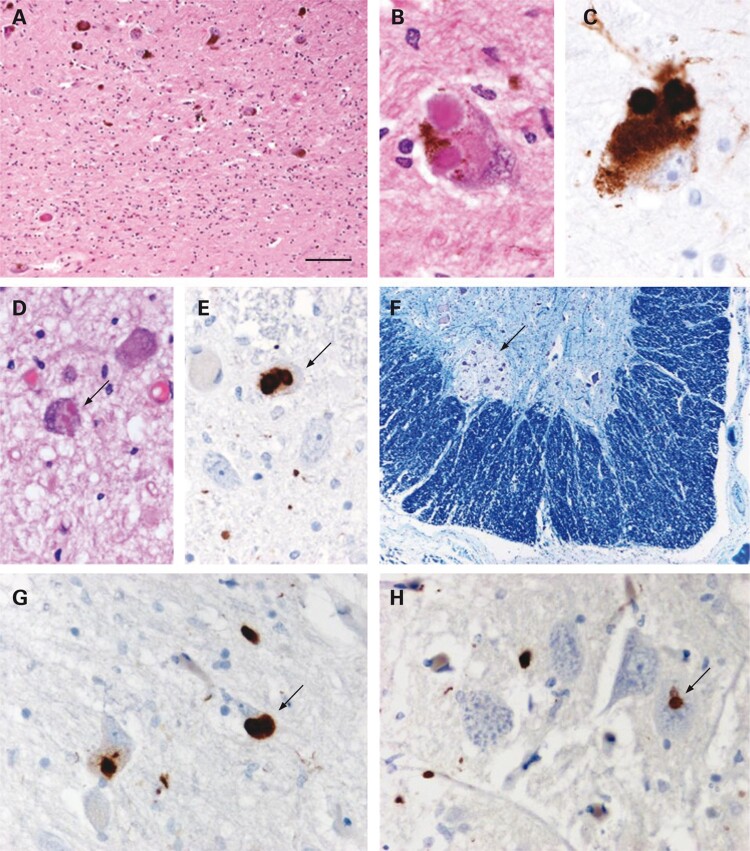
Only a few cases of patients with sporadic PD and LBAS located either in the dorsal group of the nucleus intermediolateralis of the third sacral segment30 or in the Onuf’s nucleus31 (Fig. 2) have been reported. Loss of myelinated fibers in the gracile fascicles and moderate neuronal loss in the Clark’s nucleus have been observed in parkin gene mutation with tau pathology but without LBAS.32
Figure 2.
Examination of the substantia nigra showed moderate loss of pigmented neurons with gliosis (A). Typical Lewy bodies were identified in residual neurons (B) and were confirmed using α-synuclein immunohistochemistry (C). Lewy bodies were also found in other brain stem structures, including the Xth nerve nucleus (arrow in D). Immunohistochemical staining for α-synuclein also revealed small numbers of Lewy bodies in neurons of the intermediolateral column in the thoracic spinal cord (arrow in E). In the sacral cord, the Onuf’s nucleus (ON) was identified (arrow in F) and, at this level, Lewy neurites and Lewy bodies could be identified in the gray matter (arrow in G indicates a Lewy body). In the ON, there were Lewy neurites and also a single probable Lewy body (arrow in H). A, B and D: Haematoxylin and eosin; F: Luxol fast blue and cresyl violet; C, E, G and H: α-synuclein immunohistochemistry, chromogen: diaminobenzidine. Bar in A represents 260 μm in F; 100 μm in A; 25 μm in D, E, G and H; and 17 μm in B and C. Reproduced with permission from O’Sullivan et al.31
In a study on subjects with parkin-positive autosomal recessive juvenile parkinsonism, LB-like inclusions have been described in the somata of the large anterior horn neurons of the lumbar spinal cord. These have been observed, to a lesser degree, in the IML nucleus of the upper lumbar cord as well as in the Onuf’s nucleus in a single case of autosomal recessive parkinsonism.33
In another study, the spinal cord has been examined in six patients with PD: LBs were observed in the ganglion cells of the lateral horns in four cases, and in the ganglion cells of the posterior horn in one case.17
In 2 neuropathological studies, twenty-five patients with PD (mean age 69.9 years, range 54–84 years, disease duration between 1 and 30 years) have been examined.34,35 The average number of neurons in the IML nucleus at the T2 and T9 levels was, respectively, 69 and 57% of that in the control subjects, while there was no significant difference in Clarke’s column neurons count. Notably, the neurons count was not correlated with age at death nor with the disease duration.
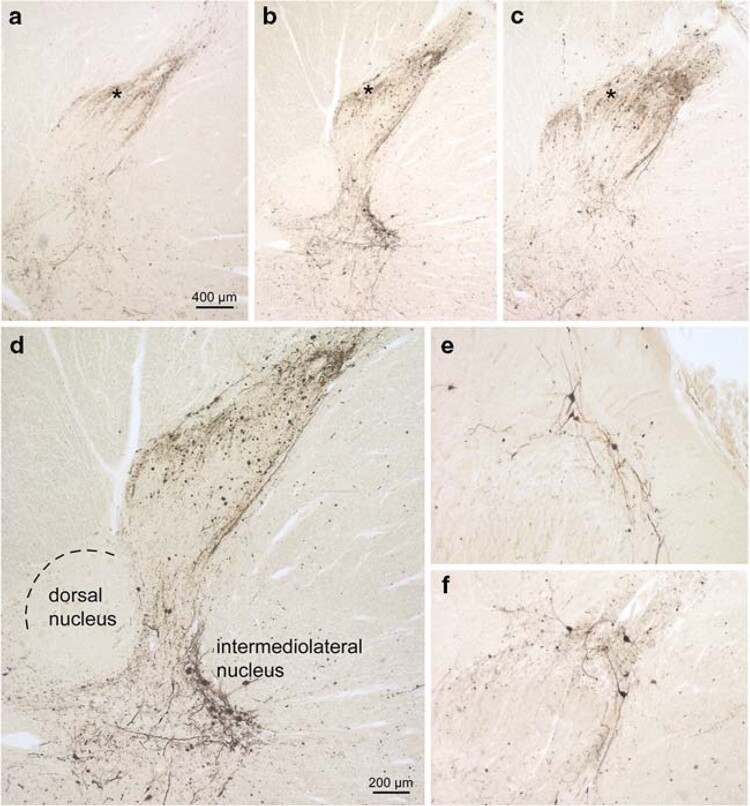
An involvement in lamina I of the spinal cord dorsal horn has been found in all PD patients36 (Fig. 3). The pathological changes were to a lesser extent in the cervical segments, and density gradually increased caudally. In a subsequent study the spine has been examined in 23 neurologically asymptomatic subjects (seven with ILBD), 17 patients with PD, 9 with DLB, 19 with AD with LB, and 19 with AD without LB.37 In 16 out of 17 patients with PD, LB were detected in all gray matter regions of the spinal cord. The lesions were predominantly located in the Th/IML horn at the base of the posterior horn of the sacral cord. Although the sample size is rather limited, these reports strongly suggest that the spinal cord and particularly of the IML columns are frequently involved. However, available data do not allow us to specify whether the involvement of the spinal cord precedes or follows LBAS of the brain or of the ganglia.
Figure 3.
PD-related involvement of the spinal cord. a Seventh cervical segment. b Twelfth thoracic segment. c Third lumbar segment. Note the increase caudally in the density of the LN network in lamina I (asterisks) and close to it. d Detailed micrograph of b showing involvement of the intermediolateral nucleus. Several multipolar preganglionic sympathetic relay neurons are Wlled with _-synuclein aggregates. Note that the dorsal nucleus (Clarke’s column) is virtually uninvolved. a–d orginate from a 76-year-old male at stage 4 (case 2). e, f Both sections show the multipolar relay neurons in lamina I as being exclusively aVected. In contrast, the nerve cells in the subjacent layer II remain intact. e Originates from the seventh cervical spinal cord segment of case 6, f from the tenth thoracic spinal cord segment of case 3. Syn-1 (Transduction Laboratories) immunoreactions in 100 _m polyethylene glycol-embedded tissue sections. Scale bar in a is valid for b and c. Scale bar in d applies to e and f. Reproduced with permission from Braak et al.36
LBAS shows a multisystemic distribution. In fact, it has been found in the sciatic nerve, in the endocrine, gastrointestinal, and respiratory systems, and in the genitourinary tract.9,17,37–39
In an autoptic study, by means of α-synuclein immunohistochemistry and lipofuscin pigment-Nissl architectonics the brains and spinal cords of 28 patients with clinically and neuropathologically confirmed PD, 6 cases with ILBD, and 12 age-matched controls have been investigated. LBAS (particulate aggregations, LNs/LBs) in the spinal cord were observed between neuropathological stages 2–6 in all cases whose brains were staged for PD-LBAS40 (Fig. 4). Only individuals with ILBD and all controls did not have Lewy pathology. The PD-related lesions were found in the spinal cord only after Lewy pathology was observed in the brain. It can therefore be supposed that, within the central nervous system, sporadic PD does not begin in the spinal cord. α-synuclein-immunoreactive axons were clearly predominant over LBs throughout the spinal cord and were observed in medial and anterior portions of the anterolateral funiculus. They formed dense α-synuclein-immunoreactive networks in the gray matter and were most remarkable in the lateral portions of layers 1, 7, and in the cellular islands of layer 9. The density of these axonal lesions increased markedly in from cervicothoracic segments to lumbosacral segments of the cord. The spinal cord α-synuclein immunoreactive axonal networks might represent descending projections from the supraspinal level setting nuclei (locus coeruleus, lower raphe nuclei, magnocellular portions of the reticular formation).
Figure 4.
Lewy pathology (LP) in the level setting nuclei of the brainstem. a Great raphe nucleus, and laterally, magnocellular portions of the reticular formation. b Locus coeruleus (a, b case 18, PD stage 4, α-syn-ir). Sections through the cervical spinal cord (case 17, PD stage 4, α-syn-ir and pigment-Nissl staining). c Overview of segment C7. d,e Globular axonal varicosities in the dorsal root and in the transition zone to Lissauer’s tract. f Spinothalamic tract cell. g Motoneurons in layer 9. Arrow indicates involved neuron. h Spinothalamic tract cell. Framed areas in c appear at higher magnification in d–h. i,k Affected neurons showing characteristics of sympathetic preganglionic nerve cells occur in lower segments of the cervical cord, located in the lateral funiculus (case 10, PD stage 5, α-syn-ir and pigment-Nissl staining). Scale bar in b is valid for a; bar in h also applies to d–g and i. Reproduced with permission from Del Tredici and Braak.40
In particular, nociceptive neurons of the dorsal horn in layer 1, sympathetic and parasympathetic preganglionic neurons in layer 7, the cellular pools of α-motoneurons in layer 9, and the smaller motoneurons in Onuf's nucleus in layer 9 (ventral horn) displayed α-synuclein-immunoreactive inclusions.
LBAS and non-motor symptoms
Spinal cord lesions due to LBAS may contribute to the occurrence of several clinical symptoms (e.g. pain, constipation, poor balance, lower urinary tract complaints, and sexual dysfunction) during both the premotor and motor phases of sporadic PD.
Findings from animal models
Most nociceptive A delta and C fibers terminate in laminae I and II. From lamina I, spinal cord neurons, which exhibit neurokinin I receptor for substance P, project to areas of the brain, in particular to the parabrachial area but also to the brainstem, mainly to the rostral medulla, which in turn has descending projections to the dorsal horn.41–43 Sympathetic postganglionic neurons represent the efferent system. Especially the disinhibition of inhibitory interneurons (GABAergic or glycinergic) might contribute to spontaneous pain.43 A pathology of the dorsal horns and in particular of lamina I may clearly alter this delicate balance.
Pelvic primary afferent fibers and secondary afferent neurons project from the sacral cord to the ventrolateral PAG.44,45
The pelvic nerve also innervates the distal one-third of the colon and rectum and these afferents share the same territory as bladder inputs in the sacral dorsal horn.46
Functional imaging studies showed that in humans the PAG projects to Barrington’s nucleus,47 which controls the detrusor muscle via descending projections to the sacral intermediolateral cell column.
Pain
Pain may precede the onset of the motor symptoms of PD and may occur in the prodromal phases as well as in the advanced stages. Its genesis is thought to be multifactorial and partly of central origin. PD patients who previously referred spontaneous pain showed an increased sensitivity to painful stimuli.48 The pain threshold is lower in more affected limbs, and did not differ between on and off periods. The dynamic sensitization of the central nervous system or by the hypersensitization of the pain pathways between the basal ganglia and the cortex may explain these data. Different neuronal structures are involved in the pathogenesis hyperalgesia or allodynia: in the brain the rostral ventromedial medulla, nucleus reticularis gigantocellularis, thalamic nuclei, anterior cingulate cortex, and lateral and ventral orbital cortex; in the spinal cord neurons in lamina I, microglia, astrocytes, the afferent C fibers and vagal fibers, dorsal columns fibers, and fibers traveling in the lateral and anterolateral funiculus.
Moreover, the poor response of the pain to the dopaminergic therapy,49,50 suggests the involvement of different neurotransmitters (GABA, glycine or other neurotransmitters).
The first relay stations of the pain system as well as parasympathetic and sympathetic pre- and postganglionic nerve cells in the lower brainstem, spinal cord, and coeliac ganglion has been investigated by using immunocytochemistry for α-synuclein, These examinations showed immunoreactive inclusions for the first time in spinal cord lamina I neurons. The lower portions of the spinal cord downwards of the fourth thoracic segment were mainly affected, while the spinal trigeminal nucleus was found to be virtually intact. An additional involvement of the parasympathetic preganglionic projection neurons of the vagal nerve, the sympathetic preganglionic neurons of the spinal cord, and the postganglionic neurons of the coeliac ganglion has been reported. The connections between these structures may explain their particular vulnerability. Lamina I neurons directly project upon sympathetic relay centers involved in the pain system; these, in turn, influence the parasympathetic regulation of the enteric nervous system.
In PD patients, abnormal accumulation of α-synuclein occurs in the projection neurons (and especially in their terminal arbors) localized in the lamina 1 of the spinal cord; these pathological findings are more consistent in the caudal than in the rostral spinal segments.
Lamina I neurons are not affected in subjects with preclinical PD, while lamina V neurons are unaffected at any disease stage. Therefore, degenerative changes occur in a selective population of centrally projecting spinal cord neurons (mainly located in the lamina I) integrating pain particularly from lower body periphery.23,36
Urinary symptoms
Urinary symptoms may occur in up to 70% of individuals with PD.51 Several authors suggested a correlation of urinary disturbances with neurological disability 52,53 or with age.54 Symptoms are mainly represented by an overactive bladder, such as nocturia, and to a lesser degree, urgency and greater frequency.
Urinary symptoms usually affect the PD patients late in the course of the disease and their pathogenesis is likely to be multifactorial. Due to the dopaminergic cell loss in the substantia nigra the most accepted hypothesis is the disinhibition of the pontine micturition center,55 which projects to the sacral spinal cord, is involved in the regulation of the peripheral reflexes and is inhibited during the storage phase. Its activity is regulated by the periaqueductal gray (PAG), the anterior cingulate gyrus, the insula, and the prefrontal cortex. The activation of these brain regions during the micturition determines the activation of the pontine micturition center and finally of the sacral segments of the spinal cord. In cats, the bladder projects afferent inputs by the lateral funiculus to the PAG that in turn sends excitatory inputs to the pontine micturition center.
However, the dopaminergic therapy often failed to improve urinary symptoms; it is therefore likely that other neurotransmitter systems are involved in their genesis.56–58
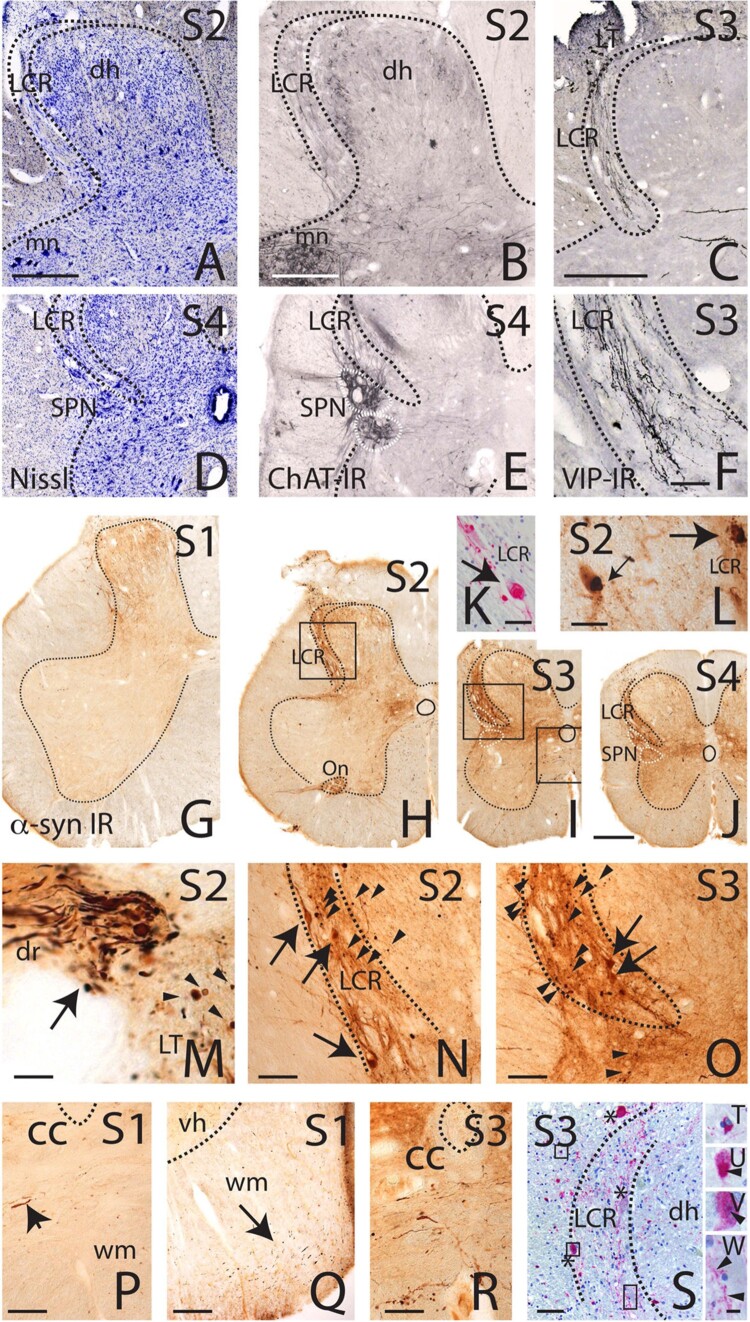
In a recent study, a prominent accumulation of α-synuclein pathology has been found in the sacral dorsal roots, Lissauer’s tract, and especially the lateral collateral region (LCR) in subjects with a primary diagnosis of PD and LBD. LCR is located lateral to the dorsal horn and is thought to be neurochemically distinct from other parts of the dorsal horn, which receives primary visceral sensory afferents from the bladder and distal colon via the pelvic nerve59 (Fig. 5).
Figure 5.
Photomicrographs of the human sacral spinal cord. Location of the LCR: Nissl stained (A and D; case 9), ChAT stained (B and E; case 9) and VIP stained sections (C and F; case 4) marking the lateral collateral pathway (LCR) in relation to the dorsal horn and sacral parasympathetic nucleus (SPN). Note that the LCR can be clearly distinguished from the dorsal horn at level S2 (A and B), rostral to the location of preganglionic ChAT-immunoreactive neurons in the SPN (D and E). Reproduced with permission from Vanderhorst et al.59
LBAS has been observed in the sacral parasympathetic nucleus,30 in lamina I of the sacral cord and sacral parasympathetic nucleus23,37,40,60 (Fig. 6), and in particular in the Onuf’s nucleus.31 The external urethral sphincter is innervated by cholinergic motor nerves arising in the Onuf’s nucleus. The guarding reflex is organized in the spinal cord and involves inhibition of the parasympathetic innervation of the detrusor as well as activation of the muscles of the urethral sphincter during the storage phase.61 LBAS of IML columns likely impairs the communication from and toward higher centers.
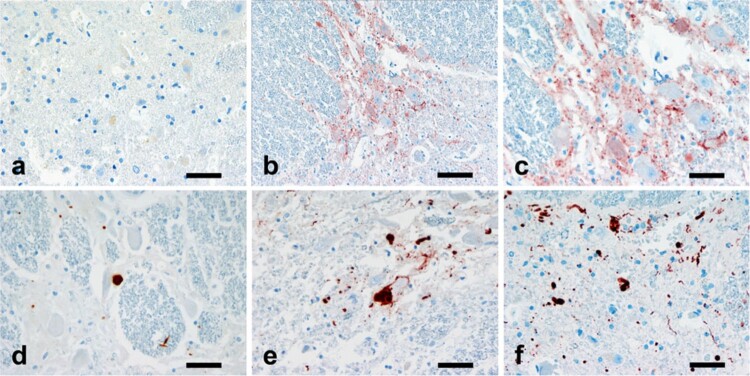
Figure 6.
A semiquantitative assessment of the αS pathology in the spinal cord. The figures illustrating the increasing severity of the immunoreactivity in the intermediolateral column of thoracocspinal cord from negative (a), to mini-aggregates with low magnification (b), mini-aggregates (c), mild (d), moderate (e) and severe (f) forms. αS immunostaining: magnification, 400× a and c–f 200×; b Scale bars, a, c–f 40 μm and b 80 μm. Reproduced with permission from Oinas et al.60
Moreover, functional imaging studies showed that in humans the PAG is important for bladder filling.62,63 It has been hypothesized that the accumulation of α-synuclein pathology in the afferent pelvic-PAG loop might contribute to disinhibition or overactivation of Barrington’s nucleus, which results in detrusor overactivity.59
Urinary frequency and incontinence are common and often early symptoms in MSA.64 In addition to pathology in Onuf’s nucleus and the area of the pontine micturition center,64,65 specific LCR pathology might contribute to urinary symptoms in MSA. Phosphorylated α-synuclein in the form of labeled neurites has been found in the LCR of MSA with prominent autonomic features, including urinary incontinence. The pathological, diffuse cytoplasmic phosphorylated α-synuclein, which is a common feature in MSA,66 was found in numerous medium-sized neurons in the LCR. Widespread glial cytoplasmic inclusions were detected in line with prior reports.67 Larger studies are necessary to assess whether the clinical features in the various subtypes of MSA correlate with the severity and type of pathology in the LCR.
Bowel dysfunction
Constipation is another frequent symptom ranging from 30% to 60% of the PD patients,68–71 and may occur during the pre-symptomatic stages and at the motor phases of PD.72 Defecatory dysfunctions also occur frequently, as much as 67% of the patients,68 sometimes since the early stage of the disease.71 According to Braak’s hypothesis, LBAS originates in the anterior olfactory structures and in the dorsal motor nucleus of the vagus.73 LBAS is also abundant in the myenteric Auerbach’s plexus and in the submucosal Meissner’s plexus;19,20,74 therefore, the frequency and importance of gastrointestinal symptoms are not surprising.
The dorsal motor nucleus of the vagus innervates the striated muscles of the pharynx, larynx, and esophagus, while the preganglionic neurons of the dorsal motor nucleus of the vagus innervate the Auerbach’s plexus and in the Meissner’s plexus. The parasympathetic excitatory input arises from spinal roots S2-S4 and is transmitted to the anus, rectum, and descending colon. Preganglionic inhibitory sympathetic neurons arise from spinal roots T10-L2, reach the celiac and superior mesenteric ganglia, and innervate the small intestine, the ascending and transverse colon and, via the pelvic ganglion, the internal anal sphincter.75 However, reflex pathways mediating defecation and -bladder control differ markedly.76,77 Large intestine innervation relies on the Auerbach's plexus, which is also affected in PD, and a spinal reflex loop.75 Pathologic involvement of the afferent portion of this spinal reflex loop is likely to contribute to constipation and a sensory deficit may contribute to inhibition of the defecation reflex in the elderly.77
Sexual dysfunction
Brown and co-workers first examined systematically sexual dysfunction in PD patients using a questionnaire. A range of problems, especially loss of ejaculation control in males and loss of libido in females, were reported by 65% of male and 36% of female patients.78 These findings were substantially confirmed in several other studies.71,79–83 The dopaminergic therapy often is often not very effective to improve sexual dysfunction.71 Several variables, such as age, drugs, motor and psychiatric symptoms, may interfere with sexuality. However, an early appearance of sexual dysfunction and the importance of the spinal pathways in controlling the sexual response suggest an early involvement of the spinal cord.
Sympathetic pathways which originate in the dorsolumbar spinal cord reach the penis via the lumbar splanchnic nerves or the paravertebral chain, while parasympathetic neurons, which are located in the sacral parasympathetic nucleus, reach the penis via the pelvic plexus. The Onuf’s nucleus which is located in the sacral cord also innervates the perineal striated muscles. Dorsolumbar sympathetic, sacral parasympathetic, and sacral pudendal neurons are coordinated by interneurons located in laminae VII and X to regulate the processes of erection and ejaculation in men. The supraspinal control is regulated by some brain areas, such as the preoptic area or paraventricular nucleus of the hypothalamus, which project directly to the spinal cord. Therefore, the spinal cord is the key structure upon which the peripheral and central inputs converge.84–86 Sexual dysfunction is common at the early stages of PD;71,87 moreover, it may also precede motor symptoms.88 Erectile dysfunction is an important and early symptom in men with MSA.89 Its earlier occurrence suggests a lack of a causal relationship to autonomic failure and hypotension.
Autonomic failure and orthostatic hypotension
In PAF autonomic failure is caused by neuronal degeneration of pre- and postganglionic sympathetic and parasympathetic neurons located in the thoracic spinal cord and paravertebral autonomic ganglia.90
Autonomic failure is also the core clinicopathological entity of MSA, and is caused by the preganglionic neuronal loss of the thoracic and sacral IML and the loss of visceromotor neurons of Onuf’s nuclei in the second sacral segment. The neuronal loss of the IML was found in 70 out of 80 cases, and was more pronounced in the middle and lower thoracic levels.66
Autonomic failure is commonly expressed through symptoms of orthostatic hypotension.
The control of arterial pressure depends on the arterial baroceptor located in the carotid sinus and in the aortic arch as well as the venous baroceptor located in the heart and lungs. The afferent inputs from the carotid sinus and from the carotid arch, which travel via the glossopharyngeal nerve and via the vagus nerve respectively, and reach the nucleus of the tractus solitarius. Unmyelinated vagal afferents from the baroceptors located in the heart and lungs reach the same nucleus. From this nucleus, polysynaptic fibers reach the nucleus ambiguus and the dorsal motor nucleus of vagus and the sinoatrial node.
Fibers originating in the rostroventral nucleus of the medulla that traveling in the Th/IML provide the efferent innervation. Of great importance is also the capacitance of the splanchnic-mesenteric bed. This region is supplied by the splanchnic nerve with cell bodies at the thoracic level and synapses at the celiac ganglion.91 Orthostatic hypotension is a chief complaint in the pure autonomic failure, in LBD and in MSA. Some cases starting as isolated autonomic failure and subsequently developing PD or LBD features were described.71,92,93 Moreover, dysfunctions of cardiac sympathetic and parasympathetic neurons were observed in the initial stages of PD.93 Even is also the genesis of orthostatic hypotension is likely to be multifactorial, the pathology of the dorsal motor nucleus of the vagus may explain the early occurrence of orthostatic hypotension.
The distribution of LBAS has been immunohistochemically evaluated in subjects with PD, DLB and PAF.94 Since the autonomic functions are diffusely controlled by central and peripheral nervous system, it is difficult to evaluate whether the autonomic nuclei in the spinal cord are related to clinical symptoms. However, the LBAS of IML nuclei may explain the clinical manifestations of autonomic failure associated with LBD.
LBAS and motor symptoms
The locomotion requires the integration of several intentional or automatic processes at different levels. Intentional locomotion initiation arises in the cerebral cortex or in the limbic-hypothalamic system when triggered emotionally. Locomotion regulation processes originate in the cerebral cortex, basal ganglia, and cerebellum. The automatic basic locomotion execution processes are located in the brain stem and in the spinal cord. Two areas located in the mesopontine tegmentum, the midbrain locomotor region and the muscle tone inhibitory region, are involved in the control of locomotion. Two pathways arise from the midbrain locomotor region, descend in the ventrolateral and dorsolateral funiculi, and activate the interneurons of the locomotor central pattern generator located in laminae VII, VIII, and X of the lumbar cord. In contrast, inputs originating from the ventrolateral part of the pedunculopontine tegmental nucleus have inhibitory effects upon the motoneurons.95 LB-like inclusions have been observed in the lumbar spinal cord’s motoneurons of a patient affected by autosomal juvenile PD.33 Unlike in animal models of PD,96 in humans the epidural electrical stimulation of the dorsal columns failed to restore locomotion.97
Mild pyramidal tract signs are a main clinical feature of MSA. α-synuclein-positive glial cytoplasmatic inclusions are diffusely distributed in the pyramidal tract;66 moreover, neuronal cytoplasmatic and neuronal nuclear inclusions have been detected in large motoneurons of the spinal cord of MSA.66
Discussion
The studies reviewed here indicate that the spinal cord pathology plays an important role in PD and other α-synucleinopathies.
In fact, the spinal cord involvement may explain the impairment of several functions such as blood pressure, heart rate, sweating, digestion, and bowel and bladder emptying, in subjects with these neurodegenerative diseases.
The α-synuclein is concentrated in the gray matter in laminae I, II, VII, and X, mainly of the lumbar regions.98 The spinal regions involved in motor control of micturition and constipation, in particular the sacral parasympathetic nucleus which innervate the smooth muscles of the bladder and distal colon and Onuf’s nucleus innervating the striated sphincters, have been mainly examined for the presence LBAS, which has been found in the spinal dorsal horn in ILBD, PD, DLB, and MSA.23,30,37,40,60,64 It has been hypothesized that spinal cord involvement might represent a common feature also in other degenerative diseases, such as the PAF, motor neuron disease (MND), and the complex frontotemporal dementia-MND-PD.99
There are several possible mechanisms through which the accumulation and spread of α-synuclein pathology in the spinal cord may lead to bladder dysfunction and constipation in PD. In particular, LBAS was found to involve the LCR, which is anatomically and neurochemically distinct from the dorsal horn proper and the sacral parasympathetic nucleus. Therefore, LBAS accumulates not only in regions that control motor aspects of micturition, but also in peripheral and central visceral afferent pathways which mediate this function.
LBAS was found in the sacral spinal cord in 2 out of 3 AD dementia cases reported by VanderHost and colleagues,59 thus indicating that α-synuclein pathology might contribute to micturition difficulties and constipation in elderly subjects who do not meet diagnostic criteria for PD.
Autonomic dysfunctions and the experience of pain represent frequently recognized clinical signs in early phases of sporadic PD. These symptoms are caused by early disease-related lesions, but are at present unknown or incompletely known.
It has been postulated that PD progression may be governed by a prion-like spread of a-synuclein throughout the nervous system.100
The pathologic substrate of non-motor manifestations in PD, many of them precede motor dysfunction and represent a preclinical phase lasting 20 or more years, are related to the widespread distribution of α-synuclein pathology, which is not restricted to the dopaminergic nigrostriatal system that is responsible for core motor features of PD. α-synuclein pathology involves, besides non-nigral brainstem nuclei, sympathetic and parasympathetic, enteric, cardiac and pelvic plexuses, as well as many other organs suggesting a topographical and chronological spread, particularly in the early stages of the disease.
The correlation between regional α-synuclein/Lewy pathology, neurodegenerative processes, and the corresponding clinical symptoms needs to be better elucidated, also because few animal models can recapitulate non-motor symptoms in PD. Controlled clinicopathologic studies might better define the relationship between presymptomatic, late-developing non-motor symptoms of PD and neuropathology. The identification of new premotor biomarkers will facilitate early diagnosis of PD, and could therefore represent a basis for more effective preventive and therapeutic options of neurodegenerative disease, in particular PD.101
The etiology of LBAS is still unknown. The Braak's theory postulates that α-synuclein inclusions appear first in peripheral nervous system to spread, following neuronal innervation, towards the central nervous system in a spatio-temporal dissemination described in a staging procedure. Some animal models have been employed in order to reproduce PD and to propose new therapeutic approaches. Different mechanisms can contribute to neurodegeneration in PD such as genetic mutations of α-synuclein gene, mitochondrial dysfunctions, and neuroinflammation.
It should be noted that in the most human studies the correlation between symptoms and autopsy findings is only speculative. There has been no direct clinico-pathological correlation between symptoms in these patients and the autopsy findings. A limitation of this review is that the clinico-pathologic correlations of the single studies have not been deeply discussed for space reasons.
Nevertheless, the present review highlights α-synuclein as the key for PD pathology and alpha synucleinopathies and a main target in PD research. Several therapeutic approaches can be proposed. However, all of them are targeted to advanced stages of the pathology. The focus of future research will be the alteration of α-synuclein physiological pathway, which allows to develop therapy for early stages intracellular or extracellular level. For example, the use of anti ER-stress compounds and innovative immunotherapy could represent promising tools to reduce neuronal degeneration and to halt PD progression.102
Disclaimer statements
Contributors None.
Funding None.
Conflicts of interest The authors declare that they have no conflict of interest.
References
- 1.Del Tredici K, Braak H.. Review: sporadic Parkinson's disease: development and distribution of α-synuclein pathology. Neuropathol Appl Neurobiol 2016;42(1):33–50. [DOI] [PubMed] [Google Scholar]
- 2.Atik A, Stewart T, Zhang J.. Alpha-Synuclein as a Biomarker for Parkinson's disease. Brain Pathol 2016;26(3):410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wakabayashi K, Tanji K, Odagiri S, Miki Y, Mori F, Takahashi H.. The Lewy body in Parkinson's disease and related neurodegenerative disorders. Mol Neurobiol 2013;47:495–508. [DOI] [PubMed] [Google Scholar]
- 4.Lee HJ, Bae EJ, Lee SJ.. Extracellular α–synuclein-a novel and crucial factor in Lewy body diseases. Nat Rev Neurol 2014;10(2):92–8. [DOI] [PubMed] [Google Scholar]
- 5.Mayo MC, Bordelon Y.. Dementia with Lewy bodies. Semin Neurol 2014;34(2):182–8. [DOI] [PubMed] [Google Scholar]
- 6.Arai Y, Yamazaki M, Mori O, Muramatsu H, Katayama Y.. Apha-synuclein positive structures in cases with sporadic Alzheimer’s disease: Morphology and its relationship to tau aggregation. Brain Res 2001;888(2):287–96. [DOI] [PubMed] [Google Scholar]
- 7.Tong J, Wong H, Guttman M, Ang LC, Forno LS, Shimadzu M, et al. Progressive supranuclear palsy: A comparative investigation. Brain 2009;133(Pt 1):172–88. [DOI] [PubMed] [Google Scholar]
- 8.Lippa CF, Schmidt ML, Lee VM, Trijanowski JQ.. Antibodies to alpha-synuclein detect Lewy bodies in many Down’s syndrome brains with Alzheimer’s disease. Ann Neurol 1999;45(3):353–7. [DOI] [PubMed] [Google Scholar]
- 9.Johnson RH, Lee J, Oppenheimer DR, Spalding R.. Autonomic failure with orthostatic hypotension due to intermediolateral column degeneration. Q J Med 1966;35(138):276–92. [PubMed] [Google Scholar]
- 10.Oppenheimer DR. Lateral horn cells in progressive autonomic failure. J Neurol Sci 1980;46:393–404. [DOI] [PubMed] [Google Scholar]
- 11.Braak H, Braak E, Yilmazer D, De Vos RA, Jansen EN, Bohl J, et al. Amygdala pathology in Parkinson’s disease. Acta Neuropathol 1994;88(3):493–500. [DOI] [PubMed] [Google Scholar]
- 12.Braak H, Sandmann-Keil D, Gai WP, Braak E.. Extensive axonal Lewy neurites in Parkinson’s disease: a novel pathological feature revealed by α-synuclein. Neurosci Lett 1999;265(1):67–9. [DOI] [PubMed] [Google Scholar]
- 13.Pouclet H, Lebouvier T, Coron E, Des Varannes SB S.B., Rouaud T, Roy M, et al. A comparison between rectal and colonic biopsies to detect Lewy pathology in Parkinson’s disease. Neurobiol Dis 2012;45(1):305–9. [DOI] [PubMed] [Google Scholar]
- 14.Braak E, Sandmann-Keil D, Rüb U, Gai WP, de Vos RA, Steur EN, et al. Alpha-synuclein immunopositive Parkinson’s disease-related inclusion bodies in lower brain stem nuclei. Acta Neuropathol 2001;101(3):195–201. [DOI] [PubMed] [Google Scholar]
- 15.Kuusisto E, Parkkinen L, Alafuzoff I.. Morphogenesis of Lewy bodies: dissimilar incorporation of α-synuclein, ubiquitin, and p62. J Neuropathol Exp Neurol 2003;62(12):1241–53. [DOI] [PubMed] [Google Scholar]
- 16.Dale GE, Probst A, Luthert P, Martin J, Anderton BH, Leigh PN.. Relationships between Lewy bodies and pale bodies in Parkinson’s disease. Acta Neuropathol 1992;83(5):525–9. [DOI] [PubMed] [Google Scholar]
- 17.Den Hartog Jager WA, Bethlem J.. The distribution of Lewy bodies in the central and autonomic nervous systems in idiopathic paralysis agitans. J Neurol Neurosurg Psychiat 1960;23:283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forno LS. The Lewy body in Parkinson’s disease. Adv Neurol 1987;45:35–43. [PubMed] [Google Scholar]
- 19.Wakabayashi K, Takahashi H, Ohama E, Ikuta F.. Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol 1990;79(6):581–3. [DOI] [PubMed] [Google Scholar]
- 20.Wakabayashi K, Takahashi H, Ohama E, Takeda S, Ikuta F.. Lewy bodies in the visceral autonomic nervous system in Parkinson’s disease. Adv Neurol 1993;60:609–12. [PubMed] [Google Scholar]
- 21.Del Tredici K, Rub U, De Vos R, Bohl JR, Braak H.. Where does Parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol 2002;61(5):413–26. [DOI] [PubMed] [Google Scholar]
- 22.Jellinger KA. Lewy body-related alpha-synucleinopathy in the aged human brain. J Neural Transm 2004;111(10–11):1219–35. [DOI] [PubMed] [Google Scholar]
- 23.Bloch A, Probst A, Bissig H, Adams H, Tolnay M.. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol 2006;32(3):284–95. [DOI] [PubMed] [Google Scholar]
- 24.Klos KJ, Ahlskog JE, Josephs KA, Apaydin H, Parisi JE, Boeve BF, et al. Alpha-synuclein pathology in the spinal cords of neurologically asymptomatic aged individuals. Neurology 2006;66(7):1100–1102. [DOI] [PubMed] [Google Scholar]
- 25.Wakisaka Y, Furuta A, Tanizaki Y, Kiyohara Y, Iida M, Iwaki T.. Age-associated prevalence and risk factors of Lewy body pathology in a general population: The Hisayama study. Acta Neuropathol 2003;106(4):374–82. [DOI] [PubMed] [Google Scholar]
- 26.Ding ZT, Wang Y, Jianf YP, Hashizume Y, Yoshida M, Mimuro M, et al. Characteristics of alphasynucleinopathy in centenarians. Acta Neuropathol 2006;111(5):450–458. [DOI] [PubMed] [Google Scholar]
- 27.Sukimura H, Takao M, Hatsuta H, Ito S, Nakano Y, Uchino A, et al. Distribution of α-synuclein in the spinal cord and dorsal root ganglia in an autopsy cohort of elderly persons. Acta Neuropathol Commun 2015;3:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamura T, Yoshida M, Hashizume Y, Sobue G.. Lewy body-related α-synucleinopathy in the spinal cord of cases with incidental Lewy body disease. Neuropathology 2012;32(1):13–22. [DOI] [PubMed] [Google Scholar]
- 29.Mendritzki S, Schmidt S, Sczepan T, Zhu XR, Segelcke D, Lübbert H.. Spinal cord pathology in alpha-synuclein transgenic mice. Parkinson Dis 2010;2010:375462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oyanagi K, Wakabayashi K, Ohama E, Takeda S, Horikawa Y, Morita T, et al. Lewy bodies in the lower sacral parasympathetic neurons of a patient with Parkinson’s disease. Acta Neuropathol 1990;80(5):558–9. [DOI] [PubMed] [Google Scholar]
- 31.O'Sullivan SS, Massey LA, Williams DR, Revesz T, Lees A, Holton J.. Parkinson’s disease with Onuf’s nucleus involvement mimicking multiple system atrophy. J Neurol Neurosurg Psychiatry 2008;79(2):232–4. [DOI] [PubMed] [Google Scholar]
- 32.van de Warrenburg BP, Lammens M, Lücking CB, Denèfle P, Wesseling P, Booij J, et al. Clinical and pathologic abnormalities in a family with parkinsonism and parkin genemutations. Neurology 2001;56(4):555–7. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki S, Shirata A, Yamane K, Iwata M.. Involvement of spinal motor neurons in parkin-positive autosomal recessive juvenile parkinsonism. Neuropathol 2008;28(1):74–80. [DOI] [PubMed] [Google Scholar]
- 34.Wakabayashi K, Takahashi H.. Neuropathology of autonomic nervous system in Parkinson’s disease. Eur Neurol 1997a;38:2–7. [DOI] [PubMed] [Google Scholar]
- 35.Wakabayashi K, Takahashi H.. The intermediolateral nucleus and Clarke’s column in Parkinson’s disease. Acta Neuropathol 1997b;94(3):287–9. [DOI] [PubMed] [Google Scholar]
- 36.Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K.. Parkinson’s disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol 2007;113(4):421–9. [DOI] [PubMed] [Google Scholar]
- 37.Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White Iii CL, et al. Arizona Parkinson's disease Consortium. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 2010;119(6):689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wakabayashi K, Takahashi H, Ohama E, Takeda S, Ikuta F.. Lewy bodies in the visceral autonomic system in Parkinson’s disease. Adv Neurol 1993;60:609–12. [PubMed] [Google Scholar]
- 39.Wakabayashi K, Mori F, Tanji K, Orimo S, Takahashi H.. Involvement of the peripheral nervous system in synucleinopathies, tauopathies and other neurodegenerative proteinopathies of the brain. Acta Neuropathol 2010;120(1):1–12. [DOI] [PubMed] [Google Scholar]
- 40.Del Tredici K, Braak H.. Spinal cord lesions in sporadic Parkinson's disease. Acta Neuropathol 2012;124(5):643–64. [DOI] [PubMed] [Google Scholar]
- 41.Pinto V, Szucs P, Lima D, Safranov B.. Multisegmental A delta- and C-fiber input to neurons in lamina I and the lateral spinal nucleus. J Neurosci 2010;30(6):2384–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takazawa T, Macdermott A.. Synaptic pathways and inhibitory gates in the spinal cord dorsal horn. Ann NY Acad Sci 2010;1198:153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 2010;11(12):11823–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanderhorst VG, Mouton LJ, Blok BF, Holstege G.. Distinct cell groups in the lumbosacral cord of the cat project to different areas in the periaqueductal gray. J Comp Neurol 1996;376(3):361–85. [DOI] [PubMed] [Google Scholar]
- 45.Vanderhorst VG, Terasawa E, Ralston HJ 3rd. Estrogen receptor-alpha immunoreactive neurons in the ventrolateral periaqueductal gray receive monosynaptic input from the lumbosacral cord in the rhesus monkey. J Comp Neurol 2002;443(1):27–42. [DOI] [PubMed] [Google Scholar]
- 46.De Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K.. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst 1981;3(2–4):135–60. [DOI] [PubMed] [Google Scholar]
- 47.Block BF, Holstege G.. Direct projections from the periaqueductal gray to the pontine micturition center (M-region). An anterograde and retrograde tracing study in the cat. Neurosci Lett 1994;166(1):93–6. [DOI] [PubMed] [Google Scholar]
- 48.Djaldetti R, Shifrin A, Rogowski Z, Sprecher E, Melamed E, Yarnitsky D.. Quantitative measurement of pain sensation in patients with Parkinson’s disease. Neurology 2001;62(12):2171–5. [DOI] [PubMed] [Google Scholar]
- 49.Lees AJ. A sustained-release formulation of L-dopa [Madopar HBS] in the treatment of nocturnal and earlymorning disabilities in Parkinson’s disease. Eur Neurol 1986;27:126–34. [DOI] [PubMed] [Google Scholar]
- 50.Snider SR, Fahn S, Isgreen WP, Cote LJ.. Primary sensory symptoms in parkinsonism. Neurology 1976;26(5):423–9. [DOI] [PubMed] [Google Scholar]
- 51.Winge K, Fowler CJ.. Bladder dysfunction in parkinsonism: mechanisms, prevalence, symptoms. Mov Disord 2006;21(6):737–45. [DOI] [PubMed] [Google Scholar]
- 52.Araki I, Kuno S.. Assessment of voiding dysfunction in Parkinson’s disease by the international prostate symptoms score. J Neurol Neurosurg Psychiatry 2000;68(4):429–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gray R, Stern G, Malone-Lee J.. Lower urinary tract dysfunction in Parkinson’s disease: changes relate to age and not disease. Age Ageing 1995;24(6):499–504. [DOI] [PubMed] [Google Scholar]
- 54.Campos-Sousa R, Quagliato E, Da Silva B, De Carvalho R, Ribiero S, De Carvalho D.. Urinary symptoms in Parkinson’s disease: Prevalence and associated factors. Arq Neuropsiquiatr 2003;61(2B):359–63. [DOI] [PubMed] [Google Scholar]
- 55.Yoshimura N, Sasa M, Yoshida O, Takaori S.. Dopamine D1 receptor-mediated inhibition of micturition reflex by central dopamine from the substantia nigra. Neurourol Urodyn 1992;11:535–45. [Google Scholar]
- 56.Aranda B, Cramer P.. Effects of apomorphine and L-dopa on the parkinsonian bladder. Neurourol Urodyn 1993;12(3):203–9. [DOI] [PubMed] [Google Scholar]
- 57.Singer C. Urinary dysfunction in Parkinson’s disease. Clin Neurosci 1998;5(2):78–86. [PubMed] [Google Scholar]
- 58.Uchiyama T, Sakakibara R, Hattori T, Yamanishi T.. Short-term effect of a single Levodopa dose on micturition disturbance in Parkinson’s disease patients with the wearing-off phenomenon. Mov Disord 2003;18(5):573–8. [DOI] [PubMed] [Google Scholar]
- 59.VanderHorst VG, Samardzic T, Saper CB, Anderson MP, Nag S, Schneider JA, et al. α-Synuclein pathology accumulates in sacral spinal visceral sensory pathways. Ann Neurol 2015;78(1):142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oinas M, Paetau A, Myllykangas L, Notkola IL, Kalimo H, Polvikoski T.. α-Synuclein pathology in the spinal cord autonomic nuclei associates with alpha-synuclein pathology in the brain: a population-based Vantaa 85+ study. Acta Neuropathol 2010;119(6):715–22. [DOI] [PubMed] [Google Scholar]
- 61.Fowler CJ. Integrated control of lower urinary tract – clinical perspective. Br J Pharmacol 2006;147:S14–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Athwal BS, Berkley KJ, Hussain I, Brennan A, Craggs M, Sakakibara R, et al. Brain responses to changes in bladder volume and urge to void in healthy men. Brain 2001;124(Pt2):369–77. [DOI] [PubMed] [Google Scholar]
- 63.Kuhtz-Buschbeck JP, Gilster R, van der Horst C, Hamann M, Wolff S, Jansen O.. Control of bladder sensations: an fMRI study of brain activity and effective connectivity. NeuroImage 2009;47(1):18–27. [DOI] [PubMed] [Google Scholar]
- 64.Cersosimo MG, Benarroch EE.. Central control of autonomic function and involvement in neurodegenerative disorders. Handb Clin Neurol 2013;117:45–57. [DOI] [PubMed] [Google Scholar]
- 65.Benarroch EE, Schmeichel AM.. Depletion of corticotrophin-releasing factor neurons in the pontine micturition area in multiple system atrophy. Ann Neurol 2001;50(5):640–5. [DOI] [PubMed] [Google Scholar]
- 66.Yoshida M. Multiple system atrophy: alpha-synuclein and neuronal degeneration. Neuropathology 2007;27(5):484–93. [DOI] [PubMed] [Google Scholar]
- 67.Dickson DW, Liu W, Hardy J, Farrer M, Mehta N, Uitti R, et al. Widespread alterations of alpha-synuclein in multiple system atrophy. Am J Pathol 1999;155(4):1241–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Edwards LL, Pfeiffer RF, Quigley EM, Hofman R, Balluff M.. Gastrointestinal symptoms in Parkinson’s disease. Mov Disord 1991;6(2):151–6. [DOI] [PubMed] [Google Scholar]
- 69.Jost WH. Gastrointestinal motility problems in patients with Parkinson’s disease. Effects of antiparkinsonian treatment and guidelines for management. Drugs Aging 1997;10(4):249–58. [DOI] [PubMed] [Google Scholar]
- 70.Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol 2003;2(2):107–16. [DOI] [PubMed] [Google Scholar]
- 71.Verbaan D, Marinus J, Visser M, Van Rooden SM, Stiggelbout AM, Van Hilten JJ.. Patient-reported autonomic symptoms in Parkinson’s disease. Neurology 2007;69(4):333–41. [DOI] [PubMed] [Google Scholar]
- 72.Abbott RD, Ross GW, Petrovitch H, Tanner CM, Davis DG, Masaki KH, Bowel movement frequency in late-life and incidental Lewy bodies. Mov Disord 2007;22(11):1581–6. [DOI] [PubMed] [Google Scholar]
- 73.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E.. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003;24(2):197–210. [DOI] [PubMed] [Google Scholar]
- 74.Kupsky WJ, Grimes MM, Sweeting J, Bertsch R, Cote LJ.. Parkinson’s disease and megacolon: Concentric hyaline inclusions [Lewy bodies] in enteric ganglion cells. Neurology 1987;37(7):1253–5. [DOI] [PubMed] [Google Scholar]
- 75.Lynch AC, Antony A, Dobbs BR, Frizelle A.. Bowel dysfunction following spinal cord injury. Spinal Cord 2001;39(4):193–203. [DOI] [PubMed] [Google Scholar]
- 76.Uher EM, Swash M.. Sacral reflexes: physiology and clinical application. Dis Colon Rectum 1988;41(9):1165–77. [DOI] [PubMed] [Google Scholar]
- 77.Varma JS, Bradnock J, Smith RG, Smith AN.. Constipation in the elderly. A physiologic study. Dis Colon Rectum 1988;31(2):111–5. [DOI] [PubMed] [Google Scholar]
- 78.Brown RG, Jahanshahi M, Quinn N, Marsden CD.. Sexual function in patients with Parkinson’s disease and their partners. J Neurol Neurosurg Psychiatry 1990;53(6):480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Celikel E, Ozel-Kizil E, Akbostanci MC, Cevik A.. Assessment of sexual dysfunction in patients with Parkinson’s disease: a case-control study. Eur J Neurol 2008;15(11):1168–72. [DOI] [PubMed] [Google Scholar]
- 80.Jacobs H, Vieregge A, Vieregge P.. Sexuality in young patients with Parkinson’s disease: a population based comparison with healthy controls. J Neurol Neurosurg Psychiatry 2000;69(4):550–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meco G, Rubino A, Carovana N, Valente M.. Sexual dysfunction in Parkinson’s disease. Parkinsonism Relat Disord 2008;14(6):451–6. [DOI] [PubMed] [Google Scholar]
- 82.Moore O, Gurevich T, Korczyn AD, Anca M, Shabtai H, Giladi N.. Quality of sexual life in Parkinson’s disease. Parkinsonism Relat Disord 2002;8(4):243–6. [DOI] [PubMed] [Google Scholar]
- 83.Welsh M, Hung L, Waters CH.. Sexuality in women with Parkinson's disease. Mov Disord 1997;12(6):923–7. [DOI] [PubMed] [Google Scholar]
- 84.Coolen LM, Allard J, Truitt WA, McLenna KE.. Central regulation of ejaculation. Physiol Behav 2004;83(2):203–15. [DOI] [PubMed] [Google Scholar]
- 85.Giuliano F, Rampin O.. Neural control of erection. Physiol Behav 2004;83(2):189–201. [DOI] [PubMed] [Google Scholar]
- 86.Marson M, Platt KB, McKenna KE.. Central nervous system innervation of the penis as revealed by the transneuronal transport of pseudorabies virus. Neuroscience 1993;55(1):263–80. [DOI] [PubMed] [Google Scholar]
- 87.Kim HJ, Park SY, Cho YJ, Hong KS, Cho JY, Seo SY.. Nonmotor symptoms in de novo Parkinson disease before and after dopaminergic treatment. J Neurol Sci 2009;287(1–2):200–204. [DOI] [PubMed] [Google Scholar]
- 88.Gao X, Chen H, Schwarzschild MA, Glasser DB, Logroscino G, Rimm EB, et al. Erectile function and risk of Parkinson’s disease. Am J Epidemiol 2007;166(12):1446–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kirchhof K, Apostolidis AN, Mathias CJ, Fowler CJ.. Erectile and urinary dysfunction may be the presenting features in patients with multiple system atrophy: a retrospective study. Int J Impot Res 2003;15(4):293–8. [DOI] [PubMed] [Google Scholar]
- 90.Thaisetthawatkul P. Pure autonomic failure. Curr Neurol Neurosci Rep 2016;16(8):74. [DOI] [PubMed] [Google Scholar]
- 91.Low PA, Singer W.. Update on management of neurogenic orthostatic hypotension. Lancet Neurol 2008;7(5):451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaufmann H, Nahm K, Purohit D, Wolfe D.. Autonomic failure as the initial presentation of Parkinson disease and dementia with Lewy bodies. Neurology 2001;63(6):1093–5. [DOI] [PubMed] [Google Scholar]
- 93.Post KK, Singer C, Papapetropoulos S.. Cardiac denervation and dysautonomia in Parkinson’s disease: A review of screening techniques. Parkinsonism Relat Disord 2008; 14(7):524–31. [DOI] [PubMed] [Google Scholar]
- 94.Hishikawa N, Hashizume Y, Hirayama M, Imamura K, Washimi Y, Koike Y.. Brainstem-type Lewy body disease presenting with progressive autonomic failure and lethargy. Clin Auton Res 2000;10(3):139–43. [DOI] [PubMed] [Google Scholar]
- 95.Takakusaki K, Tomita N, Yano M.. Substrates for normal gait and pathophysiology of gait disturbances with respect to the basal ganglia dysfunction. J Neurol 2008;255:19–29. [DOI] [PubMed] [Google Scholar]
- 96.Fuentes R, Petersson P, Siesser WB, Caron MG, Nicolelis MA.. Spinal cord stimulation restores locomotion in animal models of Parkinson’s disease. Science 2009;323(5921):1578–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thevathasan W, Mazzone P, Jha A, Djamshidian A, Dileone M, Di Lazzaro V, et al. Spinal cord stimulation failed to relieve akinesia or restore locomotion in Parkinson disease. Neurology 2010;74(16):1325–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vivacqua G, Casini A, Vaccaro R, Salvi EP, Pasquali L, Fornai F, et al. Spinal cord and parkinsonism: neuromorphological evidences in humans and experimental studies. J Chem Neuroanat 2011;42(4):327–40. [DOI] [PubMed] [Google Scholar]
- 99.Vivacqua G, Yin JJ, Casini A, Li X, Li YH, D'Este L, et al. Immunolocalization of alpha-synuclein in the rat spinal cord by two novel monoclonal antibodies. Neuroscience 2009;158:1478–87. [DOI] [PubMed] [Google Scholar]
- 100.Visanji N, Marras C.. The relevance of pre-motor symptoms in Parkinson's disease. Expert Rev Neurother 2015;15(10):1205–17. [DOI] [PubMed] [Google Scholar]
- 101.Jellinger KA. Neuropathobiology of non-motor symptoms in Parkinson disease. J Neural Transm 2015;122(10):1429–40. [DOI] [PubMed] [Google Scholar]
- 102.Miraglia F, Betti F, Paego L, Giannaccini G.. Parkinson's disease and alpha-synucleinopathies: from arising pathways to therapeutic challenge. Cent Nerv Syst Agents Med Chem 2016;15(2):109–16. [DOI] [PubMed] [Google Scholar]