Abstract
Spontaneous ovarian hyperstimulation syndrome (s-OHSS) is a rare finding that occurs in early pregnancy. There is a rapidly increasing ovarian size secreting vasoactive substances that lead to fluid shift into third spaces. This occurs in the absence of exogenous hormonal therapy. We present two cases of s-OHSS. A 35-year-old gravida 4 para 3 presented with complaints of progressive abdominal pain, distension, nausea, vomiting, and difficulty in breathing at 10 weeks gestation. On imaging, a singleton intrauterine gestation, enlarged ovaries containing multiple cysts, and moderate ascites were seen. Second, a 17-year-old primigravida presented with abdominal distension and pain and bleeding per vaginam following 4 months amenorrhea. A bulky uterus containing a large hyperechoic structure with multiple cystic spaces in keeping complete molar gestation and enlarged ovaries containing multiple cysts were seen on ultrasound imaging. The singleton gestation was managed successfully to term with conservative therapy tailored to clinical symptoms.
Keywords: Complete molar gestation, intrauterine gestation, spontaneous ovarian hyperstimulation syndrome
INTRODUCTION
Ovarian hyperstimulation syndrome (OHSS) is a potentially fatal clinical condition, occurring in early pregnancy as a result of rapidly increasing ovarian volume. The enlarged ovaries contain multiple cysts which secrete various vasoactive substances such as interleukins, tumor necrosis factor alpha, endothelin-1, and vascular endothelial growth factor (VEGF) that increase vascular permeability. The increased vascular permeability results in fluid shift from the intravascular space to the extravascular space and in the third spaces which are responsible for the complications and severity of the syndrome.1,2,3
OHSS can be iatrogenic or spontaneous. Iatrogenic OHSS follows exogenous ovulation induction using gonadotropins in assisted reproduction therapy (ART). This occurs as early as 3–5 weeks of gestation due to exogenous hormones and complicates about 1% of ARTs in developed countries and about 11.7%–15% in Nigeria.4,5,6 Rarely, OHSS can be spontaneous in the absence of any exogenous hormonal therapy and may be seen in single or multiple gestation, gestational trophoblastic disease, hypothyroidism, and pituitary adenoma with or without pregnancy.7,8 Follicle-stimulating hormone receptor (FSHR) mutation has been implicated in spontaneous ovarian hyperstimulation syndrome (s-OHSS). In the background of gestation, s-OHSS occurs at about 8–14 weeks of gestation due to increased sensitivity of mutated FSHR to the rise in endogenous human chorionic gonadotropin (HCG) hormone to support the pregnancy.9,10 Small body size and polycystic ovarian syndrome are documented risk factors for possible s-OHSS.11
Clinical presentation varies from mild to severe and depends on ovarian size, presence of symptoms, and imaging findings. Clinical symptoms include abdominal distension, abdominal pain or discomfort, nausea, vomiting, and difficulty in breathing, while imaging findings may comprise ascites, pericardial, and pleural effusions. In severe or critical cases, complications such as hemoconcentration, hypovolemic shock, ovarian torsion, thromboembolism, adult respiratory distress syndrome, and death have been encountered.12
Radiological imaging plays an important role in patient management such as establishing the diagnosis, ruling out intra-abdominal malignancies, follow-up monitoring of ovarian size, ascitic, pleural, and pericardial fluid volume. Evaluating metastasis in cases of trophoblastic disease can also be done. Ultrasonography, chest X-ray, magnetic resonance imaging, computed tomogram, and other imaging modalities may be used when clinically indicated. Serial ultrasound monitoring is the modality of choice in our local environment. Chest X-ray and computed tomogram are used when metastasis from malignant trophoblastic disease is considered.
We present two cases of s-OHSS following spontaneous singleton gestation and gestational trophoblastic disease, respectively.
CASES REPORTS
Case 1
A 35-year-old gravida 4 para 3+0, 3 alive who presented in a private hospital at 10 weeks gestation with a 1-week history of progressive abdominal distension, abdominal pains, nausea, vomiting, and difficulty in breathing. Her previous pregnancies were uneventful, last confinement was 5 years prior to presentation, and there is no history of ovarian stimulation in the index pregnancy. There is no history of previous gynecological abnormality. Her blood pressure and other vital signs were unremarkable. She was admitted for observation and blood work (full blood count, renal and liver function test, and quantitative beta HCG [βHCG]). Obstetric imaging was requested to assess the fetus, to rule out gestational trophoblastic disease, ovarian fibroma with suspected Meigs syndrome, and possible ovarian torsion. Her packed cell volume was 43%; other blood parameters were within normal ranges.
On ultrasound imaging, a single, viable, intrauterine fetus with normal fetal and cardiac activity and a crown rump length of 39.3 mm corresponding to a gestational age of 10 weeks 6 days was seen. The placental tissue, myometrium, internal os, and cervix were within normal limits. The ovaries were enlarged; they measured 17.4 cm × 10.3 cm × 12.1 cm and 15.1 cm × 9.5 cm × 16.3 cm on the right and left, respectively. They contained thick echogenic and vascularized stroma and multiple 2.0–4.0 cm thin-walled cysts, some of which show echogenic debris in keeping with hemorrhage but no demonstrable solid component [Figure 1a-c]. There was moderate ascites, but no pleural or pericardial fluid. The liver was mildly enlarged while the bowel loops were superiorly displaced. The other intra-abdominal organs were sonographically normal. A diagnosis of moderate spontaneous ovarian hyperstimulation was made.
Figure 1.
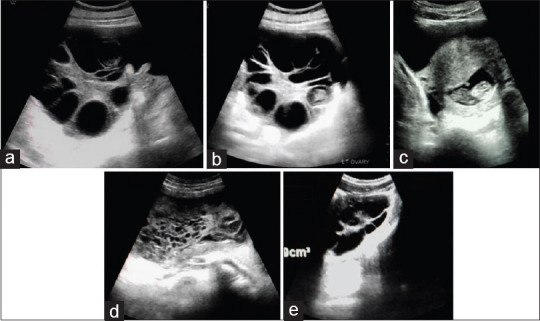
(a and b) Case 1: Transverse sections of enlarged right and left ovaries with thick vascularized echogenic stroma containing multiple peripherally placed thin-walled cysts some of which show internal echoes. (c) Case 1: Single viable intrauterine fetus with a normal anteriorly sited placenta. (d) Case 2: Bulky uterus containing a hyperechoic structure with multiple small-sized cystic spaces within its cavity. (e) Case 2: Bulky right ovary with peripherally placed thin-walled anechoic cysts and a thickened echogenic stroma giving a spoke wheel appearance
The patient was managed conservatively on admission with intravenous fluid, albumin, analgesics, 1 mg oral cabergoline (for 7 days), antibiotics, and prophylactic anticoagulation. Strict input and output chart, regular monitoring of vital signs, weight, abdominal girth, hematocrit, liver and renal function tests, and serial imaging were done until she was stable and then discharged at 13 weeks gestation. Symptoms completely resolved at 20 weeks with the abdominal girth decreasing from 110 cm to 45 cm, and the uterus became palpable. The rest of the pregnancy was uneventful. She later went into spontaneous labor at 37 weeks 4 days and delivered a healthy female neonate weighing 3.4 kg.
Case 2
A 17-year-old primigravida with a normal last menstrual period 4 months prior to presentation was sent for ultrasound imaging on account of abdominal distension, lower abdominal pain, generalized weakness, and irregular bleeding per vagina for about 3 months. There was no history of cough at presentation. A bulky, anteverted uterus measuring 15.0 cm × 7.0 cm × 13.3 cm containing large hyperechoic lesion with multiple small-sized cystic spaces within its cavity was visualized on ultrasound. There was no demonstrable fetal part, increased vascularity, or myometrial invasion [Figure 1d].
The ovaries were bulky and measure 9.0 cm × 4.4 cm × 5.5 cm and 6.6 cm × 3.5 cm × 6.1 cm. They consist of multiple thin-walled moderate-sized cysts, echogenic stroma giving a spoke wheel-appearance but no solid component. There was no ascites or pleural effusion [Figure 1e].
A diagnosis of complete molar gestation with theca lutein cysts resulting in mild OHSS was made. βHCG levels, further gynecological evaluation, chest X-ray, and tissue sampling to rule out choriocarcinoma were recommended. The patient was lost to follow-up.
DISCUSSION
s-OHSS is an uncommon phenomenon with no documented prevalence in the literature. Factors such as young age, low body mass index, polycystic ovarian syndrome, high antral follicle count, and high levels of basal estradiol levels were considered to be responsible for OHSS following ART.11 Age was the only risk factor identified in our cases.
The main pathogenesis of s-OHSS is still uncertain. Different studies have identified five heterozygous activating types of FSHR mutation that can result in the development of s-OHSS.7,8,9,10,13,14 Based on the clinical presentation and FSHR mutation, three different pathways were postulated by De Leener et al.8 In Type I s-OHSS, there is FSHR mutation with normal or low βHCG levels, which may be responsible for recurrent s-OHSS. High levels of βHCG in the background of FHSR mutation are the hallmark of Type II cases. This is the most common type and is seen in hydatidiform mole and multiple gestations. Type III s-OHSS occurs in the background of hypothyroidism with high levels of thyroid-stimulating hormone (TSH). This can occur even in the absence of pregnancy and the symptoms improve with levothyroxine therapy.15 The Type IV FSHR mutation is related to gonadotropin adenomas secreting follicle-stimulating hormone (FSH) or luteinizing hormone (LH).7
FSHR mutation can be of activating and inactivating types. Activating type results to increased sensitivity to HCG alone or with TSH and FSH while inactivating type results in sterility due to poor response of the ovaries to gonadotropins. The interplay between βHCG, FSH, LH, and TSH is because they belong to a family of glycoproteins with a similar receptor comprising two subunit: a common alpha unit and a hormone-specific beta subunit. HCG and LH normally bind to the LH receptor, while FSH and TSH bind to separate FSH and TSH receptors. One or more of these similar hormones may bind to an abnormal FSHR and cause a cascade of reaction that can lead to s-OHSS.16,17
Receptor typing was not done for our cases due to unavailability in our setting and resource constraint. Thyroid function evaluation was also not done; however, our cases appear to fall into type I and II of De Leener et al. classification.8 Our subject in case 1 had no history of s-OHSS in her previous three pregnancies to suggest recurrent s-OHSS. Similar finding has been reported in other studies.18 It is unclear if the manifestation of the FSHR mutation is transient or sporadic or if there are other genetic variations that may be responsible for a nonrecurrent expression as seen in our case.
Golan and Weissman12 classified OHSS (both spontaneous and iatrogenic) into three categories with five grades based on the clinical manifestation and imaging findings:
Mild OHSS: presence of bilateral multicystic enlarged ovaries; Grade 1 shows abdominal distention and discomfort, while Grade 2 presents with additional nausea, vomiting, and/or diarrhea and enlarged ovaries measuring 5–12 cm. Moderate OHSS: represents Grade 3 and presents with features of mild OHSS plus evidence of ascites on ultrasound. Severe OHSS: presents as Grade 4 with clinical evidence of ascites and/or hydrothorax and dyspnea and Grade 5 with change in blood volume, hemoconcentration, coagulation abnormalities, and diminished kidney perfusion and function. Our cases were in the moderate and mild categories for case 1 and case 2, respectively, based on their clinical symptoms and imaging findings.
Our case 1 patient with moderate s-OHSS was managed conservatively till delivery with a favorable outcome. Abdominal paracentesis was considered due to the rising hematocrit, but was not done as symptoms gradually regressed. Grossman et al. considered moderate-to-severe OHSS as an unrecognized compartment syndrome where the increased intra-abdominal pressure is responsible for reduced perfusion and multiorgan failure including cardiac, renal, and hepatic failure in severe cases. The resultant reduced renal perfusion, reduced hepatic proteins, and clotting factors are responsible for the oliguria, hemoconcentration, and thromboembolism. Abdominal paracentesis, low molecular weight heparin, and fluid and electrolyte balance should be part of the core management protocol.19 Increased vascular permeability with fluid shift and reduced intravascular space thought to be the final pathway for OHSS is mediated by type 2 VEGF. Cabergoline, an ergot-derived dopamine agonist used in our case 1 management, binds to and inhibits phosphorylation and signaling at the type 2 VEGF receptor, thereby improving the symptoms of OHSS.2
Evacuation, histolopathological evaluation, and adequate follow-up with imaging and biochemical of βHCG assay to rule out choriocarcinoma would have been part of our core management protocol for the second case, which was lost to follow-up.
Imaging plays a very vital role in appropriate patient management which includes diagnosis, serial monitoring of ovarian size, grading the disease, and assessing complications such as ovarian torsion and ovarian rupture. It also rules out other possibilities such as malignant ovarian masses and where necessary aid in guided interventions such as paracentesis (to avoid trauma to the enlarged ovaries). With appropriate diagnosis, unnecessary surgical interventions with poor prognosis can be avoided, especially in cases where ovarian malignancy was considered like in our index case 1.
CONCLUSION
s-OHSS occurring in the absence of ovulation induction or ovarian stimulation is a rare possibility with life threatening potential in pregnancy. Various etiologies related to FSHR mutation such as molar gestation in one of our index cases, hypothyroidism, and pituitary adenoma-induced cases have been documented. Understanding the pathophysiology, adequate imaging, and tailored management are essential to avoid misdiagnosis, exclude complications, and arrive at a successful outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): A review. Hum Reprod Update. 2002;8:559–77. doi: 10.1093/humupd/8.6.559. [DOI] [PubMed] [Google Scholar]
- 2.Hosseini MA, Aleyasin A, Mahdavi A, Nezami R, Safdarian L, Fallahi P. The effectiveness of cabergoline for the prevention of ovarian hyperstimulation syndrome. Iran J Med Sci. 2011;36:207–12. [PMC free article] [PubMed] [Google Scholar]
- 3.Soares SR, Gómez R, Simón C, García-Velasco JA, Pellicer A. Targeting the vascular endothelial growth factor system to prevent ovarian hyperstimulation syndrome. Hum Reprod Update. 2008;14:321–33. doi: 10.1093/humupd/dmn008. [DOI] [PubMed] [Google Scholar]
- 4.Smitz J, Camus M, Devroey P, Erard P, Wisanto A, Van Steirteghem AC. Incidence of severe ovarian hyperstimulation syndrome after GnRH agonist/HMG superovulation for in vitro fertilization. Hum Reprod. 1990;5:933–7. doi: 10.1093/oxfordjournals.humrep.a137223. [DOI] [PubMed] [Google Scholar]
- 5.Omokanye LO, Olatinwo AO, Saadu LO, Biliaminu SA, Durowade KA, Panti AA. Assisted reproductive technology: Experience from a public tertiary institution in north central Nigeria. Afr J Infertil Assist Concept. 2016;1:23–126. [Google Scholar]
- 6.Orhue AA, Aziken ME, Osemwenkha AP, Ibadin KO, Odoma G. In vitro fertilization at a public hospital in Nigeria. Int J Gynaecol Obstet. 2012;118:56–60. doi: 10.1016/j.ijgo.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Panagiotopoulou N, Byers H, Newman WG, Bhatia K. Spontaneous ovarian hyperstimulation syndrome: Case report, pathophysiological classification and diagnostic algorithm. Eur J Obstet Gynecol Reprod Biol. 2013;169:143–8. doi: 10.1016/j.ejogrb.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 8.De Leener A, Montanelli L, Van Durme J, Chae H, Smits G, Vassart G, et al. Presence and absence of follicle-stimulating hormone receptor mutations provide some insights into spontaneous ovarian hyperstimulation syndrome physiopathology. J Clin Endocrinol Metab. 2006;91:555–62. doi: 10.1210/jc.2005-1580. [DOI] [PubMed] [Google Scholar]
- 9.Daelemans C, Smits G, de Maertelaer V, Costagliola S, Englert Y, Vassart G, et al. Prediction of severity of symptoms in iatrogenic ovarian hyperstimulation syndrome by follicle-stimulating hormone receptor Ser680Asn polymorphism. J Clin Endocrinol Metab. 2004;89:6310–5. doi: 10.1210/jc.2004-1044. [DOI] [PubMed] [Google Scholar]
- 10.Smits G, Olatunbosun O, Delbaere A, Pierson R, Vassart G, Costagliola S. Ovarian hyperstimulation syndrome due to a mutation in the follicle-stimulating hormone receptor. N Engl J Med. 2003;349:760–6. doi: 10.1056/NEJMoa030064. [DOI] [PubMed] [Google Scholar]
- 11.Enskog A, Henriksson M, Unander M, Nilsson L, Brännström M. Prospective study of the clinical and laboratory parameters of patients in whom ovarian hyperstimulation syndrome developed during controlled ovarian hyperstimulation for in vitro fertilization. Fertil Steril. 1999;71:808–14. doi: 10.1016/s0015-0282(99)00090-4. [DOI] [PubMed] [Google Scholar]
- 12.Golan A, Weissman A. Symposium: Update on prediction and management of OHSS. A modern classification of OHSS. Reprod Biomed Online. 2009;19:28–32. doi: 10.1016/s1472-6483(10)60042-9. [DOI] [PubMed] [Google Scholar]
- 13.Vasseur C, Rodien P, Beau I, Desroches A, Gérard C, de Poncheville L, et al. A chorionic gonadotropin-sensitive mutation in the follicle-stimulating hormone receptor as a cause of familial gestational spontaneous ovarian hyperstimulation syndrome. N Engl J Med. 2003;349:753–9. doi: 10.1056/NEJMoa030065. [DOI] [PubMed] [Google Scholar]
- 14.Delbaere A, Smits G, Olatunbosun O, Pierson R, Vassart G, Costagliola S. New insights into the pathophysiology of ovarian hyperstimulation syndrome. What makes the difference between spontaneous and iatrogenic syndrome? Hum Reprod. 2004;19:486–9. doi: 10.1093/humrep/deh124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilanchezhian S, Mohan SV, Ramachandran R, Babu SR. Spontaneous ovarian hyperstimulation syndrome with primary hypothyroidsm: Imaging a rare entity. Radiol Case Rep. 2015;10:1050. doi: 10.2484/rcr.v10i1.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chae HD, Park EJ, Kim SH, Kim CH, Kang BM, Chang YS. Ovarian hyperstimulation syndrome complicating a spontaneous singleton pregnancy: A case report. J Assist Reprod Genet. 2001;18:120–3. doi: 10.1023/A:1026543027300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittal K, Koticha R, Dey AK, Anandpara K, Agrawal R, Sarvothaman MP, et al. Radiological illustration of spontaneous ovarian hyperstimulation syndrome. Pol J Radiol. 2015;80:217–27. doi: 10.12659/PJR.893536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osaikhuwuomwam JA, Osenwekha AP. Ovarian hyperstimulation syndrome in a spontaneous pregnancy: Apotential for missed diagnosis. Niger Med J. 2016;57:74–6. doi: 10.4103/0300-1652.180563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grossman LC, Michalakis KG, Browne H, Payson MD, Segars JH. The pathophysiology of ovarian hyperstimulation syndrome: An unrecognized compartment syndrome. Fertil Steril. 2010;94:1392–8. doi: 10.1016/j.fertnstert.2009.07.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]


