Supplemental Digital Content is Available in the Text.
Chronic pain fibromyalgia patients and healthy controls exhibit comparable behavioral and neural opioid-independent placebo analgesic effects that alter bottom-up nociceptive pathways.
Keywords: Placebo, Chronic pain, Fibromyalgia, fMRI, Opioid, Naloxone, Conditioning, Expectation
Abstract
Placebo analgesia is hypothesized to involve top-down engagement of prefrontal regions that access endogenous pain inhibiting opioid pathways. Fibromyalgia (FM) patients have neuroanatomical and neurochemical alterations in pathways relevant to placebo analgesia. Thus, it remains unclear whether placebo analgesic mechanisms would differ in FM patients compared to healthy controls (HCs). Here, using placebo-analgesia-inducing paradigms that included verbal suggestions and conditioning manipulations, we examined whether behavioral and neural placebo analgesic responses differed between 32 FM patients and 46 age- and sex-matched HCs. Participants underwent a manipulation scan, where noxious high and low heat were paired with the control and placebo cream, respectively, and a placebo experimental scan with equal noxious heat temperatures. Before the experimental scan, each participant received saline or naloxone, an opioid receptor antagonist. Across all participants, the placebo condition decreased pain intensity and unpleasantness ratings, decreased activity within the right insula and bilateral secondary somatosensory cortex, and modulated the neurologic pain signature. There were no differences between HCs and FM patients in pain intensity ratings or neural responses during the placebo condition. Despite the perceptual and neural effects of the placebo manipulation, prefrontal circuitry was not activated during the expectation period and the placebo analgesia was unaltered by naloxone, suggesting placebo effects were driven more by conditioning than expectation. Together, these findings suggest that placebo analgesia can occur in both HCs and chronic pain FM patients, without the involvement of opioidergic prefrontal modulatory networks.
1. Introduction
Multiple lines of evidence suggest that placebo analgesia has a neurobiological basis involving the engagement of endogenous pain inhibitory systems that likely dampen afferent nociceptive input.1,16,17,46 Experimental studies of placebo analgesia report activations during placebo-induced expectation of pain relief throughout regions involved in descending modulatory control of afferent nociceptive input, including the dorsal lateral prefrontal cortex (DLPFC) and ventral medial prefrontal cortex (VMPFC).19,43 Consistent with this idea, transient inhibition of the DLPFC using repetitive transcranial magnetic stimulation has been shown to block placebo analgesia,26 and the degeneration of the prefrontal lobes that occurs in Alzheimer disease abolishes placebo analgesic effects.7 Other studies show the endogenous release of opioids during placebo analgesia,46,49 suggesting a possible neurochemical mechanism. The role of endogenous opioids in placebo analgesia created through enhancing expectations has been directly confirmed by showing that naloxone, an opioid receptor antagonist, can block expectation-related placebo effects.1,16,29 By contrast, it seems that placebo analgesia created primarily through conditioning does not involve the opioid system as long as the unconditioned stimulus is not an opioid.5
Despite this evidence, the prevailing idea that placebo analgesia involves activation of endogenous descending-control systems and subsequent dampening of afferent nociceptive pathways has recently been challenged by a meta-analysis that revealed inconsistent effects within the neurologic pain signature (NPS) network. The authors propose that placebo treatments affect pain through brain mechanisms largely independent of effects on bottom-up nociceptive processing.50 Nevertheless, given the small sample size of many studies in the meta-analysis, differences in placebo induction methods and pain-evoking stimuli, and the large network comprising the NPS, the effects of placebo analgesia on bottom-up nociceptive processing are still unclear.
Another unresolved question involves possible differences in placebo analgesia mechanisms between chronic pain patients and healthy individuals. A recent meta-analysis concluded that pain patients have greater benefit from placebo treatment than healthy individuals and that placebo studies on only healthy individuals may underestimate the magnitude of the placebo analgesic effect.18 This finding is intriguing because evidence shows that chronic pain patients have anatomical, functional, and neurochemical alterations in brain regions involved in placebo analgesia.3,10,21,22,27,31,36,47 Furthermore, expectation levels may be altered in patients because of their experience with pain and effectiveness of medications, which could either increase or decrease placebo effectiveness.13 To date, very few studies have directly compared placebo effects in chronic pain patients to healthy people using the same experimental paradigm, and only half of the studies examined neural effects.12,24,28,30
Thus, the current study investigates perceptual effects and neural mechanisms of placebo analgesia in chronic pain patients diagnosed with fibromyalgia (FM) and matched healthy controls (HCs). We asked the following questions: Q1) Does the magnitude of perceptual placebo analgesia differ between HCs and FM patients? Q2) Does placebo analgesia involve different brain regions for HCs and FM patients? Q3) Does opioid-receptor blockade differentially affect (conditioned) placebo analgesia in HCs and FM patients?
2. Methods
2.1. Participants
Based on the sample size calculation described below for the main effect of cream on perceptual placebo effects (control cream vs placebo cream), we enrolled a minimum of 40 participants per group. A total of 96 participants were enrolled in the study. Eighteen participants were not included due to attrition, technical problems resulting in incomplete experimental sessions, or because they did not meet the inclusion criteria listed below during a second screening (eg, pain intensity rating on the day of fMRI scanning of >4 out of 10 for the patients). Thus, the final study population included 32 chronic pain patients diagnosed with FM (30 females and 2 males, mean age ± SD: 43 ± 12.3 years, range 24-62 years) and 46 age- and sex-matched HCs (39 females and 7 males, 40 ± 13 years, range 19-64; P = 0.34). Groups were further matched on race, level of education, and level of physical activity (International Physical Activity Questionnaire, IPAQ8 [Table 1]).
Table 1.
Demographic and clinical data for fibromyalgia (FM) patients and controls.
| FM patients | Controls | P | |
|---|---|---|---|
| Sex (F/M) | 30/2 | 39/7 | 0.223* |
| Age (y) | 42.53 (12.30) | 39.73 (13.01) | 0.298† |
| Symptom duration (y) | 11.91 (7.48) | ||
| Average daily pain | 6.63 (2.52) | ||
| FIQ | 41.97 (19.32) | ||
| Depressive symptoms (HADS) | 4.79 (2.98) | 1.98 (2.05) | <0.001 |
| Anxiety symptoms (HADS) | 8.10 (4.24) | 4.68 (3.07) | <0.001 |
| Medications | |||
| NSAID | 9 | 2 | |
| Antidepressants | 8 | 2 | |
| Muscle relaxants | 4 | 0 | |
| Triptans | 9 | 0 | |
| Cannabinoids | 0 | 0 | |
| Antianxiety | 4 | 0 | |
| Narcotics | 2 | 0 | |
| Amphetamines | 2 | 0 | |
| Race | 0.368* | ||
| White | 20 | 20 | |
| Black or African American | 9 | 19 | |
| Asian | 2 | 3 | |
| Hispanic | 1 | 4 | |
| Education level | 0.749* | ||
| Some high school | 0 | 0 | |
| High school graduate | 3 | 2 | |
| Some college, no degree | 5 | 6 | |
| Associates degree | 0 | 2 | |
| Bachelor's degree | 8 | 10 | |
| Master's degree | 4 | 8 | |
| Professional degree | 2 | 0 | |
| Doctoral degree | 2 | 2 | |
| Other | 0 | 0 | |
| Physical activity (IPAQ) | 4315.6 (4703.18) | 5364.76 (5106.8) | 0.478† |
Results are presented as mean (SD) for 32 FM patients and 46 HC. Categories with missing cases are listed below with corresponding sample values. Groups were compared using a 2-tailed t test unless otherwise noted. IPAQ score represents MET-min/week.
Total participants: Average daily pain and FIQ, FM = 26. HADS, FM = 29, HC = 44. Education, FM = 25, HC = 31.
Groups were compared using the χ2 test.
Groups were compared using the Mann–Whitney U test.
F, female; M, male; FIQ, Fibromyalgia Impact Questionnaire; HADS, Hospital Anxiety and Depression Scale; NSAID, nonsteroidal anti-inflammatory drug; IPAQ, International Physical Activity Questionnaires.
The inclusion criteria for patients included a diagnosis of FM (excluding other pain disorders) confirmed by medical records or directly by the treating physician, and chronic widespread pain for at least 1 year with an average daily pain intensity of at least 4 out of 10. Exclusion criteria for all participants included smoking of >10 cigarettes/week, alcohol consumption of >7 drinks/week for women and >14 drinks/week for men, use of recreational drugs, use of opioid medication, pregnancy or breastfeeding, allergies to skin creams and lotions, chronic pain conditions (other than FM for patients), major medical, neurological, or current psychiatric conditions, including severe depression and generalized anxiety disorder, and MRI contraindications. Additional exclusion criteria for HCs included taking any pain medication other than NSAIDs within the past month or for more than one month on a continual basis within the past 6 months. Patients remained on their regular pain medications (Table 1).
The study received approval from the National Institutes of Health (NIH) Institutional Review Board (IRB), and written informed consent was obtained from all participants according to the Declaration of Helsinki. As per IRB guidelines, the consent form included a general statement about deception: “At some point during the study, we will give you misleading information. After the study is finished and all participants have been tested, we will explain how the information was not true and why.” No further details regarding deception were provided, and participants were not informed that the purpose of the study was to investigate placebo analgesia. Participants were compensated for completion of the study.
2.1.1. Sample size calculation
Our primary outcome measure was the perceptual effect of placebo analgesia as measured by pain intensity ratings. Thus, an a priori sample size calculation was conducted, based on the results conducted by our group on healthy participants.35 To detect a 10-point difference with an SD of 22 on the 100-point visual analogue scale (VAS) between the placebo cream and hydrating cream, fixing the statistical power to 80% and the Type I error probability to α = 0.05, we determined that 40 participants would be needed to detect a placebo effect. The following formula was used for the sample size estimation for within-subject comparisons: n = 2 + C (s/d),2 where C is a constant of 7.85 when α = 0.05 and β = 0.80, s represents the SD of the individual measurements, and d represents the expected mean difference to be detected.
2.2. Study design
This study was patterned after a well-established placebo analgesia paradigm including both expectation and conditioning components in a between- and within-participants design,13,16,45 but participants were given additional instructions to create more neutral expectations. Participants were told that the study was designed to investigate the effect of an opiate antagonist, naloxone, on the pain-reducing effects of a powerful topical local analgesic cream (“NIH-compound,” in reality, the placebo cream), as well as on brain responses to painful and nonpainful cutaneous thermal stimuli in chronic pain patients and HC participants. They were told (truthfully) that the study was double blind and that they would have a 50% chance of receiving naloxone. They were further told that naloxone may or may not block the “analgesic” effect of the cream. These instructions are in line with instructions received in clinical trials of drug efficacy but contrast those of most other experimental placebo studies in which participants are told that they are receiving a “powerful analgesic,” without any additional stipulations, for example, the possibility that the naloxone might reverse the “analgesic” effects of the cream, as in this study.13,16,45 In addition, to minimize expectations that patients may have had about the applicability of the analgesic cream to their clinical pain states, we indicated that the analgesic cream was for applications to small skin areas and thus not suitable for the more widespread pain of deeper structures characteristic of FM.
The experimental design and paradigm are described below and detailed in Figure 1. The experiment took place across 2 separate days and included the following: day 1—medical examination and questionnaires; in the mock scanner, pain calibration and placebo manipulation 1; day 2—in an MRI scanner, a conditioning scan (placebo manipulation 2), drug administration, a high resolution anatomical scan, and 2 placebo test scans. Thermal stimuli (4-8.5 seconds) were applied to 4 different 4 × 4-cm placebo cream or control cream treated areas of the lower left leg (Fig. 1) using a contact thermode (ATS, Medoc Pathway Model, Medoc Ltd Advanced Medical System, Israel) on both days. Participants rated pain intensity and unpleasantness each on a VAS previously validated to be sensitive to subtle psychological manipulations41,42 (anchors: pain intensity, 0 = no sensation, 100 = pain threshold, 200 = intolerable pain; unpleasantness/pleasantness, −100 = extremely unpleasant, 0 = neutral, 100 = extremely pleasant).
Figure 1.
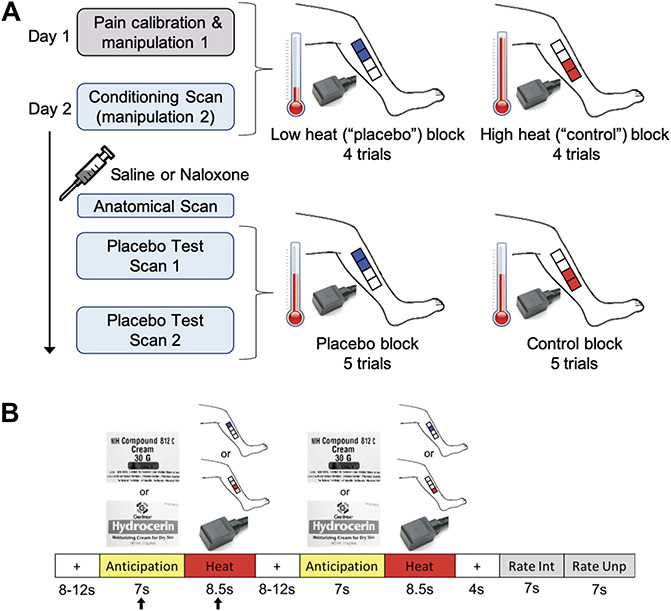
Experimental design and paradigm. (A) Day 1 included a pain calibration procedure to determine which temperatures participants would rate within the low pain range and high pain range on a 0 to 200 VAS pain intensity scale (0 = no sensation, 100 = pain threshold, 200 = intolerable pain). The low and high temperatures, differing by ∼2°C for each individual, were used during manipulation sessions 1 and 2. Manipulation session 1 was conducted in a mock scanner. Participants were presented with the powerful topical local analgesic “NIH-compound” (placebo) cream and the “hydrating” (control) cream (both creams were identical and inert). Each cream was applied to two 4 × 4-cm areas of the lower left leg (distal vs proximal placement was randomized between participants) and was left on for 5 minutes before wiping off. To condition and convince participants of the effectiveness of the “analgesic”, the individualized low and high heat stimuli were applied to the placebo and control cream locations, respectively (4 trials per condition, 2 mock scan runs). One run of the manipulation procedure took place on day 2 in an fMRI scanner followed by a bolus of saline or naloxone, an anatomical scan, and 2 placebo test scans. The heat temperature administered during the placebo test scans was midway between the individualized low and high temperatures that were used during the manipulation session, and most importantly, the same temperature was used for the placebo and control condition. Each condition block had 5 trials per scan. Participants were pseudorandomly assigned to receive either the placebo or control block first. (B) The trial paradigm during the manipulation and placebo test sessions consisted of a jittered interstimulus interval (ISI) of a black crosshair on white background, an anticipation period preceding the heat pulse (cue of either the “NIH-compound” (placebo) cream label (top image) or the “hydrating” (control) cream label (bottom image)), a heat pulse on the left leg during which a thermode image was shown, a second jittered ISI, a second anticipation cue and heat pulse, a poststimulus ISI, and a rating scale for pain intensity and pain unpleasantness. The black arrows indicate the anticipation period and heat pulse reported in the results. VAS, visual analogue scale.
2.3. Day 1—mock scanner
2.3.1. Pain calibration procedure
A sequence of brief heat stimuli (4-7seconds) between 37° and 50°C was presented to four 4 × 4-cm calibration areas on the right leg. After each stimulus, participants rated the pain intensity on the VAS. The data from this phase were used to determine, by interpolation, temperatures that the subject would rate as low-pain (VAS ∼110-130) and high-pain (VAS ∼150-180). These individually calibrated temperature intensities were used during the manipulation phases (top half of Fig. 1A).
2.3.2. Placebo manipulation 1
The placebo manipulation procedure (top half of Fig. 1A) mainly consisted of conditioning through surreptitious temperature manipulation. By contrast, we only induced limited expectations of pain relief through instructions that participants may or may not receive naloxone, which may or may not block the analgesia. The “NIH-compound” (placebo) cream was applied to two of the four 4 × 4-cm marked regions on the lower left leg and the “hydrating” (control) cream, used “to control for moisturizing effects,” was applied to the other 2 marked regions (Fig. 1A). In actuality, the 2 creams were the identical moisturizing cream. The distal vs proximal placement of the creams was randomized between participants. The creams were left on the skin for 5 minutes, with the instruction that this time insured full absorption of the “analgesic” cream. The creams were then carefully removed from the skin, and the conditioning manipulation phase started. During this phase, participants were lying in a supine position in the mock scanner (MRI Simulator, Psychology Software Tools) to allow for habituation to the scanning procedure, with eyes focused on a computer screen while undergoing the heat stimulation paradigm described below and in Figure 1B. Unbeknownst to participants, the temperature presented on the “analgesic” region was lower (VAS ∼110-130) compared to the temperature presented on the “hydrated” region (VAS ∼150-180). This served to reinforce the verbal suggestion that the placebo cream was an effective analgesic cream and to create conditioning independent of suggestions, which is also known to produce placebo analgesia without explicit manipulations of expectations of pain relief.1,5,23 Two runs were conducted in the mock scanner. Each run had 4 trials per condition (trial paradigm detailed below).
2.3.3. Trial paradigm
Each trial (Fig. 1B) consisted of a baseline period (jittered 8-12 seconds; black crosshair on white background), an anticipation period (7 seconds; grayscale picture of control cream or placebo “analgesic” cream), a heat pulse (8.5 seconds; grayscale picture of thermode), a second anticipation period and heat pulse, a poststimulus rest period (4 seconds; black crosshair on white background), and 2 rating periods (7 seconds for intensity, 7 seconds for unpleasantness; black VAS on white background). Each heat pulse was presented on one of 2 pairs of treated 4 × 4-cm regions of the lower left leg.
2.4. Day 2—fMRI scans
2.4.1. Placebo manipulation 2
On day 2, participants underwent a second placebo manipulation procedure (conditioning scan) to further reinforce conditioning. The drug administration, high-resolution anatomical scan, and placebo test phase shortly followed. Just before the conditioning scan, four 4 × 4-cm areas were marked on the left leg (Fig. 1A) and an intravenous line was inserted into the right arm. As on day 1, the 2 creams were applied in the same manner, and temperature presented for the placebo cream condition was lower compared to the temperature presented for the control cream condition. There were 4 trials per condition following the trial paradigm described above (Fig. 1B). Next, participants were given either a bolus injection of saline or naloxone, with approximately half of the participants in each group (HC or FM) randomized to receive naloxone and the other half saline. An anatomical MRI was acquired while the drug reached its peak effect.
2.4.2. Placebo test phase
The first scan of the placebo test phase then began. Each condition had 5 trials (Fig. 1B) but the heat stimuli were now the same temperature for both conditions, that is, a painfully hot temperature midway between the individualized low and high temperatures administered during the manipulation phase, following the design of Eippert et al.16 Half of the participants from each group were pseudorandomly assigned to receive the first block of 5 trials with stimuli presented on the placebo cream site and the other half on the control cream site. A second placebo test scan followed immediately after the first, with the order of cream sites reversed for each participant.
2.4.3. Drug administration
Approximately 10 minutes before the placebo test scan, some participants (23 HCs and 20 FM patients) received an infusion of naloxone, and the others (23 HCs and 12 FM patients) received an infusion of saline. Naloxone or saline was administered by the NIH Clinical Center nursing staff in a double-blinded fashion using block-stratified (age, sex) randomization. Participants were informed about naloxone, including its pharmacological properties, general clinical use, and possible side effects. Participants were also informed that they would most likely not notice that they had received naloxone because it generally has no noticeable effects in the dose used. To achieve a constant plasma level throughout the ∼40-minute testing phase, a bolus dose of naloxone (0.05 mg/kg bodyweight; generic) or saline was first administered through an intravenous line, followed by an intravenous infusion dose of 0.08 mg/kg/h naloxone or saline (diluted in 250 mL of saline), starting immediately after the bolus injection and continuing for ∼40 minutes. The total dose of naloxone could not exceed 10 mg, a dosage used clinically to reverse the effects of opiates in opiate-overdose (Micromedex 2), as required by NIH IRB guidelines. Vital signs (blood pressure, pulse, respiration, and pulse oximetry) were taken once before the intravenous bolus, once 5 minutes thereafter, and once after infusion. Participants were asked to rate naloxone-related adverse effects (dry mouth, dry skin, blurred vision, sedation, nausea, dizziness, and headache) on a scale from 0 = nonexistent to 6 = extremely strong.16 The drug administration procedure was similar to that used to block placebo analgesia in young male healthy volunteers,16 except that the dose of naloxone was lower in this study (this study vs Eippert et al.,16 bolus dose: 0.05 mg/kg vs 0.15 mg/kg; intravenous infusion dose: 0.08 mg/kg/h vs 0.2 mg/kg/h), as required by NIH IRB guidelines. Both naloxone and saline were well tolerated, and the expected side effects were nonexistent to minimal for the 2 drugs across all participants (Supplementary Table 1, available at http://links.lww.com/PAIN/B171). No significant adverse events were observed.
2.4.4. Randomization and allocation
Both the saline and naloxone solutions were prepared by the NIH pharmacy and furnished in individual subject doses on the day of the experiment for each subject. The NIH pharmacy provided subject randomization and maintained the randomization code until completion of data collection.
2.4.5. Effectiveness of cream and desire for pain relief
Numerical rating scales were used to assess the perceived effectiveness of the “NIH analgesic” (anchors: 0 = not effective at all, 10 = the most effective) and the desire for pain relief during the heat stimulation (anchors: 0=no desire for pain relief, 10 = the most intense desire for pain relief imaginable). These scales were administered after manipulation 1, after manipulation 2, and after the placebo test scans.
2.4.6. Current pain and discomfort
To assess possible interference of ongoing pain and discomfort with experimental pain ratings and fMRI findings, the intensity of current pain and discomfort during the fMRI data acquisition was assessed once immediately after the manipulation fMRI scan and once immediately after the 2 runs of the placebo-test fMRI scan. An 11-point numerical intensity rating scale from 0 to 10 was used for both pain (0 = no pain, 1 = pain threshold, 10 = worst bearable pain) and discomfort (0 = no discomfort, 1 = discomfort threshold, 10 = worst bearable discomfort). In addition, type and location of pain and of discomfort were assessed. These scales were administered after placebo manipulation 1, after placebo manipulation 2, and after the placebo test scans.
2.5. fMRI acquisition
All participants completed a 9.5-minute fMRI scan during the placebo conditioning phase, a 4.5-min high-resolution anatomical MRI scan, and two 11.8-minute fMRI scans during the placebo test phase. Throughout the session, participants wore earplugs and their heads were immobilized. Brain images were acquired using a 3 T Siemens Skyra MRI scanner (Siemens, Erlangen, Germany) with a 20-channel head and neck coil. Structural MRI images (T1-weighted) were acquired using an MPRAGE sequence (repetition time = 1900 ms, echo time = 2.07 ms, flip angle = 9°, 1 mm isotropic voxels, field-of-view = 256 × 256, 192 slices). Functional MRI data were acquired using a blood oxygenation level-dependent protocol with a T2*-weighted gradient echo planar imaging sequence (repetition time = 2000 ms, echo time = 29 ms, flip angle 70°, 3.5 mm isotropic voxels, field-of-view = 64 × 64, 38 slices). Axial slices were oriented 30° from the line between the anterior and posterior commissures, covering the entire brain, and excluding the eyes. After discarding the first 3 volumes to allow for steady-state magnetization, 285 volumes and 355 volumes were acquired for the conditioning and placebo test scans, respectively. During the fMRI scans, heart rate, blood oxygenation, and respiration were monitored.
2.6. Behavioral data analysis
Outcome measures were compared using independent-samples two-tailed t-tests or a repeated-measures analysis of variance (rm-ANOVA) with one within-subject factor (“cream”: placebo cream vs control cream) and 2 between-subject factors (“group”: FM vs HC; “drug”: saline vs naloxone) in SPSS 25 (IBM). Mann–Whitney U tests were used for nonnormally distributed data and χ2 tests were used for categorical comparisons. Correlations between behavioral measures, FM characteristics, and gray matter volume (GMV) were investigated using Pearson correlations. A significance level of P < 0.05 was used in all analyses. All results are presented as mean ± SD.
2.7. Voxel-based morphometry: preprocessing and analysis
To evaluate whether the FM patients in the current study had forebrain anatomical changes similar to those reported in other studies,10,27 voxel-based morphometry (VBM) analysis was performed. Because brain GMVs are strongly influenced by age,48 we selected a subset of the HCs who were age- and sex-matched to the patients on an individual basis. Thus, the VBM analysis included 31 FM patients and 31 HCs. Structural data were analyzed using FMRIB Software Library (FSL)37, specifically FSL-VBM.15,20 Nonbrain tissue was removed from the structural images, followed by gray matter segmentation, and nonlinear registration to the Montreal Neurological Institute 152 T1 2 mm standard space template.2 The gray matter images were averaged and flipped along the x-axis to create a left–right symmetric, study-specific gray matter template using 31 FM patients and 31 HCs. All native gray matter images were then nonlinearly registered to this study-specific template and modulated to correct for local expansion (or contraction) due to the nonlinear component of the spatial transformation. The modulated gray matter images were then smoothed with an isotropic Gaussian kernel with a sigma of 4 mm (approx. full width at half max = 8 mm). Finally, a voxelwise general linear model was applied using permutation-based nonparametric testing, correcting for multiple comparisons across space. A voxel threshold of z > 3.1 and cluster threshold of P < 0.05 was used. Age (mean-centered across all participants) was included as a nuisance regressor in the general linear model. A 6-mm sphere was used to extract the GMV values of the DLPFC cluster identified by the results of the contrast HC > FM. Placement of the sphere around the peak voxel (MNI coordinate x = 60, y = 65, z = 58) resulted in overlap with white matter. Therefore, to avoid overlapping the sphere with white matter, the sphere was moved to MNI coordinate x = 62, y = 65, z = 60, towards the center of gravity of the cluster, which included the peak voxel. Based on the a priori hypothesis that FM decreases in GMV would negatively correlate with symptom duration as reported in previous studies,10,27 a one-tailed partial correlation controlling for age was conducted between FM DLPFC GMV and symptom duration. A significance level of P < 0.05 was used.
2.8. fMRI data preprocessing and analysis
All fMRI data were preprocessed and analyzed in FSL (version 5.0.8). Preprocessing included nonbrain tissue removal, spatial smoothing (Gaussian kernel of full width at half max = 5 mm), high-pass temporal filtering, six-parameter (3 translations and 3 rotations) rigid body correction for head motion, coregistration to the T1-weighted anatomical image, and spatial normalization to the MNI152 T1 2 mm template using a 12-parameter linear registration.
Explanatory variables (EVs) were modelled at the individual level using a double-gamma hemodynamic response function. The components within each trial (Fig. 1B) were modelled separately per condition, that is, the first anticipation period, first heat pulse, second anticipation period, and second heat pulse that occurred within a trial were each modelled as separate EVs for each condition (placebo cream or control cream). The pain rating periods (“Intensity,” “Unpleasantness”) were combined into one EV for each condition (placebo cream or control cream). The fixation periods were not modelled. All anticipation periods and heat periods were included in the model. In total, the model included 10 anticipation periods and heat pulses per condition.
Higher-level group analyses were conducted using FSL's FLAME 1 + 2 mixed-effects modelling to assess group, drug, and group X drug interactions (repeated-measures ANOVA) of the following contrasts for each anticipation period and heat stimulus: placebo > baseline, control > baseline, placebo > control, control > placebo. Pain intensity ratings were included in the model as a covariate of interest for the placebo analyses. For all scans, heat pulse 1 of each trial, regardless of condition, produced significantly greater activity than heat pulse 2 despite movement of the thermode from one 4 × 4-cm marked area to the second after each heat pulse. In addition, no placebo effects were observed during the second experimental scan and all pain ratings were significantly lower during the second experimental scan compared to the first (see Supplementary section 3, available at http://links.lww.com/PAIN/B171). Thus, to account for the habituation effect observed during the second pulse of each trial and the during second scan, only the first anticipation period and first heat stimulus of each trial (black arrows in Fig. 1B) for each condition in the first experimental scan are reported in the main text, with the second pulse and second scan reported in the supplementary material (available at http://links.lww.com/PAIN/B171). Specifically, although the model included all 10 anticipation periods and heat pulses, the reported results in the main text are based on heat pulse 1 of each trial, that is, 5 anticipation periods and 5 heat pulses for each condition of the first scan. Voxelwise thresholds were set to z > 3.1 or z > 2.3 to assess subtle effects and reduce false negatives (type II error). All contrasts were cluster-corrected for multiple comparisons across the whole brain at P < 0.05.
2.9. Neurologic pain signature
Because of the meta-analysis50 reporting that placebo analgesia does not affect the NPS, we tested whether the NPS was affected by the placebo cream in our study. To compute the magnitude of the NPS response, the voxelwise pattern of regression weights was multiplied with each of the following contrast of parameter estimates (“COPEs” from the individual-level analyses) to produce dot products, which were then averaged across all voxels resulting in one value for each subject and condition: placebo test scan-control cream > placebo cream, control cream > baseline, placebo cream > baseline. The analyses were completed on MATLAB R2018a (MathWorks) using code provided by the Wager Lab (https://canlabweb.colorado.edu/). Differences between the control cream and placebo cream condition were assessed using two-tailed t-tests for dependent samples. A significance level of P < 0.05 was used.
3. Results
3.1. Patient characteristics
Fibromyalgia patients had mild to moderate FM (FIQ score mean ± SD, 41.97 ± 19.32; range 8-89), and their average symptom duration was 11.91 ± 7.48 years (range 2-30 years) with an average intensity of 6.63 ± 2.52 for daily pain. We found no significant correlations between FM characteristics and placebo effect, that is, the difference between ratings of the control and placebo creams (see Supplementary Table 2, available at http://links.lww.com/PAIN/B171). As expected, the Hospital Anxiety and Depression Scale (HADS) indicated that patients had significantly increased, but subclinical, levels of anxiety and depressive symptoms compared to HCs. A summary of the clinical and demographic information can be found in Table 1.
To further determine if the FM patients in the current study were similar to FM patients in other studies, we used VBM to determine whether FM patients had previously observed gray matter abnormalities.10,27 The whole-brain VBM analysis (corrected for age) performed on 31 FM patients and 31 age- and sex-matched HCs revealed a significant decrease in GMV within the left DLPFC of the FM patient group, as indicated by the contrast of HC > FM patients (Fig. 2A). The GMV within the left DLPFC of FM patients was negatively correlated with symptom duration (r = −0.37, P = 0.02; Fig. 2B), and showed a trend towards a negative correlation after controlling for age (partial correlation, r = −0.29, P = 0.06; Fig. 2C) as symptom duration and age correlated positively (r = 0.58, P = 0.001). The DLPFC GMV (age-corrected) of FM patients did not correlate with any other characteristics (placebo effect [control-placebo]: intensity ratings, r = 0.14, P = 0.46, unpleasantness ratings, r = −0.08, P = 0.66; FIQ, r = 0.05, P = 0.82; daily pain, r = 0.01, P = 0.63). No other brain regions showed decreased or increased GMV in FM patients compared to controls.
Figure 2.
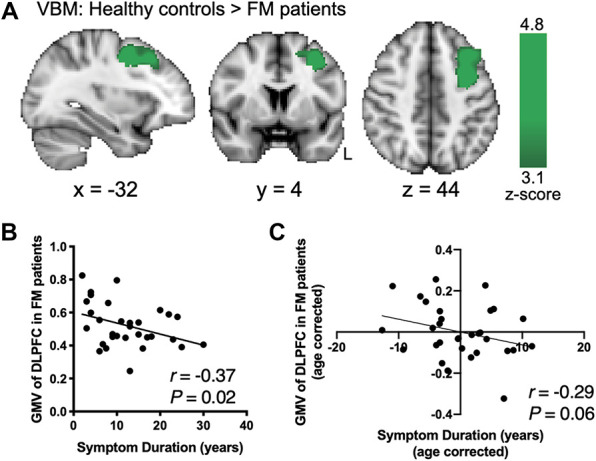
Dorsal lateral prefrontal cortex GMV reduction in FM patients is related to symptom duration. (A) VBM analysis of GMV comparing HCs and FM patients showed significant increases within the left dorsolateral prefrontal cortex (DLPFC) indicating DLPFC GMV reduction in FM patients (age-corrected, cluster forming threshold z > 3.1, cluster correction P < 0.05; 1461 voxels; peak z-score = 4.8, P = 0.002; MNI coordinates −30, 4, 44). (B) Average GMV of the DLPFC in FM patients significantly correlated negatively with the duration of fibromyalgia symptoms. (C) A trend towards a negative correlation between DLPFC GMV and symptom duration remained after correcting for age. FM, fibromyalgia; GMV, gray matter volume; HCs, healthy controls; L, left.
3.2. Q1: Magnitude of placebo analgesia did not differ between fibromyalgia and healthy control groups
During the placebo test scans, participants received the same individualized heat temperatures for the control cream and placebo cream, a temperature midway between the individualized high and low temperatures presented during the conditioning scan. The temperatures did not differ between groups (HC 45.7°C ± 1.8°C, FM 46.1°C ± 1.38°C, P = 0.29).
A significant placebo analgesic effect was observed across all participants as indicated by reduced pain intensity ratings during the placebo cream condition compared to the control cream condition (Fig. 3A; placebo 135.6 ± 28.3, control 141.2 ± 25.1, F(1,76) = 6.03, P = 0.02, = 0.08). There was no main effect of group (HC 140.2 ± 29.1, FM 135.6 ± 23, F(1,76) = 0.6, P = 0.44, = 0.01) and no interactions were observed (cream x group, F(1,76) = 0.2, P = 0.66). More specifically, the difference between pain ratings during placebo and control did not differ between FM patients and HCs (Fig. 3B; control cream—placebo cream, HC 6.7 ± 18.9, FM 4.2 ± 19.9, P = 0.52; Cohen's d = 0.146), indicating that the placebo effect in FM patients is comparable to that of HCs. A post hoc power analysis revealed that with this effect size, 938 HC and 656 FM patients would be required to detect a significant cream x group interaction. Similarly, pain unpleasantness ratings significantly decreased during placebo across all participants (Fig. 3C; control 34.8 ± 22.2, placebo 28.9 ± 26.6, F(1,76) = 4.86, P = 0.03, = 0.07). A main effect of group was observed for pain unpleasantness, with FM patients rating pain across the placebo and control conditions as significantly less unpleasant than HCs (HC 36.6 ± 30.8, FM 25.6 ± 31.7, F(1,76) = 4.3, P = 0.04, = 0.06). However, no interaction between cream and group (F(1,76) = 0.58, P = 0.45, = 0.008) was observed indicating that the difference between pain unpleasantness ratings during placebo and control did not differ between FM patients and HCs (Fig. 3D; control cream—placebo cream, HC -7.9 ± 24, FM 3.4 ± 17.1, P = 0.42, Cohen's d = 0.53).
Figure 3.
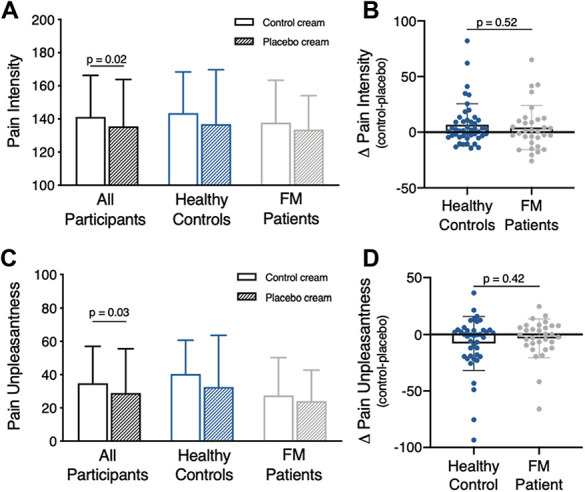
Behavioral placebo analgesic effects. Results are presented as mean ± SD. (A) Pain intensity ratings across all participants significantly decreased in response to the same temperature stimulus during the placebo cream condition compared to the control cream condition. Separate group plots show mean pain intensity ratings for each condition. (B) Comparison of the pain intensity difference score (control cream—placebo cream) between HCs and FM patients shows no group differences in placebo effect. (C) Pain unpleasantness ratings across all participants significantly decreased in response to the same temperature stimulus during the placebo cream condition compared to the control cream condition. Separate group plots show mean pain unpleasantness ratings for each condition. (D) Group comparison of the pain unpleasantness difference score (control cream—placebo cream) shows no difference. FM, fibromyalgia; HCs, healthy controls.
Consistent with ratings during the scan, when asked after the placebo test scan whether the placebo analgesic cream was effective, both groups reported slight to moderate effectiveness (HC 3.6 ± 2.9, FM 4.2 ± 2.3, P = 0.31, Cohen's d = 0.23). The reported desire for pain relief was moderate and did not differ between groups during the placebo test scan (HC 5.3 ± 3.0, FM 5.2 ± 2.5, P = 0.79, Cohen's d = 0.04).
3.3. Q2: Brain regions involved in placebo analgesia did not differ between healthy control and fibromyalgia
3.3.1. Pain-related activation
We examined pain-related activity across all participants during the placebo cream and control cream condition compared to baseline. Pain-related activations in the control cream condition showed typical patterns, including the anterior cingulate cortex , and bilateral insula, S1, and S2 (z > 2.3, P < 0.05; Table 2; Fig. 4A). Similar regions were activated in the placebo cream condition (Fig. 4A). To determine whether placebo-related reductions in brain activity occurred, we examined the contrast “control cream > placebo cream” and found significantly more activation within the right midanterior and posterior insula (contralateral to the stimuli), and bilaterally in S2 during the control cream condition compared to placebo (Fig. 4B). The placebo-related reductions in the insula and S2 were comparable between the HC and FM groups (Fig. 4B inset). No increased activations were observed in pain modulatory regions or other brain regions (placebo cream > control cream; z > 2.3, P < 0.05). Similar to the behavioral results, there were no group differences or significant findings in the F-test for the cream*group interaction in neither the control cream > placebo cream contrast nor the placebo cream > control cream contrast (z > 2.3, P < 0.05; Table 2).
Table 2.
Whole-brain blood oxygenation level-dependent responses during the first heat stimulation periods.
| Region | Voxels | MNI coordinates | Peak z-score | P | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Control > baseline | ||||||
| Insula (R) | 46,636 | 28 | 65 | 39 | 9.01 | <0.001 |
| Nucleus accumbens (R) | 39 | 68 | 34 | 5.40 | ||
| ACC | 49 | 70 | 53 | 7.07 | ||
| Insula (L) | 60 | 72 | 38 | 8.05 | ||
| Occipital ctx (L) | 53 | 21 | 27 | 8.94 | ||
| Occipital ctx (R) | 28 | 17 | 43 | 8.33 | ||
| SI (R) | 37 | 43 | 70 | 6.84 | ||
| SII (L) | 74 | 52 | 44 | 7.77 | ||
| SII (R) | 18 | 51 | 45 | 7.74 | ||
| Thalamus (R) | 40 | 59 | 38 | 6.70 | ||
| Placebo > baseline | ||||||
| Planum polare | 43,617 | 18 | 65 | 35 | 9.38 | <0.001 |
| Nucleus accumbens (R) | 40 | 67 | 33 | 6.54 | ||
| ACC | 49 | 73 | 51 | 7.16 | ||
| Insula (R) | 27 | 74 | 34 | 8.65 | ||
| Insula (L) | 64 | 54 | 39 | 7.15 | ||
| Occipital ctx (L) | 52 | 20 | 29 | 8.38 | ||
| Occipital ctx (R) | 31 | 16 | 43 | 7.68 | ||
| SI (R) | 39 | 44 | 70 | 6.15 | ||
| SII (L) | 76 | 51 | 44 | 6.50 | ||
| SII (R) | 15 | 49 | 47 | 6.81 | ||
| Control > placebo | ||||||
| Occipital ctx (L) | 1710 | 53 | 12 | 37 | 4.69 | <0.001 |
| Occipital ctx (R) | 1624 | 37 | 14 | 39 | 5.89 | <0.001 |
| Posterior insula (R) | 1474 | 26 | 55 | 42 | 4.59 | <0.001 |
| Midanterior insula (R) | 27 | 70 | 38 | 3.74 | ||
| SII (R) | 20 | 49 | 45 | 4.17 | ||
| SII (L) | 1197 | 79 | 50 | 44 | 4.34 | <0.001 |
| Placebo > control | ||||||
| — | — | — | — | — | — | — |
| Group × cream (F-test) | ||||||
| — | — | — | — | — | — | — |
| Group × cream × drug (F-test) | ||||||
| — | — | — | — | — | — | — |
(L), left; (R), right; ACC, anterior cingulate cortex; ctx, cortex; SI, primary somatosensory cortex; SII, secondary somatosensory cortex.
Figure 4.
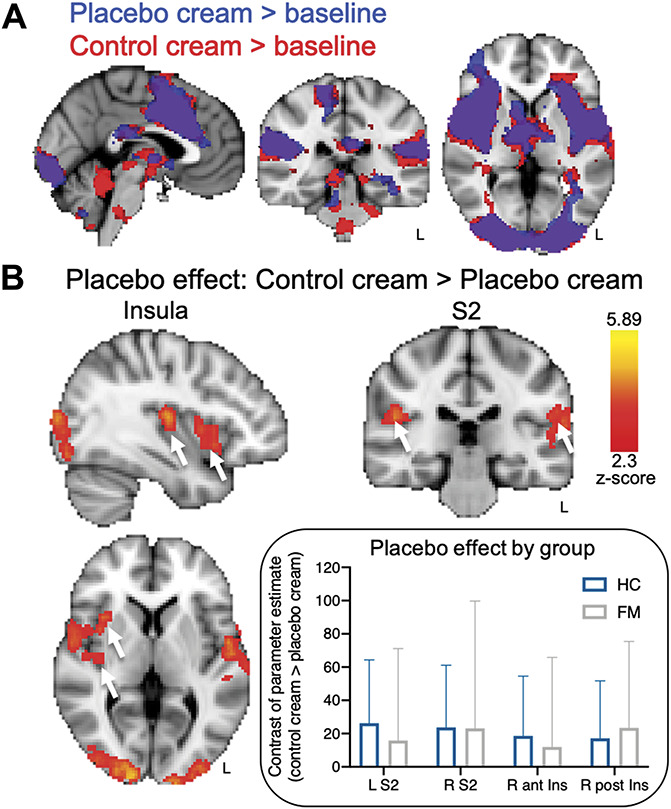
Neural placebo analgesic effects. (A) Pain-related activations in response to the same temperature stimulus during the control cream condition (red) and placebo cream condition (blue) compared to baseline (overlapping regions displayed in purple). (B) Significant placebo-related reductions (“placebo effect”, control > placebo) in brain activity were observed across all participants within the right midanterior and posterior insula and bilateral S2. Inset: The HC and FM patient contrast of parameter estimates for the placebo-modulated S2 and insula regions. All results are presented at a voxel-based threshold of z > 2.3, cluster correction of P < 0.05. ant, anterior; FM, fibromyalgia; HC, healthy controls; Ins, insula; L, left; post, posterior; R, right; S2, secondary somatosensory cortex.
3.3.2. Neurologic pain signature analysis
We then assessed whether the placebo condition modulated the NPS during the placebo test scan. We found a significantly smaller NPS response in the placebo cream condition compared to the control cream condition across all participants (control 1381.25 ± 945.08, placebo 997.83 ± 869.61, P < 0.001, Cohen's d = 0.42). This finding corroborates the placebo analgesic effects on brain activation described in the previous paragraph. No group effect was observed (control cream > placebo cream, HC 531.4 ± 745.5, FM 170.8 ± 1139.2, P = 0.1, Cohen's d = 0.4).
3.3.3. Anticipation-related activation
We examined activations during the anticipation periods (when subjects were looking at images of the cream containers) to uncover possible engagement of endogenous pain inhibiting brain networks during anticipation of pain relief. We found significant activation across all participants only in the occipital cortex during the anticipation period of the placebo cream condition compared to baseline (z > 2.3, P < 0.05; Fig. 5A) and compared to the anticipation of the control cream condition (z > 2.3, P < 0.05; Fig. 5B). No activation of pain modulatory regions previously described in the literature (eg, DLPFC, VMPFC, anterior cingulate cortex, and periaqueductal gray) was observed. During anticipation of the control cream condition compared to baseline (Fig. 5A), but not compared to anticipation of placebo, we found significant activation within the right hippocampus and right temporal cortex (z > 2.3, P < 0.05; Fig. 5A). No significant findings were observed in the F-test for the group*cream interaction for the anticipation of placebo cream > anticipation of control cream contrast or vice versa (z > 2.3, P < 0.05; Table 3).
Figure 5.
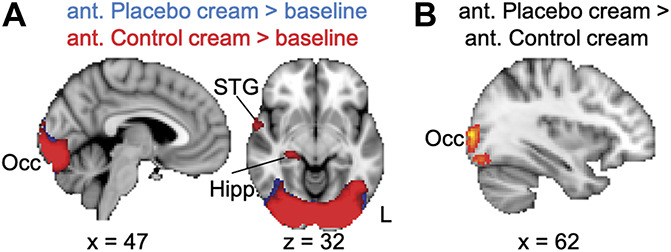
Anticipation-related activations. (A) Compared to baseline, both anticipation conditions produced activation in the occipital cortex, and the STG and hippocampus were activated only during anticipation of the control cream condition. (B) Greater activation of the occipital cortex was observed during anticipation of the placebo cream compared to anticipation of the control cream. No differences were observed in the inverse contrast. All results are presented at a voxel-based threshold of z > 2.3, cluster correction of P < 0.05. ant, anticipation; Hipp., hippocampus; L, left; STG; superior temporal gyrus.
Table 3.
Whole-brain blood oxygenation level-dependent responses during the first anticipation periods.
| Region | Voxels | MNI coordinates | Peak z-score | P | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Control > baseline | ||||||
| Occipital ctx (R) | 15,608 | 39 | 14 | 38 | 9.73 | <0.001 |
| Hippocampus (R) | 32 | 50 | 32 | 4.25 | ||
| STG (R) | 15 | 63 | 36 | 4.13 | 0.038 | |
| Placebo > baseline | ||||||
| Occipital ctx (L) | 16,235 | 55 | 14 | 38 | 12.24 | <0.001 |
| Occipital fusiform ctx (R) | 34 | 17 | 32 | 9.85 | ||
| Control > placebo | ||||||
| — | — | — | — | — | — | — |
| Placebo > control | ||||||
| Occipital ctx (L) | 1229 | 62 | 17 | 39 | 4.17 | <0.001 |
| Occipital ctx (R) | 1056 | 32 | 17 | 38 | 4.14 | <0.001 |
| Group × cream (F-test) | ||||||
| — | — | — | — | — | — | — |
| Group × cream × drug (F-test) | ||||||
| — | — | — | — | — | — | — |
(L), left; (R), right; ctx, cortex; STG, superior temporal gyrus.
3.4. Q3: Naloxone did not alter placebo analgesic responses
Naloxone did not alter the perceptual placebo effect compared to saline. There was no significant interaction for pain intensity ratings between the within-subject factor “cream” and the between-subject factor “drug” (cream × drug, F(1,76) = 0.08, P = 0.78, = 0.001). In addition, there was no interaction with group (group × drug, F(1,76) = 0.56, P = 0.46, = 0.01; cream*drug*group, F(1,76) = 0.26, P = 0.62, = 0.003). A post hoc power analysis revealed that with these effect sizes, approximately 2.5 million participants would be required to reveal a significant cream × drug × group interaction. Similar results were found for pain unpleasantness (cream × drug, F(1,76) = 0.002, P = 0.96, = 0.000; group × drug, F(1,76) = 0.67, P = 0.67, = 0.003; cream × drug × group, F(1,76) = 0.41, P = 0.52, = 0.01).
In addition, we found that naloxone compared to saline did not alter the neural placebo effect because no significant findings were observed in the F-test for the cream × drug or cream × drug × group interactions in the control cream > placebo cream contrast or vice versa (z > 2.3, P < 0.05; Table 2). Similarly, no drug effects were found on the anticipation of the placebo cream compared to the anticipation of the control cream for both interaction terms (z > 2.3, P < 0.05; Table 3).
3.4.1. Neurologic pain signature response
No significant NPS modulation by saline or naloxone was observed during placebo analgesia (control cream > placebo cream, Sal 402.43 ± 1030.36, Nal 367.96 ± 867.02, P = 0.87, Cohen's d = 0.04).
4. Discussion
This study investigated whether mechanisms of placebo analgesia in chronic pain patients with FM differed from HCs. Across all participants, we found reduced pain perception and pain-evoked functional activity within the right insula and bilateral secondary somatosensory cortex during placebo analgesia. Placebo modulation of nociceptive processing regions was further confirmed by a significant reduction in the NPS response. There were no differences between FM patients and HC participants in either pain intensity ratings or neural placebo-related effects. Across all conditions, FM patients reported the heat stimuli as less unpleasant than HCs, perhaps as a result of comparing the experimental pain to previous clinical pain episodes, as reported by over half of FM patients during a postexperimental interview session.38 Finally, there were no effects of naloxone administration nor any interactions between group and drug on anticipation-, placebo-, and NPS-related activations.
4.1. Placebo effects on experimental pain in healthy and chronic pain populations
There was no difference in placebo analgesic responses in FM patients and HCs, which is consistent with the few studies comparing placebo effects to experimental stimuli in pain patients and healthy participants. The most recent and largest behavioral study of this kind reported no differences in placebo effects between 363 chronic pain patients with temporomandibular disorder and 400 HCs.12 Comparable placebo effects have also been reported between healthy participants and patients with episodic migraine,30 irritable bowel syndrome,28 and atopic dermatitis.24 Other studies have reported placebo effects to experimental stimuli in chronic pain patients without direct comparison to healthy participants,14,33,40 and a meta-analysis concluded that pain patients have a greater benefit from placebo treatment than healthy individuals.18 These studies suggest that the presence of chronic pain might not alter placebo effects, despite the anatomical, neurochemical, and functional changes in the brain in chronic pain patients.3,10,11,21,22,27,31,36,47 Nevertheless, evidence suggests that factors such as disease chronicity in patients with moderate to severe FM inversely correlates with placebo effects.25 Although we found no relationship between placebo effects and FM characteristics, future studies should compare cohorts with greater disease burden or pain severity to determine the extent to which these factors interact with placebo analgesia.
4.2. Opioid vs nonopioid placebo analgesia
Several reports suggest that placebo analgesic responses are a result of accessing endogenous pain inhibitory opioid pathways.46,49 Here, the placebo effect was not blocked by the opioid antagonist, naloxone, suggesting that placebo analgesic effects in our study were opioid-independent. Although the dosage used in our study was lower than the dosage administered in 2 other studies with similar manipulations, that is, verbal-suggestion and conditioning,1,16 the dosage of approximately 10 mg/kg is similar to that used in the first demonstration of naloxone reversal of placebo analgesia29 and is a dose used clinically to reverse opioid-related overdoses. Furthermore, we also concurrently examined affective touch perception in a subset (n = 52) of the participants and found that touch perception was altered by our naloxone dose,9 thus confirming that our dosage was physiologically effective. Some findings suggest that longer durations of experimental pain stimuli may allow for better engagement of opioid circuitry.6,16,39 However, compared to Eippert et al.,16 we observed no naloxone effect despite having not only similar stimulus durations, but also a larger sample of healthy participants that received saline or naloxone. Another possibility that might account for the differences in results is the age and sex of the subjects. Although our population was mainly middle-aged females, the Eippert population was exclusively young males.
An explanation for our lack of naloxone effects that we find the most compelling involves placebo induction through expectation vs conditioning. Naloxone has been shown to block placebo effects when placebo analgesia is created through suggestion-related expectations of pain relief but not when created through conditioning involving repeated experiences of pain relief under the same environmental contingencies.1,40 Unlike studies that just have explicit verbal suggestions telling participants that they are receiving a powerful pain-relieving analgesic,13,16,45 we added the additional instruction that naloxone might reverse the analgesic effects of the cream. Thus, the strong customary suggestion of impending pain relief may have been neutralized by the additional instruction. Nevertheless, our conditioning procedure involved the surreptitious reduction of the pain stimulus when paired with the placebo treatment. Many placebo analgesia studies incorporate this conditioning procedure along with high expectation-inducing verbal instructions.13,16,45 However, our 2 days of conditioning, one of which occurred immediately before the experimental session, enhanced the conditioning component and simultaneously decreased the expectation component through our neutral verbal expectation instructions. Although the inherent decrease in expectation could have diminished expectation-related components of the placebo effect,32 it is plausible that the repeated, multiday conditioning-related placebo effects activated expectation-independent pathways. Thus, our observed opioid-independent placebo effects are likely influenced by learning-related conditioning that may not necessarily engage pain-relieving opioid pathways and, therefore, may neurobiologically differ from expectation-related responses.
Consistent with the idea that the current analgesic effects are not opioid-mediated is our finding that expectation-related circuitry was not significantly engaged in this study. A meta-analysis of 25 neuroimaging studies of placebo analgesia and expectancy-based pain modulation identified regions consistently activated during expectation of pain relief, including the prefrontal cortex (DLPFC, VMPFC, and orbitofrontal cortex), the midbrain surrounding the periaqueductal gray, and the rostral ACC.4 Here, none of these regions were activated during the anticipation period preceding the stimulus on the “analgesic” skin.
4.3. Does placebo analgesia involve bottom-up pain pathways?
A recent meta-analysis concluded that the mechanisms underlying placebo analgesia involve processes associated with the affective component of the pain experience, cognitive evaluation, pain-associated decision making, and mesolimbic reward processing, rather than engaging descending modulation onto afferent pain pathways.50 This conclusion was based on the minimal placebo effects on the NPS within the 20 studies included in the meta-analysis and across all the studies using only placebo responders. This finding contradicts those reported by Eippert et al. in 200917 where placebo analgesia decreased blood oxygenation level-dependent signals in the dorsal horn of the spinal cord, suggesting that placebo analgesia can modulate early nociceptive processes. Here, using the same multivariate brain activation pattern used in the meta-analysis,50 which includes brain regions involved in early nociceptive processing and is sensitive to intensities of evoked pain, we found that the NPS response44 was greater during the control cream condition than the placebo cream condition, despite using the same temperature heat stimulus for both conditions and despite pain intensity ratings that differed by only ∼5% between conditions. This suggests that placebo analgesia does, in part, alter bottom-up nociception, in contrast to the conclusion that “placebo treatments affect pain through brain mechanisms largely independent of effects on bottom-up nociceptive processing.”50 Of the 20 studies included in the meta-analysis, 65% had a placebo-induction paradigm similar to ours, that is, a combination of conditioning- and expectation-based induction, and 50% used a similar pain stimulus (heat pain), but our sample size was larger than 95% of the studies. Thus, it is possible that variations in study design (eg, experimental pain type and duration, and placebo manipulations) and sample size could account for differences in NPS responses.
Additional caveats require discussion. First, due to protocol restrictions on subject replacements, we did not fully reach the objective of having 40 usable datasets in the FM group. Thus, given the subgroup sample sizes and limited number of heat pulses, it is possible that some analyses of interest may be underpowered which limits the generalizability of the results. Nevertheless, compared to similar studies with comparable or smaller sample sizes,16,24,28,30,40 no interactions showed any indication of a trend towards significance. Indeed, post hoc power analyses indicated that we would need ∼1600 participants to detect a group difference in placebo effect and ∼2.5 million participants to detect a significant cream × group × drug interaction. Second, common verbal instructions used to induce high expectations of pain relief that engage prefrontal networks (eg, DLPFC) and opioid pathways19,34,43,49 were likely neutralized by the additional instruction that naloxone may or may not block the “analgesic” effect of the cream. Thus, given the neural aberrancies in pathways overlapping with expectation-related placebo analgesia in FM patients,19,43 for example, decreased GMV within the DLPFC as observed in the FM patients in this study, altered placebo effects under circumstances of high pain-relief expectations are still plausible.
In conclusion, the present findings provide evidence that opioid-independent predominantly conditioning-related placebo analgesia modulates pain perception and pain-evoked neural activity without accessing placebo pain modulatory networks in the prefrontal cortex. Chronic pain patients with FM did not differ from healthy participants in their behavioral or neural responses to the placebo manipulation, suggesting that the observed differences in prefrontal brain anatomy did not adversely affect conditioning-related placebo analgesia. This finding suggests that harnessing placebo effects based on conditioning in chronic pain patients might represent a relevant avenue for therapeutic strategies.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B171.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Center for Complementary and Integrative Health. The authors thank Brian Walitt, Nicole Godwin, Linda Ellison-Dejewski, Brenda Justement, Susan Goo, and Patrick Korb for subject recruitment and clinical support, Lauren Atlas for guidance with the Neurological Pain Signature scripts, and Cortney Dable for assistance with the manuscript.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
E. Frangos and M. Čeko contributed equally to this article.
References
- [1].Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci 1999;19:484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation. In: FMRIB technical report TR07JA2. 2007. www.fmrib.ox.ac.uk/analysis/techrep. Accessed 1 June 2019. [Google Scholar]
- [3].Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 2004;24:10410–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Atlas LY, Wager TD. A meta-analysis of brain mechanisms of placebo analgesia: consistent findings and unanswered questions. Handb Exp Pharmacol 2014;225:37–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Benedetti F, Amanzio M, Rosato R, Blanchard C. Nonopioid placebo analgesia is mediated by CB1 cannabinoid receptors. Nat Med 2011;17:1228–30. [DOI] [PubMed] [Google Scholar]
- [6].Benedetti F, Amanzio M, Thoen W. Disruption of opioid-induced placebo responses by activation of cholecystokinin type-2 receptors. Psychopharmacology (Berl) 2011;213:791–7. [DOI] [PubMed] [Google Scholar]
- [7].Benedetti F, Arduino C, Vighetti S, Asteggiano G, Tarenzi L, Rainero I. Pain reactivity in Alzheimer patients with different degrees of cognitive impairment and brain electrical activity deterioration. PAIN 2004;111:22–9. [DOI] [PubMed] [Google Scholar]
- [8].Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport 2000;71(suppl 2):114–20. [DOI] [PubMed] [Google Scholar]
- [9].Case LK, Ceko M, Gracely JL, Richards EA, Olausson H, Bushnell MC. Touch perception altered by chronic pain and by opioid blockade. eNeuro 2016;3. doi: 10.1523/eneuro.0138-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ceko M, Bushnell MC, Fitzcharles MA, Schweinhardt P. Fibromyalgia interacts with age to change the brain. Neuroimage Clin 2013;3:249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ceko M, Frangos E, Gracely J, Richards E, Wang B, Schweinhardt P, Catherine Bushnell M. Default mode network changes in fibromyalgia patients are largely dependent on current clinical pain. Neuroimage 2020;216:116877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Colloca L, Akintola T, Haycock NR, Blasini M, Thomas S, Phillips J, Corsi N, Schenk LA, Wang Y. Prior therapeutic experiences, not expectation ratings, predict placebo effects: an experimental study in chronic pain and healthy participants. Psychother Psychosom 2020:1–8. doi: 10.1159/000507400 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Colloca L, Benedetti F. How prior experience shapes placebo analgesia. PAIN 2006;124:126–33. [DOI] [PubMed] [Google Scholar]
- [14].Craggs JG, Price DD, Verne GN, Perlstein WM, Robinson MM. Functional brain interactions that serve cognitive-affective processing during pain and placebo analgesia. Neuroimage 2007;38:720–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, James S, Voets N, Watkins K, Matthews PM, James A. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 2007;130:2375–86. [DOI] [PubMed] [Google Scholar]
- [16].Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 2009;63:533–43. [DOI] [PubMed] [Google Scholar]
- [17].Eippert F, Finsterbusch J, Bingel U, Buchel C. Direct evidence for spinal cord involvement in placebo analgesia. Science 2009;326:404. [DOI] [PubMed] [Google Scholar]
- [18].Forsberg JT, Martinussen M, Flaten MA. The placebo analgesic effect in healthy individuals and patients: a meta-analysis. Psychosom Med 2017;79:388–94. [DOI] [PubMed] [Google Scholar]
- [19].Geuter S, Koban L, Wager TD. The cognitive neuroscience of placebo effects: concepts, predictions, and physiology. Annu Rev Neurosci 2017;40:167–88. [DOI] [PubMed] [Google Scholar]
- [20].Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001;14:21–36. [DOI] [PubMed] [Google Scholar]
- [21].Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci 2007;27:10000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jones AK, Watabe H, Cunningham VJ, Jones T. Cerebral decreases in opioid receptor binding in patients with central neuropathic pain measured by [11C]diprenorphine binding and PET. Eur J Pain 2004;8:479–85. [DOI] [PubMed] [Google Scholar]
- [23].Kaptchuk TJ, Friedlander E, Kelley JM, Sanchez MN, Kokkotou E, Singer JP, Kowalczykowski M, Miller FG, Kirsch I, Lembo AJ. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS One 2010;5:e15591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Klinger R, Soost S, Flor H, Worm M. Classical conditioning and expectancy in placebo hypoalgesia: a randomized controlled study in patients with atopic dermatitis and persons with healthy skin. PAIN 2007;128:31–9. [DOI] [PubMed] [Google Scholar]
- [25].Kosek E, Rosen A, Carville S, Choy E, Gracely RH, Marcus H, Petzke F, Ingvar M, Jensen KB. Lower placebo responses after long-term exposure to fibromyalgia pain. J Pain 2017;18:835–43. [DOI] [PubMed] [Google Scholar]
- [26].Krummenacher P, Candia V, Folkers G, Schedlowski M, Schonbachler G. Prefrontal cortex modulates placebo analgesia. PAIN 2010;148:368–74. [DOI] [PubMed] [Google Scholar]
- [27].Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci 2007;27:4004–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lee HF, Hsieh JC, Lu CL, Yeh TC, Tu CH, Cheng CM, Niddam DM, Lin HC, Lee FY, Chang FY. Enhanced affect/cognition-related brain responses during visceral placebo analgesia in irritable bowel syndrome patients. PAIN 2012;153:1301–10. [DOI] [PubMed] [Google Scholar]
- [29].Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet 1978;2:654–7. [DOI] [PubMed] [Google Scholar]
- [30].Linnman C, Catana C, Petkov MP, Chonde DB, Becerra L, Hooker J, Borsook D. Molecular and functional PET-fMRI measures of placebo analgesia in episodic migraine: preliminary findings. Neuroimage Clin 2018;17:680–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M, Laurent B, Garcia-Larrea L. Differential brain opioid receptor availability in central and peripheral neuropathic pain. PAIN 2007;127:183–94. [DOI] [PubMed] [Google Scholar]
- [32].Montgomery GH, Kirsch I. Classical conditioning and the placebo effect. PAIN 1997;72:107–13. [DOI] [PubMed] [Google Scholar]
- [33].Muller M, Kamping S, Benrath J, Skowronek H, Schmitz J, Klinger R, Flor H. Treatment history and placebo responses to experimental and clinical pain in chronic pain patients. Eur J Pain 2016;20:1530–41. [DOI] [PubMed] [Google Scholar]
- [34].Pecina M, Stohler CS, Zubieta JK. Neurobiology of placebo effects: expectations or learning? Soc Cogn Affect Neurosci 2014;9:1013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schweinhardt P, Seminowicz DA, Jaeger E, Duncan GH, Bushnell MC. The anatomy of the mesolimbic reward system: a link between personality and the placebo analgesic response. J Neurosci 2009;29:4882–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology 2010;139:48–57 e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23(suppl 1):S208–219. [DOI] [PubMed] [Google Scholar]
- [38].Stussman BJ, Nahin RL, Ceko M. Fibromyalgia patients and healthy volunteers express difficulties and variability in rating experimental pain: a qualitative study. Scand J Pain 2018;18:657–66. [DOI] [PubMed] [Google Scholar]
- [39].Vase L, Petersen GL, Riley JL, III, Price DD. Factors contributing to large analgesic effects in placebo mechanism studies conducted between 2002 and 2007. PAIN 2009;145:36–44. [DOI] [PubMed] [Google Scholar]
- [40].Vase L, Robinson ME, Verne GN, Price DD. Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. PAIN 2005;115:338–47. [DOI] [PubMed] [Google Scholar]
- [41].Villemure C, Bushnell MC. Mood influences supraspinal pain processing separately from attention. J Neurosci 2009;29:705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Villemure C, Slotnick BM, Bushnell MC. Effects of odors on pain perception: deciphering the roles of emotion and attention. PAIN 2003;106:101–8. [DOI] [PubMed] [Google Scholar]
- [43].Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci 2015;16:403–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med 2013;368:1388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science 2004;303:1162–7. [DOI] [PubMed] [Google Scholar]
- [46].Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci U S A 2007;104:11056–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, Bushnell MC, Chizh BA. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci 2007;25:3576–82. [DOI] [PubMed] [Google Scholar]
- [48].Ziegler G, Dahnke R, Jancke L, Yotter RA, May A, Gaser C. Brain structural trajectories over the adult lifespan. Hum Brain Mapp 2012;33:2377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci 2005;25:7754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zunhammer M, Bingel U, Wager TD. Placebo imaging C. Placebo effects on the neurologic pain signature: a meta-analysis of individual participant functional magnetic resonance imaging data. JAMA Neurol 2018;75:1321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B171.


