Abstract
Background:
Fucosyltransferase 2 (Fut2)-mediated intestinal α1-2-fucosylation is important in maintaining a symbiotic host-microbiota relationship and can protect against several pathogens. Intestinal dysbiosis is an important factor for the progression of experimental ethanol-induced liver disease, but the role of Fut2 in modulating the intestinal glycocalyx during alcohol-associated liver disease is unknown. We investigated the role of Fut2-mediated intestinal α1-2-fucosylation for the development of alcohol-associated liver disease.
Methods:
Immunohistochemistry staining was applied to evaluate α1-2-fucosylation in duodenal biopsies from patients with alcohol use disorder. Wild type (WT) and Fut2 deficient littermate mice were subjected to Lieber DeCarli models of chronic ethanol administration and the chronic-binge ethanol diet (NIAAA model).
Results:
Intestinal α1-2-fucosylation was down-regulated in patients with alcohol use disorder. Lack of α1-2-fucosylation in Fut2 deficient mice exacerbates chronic ethanol-induced liver injury, steatosis and inflammation without affecting ethanol metabolism. Dietary supplementation of the α1-2-fucosylated glycan 2’-fucosyllactose ameliorates ethanol-induced liver disease in Fut2 deficient mice in the NIAAA model. Despite no direct effects on growth of Enterococcus faecalis in vitro, intestinal α1-2-fucosylation reduces colonization of cytolysin-positive E. faecalis in the intestine of ethanol-fed mice.
Conclusions:
Intestinal α1-2-fucosylation acts as a host protective mechanism against ethanol-induced liver disease. 2’-FL is an oligosaccharide naturally present in human milk that could be considered as therapeutic agent for alcohol-associated liver disease.
Keywords: microbiome, alcoholic liver disease, microbiota, fucosyltransferase 2, cytolysin
Introduction
Consumption of alcohol caused 3 million deaths (5.3% of all deaths) worldwide in 2016 according to the WHO, which is higher than that caused by HIV/AIDS and diabetes. Alcohol is one of the most frequent causes of liver disease. Alcohol-associated liver disease includes alcohol-associated steatosis, steatohepatitis, fibrosis and cirrhosis, and alcoholic hepatitis (Bajaj, 2019; European Association for the Study of, 2012). Acute alcoholic hepatitis with a 90-day mortality of up to 50% (Lucey et al., 2009) and advanced liver cirrhosis with the median survival time of as low as 1–2 years (Bruha et al., 2012) are the most severe subtypes of alcohol-associated liver disease. The gut-liver axis is important for the progression of alcohol-associated liver disease in both patients and experimental models.
The Fut2 gene encodes a galactoside 2-L-fucosyltransferase (fucosyltransferase 2, Fut2) which catalyzes the process of α1-2-fucosylation by adding fucose to glycolipids and glycoproteins as well as unconjugated glycans like human milk oligosaccharides (Domino et al., 2001; Goto et al., 2014; Bode, 2012). Fut2 is expressed in epithelial cells of the digestive tract while absent in hepatocytes. Fut2 expression is high in the distal gut, which is colonized by a large amount of symbiotic microbes (Bry et al., 1996). Absence of α1-2-fucosylation at the cell surface of enterocytes and mucus in so-called “non-secretor” subjects may result in alterations in intestinal bacteria, barrier function and pathogen adhesion (Pham et al., 2014; Rausch et al., 2011). Both “non-secretors” and Fut2 deficient mice showed changes in the commensal microbiota and in microbial metabolite profiles (Rausch et al., 2011; Wacklin et al., 2011; Hurd et al., 2005). Fucosylated glycans regulate host-microbe interactions. Membrane and secreted α1-2-linked fucose can be cleaved by bacterial fucosidase and the liberated L-fucose is utilized by certain bacteria. For these reasons, Fut2 polymorphism has been implicated in pathogenesis of several diseases that are closely associated with the intestinal microbiome. Non-secretors are more susceptible to Crohn’s disease (Tong et al., 2014), chronic pancreatitis (Weiss et al., 2015), primary sclerosing cholangitis (Maroni et al., 2015) and some specific pathogens like Candida albicans, Haemophilus influenza and pathogenic Escherichia coli (Goto et al., 2016). Although intestinal dysbiosis is an important co-factor for progression of alcohol-associated liver disease, the underlying mechanism remains unclear. In this study we investigated the role of Fut2-mediated intestinal α1-2-fucosylation in development of alcohol-associated liver disease.
Materials and Methods
Animal models
Fut2 deficient (Fut2−/−) mice on a C57BL/6 background have been described (Tong et al., 2014) (kindly provided by Dr. Justin Sonnenburg, Stanford University). Immunohistochemistry with biotinylated Ulex Europaeus Agglutinin I (see below) confirmed the absence of α1-2-fucosylated glycans in the intestine of Fut2−/− mice (not shown). Heterozygous mice were used for breeding. Age-matched Fut2 knockout and wild type (WT) littermate mice (8 week, female mice) were given Lieber DeCarli diet (LD101A, TestDiet, St.Louis, MO) for 9 weeks (Llorente et al., 2017). In brief, the caloric intake from ethanol was increased gradually, 0% on day 1, 10% on day 2 and 3, 20% on day 4 and 5, 30% from day 6 to the end of week 6, 36% from week 7 to week 9. Pair-fed control mice received a diet with an isocaloric substitution of isomaltose. After weaning, age-matched WT or Fut2−/− groups with the same genotype were housed in the same cage, and are termed littermates. In the co-housing groups, equal numbers (1–2 per cage) of age-matched WT and Fut2−/− mice were housed within one cage.
In the 2’-fucosyllactose (2’-FL; a gift from Jennewein Biotechnologie GmbH, Rheinbreitbach, Germany) supplementation experiments we used a chronic-binge ethanol diet (NIAAA) model (Bertola et al., 2013) and homozygous Fut2−/− mice were used for breeding. Fut2−/− mice (10–12 weeks, male and female) were fed with Lieber-DeCarli diet with caloric intake from ethanol 0% on days 1–5 and 36% of total calories from day 6 to the end of the experiments. At day 16 in the early morning, mice were gavaged with a single dose of ethanol (5g/kg body weight) and harvested 9 hours later. In the 2’-FL treated groups 2’-FL was added in the ethanol diet at a final concentration of 2mg/ml and given continuously during the study period but not given in the binge.
Patient cohorts
Patients with alcohol use disorder (AUD) were diagnosed according to the DSM IV criteria (Ball et al., 1997) and a detailed description has been published (Badaoui et al., 2008; Yan et al., 2011; Hartmann et al., 2013). In brief, all patients reported long-term (>1year) alcohol consumption (>60g/day) and were actively drinking until the day of admission to a detoxification unit. Patients underwent a panel of investigations including transient elastography (FibroScan) (Echosens, Paris, France), Doppler ultrasound, and blood tests. We used the cut-offs for fibrosis staging in patients with AUD: 5.9, 7.8, 11, 19.5 kPa for F1, F2, F3 and F4, respectively (Nguyen-Khac et al., 2008). The characteristics of all subjects are shown in Supplementary Table 1. Patients with AUD (n=10) or non-alcoholic controls (n=11) underwent an upper gastrointestinal endoscopy (EGD) with duodenal biopsies if clinically indicated as part of routine clinical care. To preserve the mucus layer, duodenal biopsies obtained during an upper endoscopy were fixed in Carnoy’s fixative consisting of 60% Ethanol, 30% Chloroform, and 10% Glacial acetic acid for 1h. Written informed consent was obtained from all patients and controls. The protocol was approved by the ethics committee of each participating center, including the University of California San Diego, in La Jolla, CA, and the Université Catholique de Louvain, in Brussels, Belgium.
Staining procedures
Paraffin-embedded sections were deparaffinized by xylene and rehydration in concentration gradients of ethanol. The sections were immersed in 0.1% H2O2 (Sigma-Aldrich, St.Louis, MO) for 30 min and then blocked with avidin and biotin (Vector, SP-2002) for 15 min each. After blocking with 1% bovine serum albumin for 5 min, sections were incubated with biotinylated Ulex Europaeus Agglutinin I (UEA, Vector, Burlingame, CA) overnight at 4°C. Sections were then washed with TBST, and incubated with Streptavidin, Horseradish Peroxidase for 30 min. Then the sections were stained by DAB solution (Vector, Burlingame, CA) for 2 min, and hematoxylin for 1 min for counterstaining. A negative staining control was performed by using PBS instead of UEA.
Formalin-fixed and paraffin-embedded mouse livers were stained with hematoxylin-eosin (Leica Biosystems Inc., Wetzlar, Germany) using standard staining protocols. Frozen liver sections were stained with Oil Red O (Sigma-Aldrich, St.Louis, MO).
Biochemical assays
Levels of plasma alanine amino-transferase (ALT) were measured using infinity ALT kit (Thermo Fisher Scientific, Waltham, MA). Triglyceride levels were measured using the Triglyceride Liquid Reagents Kit (Pointe Scientific, Canton, MI). Levels of plasma ethanol were measured using the Ethanol Assay Kit (Bio Vision, Milpitas, CA).
Reverse transcription and real-time quantitative PCR
For reverse transcription qPCR, RNA was extracted from mouse liver, and cDNAs were generated using the High-Capacity cDNA Reverse Transcription Kit (ABI, Carlsbad, CA) as described (Llorente et al., 2017). DNA from mouse feces was extracted using QIAamp Fast DNA Stool kit (Qiagen, Venlo, Netherlands). Quantitative PCR was performed with iTaq universal SYBR Green Supermix (Bio-Rad, Hercules, CA) using a StepOnePlus thermocycler real-time PCR system. Primer sequences for mouse genes were obtained from the NIH qPrimerDepot. The values of mouse genes were normalized to 18S, while bacterial genes were normalized to 16S.
Immunoblotting
Liver tissue was homogenized in RIPA buffer, supplemented with protease inhibitor, and used for immunoblotting. Immunoblot analysis was performed as described using Anti-Cytochrome P450 Enzyme (Cyp2e1) (Millipore, Billerica, MA) and β-actin (Sigma-Aldrich, St.Louis, MO) antibodies (Inamine et al., 2016).
Enterococcus faecalis cultures
A cytolysin positive strain of E. faecalis, which was isolated from feces of an ethanol-fed Atp4aSl/Sl mouse (Llorente et al., 2017), was cultured in BHI media with 0, 0.25, 0.5, 1, 2 or 20mg/ml of 2’-FL in triplicate for each concentration. Sampling was taken at 30min intervals during the course of 6h. Cell growth was determined by measuring the optical density at 600nm for each time-point.
Statistical analysis
All data were expressed as mean ± SEM. For comparison of two groups, the Student’s unpaired t-test was used. For comparisons of > 2 groups between ethanol diet groups, two-way analysis of variance (ANOVA) was used followed by Tukey’s post-hoc test. Analysis was performed with GraphPad Prism V.7.0. (GraphPad Software Inc., San Diego, CA). A P value <0.05 was considered significant.
Results
Patients with alcohol use disorder have decreased intestinal α1-2-fucosylation
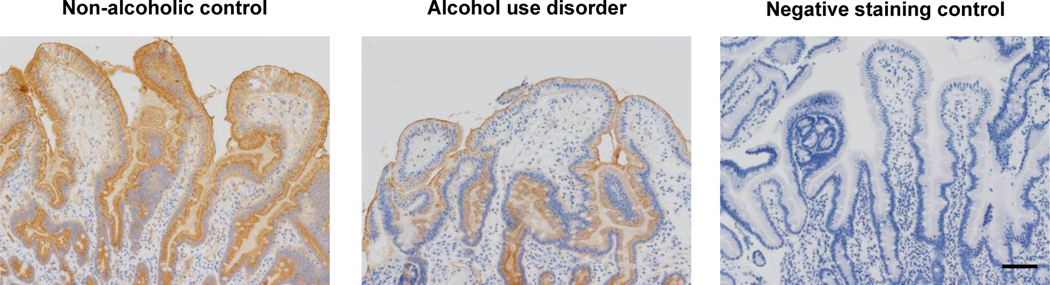
The majority of patients with alcohol use disorder had mild liver disease. Steatosis was presented in 70% of patients, and 62.5% (5/8) of patients had stage F0/F1 fibrosis as determined by FibroScan (Supplementary Table1). To evaluate the role of intestinal α1-2-fucosylation, UEA staining of duodenal biopsies from non-alcoholic controls and patients with AUD was performed. Despite the differences in laboratory and fibrosis parameters, AUD patients consistently showed an obvious lower expression of α1-2-fucosylation on duodenal biopsies as compared with non-alcoholic controls (Fig. 1). This indicates that chronic alcohol consumption down-regulates intestinal α1-2-fucosylation in humans.
Figure 1. Decreased intestinal α1-2-fucosylation in patients with alcohol use disorder.
The expression of intestinal α1-2-fucosylation was determined on duodenal biopsies obtained from patients with alcohol use disorder (n=10) and non-alcoholic controls (n=11) using Ulex Europaeus Agglutinin I (UEA) staining. A negative staining control was performed by using PBS instead of UEA. Representative intestinal sections are shown. The slide of alcohol use disorder patient shown in this figure was from a patient with alcohol use disorder who had no steatosis, liver fibrosis stage F1 and normal level of alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Scale bar = 50μm.
Fut2 deficiency exacerbates chronic ethanol-induced liver disease in mice
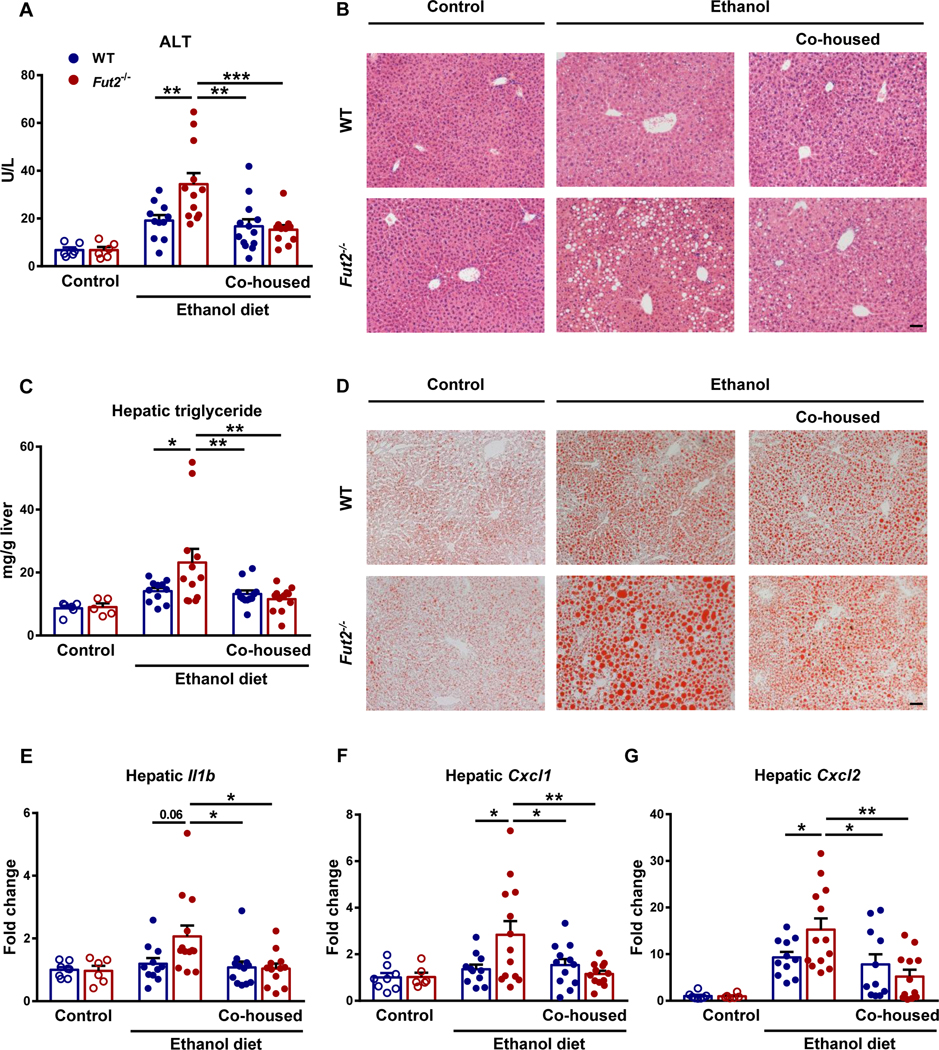
To further investigate α1-2-fucosylation for pathogenesis of chronic ethanol-induced liver disease, Fut2 deficient and WT littermate mice were subjected to feeding of an ethanol diet for 9 weeks. Fut2−/− mice had more severe ethanol-induced liver injury, indicated by elevated level of plasma ALT (Fig. 2A) and liver histopathology (Fig. 2B), and increased hepatic steatosis as evidenced by higher hepatic triglyceride (Fig. 2C) and oil red o staining (Fig. 2D) when compared with WT mice. Fut2−/− mice also had more liver inflammation as evidenced by higher expression of hepatic inflammatory genes, like Interleukin-1 beta (Il1b), chemokine (C-X-C motif) ligand 1 (Cxcl1) and Cxcl2 (Fig. 2E-2G).
Figure 2. Fut2 deficiency exacerbates chronic ethanol-induced liver disease in mice.
Fut2−/− and wild type (WT) littermates were fed with either control diet or ethanol-containing Lieber DeCarli diet for 9 weeks. (A) Plasma alanine aminotransferase (ALT). (B) Representative images of H&E-stained liver tissue. (C) Hepatic triglyceride levels. (D) Representative images of Oil Red O-stained liver tissue. (E) Hepatic Il1b mRNA. (F) Hepatic Cxcl1 mRNA. (G) Hepatic Cxcl2 mRNA. Data represent mean ± SEM; *, ** and *** indicate P<0.05, P<0.01 and P<0.001, respectively. Scale bar = 50μm. Experiments performed in n=5–8 in control diet groups and n=11–13 in ethanol diet groups. For the H&E and Oil Red O staining, n=5 per group.
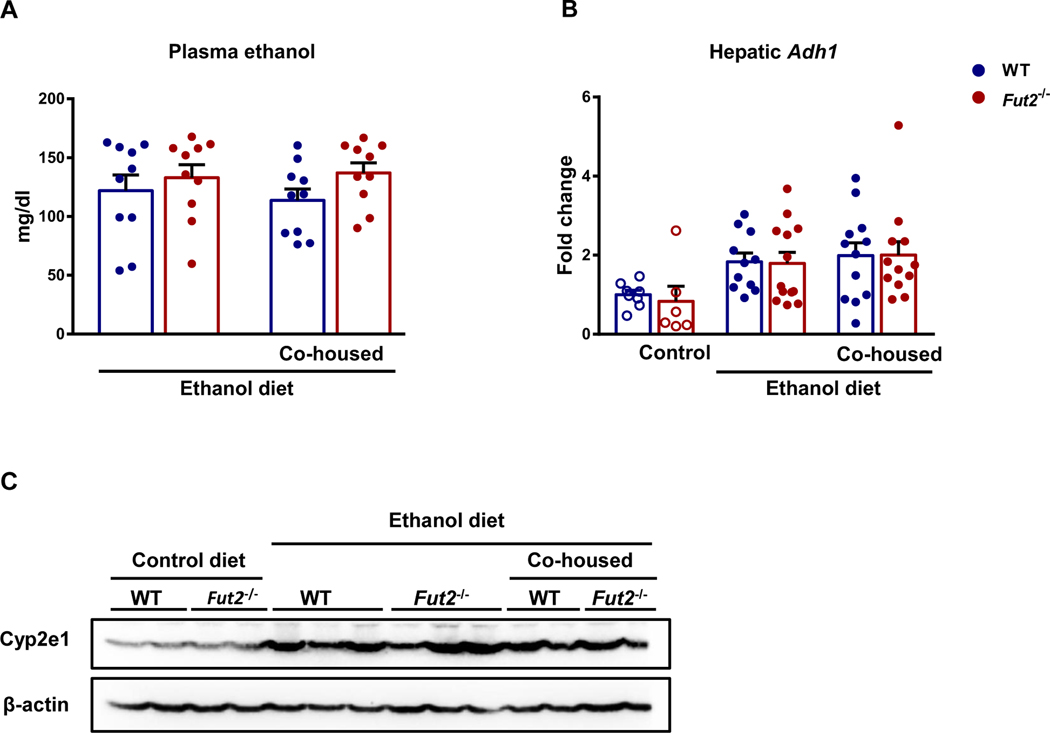
To determine whether Fut2 deficiency alters absorption and hepatic metabolism of ethanol, we measured several ethanol metabolism related parameters. Plasma ethanol levels were not different between WT and Fut2−/− ethanol diet-fed mice (Fig. 3A). Two major enzymes that metabolize ethanol in the liver are alcohol dehydrogenase-1 (Adh1) and Cyp2e1. Hepatic Adh1 mRNA and Cyp2e1 protein were similar between WT and Fut2−/− ethanol diet-fed mice (Fig. 3B and 3C).
Figure 3. Metabolism of ethanol.
Fut2−/− and wild type (WT) littermates were fed with either control diet or ethanol-containing Lieber DeCarli diet for 9 weeks. (A) Plasma ethanol. (B) Hepatic Adh1 mRNA. (C) Immunoblot analysis of hepatic Cyp2e1. Experiments performed in n=6–8 in control diet groups and n=10–13 in ethanol diet groups.
Taken together, these data demonstrate that Fut2 deficiency exacerbates chronic ethanol-induced liver disease in mice and this effect was not through an altered ethanol metabolism.
Co-housing ameliorates the disease promoting effect in ethanol-fed Fut2 deficient mice
Since intestinal α1-2-fucosylation is important for host-microbiota interaction (Kashyap et al., 2013), we co-housed WT and Fut2−/− mice after weaning and throughout the ethanol feeding period in the same cage. Co-housing results in fecal microbiota transfer between mice with different genotype in the same cage. Co-housing of WT and Fut2−/− mice conferred protection from features of ethanol diet-induced liver disease to Fut2−/− mice (Fig. 2 and 3), indicating that the phenotype is transmissible via microbiota transfer.
Supplementation of the exogenous α1-2-fucosylated glycan 2’fucosyllactose (2’FL) attenuates ethanol- induced liver disease in mice
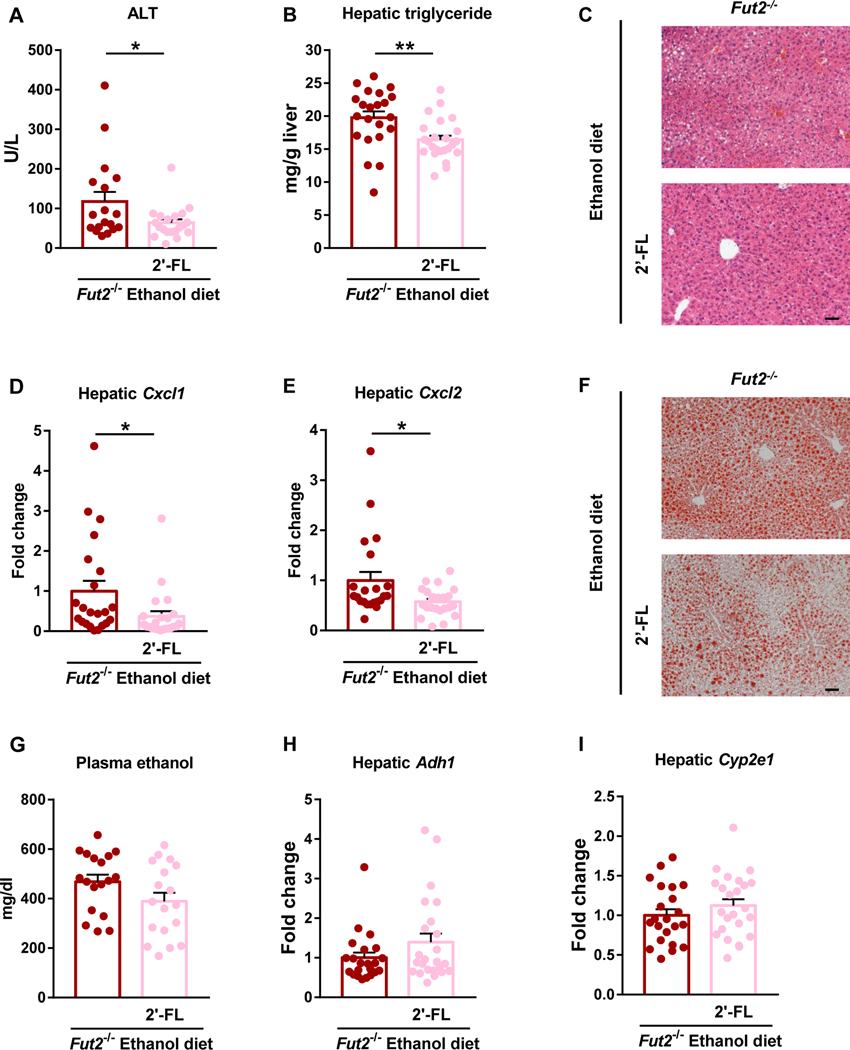
To restore intestinal α1-2-fucosylation in Fut2 deficient mice, 2’-fucosyllactose (2’-FL) was supplemented in the liquid diet of Fut2−/− mice during ethanol feeding. 2’-FL is an α1-2-fucosylated oligosaccharide that is highly abundant in breast milk of secretor women and serves as a prebiotic that can be cleaved and used as substrate and energy source by intestinal bacteria (Grabinger et al., 2019; Elison et al., 2016). Here we chose the NIAAA model which combines chronic ethanol feeding and an ethanol binge and causes significant higher level of ALT and the same extent of steatosis and inflammation compared with the 9 weeks model. Dietary supplementation of 2’-FL in Fut2−/− mice decreased ALT, hepatic steatosis and inflammation as evidenced by lower ALT (Fig. 4A), hepatic triglyceride (Fig. 4B), improvement in liver histopathology (Fig. 4C) and Oil Red O staining (Fig. 4F), and decreased mRNA level of hepatic inflammatory genes including Cxcl1 and Cxcl2 (Fig. 4D and 4E) compared with Fut2−/− mice fed an ethanol diet alone. Restoration of intestinal α1-2-fucosylation ameliorates ethanol-induced liver disease in Fut2−/− mice without affecting metabolism of ethanol (Fig 4G – 4I). These findings indicate that Fut2-mediated intestinal α1-2-fucosylation is critical in ethanol-induced liver disease.
Figure 4. Restoration of α1-2-fucosylated glycans in the intestine attenuates ethanol-induced liver disease in Fut2 deficient mice.
Fut2−/− mice were assigned to 2’-fucosyllactose (2’-FL) treated group and control group, and fed with chronic-binge ethanol diet (NIAAA model). In the 2’-FL treated group, 2’-FL (2mg/mL) was supplemented continuously in the ethanol diet. The experimental diet and 2’-FL treatment lasted for 15 days. (A) Plasma alanine aminotransferase (ALT). (B) Hepatic triglyceride levels. (C) Representative images of H&E-stained liver tissue. (D) Hepatic Cxcl1 mRNA. (E) Hepatic Cxcl2 mRNA. (F) Representative images of Oil Red O-stained liver tissue. (G) Plasma ethanol. (H) Hepatic expression of Adh1 mRNA. (I) Hepatic expression of Cyp2e1 mRNA. Data represent mean ± SEM; * and ** indicate P<0.05 and P<0.01, respectively. Scale bar = 50μm. Experiments performed in n=18–24 per group. For the H&E and Oil Red O staining, n=10 per group.
Intestinal α1-2-fucosylation prevents intestinal colonization of cytolysin-positive Enterococcus faecalis in ethanol diet-fed mice
Translocated endotoxin derived from intestinal bacteria contributes to ethanol-induced liver disease in mice. WT and Fut2−/− mice showed similar levels of plasma lipopolysaccharides (LPS) after chronic ethanol feeding (Fig. 5A). Consistently, 2’-FL supplementation did not affect plasma LPS levels (Fig. 5D). This indicates that the protective effect of α1-2-fucosylation was not through decreasing paracellular permeability in the intestine.
Figure 5. Effects of Fut2 deficiency and 2’-FL supplementation on systemic endotoxin and intestinal E. faecalis and cytolysin.
(A) – (C) Fut2−/− and wild type (WT) littermates were fed with ethanol-containing Lieber DeCarli diet for 9 weeks. (D) – (F) Fut2−/− mice were fed with chronic-binge ethanol diet with or without 2’-FL for 15 days (NIAAA model). (A) and (D) Plasma LPS. (B) and (E) Fecal E. faecalis. (C) and (F) Fecal cytolysin. (G) A cytolysin-positive E. faecalis strain was incubated with different concentrations of 2’-FL for 6hours, and OD600 was measured every 30minutes. Growth curve of E. faecalis. Data represent mean ± SEM, * indicate P<0.05. (A) – (F) Experiments performed in n=8–28 per group. (G) Experiments performed in triplicate and repeated for 3 times. A representative growth curve is shown.
Our previous study showed that experimental expansion of intestinal E. faecalis by gavage during ethanol feeding exacerbates ethanol-induced liver disease in mice (Llorente et al., 2017). To evaluate the role of E. faecalis in ethanol-induced liver disease in Fut2 deficient mice, we first quantified fecal E. faecalis by qPCR following our 9 weeks of chronic ethanol feeding. After chronic ethanol feeding, Fut2−/− mice had significantly higher level of fecal E. faecalis compared with WT mice (Fig. 5B). Dietary supplementation of the prebiotic 2’-FL decreased the intestinal amount of E. faecalis in Fut2−/− mice following the chronic-binge ethanol diet (NIAAA) model (Fig. 5E). This result was consistent with a previous study, which reported that intestinal α1-2-fucosylation enhances resistance to colonization of E. faecalis in the colonic lumen and mucosa (Pham et al., 2014).
The association of cytolysin expression with increased toxicity of E. faecalis infections had been established in many animal and clinical studies (Van Tyne et al., 2013). Importantly, mice gavaged with E. faecalis expressing the exotoxin cytolysin had more severe ethanol-induced liver disease than those gavaged with cytolysin-negative E. faecalis (Llorente et al., 2017). Based on these findings we quantified fecal cytolysin and found that Fut2−/− mice fed ethanol diet had higher levels of fecal cytolysin than ethanol diet-fed WT mice (Fig. 5C). 2’-FL supplementation decreased fecal cytolysin in Fut2−/− mice following chronic-binge ethanol feeding (Fig. 5F). Taken together, increased intestinal cytolytic E. faecalis might contribute to more severe ethanol-induced liver disease observed in Fut2−/− mice.
Growth of E. faecalis is not affected by 2’-FL
In order to investigate the direct effect of 2’-FL on the growth of cytolytic E. faecalis, we incubated a strain of cytolytic E. faecalis with different concentrations of 2’-FL in the culture medium. The results showed that E. faecalis growth was not affected by supplementation with different concentrations of 2’-FL, which indicates that 2’-FL does not directly inhibit the growth of cytolytic E. faecalis (Fig. 5G).
Discussion
Microbiota colonizing the gut has remarkable effects on host health and disease. The host developed sophisticated mechanisms to regulate composition and function of the intestinal microbiota. Fut2 is involved in maintaining a symbiotic host-microbiota relationship by modifying the intestinal glycocalyx. Fut2 mediated intestinal α1-2-fucosylation enables expression of α1-2-fucosylated carbohydrates on intestinal epithelial cells and in luminal contents, which can serve as substrates for metabolites, energy source and adhesion receptors for many symbiotic beneficial bacteria (Kashyap et al., 2013; Goto et al., 2016). Host derived α1-2-fucosylation protects against several intestinal pathogens (Goto et al., 2016). During pathogen-induced stress, rapid α1-2-fucosylation of the small intestine serves as a protective mechanism (Pickard et al., 2014). Studies in Crohn’s disease patients and animal models revealed that potentially pathogenic species, like unclassified species belonging to the family Lachnospiraceae and Prevotella, are specifically associated with Fut2 non-secretor status (Rausch et al., 2011; Nagalingam et al., 2011; Duck et al., 2007; Rho et al., 2005), while probiotic species, including Lactobacillus (Lebeer et al., 2008) and Faecalibacterium (Sokol et al., 2008) are associated with secretor status in healthy controls. Lactobacillus specifically targets α1-2-fucosylated glycans to acquire adhesion molecules to the intestinal mucosa, which can block the attachment of potential pathogens (Watanabe et al., 2010; Chan et al., 1985).
In our study, alcohol abuse is associated with decreased intestinal α1-2-fucosylation in patients with chronic alcohol use. Eliminating α1-2-fucosylation by using Fut2 deficient mice exacerbates ethanol-induced liver injury, steatosis and inflammation. A dietary approach to restore α1-2-fucosylation with prebiotic α1-2-fucosylated glycans overcomes the absence of intestinal α1-2-fucosylation in Fut2−/− mice and attenuates ethanol-induced liver disease. All these findings emphasize the important role of Fut2 and intestinal α1-2-fucosylation for the pathogenesis of ethanol-induced liver disease in mice. Intestinal α1-2-fucosylation acts as a host protective mechanism against ethanol-induced liver disease.
Fut2 mediated intestinal α1-2-fucosylation protects against intestinal colonization and translocation of the pathobiont E. faecalis in a chemical-induced colitis mouse model (Pham et al., 2014). Colonization of E. faecalis in the intestine induces mild liver disease and exacerbates ethanol-induced liver disease in mice (Llorente et al., 2017). Our recent study showed an association between E. faecalis cytolysin positivity in patients with alcoholic hepatitis and mortality (Duan et al., 2019). Cytolysin is a bacterial exotoxin produced by E. faecalis and it has lytic activity against eukaryotic cells (Shankar et al., 2004). Ethanol-fed mice gavaged with cytolytic E. faecalis had more severe liver disease compared with mice gavaged with non-cytolytic E. faecalis. Treatment with phages against cytolysin-positive E. faecalis reduces intestinal cytolysin-positive E. faecalis and ameliorates ethanol-induced liver disease in gnotobiotic mice colonized with feces from cytolysin-positive patients with alcoholic hepatitis (Duan et al., 2019). All these findings indicate that cytolysin produced by intestinal E. faecalis is important for the pathogenesis of ethanol-induced liver disease. In this study, we observed both, an increase of intestinal E. faecalis and cytolysin in Fut2−/− mice after ethanol diet feeding. This indicates that loss of intestinal α1-2-fucosylation is associated with an increase of intestinal cytolytic E. faecalis after ethanol feeding and this could contribute to an exacerbation of liver disease.
Our in vitro culture experiments did not show a direct effect of 2’-FL on growth of cytolytic E. faecalis. It is therefore likely that 2’-FL causes an increase in other beneficial bacteria in the intestine, which in turn prevents the colonization of cytolysin-positive E. faecalis. 2’-FL is an α1-2-fucosylated glycan that is highly abundant in human milk of secretor women (Bode, 2009). 2’-FL synthesized in bioengineered microbes has received FDA GRAS status for use in both infants and adults and is currently used as supplement in infant formula to support growth of the infant (Bode, 2009; Vandenplas et al., 2018; Hegar et al., 2019). 2’-FL reduces ethanol-induced liver disease in Fut2−/− mice, which warrants future studies testing whether or not the observed effects translate from mice to humans, and developing 2’FL as a safe and low-cost dietary supplement or medical food for patients suffering from alcohol-associated liver disease.
In summary, alcohol misuse in patients decreases intestinal α1-2-fucosylation. Absence of intestinal α1-2-fucosylation allows intestinal growth of cytolysin-positive E. faecalis, which likely contributes to an exacerbation of ethanol-induced liver disease in mice.
Supplementary Material
Acknowledgements:
This study was supported by Hunan Provincial Natural Science Foundation of China (2020JJ4932 to R.Z.). R.Z.’s work in the U.S. is supported by a foundation from Xiangya Hospital of China. C.L. is supported by an AASLD Pinnacle Research Award in Liver Disease and a pilot project award from Southern California Research Center for Alcoholic Liver and Pancreatic Disease (ALPD) and Cirrhosis (P50 AA011999). J.L.’s work in the U.S. is supported by a Scholarship of the Shanxi Scholarship Council of China (20161625). P.S. is supported by grants from Fond National de Recherche Scientifique Belgium (J.0146.17 and T.0217.18) and Action de recherche concertée (ARC), Université Catholique de Louvain, Belgium. This study was supported by NIH grants R01 AA020864 (to V.K.), R01 AA24726, R01AA020703, U01 AA026939, by Award Number BX004594 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development (to B.S.), services provided by NIH P30 DK120515 and P50 AA011999, and funding from the Larsson-Rosenquist Foundation Mother-Milk-Infant Center of Research Excellence at UC San Diego.
Abbreviations:
- 2’-FL
2’-fucosyllactose
- Adh
alcohol dehydrogenase
- ALT
alanine aminotransferase
- AUD
alcohol use disorder
- Cyp
cytochrome p450 enzyme
- Fut2
fucosyltransferase 2
- IL
interleukin
- LPS
lipopolysaccharides
- PCR
Polymerase chain reaction
- WT
wild-type
Footnotes
Conflict of interest: B.S. has been consulting for Ferring Research Institute, HOST Therabiomics, Intercept Pharmaceuticals and Patara Pharmaceuticals. B.S.’s institution UC San Diego has received grant support from Axial Biotherapeutics, BiomX, CymaBay Therapeutics, NGM Biopharmaceuticals, and Synlogic Operating Company.
References
- BADAOUI A, DE SAEGER C, DUCHEMIN J, GIHOUSSE D, DE TIMARY P. & STARKEL P. 2008. Alcohol dependence is associated with reduced plasma and fundic ghrelin levels. Eur J Clin Invest, 38, 397–403. [DOI] [PubMed] [Google Scholar]
- BAJAJ JS 2019. Alcohol, liver disease and the gut microbiota. Nat Rev Gastroenterol Hepatol, 16, 235–246. [DOI] [PubMed] [Google Scholar]
- BALL SA, TENNEN H, POLING JC, KRANZLER HR & ROUNSAVILLE BJ 1997. Personality, temperament, and character dimensions and the DSM-IV personality disorders in substance abusers. J Abnorm Psychol, 106, 545–53. [DOI] [PubMed] [Google Scholar]
- BERTOLA A, MATHEWS S, KI SH, WANG H. & GAO B. 2013. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc, 8, 627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BODE L. 2009. Human milk oligosaccharides: prebiotics and beyond. Nutr Rev, 67 Suppl 2, S183–91. [DOI] [PubMed] [Google Scholar]
- BODE L. 2012. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology, 22, 1147–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUHA R, DVORAK K. & PETRTYL J. 2012. Alcoholic liver disease. World J Hepatol, 4, 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRY L, FALK PG, MIDTVEDT T. & GORDON JI 1996. A model of host-microbial interactions in an open mammalian ecosystem. Science, 273, 1380–3. [DOI] [PubMed] [Google Scholar]
- CHAN RC, REID G, IRVIN RT, BRUCE AW & COSTERTON JW 1985. Competitive exclusion of uropathogens from human uroepithelial cells by Lactobacillus whole cells and cell wall fragments. Infect Immun, 47, 84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOMINO SE, ZHANG L. & LOWE JB 2001. Molecular cloning, genomic mapping, and expression of two secretor blood group alpha (1,2)fucosyltransferase genes differentially regulated in mouse uterine epithelium and gastrointestinal tract. J Biol Chem, 276, 23748–56. [DOI] [PubMed] [Google Scholar]
- DUAN Y, LLORENTE C, LANG S, BRANDL K, CHU H, JIANG L, WHITE RC, CLARKE TH, NGUYEN K, TORRALBA M, SHAO Y, LIU J, HERNANDEZ-MORALES A, LESSOR L, RAHMAN IR, MIYAMOTO Y, LY M, GAO B, SUN W, KIESEL R, HUTMACHER F, LEE S, VENTURA-COTS M, BOSQUES-PADILLA F, VERNA EC, ABRALDES JG, BROWN RS JR., VARGAS V, ALTAMIRANO J, CABALLERIA J, SHAWCROSS DL, HO SB, LOUVET A, LUCEY MR, MATHURIN P, GARCIA-TSAO G, BATALLER R, TU XM, ECKMANN L, VAN DER DONK WA, YOUNG R, LAWLEY TD, STARKEL P, PRIDE D, FOUTS DE & SCHNABL B. 2019. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature, 575, 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUCK LW, WALTER MR, NOVAK J, KELLY D, TOMASI M, CONG Y. & ELSON CO 2007. Isolation of flagellated bacteria implicated in Crohn’s disease. Inflamm Bowel Dis, 13, 1191–201. [DOI] [PubMed] [Google Scholar]
- ELISON E, VIGSNAES LK, RINDOM KROGSGAARD L, RASMUSSEN J, SORENSEN N, MCCONNELL B, HENNET T, SOMMER MO & BYTZER P. 2016. Oral supplementation of healthy adults with 2’-O-fucosyllactose and lacto-N-neotetraose is well tolerated and shifts the intestinal microbiota. Br J Nutr, 116, 1356–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUROPEAN ASSOCIATION FOR THE STUDY OF, L. 2012. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol, 57, 399–420. [DOI] [PubMed] [Google Scholar]
- GOTO Y, OBATA T, KUNISAWA J, SATO S, IVANOV II, LAMICHHANE A, TAKEYAMA N, KAMIOKA M, SAKAMOTO M, MATSUKI T, SETOYAMA H, IMAOKA A, UEMATSU S, AKIRA S, DOMINO SE, KULIG P, BECHER B, RENAULD JC, SASAKAWA C, UMESAKI Y, BENNO Y. & KIYONO H. 2014. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science, 345, 1254009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTO Y, UEMATSU S. & KIYONO H. 2016. Epithelial glycosylation in gut homeostasis and inflammation. Nat Immunol, 17, 1244–1251. [DOI] [PubMed] [Google Scholar]
- GRABINGER T, GLAUS GARZON JF, HAUSMANN M, GEIRNAERT A, LACROIX C. & HENNET T. 2019. Alleviation of Intestinal Inflammation by Oral Supplementation With 2-Fucosyllactose in Mice. Front Microbiol, 10, 1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTMANN P, CHEN P, WANG HJ, WANG L, MCCOLE DF, BRANDL K, STARKEL P, BELZER C, HELLERBRAND C, TSUKAMOTO H, HO SB & SCHNABL B. 2013. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology, 58, 108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEGAR B, WIBOWO Y, BASROWI RW, RANUH RG, SUDARMO SM, MUNASIR Z, ATTHIYAH AF, WIDODO AD, SUPRIATMO, KADIM M, SURYAWAN A, DIANA NR, MANOPPO C. & VANDENPLAS Y. 2019. The Role of Two Human Milk Oligosaccharides, 2’-Fucosyllactose and Lacto-N-Neotetraose, in Infant Nutrition. Pediatr Gastroenterol Hepatol Nutr, 22, 330–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURD EA, HOLMEN JM, HANSSON GC & DOMINO SE 2005. Gastrointestinal mucins of Fut2-null mice lack terminal fucosylation without affecting colonization by Candida albicans. Glycobiology, 15, 1002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INAMINE T, YANG AM, WANG L, LEE KC, LLORENTE C. & SCHNABL B. 2016. Genetic Loss of Immunoglobulin A Does Not Influence Development of Alcoholic Steatohepatitis in Mice. Alcohol Clin Exp Res, 40, 2604–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASHYAP PC, MARCOBAL A, URSELL LK, SMITS SA, SONNENBURG ED, COSTELLO EK, HIGGINBOTTOM SK, DOMINO SE, HOLMES SP, RELMAN DA, KNIGHT R, GORDON JI & SONNENBURG JL 2013. Genetically dictated change in host mucus carbohydrate landscape exerts a diet-dependent effect on the gut microbiota. Proc Natl Acad Sci U S A, 110, 17059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEBEER S, VANDERLEYDEN J. & DE KEERSMAECKER SC 2008. Genes and molecules of lactobacilli supporting probiotic action. Microbiol Mol Biol Rev, 72, 728–64, Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLORENTE C, JEPSEN P, INAMINE T, WANG L, BLUEMEL S, WANG HJ, LOOMBA R, BAJAJ JS, SCHUBERT ML, SIKAROODI M, GILLEVET PM, XU J, KISSELEVA T, HO SB, DEPEW J, DU X, SORENSEN HT, VILSTRUP H, NELSON KE, BRENNER DA, FOUTS DE & SCHNABL B. 2017. Gastric acid suppression promotes alcoholic liver disease by inducing overgrowth of intestinal Enterococcus. Nat Commun, 8, 837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCEY MR, MATHURIN P. & MORGAN TR 2009. Alcoholic hepatitis. N Engl J Med, 360, 2758–69. [DOI] [PubMed] [Google Scholar]
- MARONI L, VAN DE GRAAF SF, HOHENESTER SD, OUDE ELFERINK RP & BEUERS U. 2015. Fucosyltransferase 2: a genetic risk factor for primary sclerosing cholangitis and Crohn’s disease--a comprehensive review. Clin Rev Allergy Immunol, 48, 182–91. [DOI] [PubMed] [Google Scholar]
- NAGALINGAM NA, KAO JY & YOUNG VB 2011. Microbial ecology of the murine gut associated with the development of dextran sodium sulfate-induced colitis. Inflamm Bowel Dis, 17, 917–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NGUYEN-KHAC E, CHATELAIN D, TRAMIER B, DECROMBECQUE C, ROBERT B, JOLY JP, BREVET M, GRIGNON P, LION S, LE PAGE L. & DUPAS JL 2008. Assessment of asymptomatic liver fibrosis in alcoholic patients using fibroscan: prospective comparison with seven non-invasive laboratory tests. Aliment Pharmacol Ther, 28, 1188–98. [DOI] [PubMed] [Google Scholar]
- PHAM TA, CLARE S, GOULDING D, ARASTEH JM, STARES MD, BROWNE HP, KEANE JA, PAGE AJ, KUMASAKA N, KANE L, MOTTRAM L, HARCOURT K, HALE C, ARENDS MJ, GAFFNEY DJ, SANGER MOUSE GENETICS P, DOUGAN G. & LAWLEY TD 2014. Epithelial IL-22RA1-mediated fucosylation promotes intestinal colonization resistance to an opportunistic pathogen. Cell Host Microbe, 16, 504–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PICKARD JM, MAURICE CF, KINNEBREW MA, ABT MC, SCHENTEN D, GOLOVKINA TV, BOGATYREV SR, ISMAGILOV RF, PAMER EG, TURNBAUGH PJ & CHERVONSKY AV 2014. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature, 514, 638–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAUSCH P, REHMAN A, KUNZEL S, HASLER R, OTT SJ, SCHREIBER S, ROSENSTIEL P, FRANKE A. & BAINES JF 2011. Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc Natl Acad Sci U S A, 108, 19030–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHO JH, WRIGHT DP, CHRISTIE DL, CLINCH K, FURNEAUX RH & ROBERTON AM 2005. A novel mechanism for desulfation of mucin: identification and cloning of a mucin-desulfating glycosidase (sulfoglycosidase) from Prevotella strain RS2. J Bacteriol, 187, 1543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANKAR N, COBURN P, PILLAR C, HAAS W. & GILMORE M. 2004. Enterococcal cytolysin: activities and association with other virulence traits in a pathogenicity island. Int J Med Microbiol, 293, 609–18. [DOI] [PubMed] [Google Scholar]
- SOKOL H, PIGNEUR B, WATTERLOT L, LAKHDARI O, BERMUDEZ-HUMARAN LG, GRATADOUX JJ, BLUGEON S, BRIDONNEAU C, FURET JP, CORTHIER G, GRANGETTE C, VASQUEZ N, POCHART P, TRUGNAN G, THOMAS G, BLOTTIERE HM, DORE J, MARTEAU P, SEKSIK P. & LANGELLA P. 2008. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A, 105, 16731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONG M, MCHARDY I, RUEGGER P, GOUDARZI M, KASHYAP PC, HARITUNIANS T, LI X, GRAEBER TG, SCHWAGER E, HUTTENHOWER C, FORNACE AJ JR., SONNENBURG JL, MCGOVERN DP, BORNEMAN J. & BRAUN J. 2014. Reprograming of gut microbiome energy metabolism by the FUT2 Crohn’s disease risk polymorphism. ISME J, 8, 2193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN TYNE D, MARTIN MJ & GILMORE MS 2013. Structure, function, and biology of the Enterococcus faecalis cytolysin. Toxins (Basel), 5, 895–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANDENPLAS Y, BERGER B, CARNIELLI VP, KSIAZYK J, LAGSTROM H, SANCHEZ LUNA M, MIGACHEVA N, MOSSELMANS JM, PICAUD JC, POSSNER M, SINGHAL A. & WABITSCH M. 2018. Human Milk Oligosaccharides: 2’-Fucosyllactose (2’-FL) and Lacto-N-Neotetraose (LNnT) in Infant Formula. Nutrients, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WACKLIN P, MAKIVUOKKO H, ALAKULPPI N, NIKKILA J, TENKANEN H, RABINA J, PARTANEN J, ARANKO K. & MATTO J. 2011. Secretor genotype (FUT2 gene) is strongly associated with the composition of Bifidobacteria in the human intestine. PLoS One, 6, e20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE M, KINOSHITA H, NITTA M, YUKISHITA R, KAWAI Y, KIMURA K, TAKETOMO N, YAMAZAKI Y, TATENO Y, MIURA K, HORII A, KITAZAWA H. & SAITO T. 2010. Identification of a new adhesin-like protein from Lactobacillus mucosae ME-340 with specific affinity to the human blood group A and B antigens. J Appl Microbiol, 109, 927–35. [DOI] [PubMed] [Google Scholar]
- WEISS FU, SCHURMANN C, GUENTHER A, ERNST F, TEUMER A, MAYERLE J, SIMON P, VOLZKE H, RADKE D, GREINACHER A, KUEHN JP, ZENKER M, VOLKER U, HOMUTH G. & LERCH MM 2015. Fucosyltransferase 2 (FUT2) non-secretor status and blood group B are associated with elevated serum lipase activity in asymptomatic subjects, and an increased risk for chronic pancreatitis: a genetic association study. Gut, 64, 646–56. [DOI] [PubMed] [Google Scholar]
- YAN AW, FOUTS DE, BRANDL J, STARKEL P, TORRALBA M, SCHOTT E, TSUKAMOTO H, NELSON KE, BRENNER DA & SCHNABL B. 2011. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology, 53, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.