Abstract
Background:
Age-dependent differences in clinical presentation and viral loads in infants and young children with RSV infection, and their correlation with disease severity are poorly defined.
Methods:
Previously healthy children <2 years old with mild (outpatients) and severe (inpatients) RSV infection were enrolled and viral loads measured by PCR in nasopharyngeal swabs. Patients were stratified by age in 0-<3, 3–6, and >6–24 months, and multivariable analyses were performed to identify clinical and viral factors associated with severe disease.
Results:
From 2014–2018 we enrolled 534 children with RSV infection, 130 outpatients with mild RSV infection and 404 inpatients with severe RSV disease. Median duration of illness was 4 days for both groups, yet viral loads were higher in outpatients than in inpatients (p<0.001). In bivariate analyses, wheezing was more frequent in outpatients of older age (>3 months) than in inpatients (p<0.01), while fever was more common in inpatients than outpatients (p<0.01) and its frequency increased with age. Adjusted analyses confirmed that increased work of breathing and fever were consistently associated with hospitalization irrespective of age, while wheezing in infants >3 months, and higher RSV loads in children >6–24 months were independently associated with reduced disease severity.
Conclusions:
Age had a significant impact defining the interactions among viral loads, specific clinical manifestations and disease severity in children with RSV infection. These observations highlight the importance of patient stratification when evaluating interventions against RSV.
Keywords: Respiratory syncytial virus, viral loads, clinical disease severity, clinical symptoms, infants
INTRODUCTION
Respiratory syncytial virus (RSV) is the most common cause of bronchiolitis leading to hospitalization in infants worldwide, and the second cause of infant mortality in resource-limited countries (1, 2). Despite the disease burden, no vaccines for RSV are currently licensed. In addition, there are no specific therapeutic options for these patients which is due in part to our limited understanding of the pathogenesis of the disease (3).
The variability of clinical manifestations caused by RSV in young children is wide and range from mild upper respiratory tract infection to bronchiolitis when there is involvement of the lower respiratory tract, with no consensus on its definition (4, 5). Nevertheless, RSV disease represents a continuum in which the immaturity of the host immune response appears to play a fundamental role (6). In fact, studies performed in otherwise healthy infants have consistently identified young age as a risk factor for severe RSV disease (7). On the other hand, the role of viral loads on clinical disease severity has also been thoroughly studied in hospitalized infants with inconclusive results (8–12). These inconsistent findings might be related to differences in study design, but also to other factors such as not including adequate number of outpatients with mild disease, and not carefully assessing for the influence of age. A better understanding of the relationship between demographic characteristics, clinical manifestations, and RSV viral loads in young children with mild (outpatients) and severe (inpatients) disease across different ages is particularly relevant for evaluation of targeted interventions against RSV, and may help with patient stratification in the clinical setting. We sought to define differences in demographic, clinical manifestations and viral loads between children < 2 years of age with mild RSV infection evaluated as outpatients versus those hospitalized with RSV bronchiolitis, stratified by age.
METHODS
Study design
Previously healthy children < 2 years of age with RSV infection were enrolled either at Primary Pediatric Offices, the Emergency Department or Urgent Care (outpatients) or at Nationwide Children’s Hospital (NCH) hospital units (inpatients) during four respiratory seasons. RSV diagnosis was established per standard of care by rapid antigen testing (Sofia RSV FIA; Quidel®) or a PCR panel (FilmArray Respiratory Viral Panel; BioFire, BioMerieux) (12). Patients were excluded from the study if they were premature (<37 weeks’ gestation), had chronic underlying conditions, or congenital or acquired immunodeficiency.
At enrollment we collected in all study patients: a) demographic and detailed clinical manifestations, including a clinical disease severity score (CDSS) (12), b) a nasopharyngeal swab for RSV typing and quantitation by real-time PCR targeting the N gene as described (13), and c) a blood sample for white blood cell count (WBC) with differential. The CDSS ranged from 0 (normal) to 15 (most abnormal) and comprised 5 parameters: respiratory rate, auscultation, transcutaneous O2 saturation, retractions, and level of activity (see Table, Supplemental Digital Content 1) (12–14). The CDSS has been validated internally and has shown to reliably categorize patients with mild and severe RSV disease in our previous studies. It has also shown to consistently stratified patients according to standard disease severity outcomes, such as need for hospitalization.
Inpatient status was defined as a hospital admission for > 24h. Demographic and clinical information was collected by a study team member at enrollment using standardized electronic questionnaires designed for the study, and information transferred to a secure electronic database (REDCap) as described (12). As the study was conducted prospectively the amount of missing data was negligible, and if needed, data was extracted from the electronic health care records. Families were contacted at 2 and 4 weeks after enrollment to confirm the lack of subsequent need for readmission in the inpatient group or hospitalization in the outpatient cohort.
Statistical Analysis
Data are summarized using frequencies and percentages for categorical variables, means and standard deviations for normally distributed continuous variables, and medians with interquartile ranges for non-normally distributed continuous variables. Group comparisons were evaluated with chi-square or Fisher’s exact tests, two-sample t-tests or Wilcoxon rank sum tests, and one-way ANOVA or Kruskal-Wallis tests. Pairwise comparisons were adjusted for multiplicity with Tukey or Benjamini-Hochberg corrections. Correlations were computed using Spearman’s correlation coefficient.
To determine which factors were independently associated with disease severity defined by the need for hospitalization according to age (0 - <3 months, 3 −6 months, and >6–24 months) we used multivariable logistic regression. For all multivariable analyses, variables were entered into the model if they had a univariate p-value of <0.15, and were retained in the model if they had an adjusted p-value of <0.1, or if their inclusion had a substantial impact on model goodness of fit, based on Akaike’s information criterion (AIC)(15). RSV loads were retained in all models as a key risk factor of interest. In addition, we evaluated the association between age and RSV loads using polynomial regression and restricted cubic spline regression. All analyses were conducted using GraphPad Prism v.8 (GraphPad Software) and R for Statistical Computing with a two-sided p-value <0.05 considered statistically significant.
Ethical Considerations
The study was approved by the Institutional Review Board (IRB) at Nationwide Children’s Hospital and classified as a level 1 risk clinical study- no greater than minimal risk- pursuant under 45 Code Federal Regulations (CFR) 46.404 and 21 CFR 50.51. Informed consent procedures followed in compliance with Nationwide Children’s Research Responsible Conduct Guidelines.
RESULTS
Demographic and Clinical Characteristics of Study Patients
From October 2014 to March 2018 we enrolled a convenience sample of 534 previously healthy children < 2 years of age with acute RSV infection. Of those 139 were originally diagnosed with mild RSV infection in the outpatient setting (outpatients- OP-) and 395 were hospitalized (inpatients -IP-) with RSV lower respiratory tract infection (LRTI). Nine of the 139 outpatients were subsequently hospitalized within 36–40 hours of the outpatient visit and thus included in the inpatient cohort, leaving a total of 130 RSV outpatients and 404 RSV inpatients hospitalized in the inpatient ward (n=305) or pediatric intensive care unit (PICU; n=99, see Table, Supplemental Digital Content 2). Inpatients were enrolled within 20 [14–32] hours of admission. Median age of outpatients was 6.0 [3.2–10.1] months and 2.7 [1.5–5.7] months in the inpatient group (p<0.001). The majority of patients (478; 90%) were <12 months of age at the time of study enrollment. Children in the inpatient cohort were more frequently white, and those in the outpatient group had higher rates of breastfeeding and daycare attendance (Table 1).
Table 1.
Demographic, Clinical, and virologic characteristics in infants with mild (outpatients) and severe (inpatients) RSV infection
| Outpatients (n=130) | Inpatients (n=404) | p-value | |
|---|---|---|---|
| Demographic Characteristics | |||
| Age, months | 6.0 [3.2–10.1] | 2.7 [1.5–5.7] | <0.001 |
| Age groups | |||
| < 3 months | 30 (23.1) | 222 (55.0) | |
| 3–6 months | 38 (29.2) | 90 (22.3) | <0.001 |
| >6–24 months | 62 (47.7) | 92 (22.8) | |
| Sex (male) | 66 (50.8) | 219 (54.2) | 0.54 |
| Race | |||
| White | 63 (48.5) | 276 (68.3) | |
| Black | 49 (37.7) | 64 (15.8) | <0.001 |
| Other | 18 (13.8) | 64 (15.8) | |
| Vaginal delivery | 88/118 (74.6) | 278/397 (70.0) | 0.36 |
| Breastfed | 81/130 (62.3) | 166/360 (46.1) | 0.002 |
| Daycare attendance | 49/130 (37.7) | 90/360 (25.0) | 0.009 |
| Smoke exposure | 42/129 (32.6) | 128/360 (35.6) | 0.59 |
| Vaccines up to date | 112/123 (91.1) | 344/402 (85.6) | 0.13 |
| Asthma (family history) | 56/130 (43.1) | 199/404 (49.3) | 0.23 |
| Signs and Symptoms | |||
| Cough | 128 (98.5) | 380 (94.1) | 0.06 |
| Nasal congestion | 116 (89.2) | 337 (83.4) | 0.12 |
| Decreased oral intake | 58 (44.6) | 209 (51.7) | 0.19 |
| Fever | 61 (46.9) | 250 (61.9) | 0.003 |
| Rhinorrhea | 97 (74.6) | 165 (40.8) | <0.001 |
| Conjunctivitis | 20 (15.4) | 39 (9.7) | 0.08 |
| Vomiting | 46 (35.4) | 100 (24.8) | 0.02 |
| Diarrhea | 31 (23.8) | 52 (12.9) | 0.005 |
| Wheezing (parental report) | 71 (54.6) | 165 (40.8) | 0.006 |
| Wheezing (by physician) | 85 (65.4) | 208 (51.5) | 0.006 |
| Increased WOB (parental report) | 56 (43.1) | 234 (57.9) | 0.003 |
| Increased WOB (by physician) | 46 (35.4) | 278 (68.8) | <0.001 |
| Laboratory/Virology data | |||
| WBC (10*3/uL) | 9.90 [7.28–12.03] | 10.20 [7.90–13.48] | 0.075 |
| Neutrophils (%) | 29.0 [22.0–43.0] | 32.0 [22.0–45.0] | 0.42 |
| Lymphocytes (%) | 54.3 [42.5–64.5] | 53.0 [41.0–63.0] | 0.38 |
| Monocytes (%) | 11 [7–14] | 12 [9–15] | 0.24 |
| RSV viral loads (log10 copies/mL) | 7.97 [7.26–8.55] | 7.44 [6.61–8.09] | <0.001 |
| RSV viral type | |||
| A | 60 (46.1) | 223 (55.2) | 0.09 |
| B | 70 (53.8) | 181 (44.8) | |
| Viral co-detection | 17/59 (28.8) | 75/343 (21.9) | 0.24 |
| RV/EV | 8 (13.5) | 47 (13.7) | |
| Adenovirus | 3 (5.0) | 9 (2.6) | |
| hMPV | 2 (3.3) | 1 (0.3) | |
| Influenza virus | 0 (0.0) | 3 (0.9) | |
| Parainfluenza virus | 3 (5.0) | 4 (1.2) | |
| Coronavirus | 5 (8.4) | 20 (5.8) | |
Categorical data are expressed as frequencies (%) and analyzed using Fisher or chi-square test. Continuous data are expressed as median (25%-75% interquartile range) and analyzed using Man-Whitney rank U test. Values in bold indicate significant 2-sided p values. WBC values were available in 81% of infants in each cohort. The number of children who underwent multiplex testing is denoted in the denominator of the viral co-detection section. RSV, respiratory syncytial virus; CDSS, Clinical disease severity score; WOB, work of breathing; WBC, white blood cell count; RV, rhinovirus, EV, enterovirus; hMPV, human metapneumovirus
Median [25%−75% interquartile range-IQR] duration of symptoms at enrollment for outpatients (4 [3–5] days) and inpatients (4 [3–6] days) was similar. Overall, 40% (211/534) of children at enrollment had at least one previous medical visit for the same illness. The frequency of these previous medical encounters was similar between inpatients 41% (165/404) and outpatients (35% (46/130); p=0.30).
Among the signs and symptoms evaluated, cough (94%−98%) followed by nasal congestion (83%−89%) and decreased oral intake (45%−52%) were present in both groups irrespective of disease severity. Rhinorrhea (75% vs 41%; p<0.001), and presence of wheezing both by parental report (55% vs 41%; p=0.003) and physician report (65% vs 51%; p=0.006) were more common in outpatients than among inpatients. Other symptoms such as vomiting (35% vs. 25%; p=0.02), and diarrhea (24% vs. 13%; p=0.005) were more common in outpatients. On the other hand, fever (62% vs 47%; p=0.003), and increased work of breathing (69% vs 43%; p<0.005) both by parental report or documented by the evaluating physician, were more frequent in inpatients than in outpatients (Table 1). Increased work of breathing by parental report was defined as the presence of fast breathing, if muscles between the ribs were pulling inward or if parents had observed changes from basal respiratory rate, and by physician report as the presence of retractions, use of accessory muscle or nasal flaring. Hospitalized children also had higher clinical disease severity scores (CDSS) than outpatients (median [25%−75% IQR]: 6[4–8] vs 3[2–4]; p<0.001). Within hospitalized children CDSS were higher in those needing PICU vs ward care (10 [7–15] vs 5 [3–7] respectively; p<0.001) (see Table, Supplemental Digital Content 2).
RSV loads at enrollment were higher in outpatients than in inpatients (median [25%−75% IQR]: 7.97 [7.26–8.55] vs 7.44 [6.61–8.09] log10 copies/mL respectively; p<0.001; see figure, Supplemental Digital Content 3a), and within hospitalized children viral loads were higher in ward vs PICU patients (7.48 [6.69–8.14] vs 7.26 [6.11–7.87] log10 copies/mL respectively; p<0.01). In addition, based on the CDSS infants with mild disease (CDSS ≤ 5) compared with those with moderate/severe disease (CDSS >6–15) had also significantly higher viral loads (see figure, Supplemental Digital Content 3b). There were no differences in the proportion of RSV A or B types in these children or in the rates of viral co-detection in inpatients (22%) or outpatients (28%). In both RSV cohorts, rhinovirus/enterovirus and coronaviruses were the viruses most commonly co-detected.
Signs and Symptoms in Inpatients and Outpatients with RSV Infection According to Age
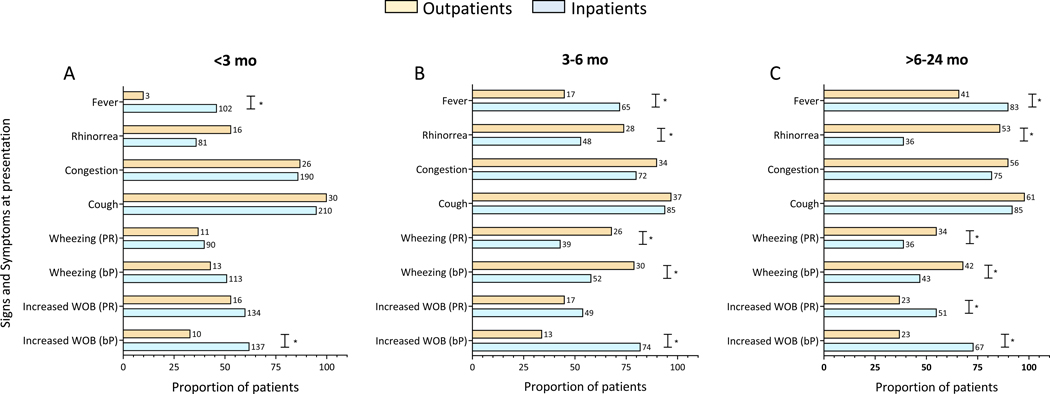
We then analyzed whether the frequency of specific signs and symptoms differed according to age. To this end, RSV outpatients and inpatients were stratified in three different groups: 0-<3 months of age (n=252 (47%); OP, n=30; IP, n=222); 3 to 6 months of age (n=128 (24%); OP, n= 38; IP n=90) and >6–24 months of age (n=154 (29%) patients; OP, n=62; IP, n=92) (Fig 1; see table, Supplemental Digital Content 4).
Figure 1. Sign and Symptoms according to disease severity and age in infants with RSV infection.
Most relevant signs and symptoms were stratified in outpatients (orange) vs inpatients (blue) by age in (A) <3 months, (B) between 3 and 6 months, and (C) > 6 to 24 months of age. The Y axis represents the signs and symptoms in the two disease severity groups and the X axis the frequency of that specific symptom (%). Numbers next to bars represent the exact number of patients with that specific sign/symptom. Comparisons by Fisher exact test. Symbol (*) indicate significant 2-sided p values. WOB: work of breathing; bP: by parental report; PR: by physician report.
Fever was significantly more frequent in inpatients, and its frequency increased proportionally with age, varying from 46% to 72% and 90% in inpatients <3, 3–6 and >6–24 months of age respectively, and from 10% to 55% and 66% in the OP age-matched counterparts (p<0.05). Rhinorrhea was more common in outpatients than inpatients older than 3 months, ranging from 74% to 85% among the older age groups. Cough, congestion and decreased oral intake were common with no differences according to disease severity or age. Vomiting in infants <6 months and diarrhea in children younger than 3 months were more commonly identified in outpatients than in inpatients. Wheezing by parental report and documented by a physician, was more frequent in outpatients >3 months, ranging from 68% to 79% in these children and from 47% to 58% in inpatients (p<0.05). Increased work of breathing was consistently identified by the evaluating physician in inpatients more frequently than in outpatients and irrespective of age.
Viral loads and age in inpatients and outpatients with RSV infection
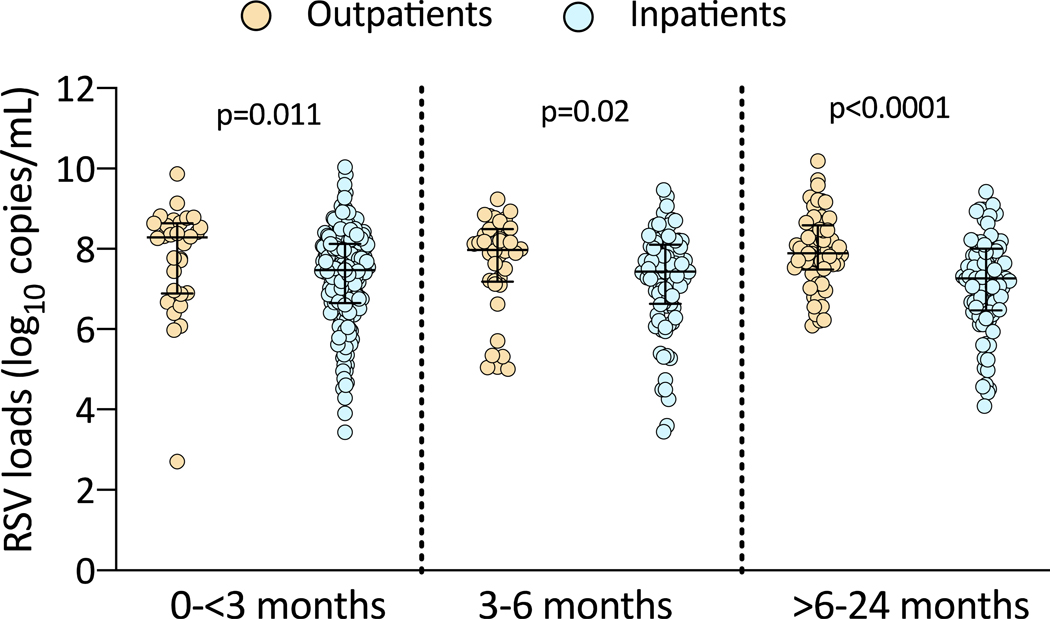
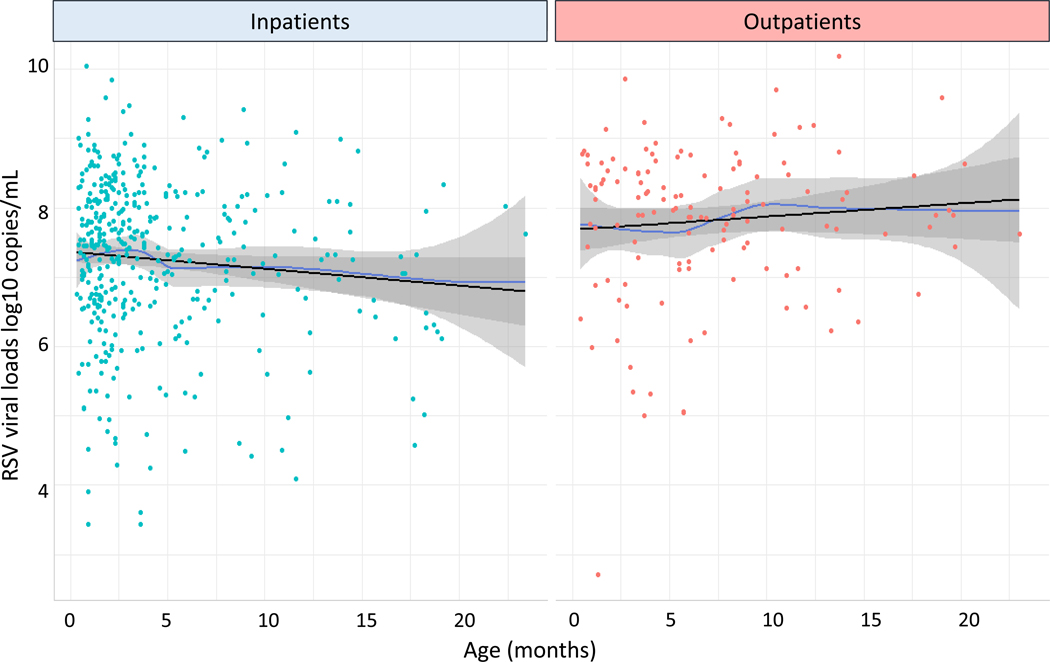
We also analyzed differences in RSV loads according to age in inpatients and outpatients, and found that RSV loads among the three age groups were significantly higher in the outpatient cohort compared with inpatients of the same age (Fig 2; see table, Supplemental Digital Content 4). Further, we explored the association between age and RSV viral loads using polynomial regression and restricted cubic spline regression. These analyses showed that the association between age and RSV loads was roughly linear (p-value for non-linearity >0.56 in all analyses) and might differ according to age (p-value for interaction = 0.059), with a decline in viral loads with increasing age among RSV inpatients and little association between age and viral loads in RSV outpatients (Fig 3).
Figure 2. Viral load differences according to age in infants with RSV infection.
The Y axis represents RSV loads in log10 copies/mL and the X axis differences in viral loads in outpatients (orange) and inpatients (blue) in the three age groups. Comparisons by Mann Whitney test, two-tailed p values are provided in the graphs.
Figure 3. Association between viral loads and age in inpatients and outpatients with RSV infection.
Viral loads for RSV inpatients (left panel; tile dots) and outpatients (right panel; orange dots) according to age are represented using locally estimated scatterplot smoothing (LOESS) curves (in blue), which summarize the association into a single line without imposing constraints such as linearity. Based on polynomial and restricted cubic spline regression there were no significant non-linearity associations, and so linear regression lines (in black) were also fitted to the data. Mean RSV loads (log10 copies/mL) are lower by 0.02 per month for the inpatient cohort, and are higher by 0.02 per month among the outpatient group.
Adjusted Odds of Hospitalization
Last, we analyzed which factors, including viral loads, were independently associated with increased disease severity defined by the need for hospitalization across the three age groups (Table 2). Fever and increased work of breathing reported by a physician across age groups were consistently associated with greater odds of hospitalization, while breastfeeding and physician reported wheezing in infants > 3 months of age, and higher RSV loads in infants > 6 months of age were independently associated with lower odds of hospitalization.
Table 2.
Adjusted odds of hospitalization in patients with RSV infection stratified by age
| < 3 months n=252 | 3 to 6 months n=128 | > 6–24 months n=154 | ||||
|---|---|---|---|---|---|---|
| OR [95% CI] | p-value | OR [95% CI] | p-value | OR [95% CI] | p-value | |
| Fever | 8.33 [2.74–36.41] | 0.001 | 2.76 [1.03–7.74] | 0.047 | 7.85 [1.65–59.48] | 0.019 |
| Increased WOB | 3.37 [1.37–8.85] | 0.010 | 12.13 [4.32–34.96] | <0.001 | 5.78 [2.09–17.82] | 0.001 |
| Breastfeeding | 0.71 [0.29–1.72] | 0.459 | 0.26 [0.09–0.72] | 0.012 | 0.19 [0.06–0.53] | 0.002 |
| Rhinorrhea | 0.30 [0.12–0.74] | 0.010 | 0.40 [0.14–1.12] | 0.130 | 0.13 [0.04–0.40] | 0.001 |
| Wheezing | 1.64 [0.69–4.01] | 0.269 | 0.25 [0.07–0.79] | 0.025 | 0.25 [0.08–0.72] | 0.013 |
| RSV loads | 0.75 [0.49–1.09] | 0.151 | 0.96 [0.61–1.50] | 0.867 | 0.32 [0.16–0.58] | 0.001 |
RSV, respiratory syncytial virus; OR, odds ratio; CI, confidence interval; WOB, work of breathing.
DISCUSSION
This study showed that age had a significant impact defining the interactions between viral loads, specific clinical manifestations, and disease severity in children with RSV infection. Overall, we found that differences in clinical signs and symptoms between mild and severe RSV infection were more evident in infants of older age. Specifically, children with mild RSV infection and older than 3 months of age, presented with wheezing more frequently. On the other hand, fever was consistently more frequent in inpatients than outpatients and proportionally increased with age. Adjusted analyses confirmed that while higher RSV loads and wheezing were independently associated with reduced disease severity in older infants, increased work of breathing and fever were consistently and independently associated with hospitalization, irrespective of age. Overall, these data suggest that clinical characteristics and viral loads differ in children with RSV infection, not only according to disease severity, but also by age.
The clinical symptoms associated with severe RSV infection in children < 2 years of age have been previously reported, and clinical scores developed with the hope of identifying clinical characteristics predictive of clinical outcomes (13, 16–23). Nevertheless, assessment of disease severity based on those scores have proven to be challenging, due to the heterogeneous patient populations of different ages evaluated, and the evolving nature of the disease. This heterogeneity likely mirrors the ability of the host immune response to modify or contain the infection, and thus varies according to age (6). By carefully stratifying otherwise healthy infants and young children according to both disease severity and age, we found that among all clinical factors analyzed, increased work of breathing and fever were independently associated with increased odds of hospitalization in all age groups. Although fever has not been consistently identified as a marker of severity, studies showed that fever may be present in children with a more severe disease phenotype, and associated with worse clinical outcomes, such as prolonged length of hospitalization (19, 20, 24–27). In our study, fever proportionally increased with age and was more common in hospitalized infants with severe disease. Among inpatients presence of fever ranged from 46% in infants <3 months to 90% in the older age group as compared to 10% in outpatients <3 months and 66% in those >6–24 months of age. The proportional increase of fever according to age has been previously reported in children with RSV infection, and thought to be related to immature innate immune responses early in infancy (17, 28, 29). Wheeze, has been classically identified as one of the main clinical criteria diagnostic for bronchiolitis, and considered as a severity marker in children with RSV infection (12, 21, 30–33). In our study however, we prospectively documented that wheezing at the time of presentation, was present in 51% of inpatients and in 65% of outpatients with mild disease. Unexpectedly, in infants >3 months of age physician-diagnosed wheezing was independently associated with lower odds of hospitalization. Whether the older infants with RSV infection represent a different endotype needs further understanding.
The spectrum of clinical manifestations in outpatients with a milder disease phenotype has been less studied. A population-based cohort study conducted in children < 5 years of age with and without risk factors for severe RSV infection, found that increased work of breathing (95% vs 73%), and wheezing (78% vs 65%) were significantly more common in inpatients than outpatients, with fever rates that ranged from 69% in inpatients to 75% in outpatients and no significant differences between groups (30). Nevertheless, none of the clinical characteristics evaluated in that study were predictive of hospitalization, which differs from our study as we found that breastfeeding, wheezing and higher RSV loads were associated with decreased odds of admission.
The interaction between RSV loads and clinical disease severity is not well understood, and data are contradictory (8, 10–12, 34–38). Most of the data have been derived from studies conducted in hospitalized children with differences in study design and disease severity outcomes. Recent data however, suggest that infants and young children with milder RSV disease have higher viral loads than those hospitalized with severe RSV infection (10, 12, 35). In agreement with those studies we also found that viral loads were higher in RSV outpatients. This observation of higher viral loads in children with mild RSV infection was confirmed when patients were classified using a standardized clinical disease severity score (6, 12–14), and by polynomial regression.
A recent study conducted in outpatients <2 years of age with RSV infection, did not find significant associations between initial viral loads and symptom duration including cough, rhinorrhea, wheezing, oral intake, increased work of breathing, or fever (29). On the other hand, another study conducted in children 1–10 years of age with RSV infection also evaluated in the outpatient setting, showed that viral loads were associated with the duration of rhinitis, cough and fever in children over 2 years of age, but not in the youngest age group (38). Although those studies are not directly comparable to ours, and we did not evaluate duration of symptoms but rather differences in clinical presentation, we also found greater differences in clinical symptoms in older patients. Nevertheless, whether higher RSV loads elicit a more robust innate immune response earlier in the course of disease which hampers the progression to severe disease (6, 12, 35, 39, 40), or whether it is the infant predisposition to develop severe disease for any given viral load is not completely understood.
Our study has limitations. Patients were enrolled and data obtained at a single time point, thus providing a snapshot of a continuous event. Families were contacted 2 and 4 weeks after the acute RSV diagnosis to assure that patient’s classification as outpatients and inpatients had not changed, but we did not capture duration of specific symptoms, limiting our ability to assess the impact of initial viral loads on longer-term clinical manifestations. Similarly, daily samples to assess viral load dynamics were not obtained, which also limited our ability to assess associations between viral shedding and symptoms duration. Also, in our study, the number of outpatients was smaller than those included in the inpatient group. Nevertheless, by conducting a comprehensive analysis of age-matched outpatients and inpatients with RSV infection, we identified important age-related differences in clinical characteristics, viral loads and disease severity.
In conclusion, we found differences in specific disease manifestations and viral loads between patients with mild and severe RSV infection. Higher RSV loads were associated with a mild clinical disease phenotype based on both the need for hospitalization and the clinical disease severity score. Also, differences in clinical findings that are routinely used in the clinical setting to differentiate between mild and severe disease, appeared more evident in infants of older age. Our data emphasize the importance of age-specific assessments in these children, and their potential utility when evaluating interventions for the prevention or treatment of RSV infection in children.
Supplementary Material
ACKNOWLEDGMENTS
We thank all members of the clinical research team at NCH and Dr Cohen’s team at NCH ED for their extraordinary efforts to help enrolling our patients; the Rush and Ireland family for their generosity and continuous support for our studies, and especially our patients and their families for their participation in the study.
Conflict of Interest and Source of Funding: AM and OR and have received research grants from NIH/NIAID (grant AI112524) and Janssen. AM has received fees for participation in advisory boards from Janssen, Roche, Merck and Reviral. OR has received fees for participation in advisory boards from Sanofi/MedImmune, Merck and Pfizer and fees for lectures from Pfizer and Merck. Those fees were not related to the research described in this manuscript. The remaining authors (HB, CGM, MMC, SM, FY, DC) have no conflicts of interest.
References
- 1.Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390:946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mejias A, Rodriguez-Fernandez R, Oliva S, Peeples ME, Ramilo O. The journey to a respiratory syncytial virus vaccine. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474–1502. [DOI] [PubMed] [Google Scholar]
- 5.Wainwright C. Acute viral bronchiolitis in children- a very common condition with few therapeutic options. Paediatr Respir Rev. 2010;11:39–45; quiz 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heinonen S, Velazquez VM, Ye F, et al. Immune profiles provide insights into respiratory syncytial virus disease severity in young children. Sci Transl Med. 2020;12. [DOI] [PubMed] [Google Scholar]
- 7.Garcia CG, Bhore R, Soriano-Fallas A, et al. Risk factors in children hospitalized with RSV bronchiolitis versus non-RSV bronchiolitis. Pediatrics. 2010;126:e1453–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Fernandez R, Tapia LI, Yang CF, et al. Respiratory Syncytial Virus Genotypes, Host Immune Profiles, and Disease Severity in Young Children Hospitalized With Bronchiolitis. J Infect Dis. 2017;217:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin ET, Kuypers J, Heugel J, Englund JA. Clinical disease and viral load in children infected with respiratory syncytial virus or human metapneumovirus. Diagn Microbiol Infect Dis. 2008;62:382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thwaites RS, Coates M, Ito K, et al. Reduced Nasal Viral Load and IFN Responses in Infants with Respiratory Syncytial Virus Bronchiolitis and Respiratory Failure. Am J Respir Crit Care Med. 2018;198:1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasegawa K, Jartti T, Mansbach JM, et al. Respiratory syncytial virus genomic load and disease severity among children hospitalized with bronchiolitis: multicenter cohort studies in the United States and Finland. J Infect Dis. 2015;211:1550–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Maurino C, Moore-Clingenpeel M, Thomas J, et al. Viral Load Dynamics and Clinical Disease Severity in Infants With Respiratory Syncytial Virus Infection. J Infect Dis. 2019;219:1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mella C, Suarez-Arrabal MC, Lopez S, et al. Innate immune dysfunction is associated with enhanced disease severity in infants with severe respiratory syncytial virus bronchiolitis. J Infect Dis. 2013;207:564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suarez-Arrabal MC, Mella C, Lopez SM, et al. Nasopharyngeal bacterial burden and antibiotics: Influence on inflammatory markers and disease severity in infants with respiratory syncytial virus bronchiolitis. The Journal of infection. 2015;71:458–469. [DOI] [PubMed] [Google Scholar]
- 15.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57:120–125. [DOI] [PubMed] [Google Scholar]
- 16.Laham FR, Mansbach JM, Piedra PA, et al. Clinical Profiles of Respiratory Syncytial Virus Subtypes A AND B Among Children Hospitalized with Bronchiolitis. Pediatr Infect Dis J. 2017;36:808–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mejias A, Dimo B, Suarez NM, et al. Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. PLoS medicine. 2013;10:e1001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia C, Soriano-Fallas A, Lozano J, et al. Decreased innate immune cytokine responses correlate with disease severity in children with respiratory syncytial virus and human rhinovirus bronchiolitis. Pediatr Infect Dis J. 2012;31:86–89. [DOI] [PubMed] [Google Scholar]
- 19.Lalani K, Yildirim I, Phadke VK, Bednarczyk RA, Omer SB. Assessment and Validation of Syndromic Case Definitions for Respiratory Syncytial Virus Infections in Young Infants: A Latent Class Analysis. Pediatr Infect Dis J. 2019;38:1177–1182. [DOI] [PubMed] [Google Scholar]
- 20.Atwell JE, Geoghegan S, Karron RA, Polack FP. Clinical Predictors of Critical Lower Respiratory Tract Illness Due to Respiratory Syncytial Virus in Infants and Children: Data to Inform Case Definitions for Efficacy Trials. J Infect Dis. 2016;214:1712–1716. [DOI] [PubMed] [Google Scholar]
- 21.Caserta MT, Qiu X, Tesini B, et al. Development of a Global Respiratory Severity Score for Respiratory Syncytial Virus Infection in Infants. J Infect Dis. 2017;215:750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Justicia-Grande AJ, Pardo-Seco J, Cebey-Lopez M, et al. Development and Validation of a New Clinical Scale for Infants with Acute Respiratory Infection: The ReSVinet Scale. PloS one. 2016;11:e0157665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marlais M, Evans J, Abrahamson E. Clinical predictors of admission in infants with acute bronchiolitis. Archives of disease in childhood. 2011;96:648–652. [DOI] [PubMed] [Google Scholar]
- 24.Masarweh K, Gur M, Leiba R, et al. Factors predicting length of stay in bronchiolitis. Respir Med. 2019;161:105824. [DOI] [PubMed] [Google Scholar]
- 25.Voets S, van Berlaer G, Hachimi-Idrissi S. Clinical predictors of the severity of bronchiolitis. Eur J Emerg Med. 2006;13:134–138. [DOI] [PubMed] [Google Scholar]
- 26.Mansbach JM, Clark S, Christopher NC, et al. Prospective multicenter study of bronchiolitis: predicting safe discharges from the emergency department. Pediatrics. 2008;121:680–688. [DOI] [PubMed] [Google Scholar]
- 27.Checchia P. Identification and management of severe respiratory syncytial virus. Am J Health Syst Pharm. 2008;65:S7–12. [DOI] [PubMed] [Google Scholar]
- 28.Kawakami C, Sato A, Sumita H, et al. Fever Responses Are Enhanced with Advancing Age during Respiratory Syncytial Virus Infection among Children under 24 Months Old. The Tohoku journal of experimental medicine. 2018;245:217–222. [DOI] [PubMed] [Google Scholar]
- 29.Utsunomiya T, Hibino A, Taniguchi K, et al. Factors Contributing to Symptom Duration and Viral Reduction in Outpatient Children With Respiratory Syncytial Virus Infection. Pediatr Infect Dis J. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall CB, Weinberg GA, Iwane MK, et al. The Burden of Respiratory Syncytial Virus Infection in Young Children. N Engl J Med. 2009;360:588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Martinez CE, Sossa-Briceno MP, Nino G. Systematic review of instruments aimed at evaluating the severity of bronchiolitis. Paediatr Respir Rev. 2018;25:43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Ortiz AM, Bernal-Silva S, Comas-Garcia A, Vega-Morua M, Garrocho-Rangel ME, Noyola DE. Severe Respiratory Syncytial Virus Infection in Hospitalized Children. Arch Med Res. 2019;50:377–383. [DOI] [PubMed] [Google Scholar]
- 33.Ma X, Conrad T, Alchikh M, Reiche J, Schweiger B, Rath B. Can we distinguish respiratory viral infections based on clinical features? A prospective pediatric cohort compared to systematic literature review. Rev Med Virol. 2018;28:e1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazur NI, Bont L, Cohen AL, et al. Severity of Respiratory Syncytial Virus Lower Respiratory Tract Infection With Viral Coinfection in HIV-Uninfected Children. Clin Infect Dis. 2017;64:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piedra FA, Mei M, Avadhanula V, et al. The interdependencies of viral load, the innate immune response, and clinical outcome in children presenting to the emergency department with respiratory syncytial virus-associated bronchiolitis. PloS one. 2017;12:e0172953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El Saleeby CM, Bush AJ, Harrison LM, Aitken JA, Devincenzo JP. Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. J Infect Dis. 2011;204:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeVincenzo JP, El Saleeby CM, Bush AJ. Respiratory syncytial virus load predicts disease severity in previously healthy infants. J Infect Dis. 2005;191:1861–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uusitupa E, Waris M, Heikkinen T. Association of Viral Load With Disease Severity in Outpatient Children With Respiratory Syncytial Virus Infection. J Infect Dis. 2020. [DOI] [PubMed] [Google Scholar]
- 39.Nicholson EG, Schlegel C, Garofalo RP, et al. Robust Cytokine and Chemokine Response in Nasopharyngeal Secretions: Association With Decreased Severity in Children With Physician Diagnosed Bronchiolitis. J Infect Dis. 2016;214:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heinonen S, Rodriguez-Fernandez R, Diaz A, Rodriguez-Pastor SO, Ramilo O, Mejias A. Infant Immune Response to Respiratory Viral Infections. Immunol Allergy Clin North Am. 2019;39:361–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.