Supplemental Digital Content is Available in the Text.
Keywords: Pain, Assessment, Neonatal
Abstract
The burden of pain in newborn infants has been investigated in numerous studies, but little is known about the appropriateness of the use of pain scales according to the specific type of pain or infant condition. This systematic review aimed to evaluate the reporting of neonatal pain scales in randomized trials. A systematic search up to March 2019 was performed in Embase, PubMed, PsycINFO, CINAHL, Cochrane Library, Scopus, and Luxid. Randomized and quasirandomized trials reporting neonatal pain scales were included. Screening of the studies for inclusion, data extraction, and quality assessment was performed independently by 2 researchers. Of 3718 trials found, 352 with 29,137 infants and 22 published pain scales were included. Most studies (92%) concerned procedural pain, where the most frequently used pain scales were the Premature Infant Pain Profile or Premature Infant Pain Profile—Revised (48%), followed by the Neonatal Infant Pain Scale (23%). Although the Neonatal Infant Pain Scale is validated only for acute pain, it was also the second most used scale for ongoing and postoperative pain (21%). Only in a third of the trials, blinding for those performing the pain assessment was described. In 55 studies (16%), pain scales that were used lacked validation for the specific neonatal population or type of pain. Six validated pain scales were used in 90% of all trials, although not always in the correct population or type of pain. Depending on the type of pain and population of infants included in a study, appropriate scales should be selected. The inappropriate use raises serious concerns about research ethics and use of resources.
1. Introduction
Newborn infants, especially those born preterm, are highly vulnerable to pain.67 Preterm birth and illness, in addition to the necessary medical treatment and nursing care, inflict pain and stress on the infants.72 Infants in neonatal intensive care undergo as many as 10 to 15 painful procedures per day.18,56 Pain leads to immediate cardiovascular changes, behavioral changes, disrupted feeding, disturbed sleep, and increased energy expenditure that may lead to complications and a need for intensified and prolonged care.1 The immature nervous system and repeated exposure to pain may lower pain thresholds, which in return can make the infant even more sensitive to subsequent painful events.13 Changes in pain sensitivity may persist beyond the neonatal period,15,35 and neonatal pain may also result in poorer brain development.14,55,71 Pharmacological pain treatment needs to be used selectively because of the infants' immature drug metabolism and well-known drug-specific negative side effects, eg, hypotension and respiratory depression, along with the neuroapoptotic effect of analgesic and sedative drugs shown in animal research6,57 and studies reporting on their influence on brain development.28,53 To minimize the pharmacological treatment, nonpharmacological and caring strategies are widely used, eg, breastfeeding and skin-to-skin contact.16,30
The fact that stress increases the pain experience is well known in all patients; in the newborn population, this relates to the degree of prematurity, and it is also very difficult to discriminate between stress and pain, especially in the premature population. To effectively treat stress and pain, as well as diminish the negative effects in the neonatal period, the severity of the painful stimuli must be adequately recognized.73 In the nonverbal newborn patient, traditional self-report is not possible. Instead, there is a need for valid and reliable tools to assess the intensity of the stress and pain. Several observational scales have been constructed and tested for different newborn populations and different types of pain. Around 40 pain assessment instruments have been published, but their validity varies widely.25,29 Clinical use of measures that are insufficiently validated poses a risk to patient safety, as they may result in both overassessment and underassessment of pain. Using a validated pain scale with appropriate cutoff values is of course desirable, and if not so, overassessment may cause unnecessary use of pain-relieving medication with potential side effects, whereas underassessment may cause unnecessary pain and suffering.51
Objective and reliable pain assessment is considered the basis for safe and adequate pain management.29,51 There are unfortunately still no fully objective pain assessment tools for the assessment of pain/stress in the very sick patients in the neonatal intensive care unit (NICU). Different neurohormonal and neurophysiological monitoring measures such as skin conductance/galvanic skin response, heart rate variability and the adaption of it (Newborn Infant Parasympathetic Evaluation), near-infrared spectroscopy, electroencephalography, and functional magnetic resonance imaging have been evaluated and proven reliable in the assessment of pain in newborn infants and are used mainly in the research context and not as much in the clinical setting. These methods of pain assessments are not addressed in this review that focuses on observational scales.
It is essential that the pain assessment instruments used in clinical studies and also in the daily care of newborns are valid and reliable for the particular patient population (preterm vs term infants) and clinical situation (procedural pain, postoperative pain, and continuous pain/stress due to, eg, ventilator support), so the result of the assessment can be trustworthy. Little is known about the reporting of pain scales in clinical trials and whether they are correctly implemented for the specific type of pain or group of infants included in the studies. The aim of this study was therefore to evaluate the characteristics and the reporting of pain scales in randomized trials where newborns are exposed to any type of painful interventions or conditions.
2. Methods
The protocol of this study was registered in PROSPERO (international prospective register of systematic reviews) before the screening of the studies.2 The Cochrane methodology for systematic reviews of interventions was used for study screening, inclusion, and data extraction with 2 independent investigators.36
2.1. Search strategy and data sources
A systematic and broad search up to March 2019 was performed in Embase, PubMed, PsycINFO, CINAHL, Cochrane Library, Scopus, and Luxid using combinations of the terms/keywords: newborn infant, pain assessment, and trials (the full search strategy for each database is shown in Appendix 1, available at http://links.lww.com/PAIN/B154). Randomized and quasirandomized trials on neonatal pain (including procedural pain, postoperative pain, and pain associated with clinical conditions) reporting at least 1 pain scale were included. We included trials in both term and preterm infants. Observational studies, study protocols, conference abstracts, and reviews were excluded. There were no limits to publication years, and articles published in English, Swedish, or Italian were included.
2.2. Data extraction and management
Screening of titles and abstracts followed by a full-text screening for eligibility was performed by 2 independent researchers using an online tool for the preparation of systematic reviews.21 Disagreements were resolved by a third researcher (one of the other coauthors) or in discussion within the group, as recommended in the Cochrane Handbook.36
A web-based extraction form for data collection was designed (see Appendix 2, available at http://links.lww.com/PAIN/B154). Data extraction and quality assessment for performance and detection bias were performed by 2 researchers independently, and when disagreements arose, a third researcher appraised the trial and solved the conflict. As the aim of this systematic review was to map the reporting of pain scales rather than assessing the effects of an intervention, pooling data and creating meta-analysis were not planned.
The extracted data were entered into SPSS version 26 (IBM Corp, Armonk, NY: IBM Corp). Analyzed data are presented with frequency in absolute numbers and percentage.
3. Results
The literature search retrieved 3715 scientific articles. In addition, 3 articles were found by expert knowledge of the field. After removing duplicates, 3580 studies were screened for title and abstract, whereas 557 were subsequently read in full text. We finally included 352 trials in the systematic review (supplementary Table 1, available at http://links.lww.com/PAIN/B154). A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart is shown in Figure 1. Characteristics of the included studies and pain scales are presented in the text and in detail in Tables 1 and 2 and supplementary Tables 1 to 3 (available at http://links.lww.com/PAIN/B154).
Figure 1.
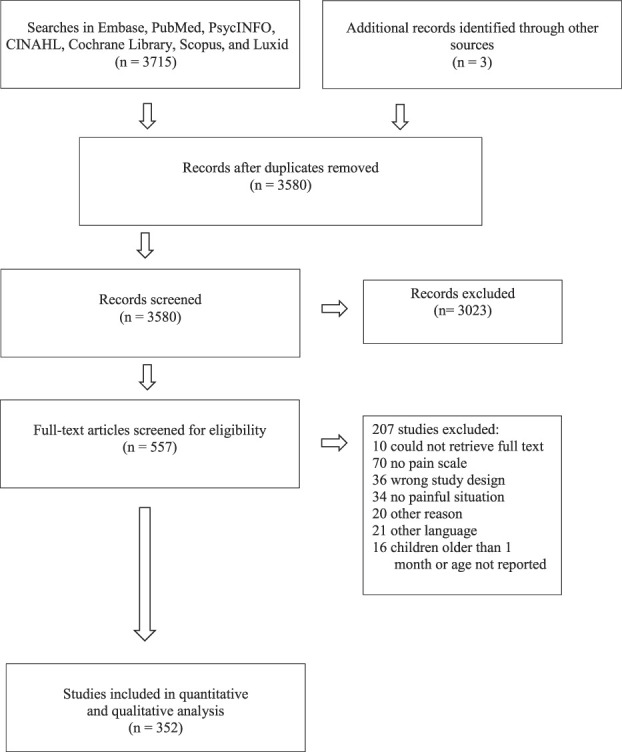
A PRISMA flowchart showing the search, screening, and inclusion procedure. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
The 22 published pain scales used in the included trials.
| Acronym or abbreviation (number of studies) | Full name | Age validation | Pain-type validation | Development studies | Validation studies | |||
|---|---|---|---|---|---|---|---|---|
| Preterm | Term | Acute procedural | Ongoing | Postoperative | ||||
| PIPP/PIPP-R (154) | Premature Infant Pain Profile/Premature Infant Pain Profile—Revised | x | x | x | 61, 63 | 26, 31, 62 | ||
| NIPS (84) | Neonatal Infant Pain Scale | x | x | x | 47 | 11, 40 | ||
| NFCS (33) | Neonatal Facial Coding System | x | x | 34, 33 | 48, 66 | |||
| DAN (20) | Douleur Aiguë Nouveau-né [Newborn Acute Pain] | x | x | x | 17 | 10, 66 | ||
| COMFORTneo/COMFORT/COMFORT-B (15) | COMFORT-B stands for Comfort Behavioral Scale | x | x | x | 69 | 5, 12, 68 | ||
| N-PASS (10) | Neonatal Pain, Agitation, and Sedation Scale | x | x | x | x | x | 41 | 42 |
| CRIES (7) | Crying, Requires oxygen, Increased vital signs, Expression, and Sleep | x | x | x | 46 | 60 | ||
| EDIN (6) | Échelle Douleur Inconfort Nouveau-Né [Neonatal Pain and Discomfort Scale] | x | x | x | 27 | |||
| ABC (3) | Acuteness of the first cry, Burst rhythmicity, and Constancy in time of cry intensity | x | x | x | 10 | 9 | ||
| BIIP (3) | Behavioral Indicators of Infant Scale Pain | x | x | x | 38 | 39 | ||
| BPSN (3) | Bernese Pain Scale for Neonates | x | x | x | 19 | 20 | ||
| ALPS-Neo/ALPS/ALPS 0 (2) | Astrid Lindgren and Lund Children's Hospital's Pain and Stress Assessment Scale for Preterm and Sick Newborn Infants | x | x | x | 49 | |||
| CHEOPS (1) | Children's Hospital of Eastern Ontario Pain Scale | x | x | x | 52 | 65 | ||
| FLACC (1) | Face, Legs, Activity, Cry, and Consolability | x | x | 54 | 23, 50 | |||
| IBCS (1) | Infant Body Coding System | x | x | x | 22 | |||
| LNPS (1) | Leuven Neonatal Pain Score | x | x | x | 3 | |||
| MAX (1) | Maximally discriminative facial movement coding system | x | 44 | 43 | ||||
| MBPS (1) | Modified Behavioral Pain Scale | 2-6 m | x | 64 | 24 | |||
| NPAS (1) | Neonatal Pain Assessment Scale | x | x | 59 | ||||
| PAT (1) | Pain Assessment Tool | x | 37 | 60 | ||||
| PCS (1) | The Postoperative Comfort Score | x | x | 7 | ||||
| RIPS (1) | Riley Infant Pain Score | x | x | 45, 58 | ||||
The pain scales are presented in descending order, showing the frequency of their use in the included studies.
Table 2.
The 6 most frequent pain scales, types of pain, studied intervention, study setting, age group at the time of the study, and study design.
| PIPP/ PIPP-R | NIPS | NFCS | DAN | Comfort-neo/Comfort / Comfort-B | N-PASS | |
|---|---|---|---|---|---|---|
| No. of studies | 154 | 84 | 33 | 20 | 15 | 10 |
| Type of pain, n (%) | ||||||
| Acute/procedural | 151 (98.1) | 78 (92.8) | 31 (93.9) | 20 (100.0) | 5 (33.3) | 9 (90.0) |
| On-going | 2 (1.3) | 3 (3.6) | 1 (3.0) | 2 (13.3) | 1 (10.0) | |
| Post-operative | 2 (2.4) | 1 (3.0) | 8 (53.3) | |||
| Other/more than one type | 1 (0.6) | 1 (1.2) | ||||
| Type of intervention, n (%) | ||||||
| Pharmacological | 53 (34.4) | 25 (29.8) | 15 (45.5) | 6 (30.0) | 14 (93.3) | 4 (40.0) |
| Non-pharmacological | 68 (44.2) | 41 (48.8) | 12 (36.3) | 6 (30.0) | 1 (6.7) | 5 (50.0) |
| Both | 33 (21.4) | 18 (21.4) | 6 (18.2) | 8 (40.0) | 1 (10.0) | |
| Study setting, n (%) | ||||||
| NICU/Neonatal unit | 132 (85,8) | 39 (46.4) | 18 (54.5) | 13 (65.0) | 6 (60.0) | 8 (80.0) |
| Nursery/Maternity unit | 11 (7.1) | 24 (28.6) | 10 (30.3) | 7 (35.0) | 3 (30.0) | 1 (10.0) |
| Other | 11 (7.1) | 21 (25.0) | 5 (15.2) | 1 (10.0) | 1 (10.0) | |
| Age group at time of the study, n (%) | ||||||
| <28 w | 1 (0.6) | |||||
| <28 w-32 w | 10 (6.5) | 3 (4.9) | 1 (6.1) | 1(5.0) | 2 (14.3) | |
| <28 w-36 w | 18 (11.7) | 3 (8.5) | 2 (9.1) | 1(5.0) | 2 (14.3) | |
| <28 w- >37 w | 8 (5.2) | 1 (6.1) | ||||
| 28 w-32 w | 3 (1.9) | 1 (1.2) | 1 (10.0) | |||
| 28 w-36 w | 31 (20.1) | 5 (6.1) | 3 (9.1) | 1 (5.0) | 1 (7.1) | 2 (20.0) |
| 28 w- >37 w | 28 (18.2) | 6 (12.2) | 4 (18.2) | 3 (15.0) | 2 (14.3) | |
| 33 w-36 w | 5 (3.2) | 2 (3.7) | ||||
| 33 w- >37 w | 14 (9.1) | 7 (15.9) | 4 (6.1) | 1 (7.1) | 2 (20.0) | |
| >37 w | 15 (9.7) | 45 (31.7) | 20 (27.3) | 11 (55.0) | 2 (14.3) | 5 (50.0) |
| Unclear | 21 (13.6) | 11 (11.0) | 3 (15.0) | 4 (28.6 | ||
| Study design, n (%) | ||||||
| Randomized | 112 (72.7) | 73 (86.9) | 27 (81.8) | 16 (80.0) | 14 (93.3) | 7 (70.0) |
| Cross-over | 39 (25.3) | 10 (11.9) | 4 (12.1) | 4 (20.0) | 3 (30.0) | |
| Other | 3 (2.0) | 1 (1.2) | 2 (6.1) | 1 (6.7) |
DAN, Douleur Aiguë Nouveau-né; NFCS, Neonatal Facial Coding System; NICU, neonatal intensive care unit; NIPS, Neonatal Infant Pain Scale; N-PASS, Neonatal Pain, Agitation, and Sedation Scale; PIPP, Premature Infant Pain Profile; PIPP-R, Premature Infant Pain Profile—Revised.
A total of 29,137 infants (ranging from 10 to 898 per trial, mean of 82.8) from 41 different countries were enrolled in the 352 included studies (supplementary Table 1, available at http://links.lww.com/PAIN/B154). Twenty-two previously published pain scales (Table 2), along with a number of scales designed locally for specific studies or instruments originally not intended for pain assessment, were adopted. Eight pain scales represented 93.7% of the use of published pain scales, and only 6 of them were used in more than 10 of the included studies each: Premature Infant Pain Profile/Premature Infant Pain Profile—Revised,61,63 43.9%; Neonatal Infant Pain Scale (NIPS),47 23.9%; Neonatal Facial Coding System (NFCS),33 9.4%; Douleur Aiguë Nouveau-né [Newborn Acute Pain] (DAN),17 5.7%; COMFORTneo (including COMFORT/COMFORT-B),4,70 4.3%; and Neonatal Pain, Agitation, and Sedation Scale (N-PASS),41 2.8%. The type of pain, intervention, study setting, age group, and study design for those 6 most frequently used scales are summarized in Table 2.
Nonvalidated, locally developed pain scales were used in 19 of the studies, eg, “revised NFCS,” “pain score developed from CHEOPS and NIPS,” or “5-item behavior scale.” In many of these studies, the description of the pain scale was vague. Another 19 studies used scales originally not intended or validated for pain assessment, such as Brazelton behavioral states or Prechtl score. Finally, 17 studies used a Visual Analog Scale (VAS) or Numeric Rating Scale (NRS) for pain assessment. In 6 of these studies, the VAS/NRS was combined with one of the COMFORT scales, which is part of that method, and in 8 studies, the VAS/NRS was combined with another pain scale. Seven studies on ongoing pain used scales that were developed and validated for procedural pain, and vice versa, 10 studies on procedural pain used scales intended for ongoing pain. Five studies used scales not validated for the studied age group, eg, CHEOPS to assess postoperative pain in preterm infants. To summarize, of the included trials, 15.6% used a pain scale that was not appropriate for the type of pain investigated in the trial or an inappropriate scale with regard to the age of the included infants.
In most studies (93%), only one pain scale was used. In 26 trials, more than 1 pain scale were used in the same infants, whereas in only 16 of these studies the scales were compared (supplementary Table 2, available at http://links.lww.com/PAIN/B154). In 111 trials, the presence and intensity of pain were assessed independently by 2 or more observers (supplementary Table 3, available at http://links.lww.com/PAIN/B154). However, in only 61 of these trials (54.5%), agreement between the assessors was reported.
Most of the studies were performed in a NICU setting (61.4%, n = 216), followed by maternity units (18.5%, n = 65) and other hospital settings such as pediatric intensive care units and postoperative care units. Most included studies used a design with 2 or more randomized parallel groups (79.2%, n = 279), whereas 18.7% (n = 66) used a cross-over design; 2.0% (n = 7) did not clearly describe a study design.
Only 4.3% (n = 15) and 3.7% (n = 12) of the trials reported on ongoing pain and postoperative pain, respectively. In those trials, any of the COMFORT scales were most frequently used (31.0%), followed by the NIPS (20.7%) and Échelle Douleur Inconfort Nouveau-Né (EDIN) (17.2%). Procedural pain was the most common type of pain being studied (91.8%, n = 323), with heel lance reported as the most common painful procedure (37%, n = 152), followed by venipuncture (15%, n = 63) and intramuscular injection (8%, n = 33). There was an equal amount of studies investigating pharmacological interventions (n = 140) and nonpharmacological interventions (n = 140), whereas 72 of the studies used a combination of both. The pharmacological interventions most frequently reported were sweet solutions such as sucrose or glucose (133 studies), followed by local anesthetics (n = 41) and morphine (n = 20). Nonpharmacological interventions included a range of different strategies where non-nutritive sucking was most frequently reported (n = 24).
Our quality assessment of the included trials focused on performance and detection bias, ie, blinding of those administering the painful procedure and of those assessing the pain scale, respectively (supplementary Table 1, available at http://links.lww.com/PAIN/B154). Only in 119 trials (33.8%), blinding was described for those performing the pain assessment; in 30 (8.5%), blinding was described for those performing the painful procedure and those performing the pain assessment; in 7 (2.0%), blinding was described for those administering the pain-relieving intervention and those performing the pain assessment, and in 78 (22.1%), blinding was described for those administering the pain-relieving intervention, those performing the painful procedure, and those performing the pain assessment. Overall, only 108 trials (30.7%) had a low risk of bias for both performance and detection bias. Complete lack of blinding and unclear description of blinding were detected in 51 (14.5%) and 61 (17.3%) trials, respectively.
4. Discussion
This is the first systematic review reporting how pain scales are used in randomized trials in the newborn. Although 22 different published pain scales and a number of nonvalidated instruments were used in the 352 included trials, most of the studies used the same pain scales.
Optimally, pain should be monitored in a completely objective way, but unfortunately such strategies are not available in the neonatal setting. Therefore, assessment of pain with the help of validated pain scales is the best available method, which underlines the necessity of the validation process. In numerous trials, the pain scales that were used were lacking validation for the studied population or the types of pain that were assessed. In our review, we report that 15.6% of the included studies used a pain scale that was validated for neither the type of pain studied nor the infant population. For example, the NIPS is validated only for acute pain; however, it was the second most used scale for ongoing and postoperative pain. It is also worth to notice that many of the validation studies are quite old and conducted before the implementation of protocols such as COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN). The type and accuracy of the validation is also important when choosing an instrument for a clinical trial.32 It could be argued that many of the scales are similar and comprise the same items in different combinations, and that using any scale is better than not trying to assess pain. Even if the argument for doing something being better than doing nothing is true, it is not a level of acceptance to stop at. There is probably no need for more scales, but that the scales that are used are valid and responsive, ie, can detect pain but also a reduction in pain after a pain-relieving intervention. More efforts should be put on examining this in existing scales, for infants with different age groups and medical conditions, and on implementing them properly in neonatal care.
Moreover, we found that most trials were conducted in newborns exposed to procedural pain, whereas there is a paucity of studies on postoperative pain and continuous pain/stress. The explanation to this is most probably that the era of pain research started with studies on procedural pain in the 1990s8 and that these studies are easier to conduct. Research focusing on procedural pain has taught us on pain physiology in preterm and term infants in a very structured way, whereas studies on the complexity of postoperative pain, painful conditions, or continuous pain/stress, eg, during ventilator support in the very sick NICU infants with a variety of different conditions and different clinical states, are more difficult to conduct in an optimal way. Continuous pain for instance due to necrotizing enterocolitis is more difficult to assess, especially in preterm infants who may react with a “shut-down,” hypotonia, and immobility, in response to strong pain and stress. It might therefore be possible that in some centers, procedural pain assessment is still used to “assess” continuous pain the way it was before the adequate pain scales for continuous pain were developed, ie, translate a high score at a procedure to indicate that the infant is also inadequately treated for its continuous pain.
In the review, we found very few studies on endotracheal intubation, which is considered a painful procedure. The explanation might be that some of the pharmacological strategies used include muscle relaxants, and as a result, pain assessment might therefore be inconclusive as the infants lose the ability to breath and move, 2 major items included in most pain assessment scales.
Recently, Giordano and colleagues conducted a systematic review on the validation of pain and sedation scales in newborns and infants.32 Their work importantly differs from our review by using broader inclusion criteria with regard to the type of studies (not only trials as in this review) and study population (not only newborns). Furthermore, their evidence synthesis included 89 studies, as they focused on the validation rate rather than on how trials report the use of pain scales. Giordano and colleagues32 also showed that only 28 of 65 scales for infant and toddlers had been tested for construct validity, internal consistency, and interrater reliability. Furthermore, they highlighted the importance of defined and tested cutoff values for pain/no pain for the clinical utility of any scale.
The strengths of our systematic review include the originality of study design and the broad search strategy. We also registered the protocol a priori to avoid intellectual bias.
The limitations include the language restriction to 3 languages and the choice to assess only 2 of the 6 items of the Cochrane risk of bias tool, ie, performance and detection bias.36 However, this is a minor issue because we did not aim to assess the benefits and harms of different interventions, but instead on how the use of neonatal pain scales is reported in trials. Of note, blinding of both those administering the painful condition and of those using the pain scale was reported in less than one-third of the trials. Whereas the lack of performance bias might be due to the nature of the interventions in some studies, researchers might easily design trials with a low risk of detection bias by ensuring blinding of outcome assessors.
In this review, we can show that there are a few validated pain assessment scales used in most clinical studies, which is positive news. In the clinical setting, it is crucial to choose an appropriate pain assessment scale, validated for the type of pain and population of infants to be able to support the infants in the best way. In research, it is crucial to get reliable results and thereby provide the best evidence-based clinical strategies. The inappropriate use of pain scales in research raises serious concerns on the ethical conduct of research and use of resources.
Furthermore, we reiterate the need for fewer but larger trials, with appropriate sample size to assess clinically relevant outcomes, in addition to the reporting of measurement properties.
Although it was not the scope of our review, the findings raise concerns whether we face a situation of excess of research in the area where studies on procedural pain can be conducted more easily and of lack of research in the more challenging clinical NICU situation with ongoing and postoperative pain.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B154.
Acknowledgements
The authors thank Matthias Bank (Library and ICT services, Lund University) for defining and running the search strategy and Thomas Evertsson (Library and ICT services, Lund University) for retrieving the full-text articles.
M. Bruschettini has received research funding from the ALF grant (nonprofit—Lund University) and the Crafoord Foundation (nonprofit) for research projects not related to Cochrane. E. Olsson and M. Eriksson performed the work as part of their university employment.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Abdulkader HM, Freer Y, Garry EM, Fleetwood-Walker SM, McIntosh N. Prematurity and neonatal noxious events exert lasting effects on infant pain behaviour. Early Hum Dev 2008;84:351–5. [DOI] [PubMed] [Google Scholar]
- [2].Ahl H, Eriksson M, Norman E, Sjöström Strand A, Olsson E, Bruschettini M. Pain assessment instruments for use in clinical studies on newborn infants—a mapping of the evidence 2018. Available at: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018081412. Accessed May 23, 2020.
- [3].Allegaert K, Tibboel D, Naulaers G, Tison D, De Jonge A, Van Dijk M, Vanhole C, Devlieger H. Systematic evaluation of pain in neonates: effect on the number of intravenous analgesics prescribed. Eur J Clin Pharmacol 2003;59:87–90. [DOI] [PubMed] [Google Scholar]
- [4].Ambuel B, Hamlett KW, Marx CM, Blumer JL. Assessing distress in pediatric intensive care environments: the COMFORT scale. J Pediatr Psychol 1992;17:95–109. [DOI] [PubMed] [Google Scholar]
- [5].Andersen RD, Bernklev T, Langius-Eklof A, Nakstad B, Jylli L. The COMFORT behavioural scale provides a useful assessment of sedation, pain and distress in toddlers undergoing minor elective surgery. Acta Paediatr 2015;104:904–9. [DOI] [PubMed] [Google Scholar]
- [6].Andropoulos DB. Effect of anesthesia on the developing brain: infant and fetus. Fetal Diagn Ther 2018;43:1–11. [DOI] [PubMed] [Google Scholar]
- [7].Attia J, Barrier G, Mayer MN, Amiel-Tison C, Shnider SM. Measurement of post-operative pain and narcotic administration in infants using a new clinical scoring system. Intensive Care Med 1989;15(suppl 1):S37–9. [DOI] [PubMed] [Google Scholar]
- [8].Banos JE, Ruiz G, Guardiola E. An analysis of articles on neonatal pain published from 1965 to 1999. Pain Res Manag 2001;6:45–50. [DOI] [PubMed] [Google Scholar]
- [9].Bellieni C, Buonocore G. Recommendations for an ethical treatment of newborns involved in clinical trials. Acta Pædiatrica 2010;99:30–2. [DOI] [PubMed] [Google Scholar]
- [10].Bellieni CV, Bagnoli F, Sisto R, Neri L, Cordelli D, Buonocore G. Development and validation of the ABC pain scale for healthy full-term babies. Acta Paediatr 2005;94:1432–6. [DOI] [PubMed] [Google Scholar]
- [11].Blauer T, Gerstmann D. A simultaneous comparison of three neonatal pain scales during common NICU procedures. Clin J Pain 1998;14:39–47. [DOI] [PubMed] [Google Scholar]
- [12].Boerlage AA, Ista E, Duivenvoorden HJ, de Wildt SN, Tibboel D, van Dijk M. The COMFORT behaviour scale detects clinically meaningful effects of analgesic and sedative treatment. Eur J Pain 2015;19:473–9. [DOI] [PubMed] [Google Scholar]
- [13].Bouza H. The impact of pain in the immature brain. J Matern Fetal Neonatal Med 2009;22:722–32. [DOI] [PubMed] [Google Scholar]
- [14].Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant R, Vinall J, Gover A, Synnes AR, Miller SP. Procedural pain and brain development in premature newborns. Ann Neurol 2012;71:385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Buskila D, Neuman L, Zmora E, Feldman M, Bolotin A, Press J. Pain sensitivity in prematurely born adolescents. Arch Pediatr Adolesc Med 2003;157:1079–82. [DOI] [PubMed] [Google Scholar]
- [16].Campbell-Yeo M, Fernandes A, Johnston C. Procedural pain management for neonates using nonpharmacological strategies: part 2: mother-driven interventions. Adv Neonatal Care 2011;11:312–8. [DOI] [PubMed] [Google Scholar]
- [17].Carbajal R, Paupe A, Hoenn E, Lenclen R, Olivier-Martin M. APN: evaluation behavioral scale of acute pain in newborn infants. Arch Pediatr 1997;4:623–8. [DOI] [PubMed] [Google Scholar]
- [18].Carbajal R, Rousset A, Danan C, Coquery S, Nolent P, Ducrocq S, Saizou C, Lapillonne A, Granier M, Durand P, Lenclen R, Coursol A, Hubert P, de Saint Blanquat L, Boelle P-Y, Annequin D, Cimerman P, Anand KJS, Breart G. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA 2008;300:60–70. [DOI] [PubMed] [Google Scholar]
- [19].Cignacco E, Mueller R, Hamers JP, Gessler P. Pain assessment in the neonate using the Bernese Pain Scale for Neonates. Early Hum Dev 2004;78:125–31. [DOI] [PubMed] [Google Scholar]
- [20].Cignacco E, Schenk K, Stevens B, Stoffel L, Bassler D, Schulzke S, Nelle M. Individual contextual factors in the validation of the Bernese Pain Scale for Neonates: protocol for a prospective observational study. BMC Pediatr 2017;17:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Covidence systematic review software. Melbourne: Veritas Health Innovations. [Google Scholar]
- [22].Craig KD, Whitfield MF, Grunau RV, Linton J, Hadjistavropoulos HD. Pain in the preterm neonate: behavioural and physiological indices. PAIN 1993;52:287–99. [DOI] [PubMed] [Google Scholar]
- [23].Crellin DJ, Harrison D, Santamaria N, Babl FE. Systematic review of the face, legs, activity, cry and consolability scale for assessing pain in infants and children: is it reliable, valid, and feasible for use? PAIN 2015;156:2132–51. [DOI] [PubMed] [Google Scholar]
- [24].Crellin DJ, Babl FE, Santamaria N, Harrison D. A systematic review of the psychometric properties of the Modified Behavioral Pain Scale (MBPS). J Pediatr Nurs 2018;40:14–26. [DOI] [PubMed] [Google Scholar]
- [25].de Melo GM, de Aguiar Lélis ALP, de Moura AF, Cardoso MVLML, da Silva VM. Pain assessment scales in newborns: integrative review. Rev Paul Pediatr 2014;32:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].de Oliveira MV, de Jesus JA, Tristao RM. Psychophysical parameters of a multidimensional pain scale in newborns. Physiol Meas 2012;33:39–49. [DOI] [PubMed] [Google Scholar]
- [27].Debillon T, Zupan V, Ravault N, Magny JF, Dehan M, ABU-SAAD HH. Development and initial validation of the EDIN scale, a new tool for assessing prolonged pain in preterm infants. Arch Dis Child Fetal Neonatal Ed 2001;85:F36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Duerden EG, Guo T, Dodbiba L, Chakravarty MM, Chau V, Poskitt KJ, Synnes A, Grunau RE, Miller SP. Midazolam dose correlates with abnormal hippocampal growth and neurodevelopmental outcome in preterm infants. Ann Neurol 2016;79:548–59. [DOI] [PubMed] [Google Scholar]
- [29].Eriksson M, Campbell-Yeo M. Assessment of pain in newborn infants. Semin Fetal Neonatal Med 2019;24:101003. [DOI] [PubMed] [Google Scholar]
- [30].Fernandes A, Campbell-Yeo M, Johnston CC. Procedural pain management for neonates using nonpharmacological strategies: part 1: sensorial interventions. Adv Neonatal Care 2011;11:235–41. [DOI] [PubMed] [Google Scholar]
- [31].Gibbins S, Stevens BJ, Yamada J, Dionne K, Campbell-Yeo M, Lee G, Caddell K, Johnston C, Taddio A. Validation of the Premature Infant Pain Profile-Revised (PIPP-R). Early Hum Dev 2014;90:189–93. [DOI] [PubMed] [Google Scholar]
- [32].Giordano V, Edobor J, Deindl P, Wildner B, Goeral K, Steinbauer P, Werther T, Berger A, Olischar M. Pain and sedation scales for neonatal and pediatric patients in a preverbal stage of development: a systematic review. JAMA Pediatr 2019;173:1186–97. [DOI] [PubMed] [Google Scholar]
- [33].Grunau RV, Craig KD. Pain expression in neonates: facial action and cry. PAIN 1987;28:395–410. [DOI] [PubMed] [Google Scholar]
- [34].Grunau RE, Oberlander T, Holsti L, Whitfield MF. Bedside application of the Neonatal Facial Coding System in pain assessment of premature neonates. PAIN 1998;76:277–86. [DOI] [PubMed] [Google Scholar]
- [35].Grunau RE. Neonatal pain in very preterm infants: long-term effects on brain, neurodevelopment and pain reactivity. Rambam Maimonides Med J 2013;4:e0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0: The Cochrane Collaboration, 2011. Available at: www.cochrane-handbook.org. Accessed May 23, 2020. [Google Scholar]
- [37].Hodgkinson K, Bear M, Thorn J, Van Blaricum S. Measuring pain in neonates: evaluating an instrument and developing a common language. Aust J Adv Nurs 1994;12:17–22. [PubMed] [Google Scholar]
- [38].Holsti L, Grunau RE. Initial validation of the Behavioral Indicators of Infant Pain (BIIP). PAIN 2007;132:264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Holsti L, Grunau RE, Oberlander TF, Osiovich H, Holsti L, Grunau RE, Oberlander TF, Osiovich H. Is it painful or not? Discriminant validity of the Behavioral Indicators of Infant Pain (BIIP) scale. Clin J Pain 2008;24:83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hudson-Barr D, Capper-Michel B, Lambert S, Palermo TM, Morbeto K, Lombardo S. Validation of the pain assessment in neonates (PAIN) scale with the Neonatal Infant Pain Scale (NIPS). Neonatal Netw 2002;21:15–21. [DOI] [PubMed] [Google Scholar]
- [41].Hummel P, Puchalski M, Creech SD, Weiss MG, Hummel P, Puchalski M, Creech SD, Weiss MG. Clinical reliability and validity of the N-PASS: neonatal pain, agitation and sedation scale with prolonged pain. J Perinatol 2008;28:55–60. [DOI] [PubMed] [Google Scholar]
- [42].Hummel P, Lawlor-Klean P, Weiss MG. Validity and reliability of the N-PASS assessment tool with acute pain. J Perinatol 2010;30:474–8. [DOI] [PubMed] [Google Scholar]
- [43].Izard CE, Hembree EA, Huebner RR. Infants' emotion expressions to acute pain: developmental change and stability of individual differences. Dev Psychol 1987;21:105–13. [Google Scholar]
- [44].Izard CE, editor. Maximally discriminative facial movement coding system. University of Delaware, DE: Instructional Resources Center, 1983. [Google Scholar]
- [45].Joyce BA, Schade JG, Keck JF, Gerkensmeyer J, Raftery T, Moser S, Huster G. Reliability and validity of preverbal pain assessment tools. Issues Compr Pediatr Nurs 1994;17:121–35. [DOI] [PubMed] [Google Scholar]
- [46].Krechel SW, Bildner J. CRIES: a new neonatal postoperative pain measurement score. Initial testing of validity and reliability. Pediatr Anaesth 1995;5:53–61. [DOI] [PubMed] [Google Scholar]
- [47].Lawrence J, Alcock D, McGrath P, Kay J, MacMurray SB, Dulberg C. The development of a tool to assess neonatal pain. Neonatal Netw 1993;12:59–66. [PubMed] [Google Scholar]
- [48].Lilley CM, Craig KD, Grunau RE. The expression of pain in infants and toddlers: developmental changes in facial action. PAIN 1997;72:161–70. [DOI] [PubMed] [Google Scholar]
- [49].Lundqvist P, Kleberg A, Edberg AK, Larsson BA, Hellstrom-Westas L, Norman E. Development and psychometric properties of the Swedish ALPS-Neo pain and stress assessment scale for newborn infants. Acta Paediatr 2014;103:833–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Manworren RC, Hynan LS. Clinical validation of FLACC: preverbal patient pain scale. Pediatr Nurs 2003;29:140–6. [PubMed] [Google Scholar]
- [51].Maxwell LG, Fraga MV, Malavolta CP. Assessment of pain in the newborn: an update. Clin Perinatol 2019;46:693–707. [DOI] [PubMed] [Google Scholar]
- [52].McGrath PJ, Johnson G, Goodman J. CHEOPS: a behavioral scale for rating postoperative pain in children. In: Fields H, Dubner R, Cervero F, editors. Advances in pain research and therapy. Vol. 9 New York, NY: Raven Press, 1985. p. 395–402. [Google Scholar]
- [53].McPherson C, Haslam M, Pineda R, Rogers C, Neil JJ, Inder TE. Brain injury and development in preterm infants exposed to fentanyl. Ann Pharmacother 2015;49:1291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs 1997;23:293–7. [PubMed] [Google Scholar]
- [55].Ranger M, Chau CMY, Garg A, Woodward TS, Beg MF, Bjornson B, Poskitt K, Fitzpatrick K, Synnes AR, Miller SP, Grunau RE. Neonatal pain-related stress predicts cortical thickness at age 7 Years in children born very preterm. PLoS One 2013;8:e76702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Roofthooft DWE, Simons SHP, Anand KJS, Tibboel D, van Dijk M. Eight years later, are we still hurting newborn infants? Neonatology 2014;105:218–26. [DOI] [PubMed] [Google Scholar]
- [57].Sanders RD, Hassell J, Davidson AJ, Robertson NJ, Ma D. Impact of anaesthetics and surgery on neurodevelopment: an update. Br J Anaesth 2013;110(suppl 1):i53-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Schade JG, Joyce BA, Gerkensmeyer J, Keck JF. Comparison of three preverbal scales for postoperative pain assessment in a diverse pediatric sample. J Pain Symptom Manage 1996;12:348–59. [DOI] [PubMed] [Google Scholar]
- [59].Spasojevic S, Bregun-Doronjski A. A simultaneous comparison of four neonatal pain scales in clinical settings. J Matern Fetal Neonatal Med 2011;24:590–4. [DOI] [PubMed] [Google Scholar]
- [60].Spence K, Gillies D, Harrison D, Johnston L, Nagy S. A reliable pain assessment tool for clinical assessment in the neonatal intensive care unit. J Obstet Gynecol Neonatal Nurs 2005;34:80–6. [DOI] [PubMed] [Google Scholar]
- [61].Stevens B, Johnston C, Petryshen P, Taddio A. Premature Infant Pain Profile: development and initial validation. Clin J Pain 1996;12:13–22. [DOI] [PubMed] [Google Scholar]
- [62].Stevens B, Johnston C, Taddio A, Gibbins S, Yamada J. The Premature Infant Pain Profile: evaluation 13 years after development. Clin J Pain 2010;26:813–30. [DOI] [PubMed] [Google Scholar]
- [63].Stevens BJ, Gibbins S, Yamada J, Dionne K, Lee G, Johnston C, Taddio A. The Premature Infant Pain Profile-Revised (PIPP-R): initial validation and feasibility. Clin J Pain 2014;30:238–43. [DOI] [PubMed] [Google Scholar]
- [64].Taddio A, Nulman I, Koren BS, Stevens B, Koren G. A revised measure of acute pain in infants. J Pain Symptom Manage 1995;10:456–63. [DOI] [PubMed] [Google Scholar]
- [65].Tyler DC, Tu A, Douthit J, Chapman CR. Toward validation of pain measurement tools for children: a pilot study. PAIN 1993;52:301–9. [DOI] [PubMed] [Google Scholar]
- [66].Uyan ZS, Bilgen H, Topuzoglu A, Akman I, Ozek E. Comparison of three neonatal pain scales during minor painful procedures. J Matern Fetal Neonatal Med 2008;21:305–8. [DOI] [PubMed] [Google Scholar]
- [67].Valeri BO, Holsti L, Linhares MB. Neonatal pain and developmental outcomes in children born preterm: a systematic review. Clin J Pain 2015;31:355–62. [DOI] [PubMed] [Google Scholar]
- [68].van Dijk M, de Boer JB, Koot HM, Tibboel D, Passchier J, Duivenvoorden HJ. The reliability and validity of the COMFORT scale as a postoperative pain instrument in 0 to 3-year-old infants. PAIN 2000;84:367–77. [DOI] [PubMed] [Google Scholar]
- [69].van Dijk M, Peters JW, van Deventer P, Tibboel D. The COMFORT Behavior Scale: a tool for assessing pain and sedation in infants. Am J Nurs 2005;105:33–6. [DOI] [PubMed] [Google Scholar]
- [70].van Dijk M, Roofthooft DW, Anand KJ, Guldemond F, de Graaf J, Simons S, de Jager Y, van Goudoever JB, Tibboel D. Taking up the challenge of measuring prolonged pain in (premature) neonates: the COMFORTneo scale seems promising. Clin J Pain 2009;25:607–16. [DOI] [PubMed] [Google Scholar]
- [71].Vinall J, Miller SP, Bjornson BH, Fitzpatrick KP, Poskitt KJ, Brant R, Synnes AR, Cepeda IL, Grunau RE. Invasive procedures in preterm children: brain and cognitive development at school age. Pediatrics 2014;133:412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Walker SM. Neonatal pain. Paediatr Anaesth 2014;24:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Walter-Nicolet E, Annequin D, Biran V, Mitanchez D, Tourniaire B. Pain management in newborns: from prevention to treatment. Paediatr Drugs 2010;12:353–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B154.


