Supplemental Digital Content is Available in the Text.
Key Words: PLHIV, COVID-19, SARS-CoV-2, HIV-1 viral load, CD4+ cell count
Background:
To describe the virologic and immunologic outcomes among people living with HIV (PLHIV) coinfected with SARS-CoV-2.
Setting:
Wuhan, China.
Methods:
Thirty-five coinfected patients were identified by matching the reported cases in National Notifiable Infectious Disease Report system for COVID-19 and HIV in Wuhan by time of April 19, 2020. Questionnaire-based survey and follow-up with blood sample collection were used to obtain characteristics before COVID-19 and after recovery. Nonparametric Mann–Whitney U test, χ2, or Fisher exact test, Mcnemar test, and Wilcoxon test were conducted.
Results:
Twenty of the 35 coinfected patients were identified as asymptomatic/mild/moderate COVID-19 (nonsevere group) and 15 were identified as severe/critical (severe group). The severe and nonsevere group had no differences in demographics, HIV baseline status, the intervals between last tests and follow-up tests for CD4+ cell count and HIV-1 viral load (all P > 0.05). Overall, there was a significantly increased number of coinfected patients with HIV-1 viral load ≥20 copies/mL after recovery (P = 0.008). The median viral load increased significantly after recovery in severe group (P = 0.034), whereas no significant change of HIV-1 viral load was observed in the nonsevere group. Limited change of CD4+ cell count was found (all P > 0.05).
Conclusion:
The coinfection of SARS-CoV-2 may put PLHIV at greater risk for HIV-1 viral rebound especially for severe/critical COVID-19, whereas it had limited impacts on CD4+ cell count. Whether continuous antiretroviral therapy against HIV infection would have significant impacts on CD4+ cell count among PLHIV coinfected with SARS-CoV-2 needs further research.
INTRODUCTION
In late December, the coronavirus disease 2019 (COVID-19) caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in Wuhan, China. It has quickly spread across China and throughout the world, bringing serious threat to public health. Several comorbidities such as diabetes and hypertension have been identified as risk factors of critical and mortal COVID-19 cases,1 but data on people living with HIV (PLHIV) coinfected with SARS-CoV-2 are scarce. Limited literature mainly focused on the description of clinical characteristic of the COVID-19 and/or the effect of HIV infection on the prognosis of COVID-19.2–5 Whether PLHIV coinfected with SARS-CoV-2 would aggravate HIV infection remains unknown.
Here, we would like to describe the HIV progression as measured by viral load and CD4+ cell count before COVID-19 and after recovery, and potential toxic side effects as measured by biomedical data such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), and serum creatinine etc. in the setting of SARS-CoV-2 infection among PLHIV.
METHODS
We retrospectively reviewed a total of 35 cases of PLHIV with SARS-CoV-2 infection in Wuhan by the time of April 19, 2020, identified by matching reported COVID-19 with registered PLHIV in the National Notifiable Infectious Disease Report System every half month since early March, 2020. According to the Diagnosis, Treatment and Prevention Scheme for COVID-19, COVID-19 was categorized into confirmed cases, clinically diagnosed cases, suspected cases, and asymptomatic cases, and the severity of COVID-19 was categorized into mild, moderate, severe, or critical.6,7 In this study, we only included PLHIV with confirmed COVID-19, clinically diagnosed COVID-19, and asymptomatic COVID-19. Considering the small number of patients in different severity, the severe/critical COVID-19 were classified into severe group and asymptomatic/mild/moderate COVID-19 were classified into nonsevere group.
Demographical data and baseline HIV status were extracted from the National Notifiable Infectious Disease Report System. Considering the regular follow-up of at least once a year for HIV-1 viral load and CD4+ cell count among PLHIV, patients' last HIV-1 viral load and CD4+ cell count in 2019, namely the tests within previous 12 months in 2019, were extracted as the data before COVID-19. A tele-survey on COVID-19-related clinical and epidemiological information was conducted in local Centers for Disease Control and Prevention (CDCs) from March 10 to April 22, 2020. Subsequent follow-up by questionnaire survey and blood sample collection was conducted in local CDCs from May 18 to May 19, 2020. Follow-up questionnaire survey mainly included information regarding history of comorbidities, alcohol consumption, smoking, and antiretroviral therapy (ART) discontinuation in the epidemic period. Alcohol consumption was generally classified as ever and current, abstainer, and never according to a meta-analysis on alcohol consumption and risk of dementia.8 Smoking was defined as ever and current, ex/former, and never, the most commonly used categories summarized in a meta-analysis on smoking and risk of pneumonia.9 ART discontinuation was measured by asking a question of “whether you had a history of discontinuing ART for at least 3 days since December, 2019.” Blood samples were tested for HIV-1 viral load, CD4+ cell count, biochemical data related to toxic side effects of ART against HIV infection, and antibody seroactivity against SARS-CoV-2 in the laboratory in Wuhan CDC. The lower limit of HIV-1 viral load detection was 20 copies/mL.
Continuous and categorical data were presented as medians with interquartile ranges (IQRs) and absolute numbers with percentages, respectively. The differences of demographics, baseline HIV status, and clinical findings related to COVID-19 among different groups were tested by nonparametric Mann–Whitney U test and χ2 or Fisher exact test. The differences of HIV-1 viral load, CD4 cell count, and biochemical data related to toxic side effects of ART before COVID-19 and after recovery were tested by McNemar test and Wilcoxon test. P < 0.05 was considered as level of significance. Analyses were performed using SPSS version 22.0 and R version 3.5.2.
Ethical approval was obtained from the Institutional Review Board of Wuhan Center for Disease Control and Prevention (WHCDCIRB-K-2020016). Oral and written informed consents were obtained for subjects willing to participate in tele-survey and follow-up study, respectively.
RESULTS
The detailed participant recruitment and survey could be seen in Figure 1, Supplemental Digital Content, http://links.lww.com/QAI/B558. The loss to follow-up rate was 15.2%.
Demographics
The median age of PLHIV coinfected with SARS-CoV-2 was 52.0 (IQR, 36.0–57.0) years, 33 (94.3%) were men. The most commonly reported comorbidities were hypertension (25.0%) and diabetes (10.7%). Most participants reported alcohol consumption and smoking in the never category (71.4% and 67.9% respectively; Table 1).
TABLE 1.
Demographics, Baseline HIV Status, and Clinical Findings Related to COVID-19 Among HIV Patients Coinfected With SARS-COV-2
| Demographics and Baseline HIV Status | Total (N = 35) | Nonsevere Group (N = 20) | Severe Group (N = 15) | P |
| Age, median (IQR), yrs | 52.0 (36.0–57.0) | 50.5 (33.3–57.0) | 55.0 (44.0–63.0) | 0.277 |
| Gender | ||||
| Female | 2 (5.7%) | 1 (5.0%) | 1 (6.7%) | 1.000 |
| Male | 33 (94.3%) | 19 (95.0%) | 14 (93.3%) | |
| History of comorbidities (n/%)* | ||||
| Pulmonary disease | 2 (7.1%) | 1 (6.7%) | 1 (7.7%) | 1.000 |
| Tuberculosis | 2 (7.1%) | 1 (6.7%) | 1 (7.7%) | 1.000 |
| Hypertension | 7 (25.0%) | 4 (26.7%) | 3 (23.1%) | 1.000 |
| Diabetes | 3 (10.7%) | 1 (6.7%) | 2 (15.4%) | 0.583 |
| HBV | 2 (6.7%) | 2 (11.8%) | 0 (0.0%) | 0.492 |
| HCV | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | / |
| Comorbidities per patient, n* | 1.0 (0.0–1.8) | 1.0 (0.0–2.0) | 1.0 (0.0–1.5) | 0.749 |
| Alcohol consumption* | ||||
| Ever and current | 5 (17.9%) | 3 (20.0%) | 2 (15.4%) | 0.741 |
| Abtainer | 3 (10.7%) | 1 (6.7%) | 2 (15.4%) | |
| Never | 20 (71.4%) | 11 (73.3%) | 9 (69.2%) | |
| Smoking* | ||||
| Ever and current | 4 (14.3%) | 2 (13.3%) | 2 (15.4%) | 0.399 |
| Former | 5 (17.9%) | 4 (26.7%) | 1 (7.7%) | |
| Never | 19 (67.9%) | 9 (60.0%) | 10 (76.9%) | |
| Time elapse since HIV diagnosis, mo | 70.0 (42.0–91.0) | 62.0 (38.3–84.5) | 87.0 (47.0–106.0) | 0.199 |
| Interval between tests for HIV viral load, mo* | 12.0 (9.0–14.0) | 10.0 (9.0–13.0) | 14.0 (9.3–15.5) | 0.133 |
| Interval between tests for CD4+ cell count, mo* | 9.0 (6.0–12.0) | 9.0 (8.0–11.0) | 8.0 (6.0–13.8) | 0.833 |
| Severity of HIV infection | ||||
| HIV | 20 (57.1%) | 11 (55.0%) | 9 (60.0%) | 1.000 |
| AIDS | 15 (42.9%) | 9 (45.0%) | 6 (40.0%) | |
| Time elapse since ART, mo | 61.0 (34.0–80.0) | 49.0 (23.8–69.3) | 70.5 (42.3–87.0) | 0.111 |
| Current ART-regimen (n/%) | ||||
| AZT/ABC+3 TC+EFV/NVP | 16 (45.7%) | 8 (40.0%) | 8 (53.3%) | 0.381 |
| TDF+3 TC/FTC+EFV/NVP/RPV | 16 (45.7%) | 11 (55.0%) | 5 (33.3%) | |
| TDF+3 TC+DTG/LPV/r | 3 (8.6%) | 1 (5.0%) | 2 (13.3%) | |
| ART discontinuation (n/%)* | ||||
| Yes | 4 (14.3%) | 2 (13.3%) | 2 (15.4%) | 1.000 |
| No | 24 (85.7%) | 13 (86.7%) | 11 (84.6%) | |
| Clinical findings related to COVID-19 | ||||
| Time at onset (n/%) | ||||
| January, 2020 | 25 (71.4%) | 12 (60.0%) | 13 (86.7%) | 0.242 |
| February, 2020 | 8 (22.9%) | 6 (30.0%) | 2 (13.3%) | |
| March, 2020 | 1 (2.9%) | 1 (5.0%) | 0 (0.0%) | |
| April, 2020 | 1 (2.9%) | 1 (5.0%) | 0 (0.0%) | |
| Symptoms at onset (n/%) | ||||
| Fever | 25 (71.4%) | 13 (65.0%) | 12 (80.0%) | 0.458 |
| Dry cough | 13 (37.1%) | 8 (40.0%) | 5 (33.3%) | 0.737 |
| Shortness of breath | 8 (22.9%) | 2 (10.0%) | 6 (40.0%) | 0.051 |
| Gastrointestinal symptoms | 4 (11.4%) | 2 (10.0%) | 2 (13.3%) | 1.000 |
| None | 3 (8.6%) | 3 (15.0%) | 0 (0.0%) | 0.244 |
| Time from onset to medical visit, d | 6.0 (1.0–8.0) | 4.0 (0.3–8.0) | 6.0 (1.0–10.0) | 0.638 |
| Ever hospitalized for COVID-19 (n/%) | ||||
| Yes | 29 (82.9%) | 15 (75.0%) | 14 (93.3%) | 0.207 |
| No | 6 (17.1%) | 5 (25.0%) | 1 (6.7%) | |
| Days of hospital stay, d | 16.0 (11.5–32.5) | 13.0 (10.0–23.0) | 21.5 (13.8–36.3) | 0.101 |
| SARS-CoV-2 testing (n/%) | ||||
| PCR positive test | 24 (68.6%) | 13 (65.0%) | 11 (73.3%) | 0.721 |
| positive IgM test* | 1 (3.6%) | 0 (0.0%) | 1 (7.7%) | 0.464 |
| positive IgG test* | 22 (78.6%) | 11 (73.3%) | 11 (84.6%) | 0.655 |
| Outcome (n/%) | ||||
| Survived | 33 (94.3%) | 20 (100.0%) | 13 (86.7%) | 0.176 |
| Died | 2 (5.7%) | 0 (0.0%) | 2 (13.3%) | |
| With family members having COVID-19 (n/%)† | ||||
| Yes | 9 (27.3%) | 6 (30.0%) | 3 (23.1%) | 0.367 |
| No | 23 (69.7%) | 14 (70.0%) | 9 (69.2%) | |
| Unknown | 1 (3.0%) | 0 (0.0%) | 1 (7.7%) | |
| With colleagues having COVID-19 (n/%)† | ||||
| Yes | 4 (12.1%) | 3 (15.0%) | 1 (7.7%) | 0.275 |
| No | 25 (75.8%) | 16 (80.0%) | 9 (69.2%) | |
| Unknown | 4 (12.1%) | 1 (5.0%) | 3 (23.1%) |
Nonsevere group included patients with asymptomatic COVID-19, virally confirmed and clinically diagnosed COVID-19 with mild and moderate COVID-19.
Severe group included patients with virally confirmed and clinically diagnosed COVID-19 with severe and critical COVID-19.
A total of 28 coinfected patients included as 5 of the 33 survived COVID-19 patients refused to participate in our follow-up survey.
A total of 33 coinfected patients included as 2 of the 35 COVID-19 patients died at tele-survey.
ABC, abacavir; AZT, Zidovudine; COVID-19, coronavirus disease 2019; DTG, dolutegravir; EFV, efavirenz; FTC, emtricitabine; HBV, Hepatitis B virus; HCV, Hepatitis C virus; LPV/r, Lopinavir/Ritonavir; NVP, nevirapine; RPV, rilpivirine; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; 3 TC, lamivudine; TDF, tenofovir disoproxifumarate.
Among the 35 subjects, 20 were identified as asymptomatic/mild/moderate COVID-19 (nonsevere group) and 15 were severe/critical COVID-19 (severe group). Age, gender, history of comorbidities, alcohol consumption, and smoking were all evenly distributed between severe and nonsevere group (all P > 0.05).
Baseline HIV Status
The identified coinfected cases have been living with HIV for 70.0 (IQR, 42.0–91.0) months at onset of COVID-19. Fifteen (42.9%) of them have progressed into AIDS stage. Thirty-four (97.1%) were on ART against HIV infection before onset of COVID-19 and the median duration from ART initiation to onset of COVID-19 was 61.0 months (IQR, 34.0–80.0). One patient (2.9%) initiated ART at the onset of COVID-19. Four PLHIV (14.3%) reported not on ART for at least 3 days since December, 2019, among which 1/4 reported the reason of city lockdown in COVID-19 epidemic, 2/4 of under COVID-19 treatment, and 1/4 of toxic side effects of ART. ART regimens mainly included the combined use of nucleoside reverse transcriptase inhibitors and non-nucleoside reverse transcriptase inhibitors (32/35, 91.4%). Only 3/35 included the use of protease inhibitors or integrase inhibitors (Table 1).
There were no statistically significant differences of duration of HIV infection (P = 0.199) and ART against HIV infection (P = 0.111) between severe and nonsevere groups (Table 1).
Clinical Findings Related to COVID-19
Twenty-one of 35 subjects were confirmed cases, 3/35 were asymptomatic cases, and 11/35 were clinically diagnosed cases. A total of 24 COVID-19 cases, namely the confirmed cases and asymptomatic cases, tested positive for SARS-CoV-2 viral RNA in respiratory samples at their diagnosis. At follow-up, 1/28 tested positive for SARS-CoV-2 IgM antibody, and 22/28 tested positive for SARS-CoV-2 IgG antibody (Table 1).
Most of the enrolled COVID-19 cases were identified in January, 2020 (71.4%) and February, 2020 (22.9%). The most common symptoms were fever (25/35, 71.4%), dry cough (13/35, 37.1%), and shortness of breath (8/35, 22.9%). The severe group was significantly more likely to have shortness of breath than the nonsevere group (40.0% vs. 10.0%, P = 0.051). The median time from symptoms onset to medical visit were 6.0 (IQR, 1.0–8.0) days. Twenty-nine (82.9%) reported to have history of hospital stay, among which 4 were admitted to and discharged from Fangcang shelter hospital, 2 were transferred from Fangcang shelter hospital to other medical facilities for severe progression of COVID-19. The median time of hospital stay was 16 (IQR, 11.5–32.5) days. At the point of tele-survey, 33 patients recovered and were discharged, whereas the other 2 died due to critical COVID-19 at 52 and 68 years old. Nine of the 33 survived patients reported to have family members with COVID-19 and 4/33 reported to have colleagues with COVID-19.
Viral Load and CD4+ Cell Count Before COVID-19 and After Recovery
The intervals between last tests and follow-up tests for CD4+ cell count and HIV-1 viral load were 9.0 (IQR, 6.0–12.0) months and 12.0 (IQR, 9.0–14.0) months, respectively. And there were no statistically significant difference for the intervals for tests of CD4+ cell count (P = 0.833) and HIV-1 viral load (P = 0.133) between severe and nonsevere groups (Table 1 and see Table 2, Supplemental Digital Content, http://links.lww.com/QAI/B558).
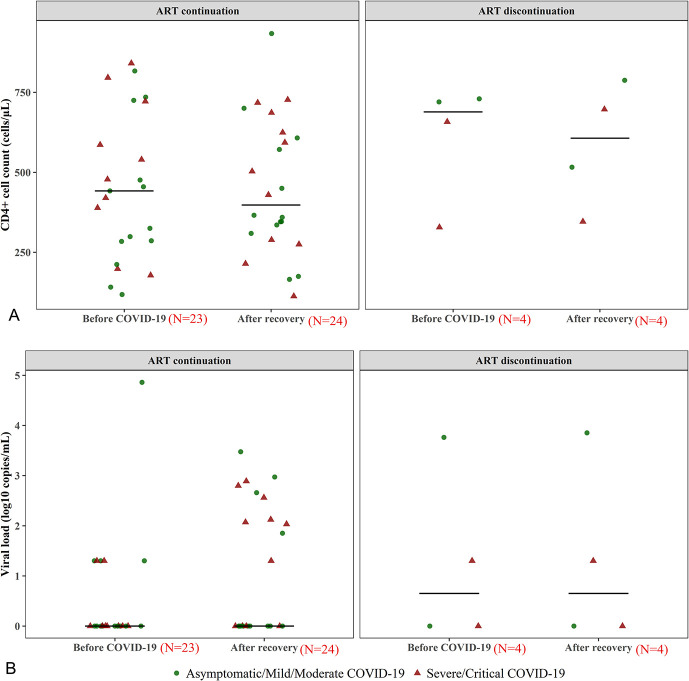
As shown in Table 1, Supplemental Digital Content, http://links.lww.com/QAI/B558 and Figure 1, coinfected patients experienced a statistically insignificant decline of CD4+ cell count after COVID-19 with different severity of COVID-19 and with continuous ART (all P > 0.05). There was a trend of increasing HIV-1 viral load for coinfected patients overall (P = 0.051), and the number of coinfected patients with HIV-1 viral load ≥20 copies/mL increased significantly after recovery (P = 0.008). The trend of increasing viral load was observed in coinfected patients with severe/critical COVID-19 but not in nonsevere COVID-19 (Table 1, Supplemental Digital Content, http://links.lww.com/QAI/B558). When excluding those with ART discontinuation, the trend of increasing viral load still existed (Fig. 1).
FIGURE 1.
The virologic and immunologic outcomes for HIV patients before COVID-19 and after recovery. A, Presenation of immunologic outcomes, (B) presentation of virologic outcomes. Twenty-eight of 33 survived coinfected patients participated in our follow-up survey; one of 28 coinfected patients initiated ART against HIV infection at onset of COVID-19, thus no HIV-1 viral load and CD4+ cell count were available before COVID-19. Finally, a total of 27 coinfected patients were included in the scatter diagram before COVID-19 and 28 included in the scatter diagram after recovery. To facilitate presentation, log10 copies/mL was calculated based on raw data where HIV-1 viral load <20 copies/mL was regarded as 20 copies/mL.
Toxic Side Effects of ART Against HIV Infection Before COVID-19 and After Recovery
Limited change was found in leukocytes, hemoglobin, blood platelets, serum creatinine, total bilirubin, ALT (all P > 0.05), except for aspartate aminotransferase (AST) and AST/ALT ratio (see Table 1, Supplemental Digital Content, http://links.lww.com/QAI/B558). There was a statistically and significantly increased proportion of abnormal AST and AST/ALT ratio in both nonsevere group (P = 0.002 and P = 0.001, respectively) and severe group (P = 0.008 and P = 0.008, respectively).
DISCUSSION
We observed a greater number of males, underlying comorbidities of diabetes and hypertension, and older age in the 35 PLHIV coinfected with SARS-CoV-2. Those with severe COVID-19 may be more likely to have shortness of breath. Although we did not observe the role of other risk factors in the severe progression of COVID-19 among PLHIV as current literature found in general population.10 The proportion of severe COVID-19 among PLHIV was much higher than that reported among general population in China (42.8% vs. 15.7%, P < 0.001).11 However, the case fatality rate was comparable to that observed in general population with COVID-1912 (5.7% vs. 5.0%, P = 0.694). It may be explained by the possibility that PLHIV had a deficient immune system and showed some persistent immune activation that may hinder the lethal progression of COVID-19 caused by severe cytokine storm.11 Age, gender, and death rate we found were similar to those reported in PLHIV with COVID-19 in Madrid and Italy.5,13
Our data indicated that COVID-19 may put PLHIV at a risk for higher viral load despite continued ART against HIV infection, especially for those with severe/critical COVID-19. As we know, HIV could not be completely eliminated despite successful suppression of viral replication under ART, and it lurks in latent and active reservoirs.14 CD8+ cells are required for maintenance of HIV viral reservoir suppression under ART.15 However, CD8+ cells were gradually decreased with severity of COVID-19.16 The source of the virus rebound in PLHIV may be probably the reactivation of virus transcription from a pool of latently infected cells, namely HIV latent reservoirs, after the depletion of CD8+ cells caused by infection of SARS-CoV-2.
Although both SARS-CoV-2 and HIV infection would lead to the decrease of CD4+ cell count, we did not find their additive effects on CD4+ cell count as we hypothesized. The reason remains unknown. We observed an insignificant decline of CD4+ cell count after COVID-19 among those with continuous ART against HIV infection. Whether continuous ART would play a significant role in maintaining stable immune status remains to be elucidated in further studies.
There are several limitations in our study. First, there is a lack of CD8+ measurement before COVID-19, so as to the CD4/CD8 ratio, which is also an important immunologic indicator in HIV infection and COVID-19. Second, we used a self-designed questionnaire to collect clinical findings related to COVID-19 because of limited access to relevant data in hospital, which may make our study susceptible to information bias. Third, there is a lack of treatment strategy for COVID-19, which may affect the observed outcomes in our study. Finally, because this is an observational study with small sample size in short time, the observed outcomes may need to be confirmed in larger cohort studies with longer observation time. However, given the short of data regarding the impact of COVID-19 on HIV progression, we believe that our data would provide useful information for concerning the management of PLHIV coinfected with SARS-CoV-2.
In conclusion, PLHIV coinfected with SARS-CoV-2 may be more likely to progress into severe COVID-19, but their risk of death is comparable to the general population with COVID-19. The coinfection of SARS-CoV-2 may put PLHIV at greater risk for HIV-1 viral rebound especially for those with severe/critical COVID-19, while having limited impacts on CD4+ cell count. Whether continuous ART against HIV infection would have significant impacts on CD4+ cell count among PLHIV coinfected with SARS-CoV-2 needs further research.
ACKNOWLEDGMENTS
The authors would like to acknowledge the work of public health workers in local CDCs involved in completing patient reviews.
Footnotes
The authors have no funding or conflicts of interest to disclose.
N.H.X. & X.W. conceived the study design. R.H., H.Y., N.H.X. & X.W. conducted the data analysis and interpretation of data analysis. R.H. & H.Y. wrote the first draft of the manuscript and conducted the revision of the manuscript. H.Y., W.J.B., X.J.H. & J.D. collected data and conducted the revision of the manuscript. M.Q.L., L.T., W.H.K., Z.R.Z. & P.L. conducted data collection, HIV-1 viral load tests, CD4+ cell count tests and revision of the manuscript. R.H. and H.Y. contributed equally to this manuscript. X.W. and N.H.X. are co-senior authors and contributed equally to this manuscript.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81:e16–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanco JL, Ambrosioni J, Garcia F, et al. COVID-19 in patients with HIV: clinical case series. Lancet HIV. 2020;7:e314–e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Härter G, Spinner CD, Roider J, et al. COVID-19 in people living with human immunodeficiency virus: a case series of 33 patients. Infection. 2020;48:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Childs K, Post FA, Norcross C, et al. Hospitalized patients with COVID-19 and HIV: a case series. Clin Infect Dis. 2020:ciaa657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vizcarra P, Pérez-Elías MJ, Quereda C, et al. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV. 2020;7:e554–e564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.General Office of National Health Commission, General Office of National Administration of Traditional Chinese Medicine. Diagnostic and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 5). 2020. Available at: http://www.nhc.gov.cn/xcs/zhengcwj/202002/d4b895337e19445f8d728fcaf1e3e13a.shtml. Accessed May 9, 2020. [Google Scholar]
- 7.General Office of National Health Commission. Prevention Protocol for Novel Coronavirus Pneumonia (Trial Version 6). 2020. Available at: http://www.nhc.gov.cn/jkj/s3577/202003/4856d5b0458141fa9f376853224d41d7.shtml. Accessed June 3, 2020. [Google Scholar]
- 8.Xu W, Wang HF, Wan Y, et al. Alcohol consumption and dementia risk: a dose-response meta-analysis of prospective studies. Eur J Epidemiol. 2017;32:31–42. [DOI] [PubMed] [Google Scholar]
- 9.Baskaran V, Murray RL, Hunter A, et al. Effect of tobacco smoking on the risk of developing community acquired pneumonia: a systematic review and meta-analysis. PLoS One. 2019;14:e0220204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harapan H, Itoh N, Yufika A, et al. Coronavirus disease 2019 (COVID-19): a literature review. J Infect Public Health. 2020;13:667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li LQ, Huang T, Wang YQ, et al. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92:577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gervasoni C, Meraviglia P, Riva A, et al. Clinical features and outcomes of HIV patients with coronavirus disease 2019. Clin Infect Dis. 2020:ciaa579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sung JM, Margolis DM. HIV persistence on antiretroviral therapy and barriers to a cure. Adv Exp Med Biol. 2018;1075:165–185. [DOI] [PubMed] [Google Scholar]
- 15.McBrien JB, Kumar NA, Silvestri G. Mechanisms of CD8(+) T cell-mediated suppression of HIV/SIV replication. Eur J Immunol. 2018;48:898–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang F, Hou H, Luo Y, et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020;5:137799. [DOI] [PMC free article] [PubMed] [Google Scholar]