Abstract
Xenotransplantation of human tissues into immunodeficient mice has emerged as an invaluable preclinical model to study human biology and disease progression and predict clinical response. The most common anatomical site for tissue transplantation is the subcutaneous pocket due to simple surgical procedures and accessibility for gross monitoring and advanced imaging modalities. However, subcutaneously implanted tissues initially experience a sharp change in oxygen and nutrient supply and increased mechanical deformation. During this acute phase of tissue integration to the host vasculature, substantial cell death and tissue fibrosis occur limiting engraftment efficiency. Previously, we demonstrated that the implantation of inverted colloidal crystal hydrogel scaffolds triggers proangiogenic and immunomodulatory functions without characteristic foreign body encapsulation. In this study, we examine the use of this unique host response to improve the ectopic transplantation of tissues to the subcutaneous site. Scaffold-assisted tissues preserved morphological features and blood vessel density compared to native tissues, whereas scaffold-free tissues collapsed and were less vascularized. Notably, the supporting biomaterial scaffold modulated the foreign body response to reduce the localization of Ly6G+ cells within the transplanted tissues. Cotransplantation of patient-derived gastric cancer with a scaffold resulted in a comparable level of engraftment to conventional methods; however, detailed immunohistological characterization revealed significantly better retention of proliferative cells (Ki67+) and human immune cells (CD45+) by the end of the study. We envision that leveraging the immunomodulatory properties of biomaterial interfaces can be an attractive strategy to improve the functional engraftment of xenotransplants and accelerate individualized diagnostics and the development of novel therapeutic strategies.
Keywords: tissue transplantation, biomaterial, immune modulation, patient-derived xenograft, tissue engineering, gastric cancer
Graphical Abstract

1. INTRODUCTION
Mice have been used as an important model to understand the basic biology of health and disease and serve as a vital link in the preclinical development of therapies for human diseases.1–5 The development and advancement of immunodeficient mouse models have enabled the xenotransplantation of human cells and tissues, significantly improving the clinical relevance of animal studies.6 For example, the xenotransplantation of human tumor cell lines, patient-derived immune cells, fetal tissue, organoids, and tumor fragments has been performed to understand human cell and tissue biology in health and disease.7–13 In particular, patient-derived xenograft (PDX) tumor models have demonstrated their capability to reflect the human tumor microenvironment, predict patient response to chemotherapy, and accelerate the discovery and validation of preclinical drugs.14–17 A coclinical trial in small lung cell cancer patients and corresponding PDX models showed consistency in responsiveness to cisplatin therapy, demonstrating the screening potential of PDX models.18 Nevertheless, the fidelity, stability, engraftment efficiency, and engraftment kinetics of PDX tumors and mature tissues remain critical barriers to overcome for practical application.19 For example, PDX models frequently fail to progress to form metastatic lesions in mice.20 Additionally, engraftment rates range widely between studies, from 10 to 95% depending on the tumor type, and the time to engraft can take several months to establish proliferative tumors.9
Orthotopic transplantation of human tissues or tumors is most desirable but often limited by a notable size difference between the mouse and humans, and technical challenges to surgically access organs. The subcapsular region of the kidney is an attractive transplant site because of the richly vascularized environment, yet the small volume limits the size of transplanted tissues and requires technical expertise.21 Alternatively, the subcutaneous space has been commonly used for transplantation because of the large tissue volume capacity, proximity to the blood supply, simple surgical procedure, and ease of accessibility for various imaging modalities.22,23 However, the engraftment efficiency of subcutaneously transplanted tissues remains suboptimal because of acute adaptation processes experienced by the donor tissue. First, the physical stresses beneath the skin caused by compressive forces from skin elasticity and host motion can differ greatly from the donor tissue microenvironment.24,25 This additional strain can cause soft tissue deformation and trigger abnormal mechanotransduction signaling.26 Second, transplanted tissues suffer from limited oxygen and nutrient supply until blood vessels can reconnect to the host vasculature or neoangiogenesis occurs. Delayed vascularization is accompanied by cell death and transformation of cellular phenotypes.27 Third, the host wound response generates robust cellular recruitment from resident and systemic immune cells.28 Acute local inflammation and fibrosis resultantly decrease donor tissue cell number and cellular heterogeneity as host cells infiltrate the graft and gradually replace the donor tissue.29 It is imperative to develop strategies to overcome these acute detrimental effects on the donor tissue to improve the engraftment efficiency of current xenograft models to the subcutaneous space.
Accumulating data indicate that biomaterial implantation is a compelling route to modulate the host immune response.30–36 Implant geometry, surface topology and chemistry, material composition, and bioactive delivery of drugs or cells have been explored to reduce fibrosis around implants and improve the longevity of biomedical devices.37–41 For example, implantation of medical-grade polymers with circular-, triangular-, and pentagonal-shaped cross sections revealed a circular rod had the lowest host response.42 The degree of foreign body response against spherical biomaterials was shown to be modulated as a function of implant size.37 Synthetic biomaterials composed of zwitterionic hydrogels that alternate positively and negatively charged functional groups significantly delayed the foreign body response and resultant fibrotic encapsulation.43 Mechanical properties of synthetic zwitterionic hydrogels have been identified as a potent parameter to modulate the foreign body response after subcutaneous implantation.41 Extracellular matrix scaffolds have shown to trigger a notably different pattern of local and systemic immune responses compared to synthetic materials by switching from fibrotic encapsulation to wound-healing and tissue repair mechanisms.44,45 Hydrogels embedded with mesenchymal stem cells attenuated macrophage response and reduced the fibrotic capsule thickness.46 These results collectively indicate that implantable biomaterials represent an opportunity to modulate the fate of implanted human tissues to support better functional engraftment while retaining the original tissue complexity.
In the presented work, we hypothesized that an implantable biomaterial approach could be utilized to enhance the ectopic engraftment of mature tissue by improving vascularization and altering the migration of immune cells. Furthermore, the biomaterial would act as a mechanical support to suppress mechanical deformation. We tested these hypotheses using inverted colloidal crystal (ICC) hydrogel scaffolds consisting of fully interconnected macroscale spherical pore arrays that have demonstrated mechanical robustness, and proangiogenic and immunomodulatory features after subdermal implantation.47–49 We first examined if coimplanted ICC hydrogel scaffolds promote the integration of liver and lung tissues retrieved from immunocompetent mice genetically engineered with a DsRed fluorescent reporter protein to the immunodeficient nonobese diabetic (NOD) scid γ (NSG) host mice. We also tested the impact of preseeded human bone marrow stromal cells (BMSCs) in ectopic tissue engraftment, which have been shown to further enhance angiogenesis and reduce inflammation.47–50 Finally, we extended the study to examine the effects of stromal cell-seeded ICC hydrogel scaffolds on the engraftment efficiency and cellular heterogeneity of human patient-derived gastric cancer samples. We envision that this proof-of-concept study for biomaterial-assisted ectopic tissue and tumor transplantation may improve the usage of mouse models for preclinical cancer research.
2. MATERIALS AND METHODS
All chemicals and materials were purchased from Sigma-Aldrich or Fisher Scientific unless specified. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Massachusetts—Amherst and Ajou University Medical Center. Patient-derived samples were approved by the institutional review board of the Ajou University Hospital. Informed consent was received from all patients.
2.1. Porous Hydrogel Scaffold Fabrication.
Interconnected porous hydrogel scaffolds were fabricated as previously described.49 Soda lime glass beads were sorted using an Advantech Sonic Sifter using 250 and 300 μm collection trays. The resulting beads had an average diameter of 275 ± 21 μm. Glass beads were dispersed in deionized (DI) water and gradually loaded into an 8 × 35 mm or 12 × 35 mm glass vial to a height of 2 mm. A dense lattice structure was achieved utilizing an ultrasonic water bath. Water was removed in a 60 °C oven prior to thermal annealing of the glass beads in a 664 °C furnace for 4 h. The annealed glass bead templates were infiltrated with a hydrogel precursor solution via centrifugation. The precursor solution was composed of a 30 wt % acrylamide monomer, a 1.5 wt % bisacrylamide cross-linker, a 0.2 vol % N,N,N′,N′-tetramethylethylenediamine accelerator, and a 0.2 vol % 2-hydroxy-2-methylpropiophenone photoinitiator in nitrogen-purged DI water. The precursor solution was immediately polymerized under a 15 W ultraviolet light source for 15 min. The next day, excess hydrogel was removed by scraping the glass bead template with a razor blade on all surfaces. Glass beads were selectively dissolved in alternating washes of an acid solution containing a 1:5 dilution of hydrofluoric acid in 1.2 M hydrochloric acid and 2.4 M hydrochloric acid. Washes were performed on a shake plate, and the solutions were changed every 4 h until the beads were removed. Scaffolds were washed with DI water to remove residual acid and lyophilized. Cracked or misshapen scaffolds were removed before proceeding. Scaffolds were sterilized with 70% ethanol, and the scaffolds were surface-treated with sulfosuccinimidyl 6-(4′-azido-2′-nitrophenylamino)hexanoate to covalently cross-link rat-tail collagen. Scaffolds were stored at 4 °C in sterile phosphate-buffered saline (PBS).
2.2. Cell Culture of BMSCs and Seeding onto ICC Scaffold.
Human bone marrow aspirates were acquired from Lonza, and isolation was performed as previously described.49 Cells were cryogenically frozen in a medium containing 10% dimethyl sulfoxide. Cells were cultured with α-modified, minimal essential medium supplemented with 10% fetal bovine serum (FBS), 2% penicillin–streptomycin, 0.2% gentamicin, and a 1 μg/L recombinant human fibroblast growth factor. Half a million BMSCs were seeded onto partially dehydrated scaffolds and kept in culture for up to 1 week prior to use. BMSCs were not used beyond passage 4, and the media was changed every 3 days.
2.3. Sterile DsRed Tissue Collection and Integration into BMSC Scaffold.
A breeding pair of DsRed (005441) was obtained from Dr. Barbara Osbourne. Mice were housed in sterile conditions with unrestricted access to food and water. Mice used in this study were between 6 and 13 weeks old. DsRed mice were euthanized via carbon dioxide, and the liver and lung tissues were harvested in a biosafety cabinet and kept in sterile PBS over ice. A sterile 3 mm biopsy punch was used on the harvested liver and lung to generate tissue inserts. Additionally, a 3 mm hole was created in the center of the BMSC-seeded scaffolds. The tissue piece was gently inserted inside the hole of the scaffold using sterile forceps. Completed scaffold-assisted implants were kept in PBS on ice until implantation surgery.
2.4. Subdermal Transplantation into NSG Mice.
A breeding pair of NOD-scid IL2Rgnull mice (005557) was initially obtained from the Jackson Laboratories. Mice were housed in sterile conditions with unrestricted access to food and water. Mice used in this study were between 6 and 13 weeks old. NSG mice were anesthetized with 1.5% isoflurane before removing the dorsal hair with electric clippers and Nair. The skin was sterilized using 70% isopropyl alcohol prep wipes. Mice received 2 mg meloxicam/kg mouse weight subcutaneously prior to surgery. A subcutaneous pocket was created by making a 2 mm horizontal incision in the dorsal space followed by expansion of surgical scissors into the incision and expansion. One scaffold-free or scaffold-assisted tissue with or without BMSCs was inserted into the pocket, and the incisions were closed with two 7 mm wound clips. Each mouse received four implants. Wound clips were removed after 1 week.
2.5. Evans Blue Perfusion and Quantitative Imaging.
Prior to euthanization, mice were injected with a 2 wt % Evans blue dye at a dosage of 2 mL/kg mouse weight. Dye was allowed to perfuse for 20 min before euthanization via carbon dioxide. Tissues were harvested from the mice and imaged on an IVIS Spectrum CT using an excitation wavelength of 640 nm and an emission wavelength of 680 nm.
2.6. MDA-MB-231 Cell Culture and Intravenous Injection.
MDA-MB-231 human breast cancer cells were cultured with Dulbecco’s modified Eagle’s medium supplemented with 10% FBS and 1% penicillin–streptomycin. For intravenous cell injections, 2 × 106 cells were suspended in 200 μL of PBS and injected into the lateral tail vein using a 27-gauge needle.
2.7. Frozen Tissue Preparation and Sectioning.
At the conclusion of the in vivo experiment, implants were frozen by embedding the tissue in Cryomatrix and snap-frozen in 2-methylbutane cooled on dry ice. The frozen tissue was cut to 20 μm using a NX70 Cryostat. Frozen tissue blocks and sectioned slides were stored at −80 °C.
2.8. Immunohistostaining (IHS).
Frozen slides were fixed in −20 °C acetone for 10 min. Slides were washed with a wash buffer consisting of 0.5% Tween 20 in PBS (PBST) three times before blocking in a solution containing 10% normal goat or donkey serum and 1% bovine serum albumin for 1 h. Following blocking, primary antibodies diluted in a blocking solution were added to the slides and left in a humidified chamber overnight at 4 °C. Slides were washed three times with PBST and then incubated with secondary antibodies diluted in a blocking solution for 2 h at room temperature. Prior to imaging, slides were washed three times in PBST and stained with a 10 ng/μL solution of 4′,6-diamidino-2-phenylindole (DAPI). Fluorescent images were taken on a Zeiss Cell Observer SD. A table of primary and secondary antibodies used in this study has been provided (Table S1).
2.9. Human Cancer and Fibroblast Isolation and Transplantation.
Human gastric cancer specimens were obtained from patients undergoing tumor resection surgery at the Ajou University Hospital as previously described.51 Fibroblasts were isolated from gastric cancer tissues and used within six passages. Cells (2.5 × 105) were seeded onto 12 mm scaffolds. A human gastric cancer specimen (3–5 mm) was placed on top of the cancer-associated fibroblast (CAF)-seeded scaffolds. After removing excess hydrogel matrix, implants were inserted into a subcutaneous pocket made on the dorsal side of Balb/c nude mice. The incision was closed using sutures.
2.10. Tissue Histology.
Formalin-fixed paraffin-embedded tissue samples from PDX experiments were sectioned using a microtome. Sections were cut to a thickness of 6 μm. To deparaffinize sections, slides were treated sequentially with xylenes, 100% ethanol, 95% ethanol, and 50% ethanol before washing with deionized water. For antigen retrieval, citrate buffer (10 mM, pH 6.0) was preheated to 90–95 °C on a hot plate before slides were immersed and incubated for 20 min. The antigen retrieval solution was then removed from the hot plate and allowed to cool down to room temperature. Slides were washed with the PBST solution before beginning the standard blocking and antibody staining procedure. Hematoxylin and eosin (H&E) staining was performed as previously described.49 Brightfield images were taken on an EVOS FL Auto microscope.
2.11. Image Analysis.
All image processing and quantitative analysis were performed in ImageJ. For overlapping quantification, DsRed images were used to identify the transplanted tissue area.
2.12. Statistics.
Unpaired Studen’s t tests were performed for comparison of the mean values between groups in mouse tissue implantation studies. A logistic regression analysis was used to evaluate the effect of scaffold on PDX engraftment. The Mann–Whitney U test was performed to analyze the statistical significance of immunohistostaining measurements of markers in PDX tumor experiments. Statistical significance was determined if p < 0.05 for the two-tailed analysis. All quantitative data represent mean and standard deviation.
3. RESULTS
3.1. Subdermal Coimplantation of Mature Liver Tissue within an ICC Hydrogel Scaffold Reduces Tissue Deformation.
ICC scaffolds consist of an interconnected spherical pore array providing a high surface-area-to-volume ratio and elicit a unique foreign body response that results in a vascularized tissue that does not undergo endogenous host rejection. We hypothesized that the coimplantation of a mature tissue with an ICC hydrogel scaffold may improve the engraftment of transplanted tissues (Figure 1a). A 30 wt % polyacrylamide hydrogel scaffold consisting of 300 ± 15 μm pores was used for this study, which has been shown to induce a richly vascularized tissue within 3 weeks of subdermal implantation.49 To accommodate transplanted tissue samples, a cylindrical ICC scaffold (height = 2 mm and diameter = 6.5 mm) was modified by punching out a 3 mm diameter hole centrally. Human BMSCs were homogeneously seeded at a density of 5 × 105 cells/scaffold (Figure 1b). We employed DsRed immunocompetent mice as tissue donors and NSG immunodeficient mice as ectopic tissue recipients. Cells in DsRed mice ubiquitously express red fluorescence that facilitate the rapid identification of surviving donor tissues in the recipient without staining. Immediately prior to surgery, a 3 mm piece of freshly harvested DsRed mouse liver tissue was collected using a biopsy punch and placed in the center of a scaffold prior to subcutaneous implantation. Three different groups of implants were used in the study, scaffold-free liver (scaffold-free), unseeded scaffold-assisted liver (blank-scaffold), and BMSC-seeded scaffold-assisted liver (BMSC-scaffold). Transplanted tissues were retrieved after 1 or 2 weeks of implantation (Figure 1c). Gross images of the explanted tissue after 1 week of implantation showed that the color of the liver tissue turned pale due to the significant loss of red blood cells, whereas the liver tissue with a scaffold retained dark red color, a sign of vasculature (Figure 1d). Hematoxylin and eosin (H&E) staining revealed that blank-scaffold and BMSC-scaffold transplanted hepatic tissues retained morphological features present in the native liver such as portal veins. A fibrotic capsule was observed around all sides of scaffold-free transplants and the host–transplant interface of blank-scaffold and BMSC-scaffold transplants. Interestingly, the liver tissue at the interface of the biomaterial scaffold showed less fibrotic tissue formation (Figure 1e,f). Characterization of albumin secretion via immunohistochemistry revealed the maintenance of liver function in all transplanted tissues (Figure S1). We repeated the experiment with DsRed mouse lung tissue that has more than 25-fold weaker mechanical strength than a liver tissue.52 The result revealed a clear collapse of alveolar structures in scaffold-free transplants (Figure S2). Collectively, these results indicate that the coimplantation of mature mouse tissues with an ICC hydrogel scaffold better preserves the intrinsic tissue structure by preventing tissue collapse and the reduction of fibrotic capsule formation directly at the transplant interface compared to that of control scaffold-free transplants.
Figure 1.

(a) Schematic of the general strategy to improve the ectopic engraftment of mature tissues using ICC hydrogel scaffolds. (b) Representative brightfield (top) and fluorescence (bottom) microscopy images of ICC hydrogel scaffold pore structure and homogeneous seeding of fluorescently labeled BMSCs on the surface of the scaffold pores. (c) Experimental schematic of DsRed mouse liver tissue transplantation into NSG immunodeficient mice with an ICC hydrogel scaffold. (d) Gross images of mouse liver tissue and mouse liver tissue integrated with a hydrogel scaffold and after 1 week of subcutaneous transplantation. (e) H&E images of the native mouse liver tissue structure. (f) H&E images of scaffold-free, blank-scaffold, and BMSC-scaffold transplants after 1 week of implantation. White arrows highlight large portal veins, a key anatomical structure in the liver.
3.2. ICC Hydrogel Scaffold Promotes Vascularization to Transplanted Tissue.
We next examined if coimplanted ICC hydrogel scaffolds promote the formation of host blood vessels and vascular connection with the transplanted liver tissue. To validate the functional vascular connection between the transplanted liver tissue and the host, we intravenously perfused Evans blue dye in mice carrying 1 week implants, 20 min before sacrifice. A gross examination confirmed rich perfusion of Evans blue dye in scaffold-assisted transplants, whereas limited perfusion was evident in scaffold-free transplants (Figure 2a). Spatial distribution of the perfused dye was substantiated with IVIS fluorescent imaging. In scaffold-assisted transplants, strong fluorescence was localized to the area containing the liver tissue (Figure 2b). Quantitative analysis revealed that scaffold-assisted liver tissue implants showed at least 2.5-fold higher fluorescence signal than scaffold-free implants. Blank-scaffolds showed a 25% higher fluorescence than BMSC-scaffolds (Figure 2c).
Figure 2.

(a) Experimental schematic of the Evans blue perfusion experiment and gross image of the transplanted liver tissue and surrounding subcutaneous tissue. (b) Gross and fluorescent images of explanted tissues substantiating localized Evans blue dye within the transplanted liver tissue. (c) Quantitative comparison of Evans blue dye fluorescence in hepatic tissues, 1 week after transplantation. (N = 3 implants per group, *P < 0.05.)
Small sinusoidal blood vessels present in the transplanted liver tissues were visualized via endomucin antibody staining. In all transplants, intrinsic hepatic sinusoidal features were preserved. Blood vessels in the surrounding fibrotic capsule were less organized with a branched morphology (Figure 3a–d). Quantitative analysis of endomucin expression in the liver tissue revealed that the overlapping regions of endomucin and endogenous DsRed fluorescence in BMSC-scaffolds were 63 and 35% higher compared to those in scaffold-free and blank-scaffold transplants, respectively (Figure 3e). In the surrounding fibrotic tissue area, the blood vessel density was significantly higher in scaffold-assisted transplants regardless of BMSCs and at least 2-fold higher than scaffold-free controls (Figure 3f). The discrepancy between Evans blue characterization and immunohistostaining results may be due to immature vasculature and increased leakiness in blank-scaffold samples.
Figure 3.
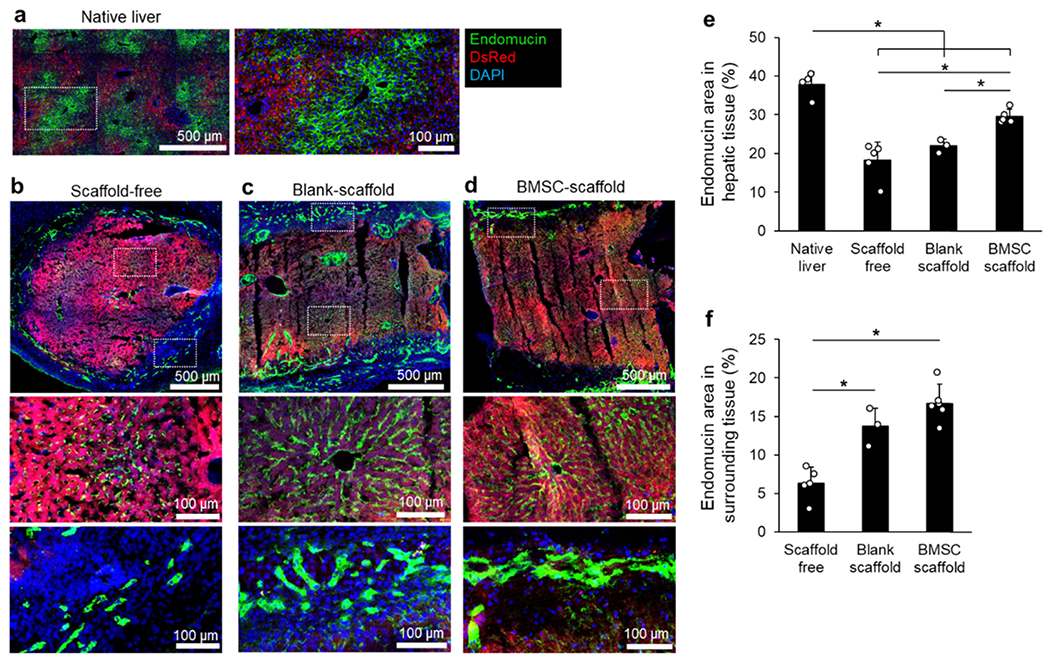
(a–d) Immunohistostaining (IHS) images of native DsRed liver (a), scaffold-free (b), blank-scaffold (c), and BMSC-scaffold (d) transplanted tissues. Higher-magnification images show hepatic sinusoids and newly generated blood vessels that surround the transplanted tissue. (e) Quantitative comparison of endomucin+ hepatic sinusoid retention in ectopic tissue transplants. (f) Quantitative comparison of endomucin+ blood vessel density in the surrounding host tissue. (N = 3–5 implants per group, *P < 0.05.)
We further characterized vascular reconnection via injecting MDA-MB-231 human breast cancer cells that stably express eGFP into mice bearing transplanted liver tissues. Three days after injection, the tissue was removed and analyzed for dissemination to the transplanted liver tissue (Figure 4a). Imaging for endogenous eGFP disseminated tumor cells (DTCs) confirmed the presence of tail vein delivered human breast cancer cells in liver transplants in proximity to positively stained endomucin blood vessels (Figure 4b). Scaffold-free tissues had significantly fewer DTCs compared to blank-scaffold and BMSC-scaffold hepatic tissues (Figure 4c). Taken together, these results demonstrated that BMSC-scaffolds improved the preservation of intrinsic hepatic sinusoids and assisted in reaching stably reconnected vasculature compared to scaffold-free and blank-scaffold.
Figure 4.

(a) Experimental schematic of functional vascular reconnection assay in scaffold-assisted ectopic transplant via intravenous delivery of human MDA-MB-231 cells. (b) IHS of endogenous eGFP MDA-MB-231 cells, endomucin+ hepatic sinusoids, and endogenous DsRed+ hepatocytes. White arrow highlights DTC. (c) Quantitative comparison of DTCs in transplanted liver tissues. [N = 9 sections from three implants per group except scaffold-free (N = 2), *P < 0.05.]
3.3. Biomaterial Scaffold Modulates Innate Immune Cell Migration.
Transplantation evokes a foreign body response, during which innate immune cells are recruited to the transplanted tissue and wound-healing mechanisms act to resolve tissue disruption. We investigated the recruitment of mouse neutrophils and macrophages in the transplanted tissue microenvironments via immunohistostaining (IHS) of Ly6G and F4/80, respectively (Figure 5a–d). Ly6G+ neutrophils are early responders in the foreign body response. Ly6G+ cell infiltration was significantly higher in transplanted liver tissue compared to that in native liver regardless of the presence of a scaffold. However, scaffold-free hepatic tissue had a 118-fold increase in Ly6G+ cells over native tissue compared to 49-fold and 20-fold increases in blank-scaffold and BMSC-scaffold groups, respectively. Samples collected 2 weeks after transplantation had no significant differences in Ly6G+ cell infiltration, indicating potential resolution of acute response by Ly6G+ cells. Interestingly, the surrounding tissue around blank-scaffold and BMSC-scaffold transplants displayed a 10-fold and 21-fold increase in Ly6G+ cells compared to that of scaffold-free transplants after 1 week, respectively. The surrounding tissue of BMSC-scaffolds had the highest infiltration of Ly6G+ cells 1 week after transplantation; however, by week 2, the Ly6G+ cell significantly decreased and was not significantly different from scaffold-free and blank-scaffold values (Figure 5e). Unlike neutrophils, macrophages are abundant in native liver tissue. Scaffold-free, blank-scaffold, and BMSC-scaffold transplants contained at least 4-fold fewer F4/80+ cells than native liver tissue. There were no differences in macrophage recruitment to the surrounding tissue in any of the experimental groups. The skewed distribution of F4/80+ cells between the hepatic and the surrounding tissues remained 2 weeks after transplantation (Figure 5f). Examination of the transplanted lung tissue in scaffold-free and BMSC-scaffold transplants showed similar modulation of Ly6G+ cell recruitment and macrophage response (Figure S3). These results demonstrate that the presence of the scaffold altered the pattern of Ly6G+ cell recruitment during the foreign body response, while increasing the vascular availability to the transplanted tissue (Figure 5g).
Figure 5.

(a–d) IHS images of Ly6G+ neutrophils and F4/80+ macrophages with nucleus (DAPI) and hepatic tissue (DsRed) in native (a), scaffold-free (b), blank-scaffold (c), and BMSC-scaffold (d). High-magnification images show the interface between the hepatic tissue implant and host tissue. (e) Quantitative comparison of Ly6G+ neutrophil density in native liver tissue and ectopic tissue transplants and their spatial distribution between the transplanted liver and surrounding biomaterials 1 and 2 weeks after transplantation. (f) Quantitative comparison of F4/80+ macrophage density in native liver tissue and ectopic tissue transplants and their spatial distribution between the transplanted liver and surrounding biomaterials 1 and 2 weeks after transplantation. (g) Proposed mechanism of improved ectopic tissue engraftment via proangiogenic and immunomodulatory scaffold that improves vascular reconnection while diverting immune cell recruitment to the biomaterial. [N = 3–7 implants per group except scaffold-free 2 week (N = 2), *P < 0.05.]
3.4. Scaffold-Assisted Transplantation of Patient-Derived Gastric Cancers Aids Maintenance of Intrinsic Tissue Heterogeneity.
Next, we investigated the translational potential of scaffold-assisted xenotransplantation by transplanting seven human patient-derived gastric cancers into immunodeficient BALB/c nude mice. Patient-derived gastric cancer poorly engrafts when transplanted into immunocompromised mice with reported engraftment rates ranging from 15 to 28%.53–55 As with most PDX models, noncancerous human cells are often lost during xenotransplantation into mice. We hypothesized that the coimplantation of patient-derived gastric cancers with a biomaterial scaffold would assist in tissue engraftment and, in doing so, maintain intratumoral cellular heterogeneity (Figure 6a). To enhance the effect of the ICC scaffold, we seeded them with patient-derived cancer-associated fibroblasts (CAFs). CAFs have been documented to secrete numerous molecules that can stimulate cancer cells and noncancerous cells in the tumor microenvironment.51,56 The ICC scaffold described above was altered to accommodate larger tissue pieces by increasing the diameter to 12 mm while maintaining the same chemical composition, pore diameter, and surface coating. Round, 2 cm pieces of gastric cancer tissue were removed from patients and stored in complete media with antibiotics. The tumor pieces were split into smaller 3–5 mm pieces and then placed on top of the prepared scaffold. Scaffold material that exceeded the diameter of the tumor tissue was carefully removed using a razor blade. Placement on top of the scaffold rather than within the scaffold was chosen to increase the surface area between the scaffold and PDX samples, increasing the effect of CAF-related soluble factors (Figure 6b). One scaffold-assisted or scaffold-free tumor was implanted into 4–6 week old male BALB/c nude mice (Figure 6c). In each PDX study, engraftment was checked after 2 weeks via palpation. If at least one tumor from the patient grew, we continued the experiment for a maximum of 6 weeks (Figure 6d). Engraftment was considered successful if tumors remained by the end of the study. At the end of the experiment, mice were sacrificed and the samples were prepared for histological evaluation. Tumor morphology appeared to be conserved following transplantation in successfully engrafted tissues (Figure 6e). Overall, 11 scaffold-free and 30 scaffold-assisted cancer pieces were transplanted into mice (Figure 6f). The frequency of transplanted tumors that displayed incremental growth was slightly higher in scaffold-free xenografts than in scaffold-assisted xenografts (43.3 vs 36.4%) but statistical analysis using a logistic regression did not show a significant effect of scaffold on PDX engraftment (P = 0.5965) (Figure 6g).
Figure 6.

(a) Experimental schematic of scaffold-assisted human gastric tumor xenograft strategy. (b) Gross images of scaffold-assisted patient gastric tumor biopsy and subcutaneous transplantation into Balb/c nude mice. (c) Gross images of PDX implants beneath the skin with and without scaffold. (d) Gross image of successful PDX engraftment and extracted sample with scaffold removed. (e) Histological comparison of gastric tumor tissue structure between the primary and PDX tumors with H&E staining. (f) Table of patient tumor information, implantation duration, and engraftment success. (g) Summary of PDX engraftment with and without scaffold.
Multiple microenvironment [mLy6G, endomucin, α-smooth muscle actin (α-SMA)], human tumor (hVimentin, hCytokeratin, hCD45, hCD44), and mitogenic (Ki67) markers were evaluated in scaffold-assisted and scaffold-free tumor transplants (Figure 7a). α-smooth muscle actin (α-SMA) is expressed by activated fibroblasts.57 Vimentin and cytokeratin are mesenchymal and epithelial cell markers, respectively.58 hCD45 is a pan-leukocyte marker, and hCD44 is a marker associated with cancer stem cells.59 Tumors implanted with a scaffold had significantly more proliferative cells (Ki67+) and human immune cells (hCD45+) (Figure 7b). There was no difference in tumor-related markers between the two groups, indicating that scaffold-assisted transplantation did not negatively affect the tumor phenotype compared to the control. Proliferative human immune cells (i.e., double-positive Ki67 and hCD45) were rare in both scaffold-free and scaffold-assisted PDX transplants (Figure S4). Taken together, IHS results demonstrate higher mitogenic and human immune cell activities in human tumors coimplanted with biomaterials compared to that of scaffold-free controls.
Figure 7.

(a) IHS comparison of mouse cells, human cells, and mitogenic marker expression of patient-derived gastric tumor microenvironments engrafted without and with scaffolds. (b) Quantitative comparison of each marker in PDX tumors transplanted without and with scaffold. (N = 4–5 tumors per group, *P < 0.05.)
4. DISCUSSION
The ability to modulate the biomaterial–host immune response is an exciting enabling step in the realization of many biomedical technologies.33,35 For example, biomaterials have widespread success in modulating osteoblast and osteoclast activity during bone tissue repair.60 Biomaterial encapsulation of islet cells has shown promise in being a functional treatment for diabetic patients.61 Immunomodulatory nanomaterials have been developed to target inflammatory monocytes and alter lymph node activity in vivo.62 Implantable biomaterial-based cancer vaccines take advantage of the influx of dendritic cells to boost the host immune response against cancer.63,64 A removable biomaterial strategy after subcutaneous implantation leaves behind a fibrotic tissue pocket that has been exploited for islet cell delivery.22 Implantable polymeric biomaterials that attract circulating tumor cells have shown the feasibility to reduce the risk of metastasis in vital organs.65,66 The inversed spherical pore geometry of ICC hydrogel scaffolds directs the formation of distinct inflammatory microenvironments, with a proinflammatory milieu at the pore surface and an immune-suppressive niche within the pore cavity.67 This phenomenon enables enhanced vascularization with reduced fibrotic encapsulation while maintaining proinflammatory immune cell activity.68–70 Previously, we exploited these features in creating humanized metastatic tumor microenvironments that consist of human immune, stromal, and tumor cells to study the systemic investigation of the humanized tumor microenvironment and identify critical events in metastatic relapse.47–49 In the present study, we demonstrate a new translational opportunity of biomaterials to improve the ectopic and xenogeneic transplantation of tissues and tumors. The findings of this work can be leveraged to improve preclinical mouse models to better reflect human biology and disease.
A foreign body reaction is a physiological host response to a foreign object within the tissue, whereby the object is encapsulated with a fibrotic capsule, effectively sealing it off from the host.28 We intended to exploit the angiogenic activity of the foreign body response to improve the transplantation of mature tissue into the subcutaneous space by coimplanting a porous biomaterial scaffold. The effects of this coimplantation strategy were threefold. First, the mechanical deformation of the transplanted tissue was minimized due to the structural stability of the scaffold. ICC scaffolds composed of at least 30 wt % polyacrylamide do not deform when implanted.49 This property enabled the preservation of original tissue structures, maintaining tissue complexity and heterogeneity compared to collapsed scaffold-free transplanted tissues. Second, increased inflammatory signaling due to the presence of the biomaterial scaffold modulated the recruitment of innate immune cells by attracting them to the biomaterial rather than within the transplanted tissue. Third, the implantation of biomaterials accelerated vascularization to the transplant by increasing proangiogenic signaling molecules such as vascular endothelial growth factor.71 The proinflammatory and proangiogenic milieu generated by the coimplanted biomaterial demonstrated a distinct advantage over scaffold-free transplants. The addition of hBMSCs on the surface of the ICC hydrogel scaffolds further enhanced the results of the scaffold-assisted approach. hBMSCs are known to secrete various immunomodulatory and angiogenic molecules.49,50 We have previously demonstrated that hBMSCs can maintain secretory function after long-term (12 week) subcutaneous implantation.49 The large surface area of ICC hydrogel scaffolds permits the accommodation of a high density of stromal cells. Seeding of the tissue-specific stroma may provide further supportive functions to transplanted tissues.
Neutrophils and macrophages are innate immune cells that play an important role in inflammation, tissue remodeling, and fibrosis. As an early responder, neutrophils create an inflammatory environment through the release of cytokines, chemokines, reactive oxygen species, and neutrophil extracellular traps (NETs).72,73 The release of reactive oxygen species and tissue digesting enzymes can be particularly disruptive and cause graft tissue damage.74 However, neutrophil-derived matrix metalloproteases and NETs have been implicated in enhancing angiogenesis.75,76 Synthetic polymer scaffolds have been shown to elicit a strong immunological response compared to extracellular matrix scaffolds.34 In the presented study, scaffold-assisted samples have benefited from proangiogenic features of neutrophils without a direct, potentially disruptive physical interaction. A recent report suggests that hBMSCs alter neutrophil response toward an immunosuppressive phenotype, which in the context of this study may have further enhanced the viability of transplanted tissues.77 Our findings suggest that hBMSCs were the cause of an increased neutrophil recruitment compared to the biomaterial alone, but it is difficult to conclude if this was linked to the increase in angiogenesis. Further transient studies of neutrophils and biomaterial may elucidate their biological function. Macrophages are another important innate immune cell type in the foreign body response and secrete a variety of inflammatory signals that can lead to fibrotic encapsulation.78 Here, macrophages were predominantly localized to the surrounding tissues regardless of the presence of a biomaterial. Interestingly, we observed a notable reduction in macrophages derived from donor liver tissue after transplantation, which is potentially due to a migratory response to the strong inflammatory signals in the surrounding tissue. The functional consequence of macrophage attrition in ectopically engrafted liver remains to be determined as tissue resident macrophages play diverse roles in liver function.
In the presented study, we explored the application of scaffold-assisted transplantation to improve human PDX models in mice. Due to technical challenges, the orthotopic transplantation of PDX tumors is rarely performed; therefore, subcutaneous implantation is primarily utilized. Although engraftment can still occur, the difference in the local stroma can change tumor behavior and growth. PDX models are also hampered by the rate of engraftment and overall engraftment efficiency. The rate of engraftment normally takes between 2 and 8 months before developing a preclinical model.9 The efficiency of gastric cancer engraftment is also low, with over 70% of xenografts failing to grow.53–55 Finally, the local stroma of PDX models gradually becomes replaced with mouse cells, leading to a loss of heterogeneity, which can skew preclinical testing and result in poor patient efficacy. Based on the ectopic mouse liver tissue transplant experiments, we adjusted the strategy for the scaffold-assisted PDX model. First, we utilized nude mice, which are only deficient in mature T cells to minimize the loss of immunological response on the tumor. A recent study showed that mice reconstituted with human immune progenitor cells prevented genetic drift within engrafted PDX tumors by better recapitulating the tumor microenvironment.12 Second, we changed the configuration of the biomaterial and transplant to increase the interface and subsequent effect of CAFs. The presence of CAFs seeded on the scaffold may have provided supportive cytokines and growth factors to resident human immune cells in addition to tumor cells to maintain a heterogeneous microenvironment. Although the presented results of gastric cancer engraftment were suboptimal, as tumor engraftment between the two groups was comparable, detailed IHS characterization distinguished higher percentages of hCD45+ and Ki67+ cells within the tumor tissue in scaffold-assisted transplants compared to that in scaffold-free controls. We believe that further exploration into biomaterial strategies of coimplantation with PDX tumors is warranted.
5. CONCLUSIONS
Our results demonstrate a new approach to aid in the transplantation of mature tissues to an ectopic, subcutaneous location. By leveraging the acute host response to biomaterial implantation, we improved vascularization while keeping potentially disruptive immune cells away from the transplant. The presented biomaterial strategy may be applicable for the xenotransplantation of human tissues such as the liver and lung, potentially enabling the study of human disseminated tumors in the context of fully mature human tissues. Additionally, we have highlighted a modulatory effect on the tumor microenvironment of human gastric cancer PDXs via this coimplantation strategy. We anticipate that this approach is an important step in expanding the preclinical value of mouse models and achieve a better understanding of the human disease and the discovery of effective therapies.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Shelly Peyton for assistance with fluorescent image acquisition. We thank the University of Massachusetts-Amherst Animal Care Services and Animal imaging Core. This work was supported by the National Cancer Institute (R00CA163671 and R01CA237171) and the National Science Foundation Research Traineeship (1545399).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsbiomaterials.9b00978.
List of antibodies used for immunohistostaining (Table S1); albumin secretion in transplanted liver tissues (Figure S1); ectopic engraftment of mature mouse lung tissue into NSG mice using ICC hydrogel scaffolds (Figure S2); immunomodulatory effects of ICC hydrogel scaffolds and immune cell migration on the transplanted lung tissue (Figure S3); and proliferative status of hCD45 cells in PDX samples (Figure S4) (PDF)
REFERENCES
- (1).Takao K; Miyakawa T Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. U.S.A 2015, 112, 1167–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Day CP; Merlino G; Van Dyke T Preclinical mouse cancer models: a maze of opportunities and challenges. Cell 2015, 163, 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Gould SE; Junttila MR; de Sauvage FJ Translational value of mouse models in oncology drug development. Nat. Med 2015, 21, 431–439. [DOI] [PubMed] [Google Scholar]
- (4).Webster SJ; Bachstetter AD; Nelson PT; Schmitt FA; Van Eldik LJ Using mice to model Alzheimer’s dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front. Genet 2014, 5, No. 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Gkouskou KK; Deligianni C; Tsatsanis C; Eliopoulos AG The gut microbiota in mouse models of inflammatory bowel disease. Front. Cell. Infect. Microbiol 2014, 4, No. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Brehm MA; Shultz LD; Greiner DL Humanized mouse models to study human diseases. Curr. Opin. Endocrinol., Diabetes Obes 2010, 17, 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Spade DJ; McDonnell EV; Heger NE; Sanders JA; Saffarini CM; Gruppuso PA; De Paepe ME; Boekelheide K Xenotransplantation models to study the effects of toxicants on human fetal tissues. Birth Defects Res. Part B 2014, 101, 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Wimmer RA; Leopoldi A; Aichinger M; Wick N; Hantusch B; Novatchkova M; Taubenschmid J; Hammerle M; Esk C; Bagley JA; Lindenhofer D; Chen G; Boehm M; Agu CA; Yang F; Fu B; Zuber J; Knoblich JA; Kerjaschki D; Penninger JM Human blood vessel organoids as a model of diabetic vasculopathy. Nature 2019, 565, 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Jung J; Seol HS; Chang S The Generation and Application of Patient-Derived Xenograft Model for Cancer Research. Cancer Res. Treat 2018, 50, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Brehm MA; Wiles MV; Greiner DL; Shultz LD Generation of improved humanized mouse models for human infectious diseases. J. Immunol. Methods 2014, 410, 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Karpel ME; Boutwell CL; Allen TM BLT humanized mice as a small animal model of HIV infection. Curr. Opin. Virol 2015, 13, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Morton JJ; Bird G; Keysar SB; Astling DP; Lyons TR; Anderson RT; Glogowska MJ; Estes P; Eagles JR; Le PN; Gan G; McGettigan B; Fernandez P; Padilla-Just N; Varella-Garcia M; Song JI; Bowles DW; Schedin P; Tan AC; Roop DR; Wang XJ; Refaeli Y; Jimeno A XactMice: humanizing mouse bone marrow enables microenvironment reconstitution in a patient-derived xenograft model of head and neck cancer. Oncogene 2016, 35, 290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Lee J; Heckl D; Parekkadan B Multiple genetically engineered humanized microenvironments in a single mouse. Biomater. Res 2016, 20, No. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Das Thakur M; Salangsang F; Landman AS; Sellers WR; Pryer NK; Levesque MP; Dummer R; McMahon M; Stuart DD Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature 2013, 494, 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Jimeno A; Amador ML; Kulesza P; Wang X; Rubio-Viqueira B; Zhang X; Chan A; Wheelhouse J; Kuramochi H; Tanaka K; Danenberg K; Messersmith WA; Almuete V; Hruban RH; Maitra A; Yeo CJ; Hidalgo M Assessment of celecoxib pharmacodynamics in pancreatic cancer. Mol. Cancer. Ther 2006, 5, 3240–3247. [DOI] [PubMed] [Google Scholar]
- (16).Heid I; Steiger K; Trajkovic-Arsic M; Settles M; Esswein MR; Erkan M; Kleeff J; Jager C; Friess H; Haller B; Steingotter A; Schmid RM; Schwaiger M; Rummeny EJ; Esposito I; Siveke JT; Braren RF Co-clinical Assessment of Tumor Cellularity in Pancreatic Cancer. Clin. Cancer Res 2017, 23, 1461–1470. [DOI] [PubMed] [Google Scholar]
- (17).Choi Y; Lee S; Kim K; Kim SH; Chung YJ; Lee C Studying cancer immunotherapy using patient-derived xenografts (PDXs) in humanized mice. Exp. Mol. Med 2018, 50, No. 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Owonikoko TK; Zhang G; Kim HS; Stinson RM; Bechara R; Zhang C; Chen Z; Saba NF; Pakkala S; Pillai R; Deng X; Sun SY; Rossi MR; Sica GL; Ramalingam SS; Khuri FR Patient-derived xenografts faithfully replicated clinical outcome in a phase II co-clinical trial of arsenic trioxide in relapsed small cell lung cancer. J. Transl. Med 2016, 14, No. 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Pompili L; Porru M; Caruso C; Biroccio A; Leonetti C Patient-derived xenografts: a relevant preclinical model for drug development. J. Exp. Clin. Cancer Res 2016, 35, No. 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Siolas D; Hannon GJ Patient-derived tumor xenografts: transforming clinical samples into mouse models. Cancer Res. 2013, 73, 5315–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Shultz LD; Goodwin N; Ishikawa F; Hosur V; Lyons BL; Greiner DL Subcapsular transplantation of tissue in the kidney. Cold Spring Harb. Protoc 2014, 2014, 737–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Pepper AR; Gala-Lopez B; Pawlick R; Merani S; Kin T; Shapiro AM A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat. Biotechnol 2015, 33, 518–523. [DOI] [PubMed] [Google Scholar]
- (23).Ward WK; Slobodzian EP; Tiekotter KL; Wood MD The effect of microgeometry, implant thickness and polyurethane chemistry on the foreign body response to subcutaneous implants. Biomaterials 2002, 23, 4185–4192. [DOI] [PubMed] [Google Scholar]
- (24).Helton KL; Ratner BD; Wisniewski NA Biomechanics of the sensor-tissue interface-effects of motion, pressure, and design on sensor performance and the foreign body response-part I: theoretical framework. J. Diabetes Sci. Technol 2011, 5, 632–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Griffin MF; Leung BC; Premakumar Y; Szarko M; Butler PE Comparison of the mechanical properties of different skin sites for auricular and nasal reconstruction. J. Otolaryngol., Head Neck Surg 2017, 46, No. 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Jaalouk DE; Lammerding J Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol 2009, 10, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Akhtar MZ; Sutherland AI; Huang H; Ploeg RJ; Pugh CW The role of hypoxia-inducible factors in organ donation and transplantation: the current perspective and future opportunities. Am. J. Transplant 2014, 14, 1481–1487. [DOI] [PubMed] [Google Scholar]
- (28).Anderson JM; Rodriguez A; Chang DT Foreign body reaction to biomaterials. Semin. Immunol 2008, 20, 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Ben-David U; Ha G; Tseng YY; Greenwald NF; Oh C; Shih J; McFarland JM; Wong B; Boehm JS; Beroukhim R; Golub TR Patient-derived xenografts undergo mouse-specific tumor evolution. Nat. Genet 2017, 49, 1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Vishwakarma A; Bhise NS; Evangelista MB; Rouwkema J; Dokmeci MR; Ghaemmaghami AM; Vrana NE; Khademhosseini A Engineering Immunomodulatory Biomaterials To Tune the Inflammatory Response. Trends Biotechnol. 2016, 34, 470–482. [DOI] [PubMed] [Google Scholar]
- (31).Hubbell JA; Thomas SN; Swartz MA Materials engineering for immunomodulation. Nature 2009, 462, 449–460. [DOI] [PubMed] [Google Scholar]
- (32).Singh A; Peppas NA Hydrogels and scaffolds for immunomodulation. Adv. Mater 2014, 26, 6530–6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Hotaling NA; Tang L; Irvine DJ; Babensee JE Biomaterial Strategies for Immunomodulation. Annu. Rev. Biomed. Eng 2015, 17, 317–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Sadtler K; Wolf MT; Ganguly S; Moad CA; Chung L; Majumdar S; Housseau F; Pardoll DM; Elisseeff JH Divergent immune responses to synthetic and biological scaffolds. Biomaterials 2019, 192, 405–415. [DOI] [PubMed] [Google Scholar]
- (35).Boehler RM; Graham JG; Shea LD Tissue engineering tools for modulation of the immune response. Biotechniques 2011, 51, 239–240 242, 244 passim. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Wang H; Mooney DJ Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nat. Mater 2018, 17, 761–772. [DOI] [PubMed] [Google Scholar]
- (37).Veiseh O; Doloff JC; Ma M; Vegas AJ; Tam HH; Bader AR; Li J; Langan E; Wyckoff J; Loo WS; Jhunjhunwala S; Chiu A; Siebert S; Tang K; Hollister-Lock J; Aresta-Dasilva S; Bochenek M; Mendoza-Elias J; Wang Y; Qi M; Lavin DM; Chen M; Dholakia N; Thakrar R; Lacik I; Weir GC; Oberholzer J; Greiner DL; Langer R; Anderson DG Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater 2015, 14, 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Vegas AJ; Veiseh O; Doloff JC; Ma M; Tam HH; Bratlie K; Li J; Bader AR; Langan E; Olejnik K; Fenton P; Kang JW; Hollister-Locke J; Bochenek MA; Chiu A; Siebert S; Tang K; Jhunjhunwala S; Aresta-Dasilva S; Dholakia N; Thakrar R; Vietti T; Chen M; Cohen J; Siniakowicz K; Qi M; McGarrigle J; Graham AC; Lyle S; Harlan DM; Greiner DL; Oberholzer J; Weir GC; Langer R; Anderson DG Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat. Biotechnol 2016, 34, 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Chen S; Jones JA; Xu Y; Low HY; Anderson JM; Leong KW Characterization of topographical effects on macrophage behavior in a foreign body response model. Biomaterials 2010, 31, 3479–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Lee J; Cuddihy MJ; Kotov NA Three-dimensional cell culture matrices: state of the art. Tissue Eng., Part B 2008, 14, 61–86. [DOI] [PubMed] [Google Scholar]
- (41).Jansen LE; Amer LD; Chen EY; Nguyen TV; Saleh LS; Emrick T; Liu WF; Bryant SJ; Peyton SR Zwitterionic PEG-PC Hydrogels Modulate the Foreign Body Response in a Modulus-Dependent Manner. Biomacromolecules 2018, 19, 2880–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Matlaga BF; Yasenchak LP; Salthouse TN Tissue response to implanted polymers: the significance of sample shape. J. Biomed. Mater. Res 1976, 10, 391–397. [DOI] [PubMed] [Google Scholar]
- (43).Zhang L; Cao Z; Bai T; Carr L; Ella-Menye JR; Irvin C; Ratner BD; Jiang S Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol 2013, 31, 553–556. [DOI] [PubMed] [Google Scholar]
- (44).Sadtler K; Estrellas K; Allen BW; Wolf MT; Fan H; Tam AJ; Patel CH; Luber BS; Wang H; Wagner KR; Powell JD; Housseau F; Pardoll DM; Elisseeff JH Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 2016, 352, 366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Sadtler K; Allen BW; Estrellas K; Housseau F; Pardoll DM; Elisseeff JH The Scaffold Immune Microenvironment: Biomaterial-Mediated Immune Polarization in Traumatic and Non-traumatic Applications. Tissue Eng. Part A 2017, 23, 1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Swartzlander MD; Blakney AK; Amer LD; Hankenson KD; Kyriakides TR; Bryant SJ Immunomodulation by mesenchymal stem cells combats the foreign body response to cell-laden synthetic hydrogels. Biomaterials 2015, 41, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Lee J; Li M; Milwid J; Dunham J; Vinegoni C; Gorbatov R; Iwamoto Y; Wang FJ; Shen KY; Hatfield K; Enger M; Shafiee S; McCormack E; Ebert BL; Weissleder R; Yarmush ML; Parekkadan B Implantable microenvironments to attract hematopoietic stem/cancer cells. Proc. Natl. Acad. Sci. U.S.A 2012, 109, 19638–19643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Bersani F; Lee J; Yu M; Morris R; Desai R; Ramaswamy S; Toner M; Haber DA; Parekkadan B Bioengineered Implantable Scaffolds as a Tool to Study Stromal-Derived Factors in Metastatic Cancer Models. Cancer Res. 2014, 74, 7229–7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Carpenter RA; Kwak JG; Peyton SR; Lee J Implantable pre-metastatic niches for the study of the microenvironmental regulation of disseminated human tumour cells. Nat. Biomed. Eng 2018, 2, 915–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Parekkadan B; Milwid JM Mesenchymal stem cells as therapeutics. Annu. Rev. Biomed. Eng 2010, 12, 87–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Ham IH; Oh HJ; Jin H; Bae CA; Jeon SM; Choi KS; Son SY; Han SU; Brekken RA; Lee D; Hur H Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer. Mol. Cancer 2019, 18, No. 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Tan Z; Dini D; Rodriguez y Baena F; Forte AE Composite hydrogel: A high fidelity soft tissue mimic for surgery. Mater. Des 2018, 160, 886–894. [Google Scholar]
- (53).Zhang T; Zhang L; Fan S; Zhang M; Fu H; Liu Y; Yin X; Chen H; Xie L; Zhang J; Gavine PR; Gu Y; Ni X; Su X Patient-Derived Gastric Carcinoma Xenograft Mouse Models Faithfully Represent Human Tumor Molecular Diversity. PLoS One 2015, 10, No. e0134493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Choi YY; Lee JE; Kim H; Sim MH; Kim KK; Lee G; Kim HI; An JY; Hyung WJ; Kim CB; Noh SH; Kim S; Cheong JH Establishment and characterisation of patient-derived xenografts as paraclinical models for gastric cancer. Sci. Rep 2016, 6, No. 22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Wang H; Lu J; Tang J; Chen S; He K; Jiang X; Jiang W; Teng L Establishment of patient-derived gastric cancer xenografts: a useful tool for preclinical evaluation of targeted therapies involving alterations in HER-2, MET and FGFR2 signaling pathways. BMC Cancer 2017, 17, No. 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Ham I-H; Lee D; Hur H Role of Cancer-Associated Fibroblast in Gastric Cancer Progression and Resistance to Treatments. J. Oncol 2019, 2019, No. 6270784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Fuyuhiro Y; Yashiro M; Noda S; Kashiwagi S; Matsuoka J; Doi Y; Kato Y; Muguruma K; Sawada T; Hirakawa K Myofibroblasts are associated with the progression of scirrhous gastric carcinoma. Exp. Ther. Med 2010, 1, 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Mani SA; Guo W; Liao MJ; Eaton EN; Ayyanan A; Zhou AY; Brooks M; Reinhard F; Zhang CC; Shipitsin M; Campbell LL; Polyak K; Brisken C; Yang J; Weinberg RA The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Takaishi S; Okumura T; Tu S; Wang SS; Shibata W; Vigneshwaran R; Gordon SA; Shimada Y; Wang TC Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 2009, 27, 1006–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Lee J; Byun H; Madhurakkat Perikamana SK; Lee S; Shin H Current Advances in Immunomodulatory Biomaterials for Bone Regeneration. Adv. Healthcare Mater 2019, 8, No. e1801106. [DOI] [PubMed] [Google Scholar]
- (61).Soon-Shiong P; Heintz RE; Merideth N; Yao QX; Yao Z; Zheng T; Murphy M; Moloney MK; Schmehl M; Harris M; Mendez R; Mendez R; Sandford PA Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet 1994, 343, 950–951. [DOI] [PubMed] [Google Scholar]
- (62).Getts DR; Terry RL; Getts MT; Deffrasnes C; Muller M; van Vreden C; Ashhurst TM; Chami B; McCarthy D; Wu H; Ma J; Martin A; Shae LD; Witting P; Kansas GS; Kuhn J; Hafezi W; Campbell IL; Reilly D; Say J; Brown L; White MY; Cordwell SJ; Chadban SJ; Thorp EB; Bao S; Miller SD; King NJ Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci. Transl Med 2014, 6, No. 219ra7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Ali OA; Emerich D; Dranoff G; Mooney DJ In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci. Transl. Med 2009, 1, No. 8ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Kim J; Li WA; Choi Y; Lewin SA; Verbeke CS; Dranoff G; Mooney DJ Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat. Biotechnol 2015, 33, 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Azarin SM; Yi J; Gower M; Aguado BA; Sullivan ME; Goodman AG; Jiang EJ; Rao SS; Ren YY; Tucker SL; Backman V; Jeruss JS; Shea LD In vivo capture and label-free detection of early metastatic cells. Nat. Commun 2015, 6, No. 8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Rao SS; Bushnell GG; Azarin SM; Spicer G; Aguado BA; Stoehr JR; Jiang EJ; Backman V; Shea LD; Jeruss JS Enhanced Survival with Implantable Scaffolds That Capture Metastatic Breast Cancer Cells In Vivo. Cancer Res. 2016, 76, 5209–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Sussman EM; Halpin MC; Muster J; Moon RT; Ratner BD Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann. Biomed. Eng 2014, 42, 1508–1516. [DOI] [PubMed] [Google Scholar]
- (68).Madden LR; Mortisen DJ; Sussman EM; Dupras SK; Fugate JA; Cuy JL; Hauch KD; Laflamme MA; Murry CE; Ratner BD Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc. Natl. Acad. Sci. U.S.A 2010, 107, 15211–15216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Galperin A; Oldinski RA; Florczyk SJ; Bryers JD; Zhang M; Ratner BD Integrated bi-layered scaffold for osteochondral tissue engineering. Adv. Healthcare Mater 2013, 2, 872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Choi SW; Zhang Y; Macewan MR; Xia Y Neo-vascularization in biodegradable inverse opal scaffolds with uniform and precisely controlled pore sizes. Adv. Healthcare Mater 2013, 2, 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Dondossola E; Holzapfel BM; Alexander S; Filippini S; Hutmacher DW; Friedl P Examination of the foreign body response to biomaterials by nonlinear intravital microscopy. Nat. Biomed. Eng 2017, 1, No. 0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Wynn TA; Ramalingam TR Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med 2012, 18, 1028–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Kolaczkowska E; Kubes P Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol 2013, 13, 159–175. [DOI] [PubMed] [Google Scholar]
- (74).Scozzi D; Ibrahim M; Menna C; Krupnick AS; Kreisel D; Gelman AE The Role of Neutrophils in Transplanted Organs. Am. J. Transplant 2017, 17, 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Aldabbous L; Abdul-Salam V; McKinnon T; Duluc L; Pepke-Zaba J; Southwood M; Ainscough AJ; Hadinnapola C; Wilkins MR; Toshner M; Wojciak-Stothard B Neutrophil Extracellular Traps Promote Angiogenesis: Evidence From Vascular Pathology in Pulmonary Hypertension. Arterioscler., Thromb., Vasc. Biol 2016, 36, 2078–2087. [DOI] [PubMed] [Google Scholar]
- (76).Christoffersson G; Vagesjo E; Vandooren J; Liden M; Massena S; Reinert RB; Brissova M; Powers AC; Opdenakker G; Phillipson M VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood 2012, 120, 4653–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Su X; Yang L; Yin Y; Huang J; Qiao F; Fang Y; Yu L; Wang Y; Zhou K; Wang J Bone marrow mesenchymal stem cells tune the differentiation of myeloid-derived suppressor cells in bleomycin-induced lung injury. Stem Cell Res. Ther 2018, 9, No. 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Wynn TA; Vannella KM Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


