Supplemental Digital Content is Available in the Text.
Two weeks of prism adaptation treatment for unilateral upper-limb complex regional pain syndrome I showed no benefits for pain or symptom severity compared with sham treatment.
Keywords: Complex regional pain syndrome, Prism adaptation, Randomized controlled trial, Attention, Pain, CRPS symptom severity, Body representation, Neuropsychology, Neglect
Abstract
Initial evidence suggested that people with complex regional pain syndrome (CRPS) have reduced attention to the affected side of their body and the surrounding space, which might be related to pain and other clinical symptoms. Three previous unblinded, uncontrolled studies showed pain relief after treatment with prism adaptation, an intervention that has been used to counter lateralised attention bias in brain-lesioned patients. To provide a robust test of its effectiveness for CRPS, we conducted a double-blind randomized controlled trial of prism adaptation for unilateral upper-limb CRPS-I. Forty-nine eligible adults with CRPS were randomized to undergo 2 weeks of twice-daily home-based prism adaptation treatment (n = 23) or sham treatment (n = 26). Outcomes were assessed in person 4 weeks before and immediately before treatment, and immediately after and 4 weeks after treatment. Long-term postal follow-ups were conducted 3 and 6 months after treatment. We examined the effects of prism adaptation vs sham treatment on current pain intensity and the CRPS symptom severity score (primary outcomes), as well as sensory, motor, and autonomic functions, self-reported psychological functioning, and experimentally tested neuropsychological functions (secondary outcomes). We found no evidence that primary or secondary outcomes differed between the prism adaptation and sham treatment groups when tested at either time point after treatment. Overall, CRPS severity significantly decreased over time for both groups, but we found no benefits of prism adaptation beyond sham treatment. Our findings do not support the efficacy of prism adaptation treatment for relieving upper-limb CRPS-I. This trial was prospectively registered (ISRCTN46828292).
1. Introduction
Complex regional pain syndrome (CRPS) is associated with continuous pain in one or more limbs accompanied by sensory, motor, and autonomic disturbances that are disproportionate to any inciting injury.35 Individuals with CRPS can also show neuropsychological symptoms reminiscent of hemispatial neglect after brain injury.33 These can present as distorted cognitive representations of the CRPS-affected limb(s),45,50,72,85,94 reduced attention to the affected limb(s) and the corresponding side of external space,11,21,25,27,76,84 poorer mental representation of the affected side of space,99 and spatially defined motor deficits.84 The extent of these neuropsychological changes has been associated with the severity of clinical signs of CRPS25,46,50,75,76,84,85,105 and could pertain to its central mechanisms.86
Prism adaptation (PA) is a sensorimotor training technique used to reduce lateralised biases in attention, spatial representations, and (ocular)motor performance in hemispatial neglect after brain injury.55,69,90 Considering similar neuropsychological deficits in CRPS, 3 previous studies tested the efficacy of PA in a total of 13 patients with this condition. They reported significant relief of pain and other CRPS symptoms following 8 to 20 PA sessions performed with the affected arm when participants adapted towards their affected side.9,12,100 The reduction in pain lasted up to 2 weeks. Thus, PA has the potential to durably relieve pain and other symptoms of CRPS. Because PA is quick (5-10 minutes a day), inexpensive, and self-administered, it is an appealing intervention compared with more intensive neurocognitive treatments such as graded motor imagery.73 However, the strength of available evidence for PA is limited because it was only tested in small samples, without any control treatments or blinding.
The mechanisms through which PA could relieve pain are unclear. One possibility is that it increases attention to the CRPS-affected side relative to the unaffected side. Indeed, when one patient underwent adaptation in the opposite direction such that the theoretical attention bias away from the affected side would be exacerbated, their pain increased.100 More severe self-reported “neglect-like” symptoms and spatial attention and motor biases have been related to greater pain intensity and worse long-term pain outcomes.25,84,85,105 A potential second mechanism is that PA restores normal sensorimotor integration, the disruption of which is thought to contribute to pathological pain, including CRPS.8,38,63,100 This is consistent with findings that individuals without spatial biases can also benefit from PA.12
We conducted a double-blind, randomized, sham-controlled trial of PA for upper-limb CRPS-I. We hypothesised that 2 weeks of twice-daily PA treatment would reduce the primary outcomes of pain intensity and CRPS symptom severity more than sham treatment of the same intensity. We also predicted greater reductions in the secondary outcomes of neuropsychological symptoms (ie, biases in spatial cognition, motor control, and body representation), clinical signs of CRPS, and self-reported CRPS-related and psychological disturbances after PA compared with sham treatment. The outcomes were assessed at 6 time points: to establish a 1-month pretreatment baseline and to examine any immediate effects of PA and their retention at 1, 3, and 6 months after treatment.
2. Methods
2.1. Study design and participants
The study was a 2-arm parallel group randomized controlled trial. It was prospectively registered (ISRCTN4682829242), and the full details of the study are reported in the study protocol and analysis plan.34 Any protocol deviations are specified in the relevant sections of the article. Anonymised participant-level data generated during the current trial (https://osf.io/ba6fq/), digital study materials (training protocol and video, PsychoPy experiment files, and stimuli; https://osf.io/7fk2v/), and analysis scripts (https://osf.io/w67rx/) are publicly available in an Open Science Framework repository. The study was approved by the UK National Health Service (NHS) Oxfordshire Research Ethics Committee A and Health Research Authority (reference 12/SC/0557).
We aimed to address the following research questions:
(1) Is 2 weeks of twice-daily PA treatment more effective in reducing pain and CRPS symptom severity than sham treatment?
(2) Are there any improvements in other clinical signs of CRPS, psychological functioning, and neuropsychological symptoms after PA treatment?
(3) How long are any benefits sustained for after the cessation of PA treatment?
(4) Are there factors that can predict the CRPS progression over time and/or the response to PA treatment?
(5) Are the neuropsychological abnormalities in CRPS (as compared to pain-free controls) related to clinical signs of CRPS?
Participants with CRPS were primarily recruited for the current randomized controlled trial of PA treatment (questions 1-4), but we also collected measures of spatial cognition, motor control, and body representation at baseline [research session 1 (RS1)] for comparison with pain-free controls and correlational analysis with clinical signs of CRPS (question 5; data reported elsewhere32).
Recruitment was conducted via post through the National CRPS-UK Registry, internal registries of the Royal United Hospitals and Walton Centre NHS Foundation Trusts, and clinicians' referrals through the Oxford University Hospitals NHS Foundation Trust and other NHS pain clinics in the United Kingdom. Word of mouth, print and online advertisements, and social media were further used to disseminate information about the study. Participants were recruited between March 2017 and December 2018, and the final long-term follow-up took place in July 2019.
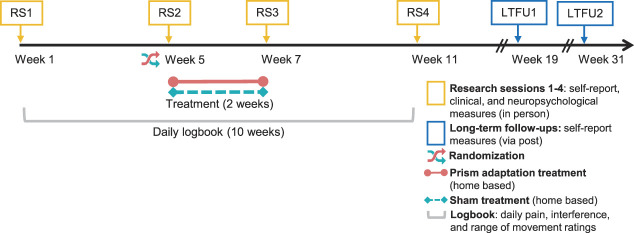
Following provisional assessment of eligibility through a phone interview, recruited participants took part in 4 RSs at the University of Bath (n = 33), at the University of Liverpool (n = 9), or in the participant's home (for participants who were unable to travel; n = 7). Participants gave written informed consent at the beginning of RS1, before any study-related procedures. The RSs involved in-person assessment of eligibility criteria and of the primary and secondary outcomes, including self-report questionnaires, clinical assessments, and tests of neuropsychological functions. Each RS lasted from 2 to 4 hours, including breaks between the assessments. The data collection schedule is presented in Figure 1. The baseline was measured over 2 RSs (RS1 and RS2) separated by 4 weeks. Immediately after RS2, participants commenced a 2-week home-based treatment period. Treatment outcomes were measured over 2 RSs, one immediately (RS3) and one 4 weeks (RS4) after completing the treatment. Two long-term follow-ups (LTFUs) were conducted through post—1 at 12 weeks (LTFU1) and 1 at 24 weeks (LTFU2) after completing the treatment. The flow of participants through each stage of the study is displayed in a CONSORT diagram (Fig. 2).
Figure 1.
Schedule of data collection and interventions. LTFU, long-term follow-up; RS, research session.
Figure 2.
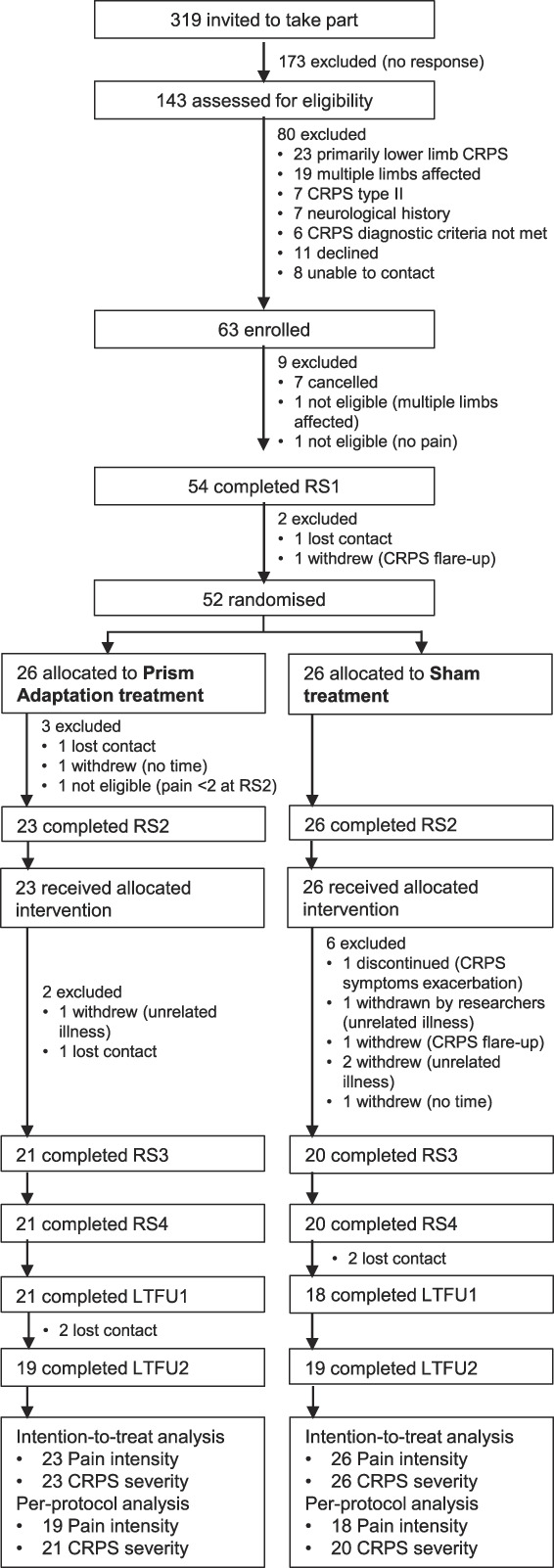
CONSORT diagram. Flow of participants through the study. CRPS, complex regional pain syndrome; RS1, research session 1; RS2, research session 2; RS3, research session 3; RS4, research session 4; LTFU1, long-term follow-up 1; LTFU2, long-term follow-up 2; intention-to-treat analysis, participants who received allocated intervention; per-protocol analysis, participants who completed allocated intervention, RS3-4 (CRPS severity), and LTFU1-2 (pain intensity). Note that 3 participants who were allocated to prism adaptation treatment did not attend RS2 or did not meet the eligibility criteria in RS2, and thus, they were not trained and did not receive any treatment and were not included in the intention-to-treat analysis.
Participant inclusion criteria were being aged 18 to 80 years, having a diagnosis of CRPS-I primarily affecting 1 upper limb based on the Budapest research criteria,35 having a CRPS diagnosis for ≥3 months at the time of RS1, and having a current pain intensity ≥2 on a 0 to 10 Numeric Rating Scale. Exclusion criteria were lacking sufficient English language ability to provide informed consent, being classified as legally blind, reporting a history of neurological disorder (eg, stroke, neurodegenerative disease, or traumatic brain injury), having CRPS in the opposite limb meeting the Budapest clinical or research criteria, reporting confirmed nerve damage (CRPS-II), reporting or showing dystonia or other physical impairment that would prevent satisfactory execution of PA/sham treatment, or reporting severe psychiatric comorbidity (eg, schizophrenia102) that could be associated with perceptual changes. Inclusion and exclusion criteria were assessed in RS1 and RS2.
2.2. Interventions
Both groups were instructed to continue any usual treatments (including medications) but were asked not to change their treatment regimens throughout the duration of the trial if possible. Current treatments and any changes are reported in Supplemental Table S1 (available at http://links.lww.com/PAIN/B162).
2.2.1. Prism adaptation treatment
Participants randomised to the PA treatment used welding goggles fitted with 35-diopter Fresnel lenses that induced approximately 19° lateral optical deviation (visual shift) away from the CRPS-affected side. In each treatment session, participants were seated approximately 50 cm from a wall or other vertical surface (the actual distance was adjusted individually to correspond to the participant's almost fully extended arm, and thus, it differs from the 60-cm distance anticipated in the trial protocol34). An A4 sheet was positioned on the wall in a landscape orientation at the eye level and in line with their body midline. There were 2 targets (2-cm diameter red circles) on the pointing sheet, located 12.5 cm to the left and 12.5 cm to the right of the participant's body midline. While wearing the prism goggles, participants used their CRPS-affected arm to perform 50 pointing movements, as fast as possible, alternating between the left and right target.
An example of PA is illustrated in Figure 3. Prismatic shifts were directed away from the CRPS-affected side, and thus, participants with left-CRPS would use rightward-shifting prism goggles as illustrated in the figure. Owing to the rightward visual shift, pointing would initially err to the right. However, with repeated movement execution and motor learning, the pointing would become increasingly accurate, as the movements would adjust in the opposite direction (to the left). This adaptive realignment of sensorimotor reference frames82,101 would produce movement after-effects towards the left (affected) side. That is, once the goggles were removed, if participants were to point to the target again, their pointing would temporarily err to the left. Conversely, participants with right-CRPS would use leftward-shifting prismatic goggles to induce adaptive realignment (movement after-effects) towards their affected side. Studies from neurologically healthy individuals and stroke patients show that these short-term movement after-effects are accompanied by a longer-lasting realignment of attention, spatial representations, and lateralised (ocular)motor performance in the same direction as the after-effect.3,14,24,43,47,53–55,65,66,69,89,90,95,96,98,104
Figure 3.
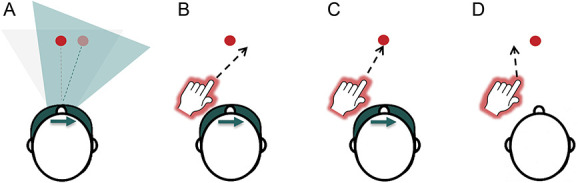
Prism adaptation procedure: In this example, a participant with left-CRPS is using rightward-shifting prisms (A–C), which induce adaptation towards the left (affected) side. For clarity of illustration, only one target (red circle) is represented in the figure. However, the treatment procedure involved 2 targets presented in the left and right side of space, and participants' pointing movements alternated between the left and right targets. (A) Prism goggles shift visual image to the right. The blue triangle represents a shift of visual perspective and perceived target location (pale red circle), relative to the real location of the target (light gray triangle, dark red circle). (B) Pointing movements initially err to the right. (C) Adaptive realignment results in correct pointing movements. (D) Goggles are removed and pointing movements err to the left (after-effect). CRPS, complex regional pain syndrome.
The chosen direction of PA, inducing a visual shift away from the affected side and thereby an after-effect towards the affected side, is consistent with previous PA studies in CRPS9,12,100 and the technique's application in rehabilitation of hemispatial neglect after brain injury.55,90 To enhance the effects of PA, welding goggles occluded the first half of the arm movement, and participants were encouraged to point as quickly as possible. Both of these measures are thought to reduce any deliberate misaiming on behalf of the participants and encourage greater adaptive realignment (ie, “true” sensorimotor adaptation).19,82,83 Previous studies in hemispatial neglect and CRPS demonstrated that the chosen number of movements (50 per session) is sufficient to induce adaptation measured as pointing after-effects and changes in spatial cognition.9,90,100 Note that the immediate movement after-effects were not measured in this trial.
Immediately after RS2, participants were trained in person in how to perform the treatment by a research psychologist J.H.B. or A.D.V. (neither of whom was involved in any data collection) according to a standardised protocol (available in study materials). Once the researcher was satisfied that the participant understood the treatment procedure, they performed the first treatment during this training session under the guidance of the researcher. At the end of the training session, participants received a pair of prism goggles in a sealed opaque bag, a pointing sheet, written instructions, and a link to a video tutorial to take home. In addition to the treatment that they underwent during training, participants were instructed to perform twice-daily self-guided treatment sessions at home for 2 weeks, resulting in 29 treatment sessions in total. The number of sessions per day and days of treatment were based on regimens that have previously been shown to reduce hemispatial neglect after stroke.23,24,47,69,95,96 This regimen was also more intense than that used in previous studies demonstrating CRPS reduction after PA treatment9,12,100; however, we also considered that it would not be too much of a burden for participants. They were instructed to commence the home-based treatment on the day after RS2, perform 1 session in the morning and 1 in the evening, and record the start and end time of each session in a provided logbook.
2.2.2. Sham treatment
Participants randomized to the sham treatment performed exactly the same procedure as described above, except they used welding goggles fitted with neutral lenses that did not induce any lateral visual shift.10,69 The neutral lenses distorted the acuity and clarity of vision to a similar extent as prism lenses (only without any lateral shift); therefore, the 2 treatment arms were similar aside from the sensorimotor adaptation.
2.3. Randomisation and blinding
Participant randomization was performed 1 to 5 days before RS2 by J.H.B., who was not involved in any data collection. Participants were randomly assigned to either the PA or sham treatment group with an equal allocation ratio, using MINIM68 software to minimize baseline (RS1) group differences in current pain intensity, CRPS severity score, primarily affected arm, pre-CRPS dominant hand, sex, age, presence of CRPS in other body parts, presence of other non-CRPS pain, and CRPS duration. The primary outcome measures (current pain intensity and CRPS severity score) were given double weighting compared with the other minimisation characteristics as we considered matching the 2 groups for these factors to be a higher priority. Note that this allocation procedure was updated in the trial registration42 after the first 2 participants were enrolled and provided RS1 data, but before they were allocated to treatment. The update reflects the use of MINIM software instead of blocked minimization to automate the allocation procedure. As per the trial protocol,34 participants excluded between treatment allocation and RS3 (Fig. 2) were removed from the minimization procedure so that subsequently recruited participants could be allocated independent of these exclusions and according to the current pool of participants remaining in each arm.
The only researchers who were aware of individual treatment allocations were those who randomised the participants and/or trained them in performing PA or sham treatments and provided them with prism or neutral goggles (J.H.B. and/or A.D.V.). These researchers were not involved in the assessments of any outcomes at any point in the trial. In RS3, the participants returned the goggles in a sealed opaque bag to M.H., which she handed unopened to J.H.B. The researcher responsible for enrolment and all data collection (M.H.) remained blinded to participants' treatment allocation until the last participant completed their RS4. After RS4, there were no further in-person assessments, as the long-term follow-up was conducted through postal questionnaires and scored by blinded research assistants. The participants were blinded to their treatment allocations throughout the entire duration of the trial. They were informed that they might receive real or sham treatment, and that both involved reaching out to touch visual targets with their affected arm while wearing goggles that distort vision. However, participants were not made aware of the specific nature of the intervention nor the differences between the types of goggles used in the 2 treatment arms. All documentation and instructions referred to the treatment arm as “sensorimotor training.”
2.4. Measures
2.4.1. Demographics
In RS1, participants reported on demographic characteristics, including age, sex, and handedness before CRPS onset. They completed 2 versions of Edinburgh Handedness Inventory78: rating their recalled (before CRPS onset) and current hand preference. Total scores can range from −100 (extreme left-handedness) to 100 (extreme right-handedness). To approximate the functional impact of CRPS, we calculated an absolute difference between current and recalled handedness scores, that is, change in handedness. We also interviewed the participants regarding their clinical history, including the date and type of any inciting injury, CRPS duration (time since diagnosis), any comorbidities, and any ongoing treatments for CRPS.
2.4.2. Primary outcomes
The primary research question was (1) whether 2 weeks of twice-daily PA treatment is more effective in reducing pain and CRPS symptom severity than sham treatment. Change between RS2 and RS3 in current pain intensity and the CRPS symptom severity score were the primary outcomes. In RS1-RS4 and LTFU1-LTFU2, participants rated their current pain intensity in the CRPS-affected limb on a Numerical Rating Scale from 0 (no pain) to 10 (pain as bad as you can imagine). This measure was taken from the Brief Pain Inventory (item 6)13 and has been recommended as a core outcome for chronic pain trials.17,31 Complex regional pain syndrome severity was assessed in RS1-RS4 according to a standardised protocol.34,37 Eight self-reported symptoms and 8 signs evaluated on clinical examination were scored as 0 (absent) or 1 (present) based on sensory testing and visual and manual examination. The summed CRPS severity score can range from 0 (no CRPS symptoms) to 16 (most severe CRPS symptoms). The CRPS severity score has good discrimination abilities, concurrent validity, and adequate sensitivity to change36,37 and was recommended as the core outcome measure for CRPS clinical studies.31
2.4.3. Secondary outcomes
Our secondary research question was (2) whether there are any improvements in other clinical signs of CRPS, psychological functioning, and neuropsychological symptoms after PA treatment. Detailed description of and rationale for the secondary outcome measures is available in a published study protocol.34 Below, we provide the basic details of how these outcomes were quantified.
2.4.3.1. Self-report measures
Self-reported secondary outcomes measured in RS1-RS4 and LTFU1-LTFU2 included questionnaires about pain, body representation, and emotional functioning. These were chosen based on recommendations for core outcome measures for chronic pain trials17 and the existing literature on CRPS implicating other relevant measures.30 For pain-related outcomes, we used the Brief Pain Inventory (0-10 scale for each subscale; higher scores indicate greater pain intensity/interference13) and Pain Detect Questionnaire (−1 to 38 scale; higher scores indicate a greater neuropathic component of experienced pain26). Body representation was measured using the Bath CRPS Body Perception Disturbance Scale (0-57 scale; higher scores indicate greater distortions49). For emotional functioning, we used the Tampa Scale for Kinesiophobia (17-68 scale; higher scores indicate more severe pain-related fear of movement and reinjury67) and Profile of Mood States (17-229 scale; higher scores indicate greater mood disturbance64). In RS1, participants also completed the Revised Life Orientation Test (0-24 scale; higher scores indicate a higher optimism level93) and Patient Centred Outcomes Questionnaire (each item rated on a 0-10 scale; higher scores indicate higher usual, desired, expected, and considered successful in terms of the treatment outcome levels of pain, fatigue, emotional distress, and interference and higher importance of improvement in each of these areas87). These 2 measures were included to assess whether 2 treatment groups were matched on their average optimism and expectations of outcomes because these factors can affect the success of novel treatments.4,51,103 In posttreatment RS3-4 and LTFU1-LTFU2, participants rated their impression of how much their activity limitations, symptoms, emotions, and overall quality of life related to CRPS changed due to treatment, using the Patient Global Impression of Change questionnaire (1-7 scale; 1 indicates no change or worsening of symptoms; higher scores indicate greater improvement40).
Throughout the first 10 weeks of the trial (RS1-RS4), participants rated their average level (over the past 24 hours) of pain intensity, the degree to which their symptoms interfered with their daily life, and range of movement in the affected limb, using daily logbooks (0-10 Numerical Rating Scales; higher scores indicate greater pain intensity, symptoms interference, and better range of movement), to track the precise time course of any changes on these outcomes.
2.4.3.2. Clinical assessments
In RS1-RS4, we assessed participants' CRPS signs and symptoms to determine whether the Budapest research criteria were met and to calculate the CRPS severity score. In addition to in-person assessments, photographs and videos of both limbs were double-scored for the presence of colour asymmetry, dystrophic changes, and motor abnormalities by a trained research assistant who was blind to treatment allocation, affected limb, and time point of assessment. Cohen's kappa statistics for interrater agreement were significantly different from zero, indicating fair agreement for colour asymmetry (κ = 0.21, P = 0.004) and dystrophic changes (κ = 0.23, P < 0.001) and borderline slight/fair agreement for motor impairment (κ = 0.20, P < 0.001). We also objectively quantified sensory, autonomic, and motor functions. Sensory tests were performed on the most painful site on the CRPS-affected limb and the corresponding site on the unaffected limb (assessed first), unless specified otherwise.
Secondary outcomes of sensory function of the affected relative to unaffected limb included elements of quantitative sensory testing administered according to the standardised protocol.34,88 Specifically, we measured mechanical detection thresholds using von Frey filaments. A positive threshold ratio [(affected − unaffected)/affected] indicates an increased tactile detection threshold (hypoesthesia) on the affected side. We measured mechanical pain thresholds using pinprick stimulators. A positive threshold ratio [(unaffected − affected)/unaffected] indicates a decreased pain threshold (hyperalgesia) on the affected side. Allodynia was assessed using a cotton ball, a Q-tip, and a brush. An arithmetic mean of 15 ratings for these sensations from 0 (no sharp, pricking, stinging, or burning sensation) to 100 (most intense pain sensation imaginable) quantifies the severity of allodynia on the affected limb. We also measured two-point discrimination thresholds on participants' index fingertips according to a staircase procedure using a disk with 1 and 2 plastic tips separated by 2 to 15 mm distance. A positive threshold ratio ([affected − unaffected]/affected) indicates a higher tactile discrimination threshold (less precise discrimination ability) on the affected hand.
In addition to contributing to the CRPS severity score, the following measures were used as secondary outcomes of autonomic and motor function of the affected relative to unaffected limb: temperature difference (affected − unaffected; a negative score indicates that the affected limb was colder; absolute values were also analysed); oedema (affected − unaffected; higher scores indicate greater swelling of the affected limb); grip strength (affected/unaffected; scores <1 indicate weaker strength of the affected hand); and delta finger-to-palm distance (affected/unaffected; scores <1 indicate a lower range of movement of the affected hand).
2.4.3.3. Tests of neuropsychological functions
In RS1-RS4, the participants completed 6 experimental tests of the following neuropsychological functions: visuospatial attention in near space (Temporal Order Judgement, Landmark, and Grayscales tasks); mental representation of space (Mental Number Line Bisection task); spatially defined motor function; and body representation (Hand Laterality Recognition task). A comprehensive battery of sensitive tests of distinct aspects of spatial cognition, motor control, and body representation deemed appropriate to fully capture any neuropsychological biases, how they would be affected by PA, and how they would relate to any changes in pain and other CRPS symptoms. Below, we summarise how the neuropsychological functions were measured and quantified, whereas detailed descriptions of the experimental materials and procedures can be found in the trial protocol.34
All experimental tasks were programmed and administered using PsychoPy software.79 Those involving the presentation of visual stimuli on a computer screen used a 34.5 × 19.4 cm touchscreen positioned at 50 cm viewing distance. In all tasks (except the Mental Number Line Bisection), participants used a chinrest and fixated on a cross aligned with their body midline. When a manual response was required, participants used their unaffected hand to press the buttons, which were aligned orthogonally to the required response format (ie, for left/right responses, participants pressed colour-coded bottom/top buttons). A short practice session was completed before each task. Data for stimuli/responses in the left and right sides of space for all tasks were recoded after collection in terms of the affected and unaffected space relative to each participant's CRPS-affected side.
The Temporal Order Judgement task measures covert spatial attention. Participants saw pairs of brief, identical light flashes, presented with different temporal offsets (±10 to 240 ms range) onto a white table surface, one on each side of space. In one block, participants reported which of the 2 lights they perceived first by saying “left” or “right.” In another block, they reported which light they perceived second. The order of response type (first or second) was counterbalanced, and results were averaged across these to account for any response bias.22 We calculated the Point of Subjective Simultaneity, which expresses by how many milliseconds the light in the affected side of space had to precede (negative score) or follow (positive score) the light in the unaffected side of space for both lights to be perceived as simultaneous. Information that receives greater attention is perceived earlier than information that receives lesser attention.97 Thus, a negative Point of Subjective Simultaneity indicates lower attention to the affected side of near space relative to the unaffected side.
The Landmark task57 measures the visual representation of relative horizontal distance in near space. Participants saw pairs of landmarks (white circles) presented simultaneously, one on each side of space. While the distance between 2 landmarks was constant across all trials, their relative distance from the central fixation cross varied by 0.1° increments from ±8.1° to ±6.9° to the left and to the right. Participants indicated through a button press whether the left or the right landmark appeared closer to or further from the fixation cross. Results were averaged across 2 separate response blocks to account for any response bias. We calculated the Point of Subjective Equality, which expresses the relative distance (°) at which the landmark on the affected side of space had to be further from (negative score) or closer to (positive score) the fixation cross to perceive the 2 landmarks to be equidistant. A negative Point of Subjective Equality value indicates underestimation of the distance on the affected relative to the unaffected side and thus underrepresentation of the affected side of near space.
The Grayscales task77 measures overt spatial attention. Participants saw pairs of horizontal bars filled with grayscales presented one above the other. The 2 bars were mirror images of each other so that one bar was darker on the left side and the other bar was darker on the right side. Participants pressed a button to indicate which bar appeared to be darker overall. A negative value of the calculated index of spatial bias indicates that a higher proportion of overall darkness judgements were made based on the unaffected sides of the stimuli, consistent with lower attention to the affected relative to unaffected side.
The Mental Number Line Bisection task99 measures mental representation of space, based on an implicit representation of numbers in a left-to-right linear arrangement.15 The experimenter read aloud pairs of numbers that were separated by an interval of 9 to 64 digits. They were presented in ascending and descending order (eg, 54 and 70, or 70 and 54) to account for any response bias. Participants were instructed to verbally report the subjective midpoint between the given pair of numbers, without making any calculations. A negative value of the calculated index of spatial bias is consistent with overestimating the subjective midpoint towards larger numbers (ie, a rightward bias). Expressed relative to each participant's CRPS-affected side, a negative index indicates a bias away from the affected side of the mental representation of space or underrepresentation of the affected side of mental space.
The spatially defined motor function task59 measures directional hypokinesia and directional bradykinesia, that is, slowing of initiation and execution of movements directed towards the affected relative to unaffected side. On each trial, participants held down a button with their finger until the target appeared either on the left or on the right side of a computer screen. Then, participants were required to release the button, touch the target on the screen, and return their finger to the button as quickly as possible. Participants completed the task from 3 starting positions (button positioned to the left, right, or aligned with their body midline; order counterbalanced), alternating between their unaffected and affected hands, across 6 blocks in total. We used average movement initiation times (from the target onset to button release) and movement execution times (from the button release to touch on the computer screen) for each combination of the hand starting position and target location to calculate indices of directional hypokinesia and bradykinesia towards the affected side,92 separately for each hand used to complete the task. Index A quantifies the speed of initiating/executing movements towards the affected side relative to the unaffected side. This index was calculated as: [central starting position (affected − unaffected target location) − affected starting position (affected − unaffected target location)]. Index A allows to dissociate motor and perceptual neglect (ie, effect of the target location); however, it involves movement trajectories of different lengths. Thus, we also derived index B that directly quantifies the speed of initiating/executing movements of the same physical length towards the affected side relative to the unaffected side. Index B was calculated as: (central starting position [affected target location] − affected starting position [affected target location]). Positive values of indices A and B indicate slowing of initiation/execution of movements directed towards the affected relative to the unaffected side, suggestive of directional hypokinesia/bradykinesia towards the affected side.
The Hand Laterality Recognition task94 measures body representation. Participants saw images of hands appearing either to the left or to the right of central fixation. The images depicted left or right hands in different postures and rotations from upright (0°, 90°, 180°, or 270°). Participants were required to indicate as fast and as accurately as possible (through a button press) whether each image depicted a left or a right hand. Accuracy rates and average reaction times to correctly responded to trials for each depicted hand were averaged across 2 image locations because the side of space effects were not the primary interest of this trial and will be reported elsewhere. We calculated 2 indices of hand laterality recognition as the differences between the depicted hands: accuracy index (unaffected hand − affected hand) and reaction time index (affected hand − unaffected hand). Positive scores indicate less accurate and slower recognition of depicted hands corresponding to the participant's affected hand, relative to depicted hands corresponding to their unaffected hand. Thus, positive accuracy and reaction time indices suggest distorted representation of the CRPS-affected limb.
2.5. Statistical analyses
2.5.1. Sample size calculation
The study was powered to evaluate the effects of PA treatment on a change in the primary outcome of pain intensity between RS2 and RS3. We estimated18 that a sample of 21 participants with CRPS per treatment group would provide 90% power to detect a minimal clinically significant reduction of 2 points on the primary outcome of pain intensity (0-10 Numerical Rating Scale20), with an SD of 1.98 (based on our previous research11) and a 2-tailed alpha of 0.05. Although we aimed to obtain 42 complete data sets for RS1-RS4, one participant in the sham treatment group withdrew after we terminated recruitment, and thus, the total number of participants who completed these sessions equals 41.
2.5.2. Analyses
The analysis plan can be found in the trial protocol,34 and thus below, we only describe the main steps, any details not previously specified, and any deviations from the protocol. We used IBM SPSS Statistics 25,41 R 3.5.3,81 and MATLAB 2018b58 software to process and analyse the data. Data preparation procedures are reported in Supplemental Text S1 (available at http://links.lww.com/PAIN/B162). Throughout, we reported bootstrapped bias-corrected and accelerated 95% confidence intervals (BCa 95% CIs) around all mean and median values. We used bootstrapped χ2 tests, bootstrapped t tests (or their nonparametric alternatives in case of violation of parametric assumptions), and analyses of variance (ANOVAs) to compare mean values between treatment groups and between data collection time points. Analysis of variance is robust to moderate violations of normality and homogeneity of variance,6,7 and we used Greenhouse–Geisser corrections if the sphericity assumption was violated. However, where severe (ie, more than borderline significant and in multiple conditions) violations of the assumptions of normality, homogeneity of variance, and sphericity were found, we used linear mixed models analyses with nonparametric bootstrapping procedures (n = 1000). For linear mixed models analyses, a model term made a significant contribution to predicting an outcome when the 95% CI around the coefficient estimate (B) did not include zero. For the remaining analyses, statistical significance was defined as P < 0.05. We used 1-tailed tests for comparisons for which we had directional hypotheses (ie, RS2 vs RS3 comparisons, as we predicted greater reductions on the outcome measures in the PA than the sham treatment group) and 2-tailed tests for the remaining comparisons. We controlled for type I errors in the primary (but not exploratory) analyses by using Holm–Bonferroni correction for multiple comparisons within the analysis of each outcome and reported adjusted P values (Padj).
Our primary analysis involved the intention-to-treat population, that is, participants who received their allocated intervention (ie, received in-person training immediately after RS2), regardless of their treatment adherence or completion of the outcome assessments (PA treatment = 23 and sham treatment = 26). Note that the trial protocol defined this population as all participants allocated to treatment, which did not account for the possibility that they could withdraw before being trained in how to perform their allocated intervention. This was the case for 3 participants (Fig. 2) who were not included in the intention-to-treat sample as per an updated definition. In Supplemental Text S2 (available at http://links.lww.com/PAIN/B162), we report a supportive per-protocol analysis of those participants who provided complete outcome data and completed their allocated treatment (missed no more than 6 treatment sessions). The results of the per-protocol analysis are broadly consistent with the intention-to-treat analysis.
2.5.2.1. Effects of prism adaptation treatment on the primary outcomes
To evaluate the effects of PA treatment on the first primary outcome of pain intensity (research question 1) and the time course of any changes (research question 3), we conducted a 2 (Group: PA treatment and sham treatment) × 6 (Time: RS1, RS2, RS3, RS4, LTFU1, and LTFU2) ANOVA. We planned 16 a priori contrasts to compare RS1 vs RS2, RS2 vs RS3, RS3 vs RS4, RS2 vs RS4, RS2 vs LTFU1, RS4 vs LTFU1, LTFU1 vs LTFU2, and RS2 vs LTFU2 within each treatment group.
To evaluate the effects of PA treatment on the second primary outcome of the CRPS severity score (research question 1) and the time course of any changes (research question 3), we conducted a 2 (Group: PA treatment and sham treatment) × 4 (Time: RS1, RS2, RS3, and RS4) ANOVA. We planned 8 a priori contrasts to compare RS1 vs RS2, RS2 vs RS3, RS3 vs RS4, and RS2 vs RS4 within each treatment group.
2.5.2.2. Effects of prism adaptation treatment on the secondary outcomes
To evaluate the effects of PA treatment on self-reported pain and psychological functioning, sensory, motor, and autonomic function, and neuropsychological functions (research question 2) and the time course of any changes (research question 3), we conducted 2 × 6 and 2 × 4 ANOVAs and planned the same contrasts as described for the analyses of the primary outcomes.
2.5.2.3. Predictors of the complex regional pain syndrome progression over time
To investigate whether any baseline factors could predict CRPS progression over time (research question 4), independent of the treatment, we used the data from the total sample (N = 49) to perform exploratory best subsets regression analyses on the overall change in pain intensity and the CRPS severity score throughout the course of the study. This analysis differed from that proposed in the trial protocol in terms of (1) operationalisation of the pain outcome, (2) selection of potential predictors, and (3) regression model used. (1) Change on the primary outcomes was quantified as individual regression slopes fitted to each participant's ratings of current pain intensity across RS1-LTFU2 (instead of planned RS1-RS4 comparisons, to capture change over a longer period) and to each participant's CRPS severity scores across RS1-RS4. More negative slopes indicate greater improvement over time (ie, reduction in pain and CRPS severity). (2) In the protocol, when specifying the selection of potential predictors, we planned to prioritize those factors on which participants with CRPS significantly differed from pain-free participants at baseline (research question 534). However, they showed no differences on the tests of neuropsychological functions (results reported elsewhere32). Thus, we instead included all primary and secondary outcomes as potential explanatory variables. That is, we included participants' demographic characteristics, self-reported pain and psychological functioning, sensory, motor, and autonomic function, and neuropsychological functions, as measured in RS1. We restricted the pool of potential predictors by excluding factors that lacked linear relationships with each outcome or were collinear with other predictors (see Supplemental Text S1, available at http://links.lww.com/PAIN/B162). (3) Instead of the planned linear mixed model regression, best subsets regression was deemed more appropriate to allow unbiased selection of the best combination of explanatory variables. Best subsets regression is an automated approach that performs an exhaustive search for the best subset of factors for predicting the outcome and returns the best model of each size (up to a specified number of predictors).56 Considering our sample size (N = 49), we compared best subsets models that included 1 up to 5 predictors of each outcome. From the 5 models, the one with the lowest Akaike Information Criterion was preferred as best fit. To address a potential issue of overfitting, we also performed a 5-fold cross-validation48 of each of the 5 models suggested by best subsets regression analyses. This approach randomly splits the data set into 5 folds (subsets of observations). Each model is trained using the 80% of the data (4 folds) and then tested on the remaining 20% of the data (1 fold). This process is repeated until each fold has served as a test subset. The average of errors recorded in each repetition is a cross-validation error. The lowest cross-validation error indicates best model performance.
3. Results
3.1. Participant characteristics
Table 1 presents baseline characteristics and comparisons between PA and sham treatment groups. On average, participants reported moderate pain intensity (6/10), comparable with previous studies on PA (5.8-6/1012,100) and other neurocognitive treatments (5.3-7/1045,61,73) for CRPS. The median CRPS severity score in our sample was higher than the average severity reported for individuals with stable CRPS in the validation study of this tool (13 vs 11.2/1637), possibly because we used stricter inclusion criteria (Budapest research diagnostic criteria35). Our participants on average had longer CRPS duration compared with other studies of neurocognitive treatments for CRPS (58 vs 5-24 months9,12,45,61,73,100). The proportion of participants with CRPS affecting their right side of the body was consistent with a large population study,70 although it was lower than in small-sample studies on PA (41% vs 71%-80%12,100). Both the mean age and proportion of females were consistent with those previously reported in CRPS.12,37,70,91,100 The most common comorbidities in our participants were depression (37%), anxiety (22%), migraines (16%), fibromyalgia (14%), and asthma (14%). These conditions were found to be prevalent in CRPS in previous population studies.52,71 The most common treatments in the current sample included weak or strong opioids (57%), anticonvulsants (47%), paracetamol (45%), antidepressants (45%), physiotherapy, hydrotherapy, or occupational therapy (39%), and nonsteroidal anti-inflammatory drugs (35%; see Supplemental Table S1, available at http://links.lww.com/PAIN/B162). Overall, demographic and clinical characteristics of our sample appear to be representative of the general population of people with CRPS2,37,70,91 and comparable with those reported in previous research investigating neurocognitive treatments for CRPS,9,12,45,61,73,100 except for the longer average disease duration in our study.
Table 1.
Baseline (RS1) participant characteristics by treatment group (intention-to-treat analysis).
| Measure | Prism adaptation treatment (n = 23) | Sham treatment (n = 26) | Contrast |
|---|---|---|---|
| Minimisation factors | |||
| Current pain intensity (/10) M | 5.96 (5.02 to 6.80) | 6.15 (5.26 to 7.00) | t(47) = −0.33, P = 0.741, d = 0.10 |
| CRPS severity score (/16) Mdn | 13.00 (12.07 to 13.93) | 12.50 (11.00 to 13.00) | U = 287.50, P = 0.809, d = 0.07 |
| Primarily affected arm (% right) | 48% | 35% | χ2(1) = 0.88, P = 0.348, ϕ = −0.13 |
| Pre-CRPS dominant hand (% right) | 91% | 92% | χ2(1) = 0.16, P = 0.898, ϕ = 0.02 |
| Sex (% female) | 83% | 85% | χ2(1) = 0.04, P = 0.850, ϕ = −0.03 |
| Age (y) M | 47.35 (43.20 to 51.95) | 45.31 (39.85 to 50.85) | t(47) = 0.53, P = 0.601, d = −0.15 |
| CRPS in other body parts (% present) | 13% | 8% | χ2(1) = 0.38, P = 0.537, ϕ = −0.09 |
| Other non-CRPS pain (% present) | 44% | 39% | χ2(1) = 0.13, P = 0.721, ϕ = −0.05 |
| CRPS duration (months since diagnosis) M | 61.26 (47.15 to 75.12) | 52.31 (39.49 to 66.35) | t(47) = 0.84, P = 0.388, d = −0.24 |
| Other control measures | |||
| Optimism (Revised Life Orientation Test[/24]) M | 13.00 (10.97 to 15.07) | 12.31 (11.00 to 13.61) | t(47) = 0.59, P = 0.560, d = −0.17 |
| Mood disturbance (Profile of Mood States[/229]) M | 94.81 (79.96 to 109.93) | 84.22 (70.94 to 98.08) | t(47) = 0.97, P = 0.349, d = −0.28 |
| Fear of movement (Tampa Scale for Kinesiophobia[/68]) M | 38.79 (35.45 to 41.95) | 40.38 (37.17 to 43.35) | t(47) = −0.65, P = 0.502, d = 0.19 |
| No. of logged treatment sessions (/29) Mdn | 29.00 (28.54 to 29.46) | 29.00 (28.55 to 29.45) | U = 297.00, P = 0.965, d = 0.01 |
CRPS, complex regional pain syndrome; RS1, research session 1.
Bootstrapped bias-corrected and accelerated 95% confidence intervals are reported in parenthesis (BCa 95% CI). There were no significant differences between groups on any measures.
The randomization procedure successfully equated the 2 treatment groups on the minimization factors (Table 1). The 2 groups were also matched on baseline mean levels of optimism, mood disturbance, fear of movement, and expectations and criteria for success of the treatment (there were no significant differences between PA and sham treatment groups on any of the Patient Centred Outcomes Questionnaire items, Us ≥ 212.00, Psadj ≥ 0.27, ds ≤ 0.51).
Eight participants (16%) withdrew from the study after treatment allocation. They were excluded from the per-protocol analysis (Supplemental Text S2, available at http://links.lww.com/PAIN/B162), but their RS2 data were carried forward for the purpose of the primary intention-to-treat analysis. We compared their baseline (RS1) pain intensity and CRPS severity against confidence intervals around the mean pain intensity and CRPS severity score of participants who remained in the trial. Of those who dropped out, 5 participants had greater pain intensity and 4 participants had greater CRPS severity compared with those who remained. However, the same or lower pain intensity and CRPS severity scores were found in another 3 and 4 participants who dropped out, respectively.
3.2. Treatment adherence and participant blinding
Twenty-one of 23 participants (91%) in the PA treatment group and 20 of 26 participants (77%) in the sham treatment group missed no more than 6 treatment sessions according to their logbooks (see Supplemental Table S1, available at http://links.lww.com/PAIN/B162). Two participants in the PA and 6 participants in the sham treatment group missed more than 6 treatment sessions and/or did not provide posttreatment outcome data. No other deviations from the treatment protocol were identified among the remaining participants. The extent of exposure to treatment (ie, average number of logged treatment sessions) was not significantly different between the 2 treatment groups (Table 1). The median recorded durations of the treatment sessions according to the participants' logbook entries were 2 minutes 25 seconds in the PA group and 2 minutes in the sham treatment group.
At the end of RS4, we asked each participant (N = 41) which treatment they thought they received. They could respond “real [PA],” “sham,” or “no idea.” Similar proportions of participants in each group made correct (real: 12.2%; sham: 12.2%) or wrong (real: 12.2%; sham: 7.3%) guesses as to their actual treatment allocation or responded that they had no idea (real: 26.8%; sham: 29.3%), χ2(2) = 0.52, P = 0.771, Cramer's V = 0.11. Only 12% of participants in each group correctly guessed their treatment allocation, and therefore, participant blinding was successful.
3.3. Effects of prism adaptation treatment on the primary outcomes
Figure 4 illustrates any changes on the primary outcomes and their time course in each treatment group (research questions 1 and 3). Despite the PA group showing some reduction in the current pain intensity scores immediately after treatment (RS3; Fig. 4A), the ANOVA did not reveal any significant main effects of Time, F(4.04, 189.81) = 1.82, P = 0.126, = 0.04, or Group, F(1, 47) = 0.26, P = 0.615, = 0.01, nor did it show any significant interaction between these factors, F(4.04, 189.81) = 0.66, P = 0.624, = 0.01. This indicates that there were no significant changes in pain intensity over time in either treatment group. Thus, contrary to our hypothesis, PA treatment did not reduce pain intensity more than sham treatment.
Figure 4.
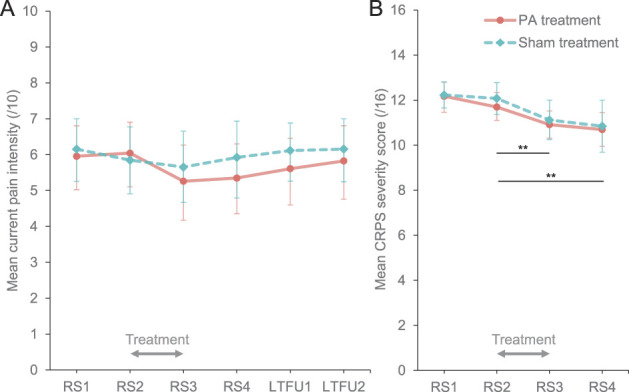
Primary outcomes (intention-to-treat analysis). Mean (BCa 95% CI) current pain intensity (A) and CRPS severity scores (B) in prism adaptation (PA; orange circles) and sham treatment (blue diamonds) groups in each time point. LTFU1 and LTFU2, long-term follow-ups 1 and 2; RS1, RS2, RS3, and RS4, research sessions 1, 2, 3, and 4. Gray arrows indicate the treatment period. **Significant decrease in CRPS severity between RS2 and RS3, maintained at RS4, regardless of treatment, Psadj < 0.01. BCa 95% CI, bootstrapped bias-corrected and accelerated 95% confidence interval, CRPS, complex regional pain syndrome; PA, prism adaptation.
Analysis of the CRPS severity scores (Fig. 4B) showed a large significant main effect of Time, F(2.28, 107.08) = 17.57, P < 0.001, = 0.27, indicating that regardless of treatment, CRPS severity decreased over time (Fig. 4B). Contrasts revealed a significant reduction in CRPS severity immediately after treatment [RS3; Mdn = 11.00, BCa 95% CI (11.00-11.00)] compared with immediately before treatment [RS2; Mdn = 12.00, BCa 95% CI (12.00-12.00)], Z = −3.91, Padj = 0.002, d = 0.86. This reduction relative to RS2 was maintained 4 weeks after completing the treatment [RS4; Mdn = 11.00, BCa 95% CI (11.00-11.00)], Z = −3.70, Padj = 0.002, d = 0.81, but without further significant change from RS3, Z = −0.81, Padj = 0.433, d = 0.16. Complex regional pain syndrome severity did not change significantly between the first [RS1; Mdn = 13.00, BCa 95% CI (13.00-13.00)] and the second baseline session, Z = −1.71, Padj = 0.170, d = 0.35. There was no significant effect of Group, F(1, 47) = 0.17, P = 0.685, < 0.01, nor was there any significant interaction effect, F(2.28, 107.08) = 0.17, P = 0.886, < 0.01, on the CRPS severity scores. Thus, contrary to our hypothesis, CRPS severity did not decrease more after PA compared with sham treatment, but both groups improved over the treatment period.
We compared mean changes in pain intensity and CRPS severity over the treatment period (RS3 − RS2) between PA and sham treatment groups. Effect sizes of these differences might be important for planning future studies. For current pain intensity, the effect size was small, d = 0.37, 95% CI (−0.20 to 0.94). Mean pain reduction in the PA treatment group was −0.78 points on a 0 to 10 Numerical Rating Scale, BCa 95% CI (−1.55 to −0.15). In the sham treatment group, mean pain reduction was −0.19 points, BCa 95% CI (−0.68 to 0.28). For the CRPS severity score, the effect size was negligible, d = −0.13, 95% CI (−0.69 to 0.43). Mean CRPS severity reduction in the PA treatment group was −0.78 points on a 0 to 16 scale, BCa 95% CI (−1.19 to −0.38). In the sham treatment group, the mean CRPS severity reduction was −0.96 points, BCa 95% CI (−1.54 to −0.38). On an individual level (Supplemental Fig. S1, available at http://links.lww.com/PAIN/B162), 5 participants in the PA group and 4 in the sham group achieved clinically significant reductions in pain (ie, at least 2-point decrease on a 0-10 Numerical Rating Scale20) over the treatment period. None of the participants achieved clinically significant reduction in CRPS severity (ie, at least 4.9-point decrease on a 0-16 scale, although this threshold is quite conservative37).
3.4. Effects of prism adaptation treatment on the secondary outcomes
Group average scores on the self-report questionnaires, clinical assessments, and neuropsychological tasks in each time point are reported in Table 2. Note that the confidence intervals around average baseline indices of the neuropsychological functions include zero, indicating that participants did not show significant biases in visuospatial attention or the mental representation of space, nor any differences in the recognition of the affected relative to unaffected hands. Furthermore, average indices of directional hypokinesia and bradykinesia vary between positive and negative values, suggesting that there were no systematic spatially defined motor deficits at baseline. Complete results of a series of ANOVAs conducted to test the effects of PA on the secondary outcomes and their time course (research questions 2 and 3) are reported in the tables, and below, we only refer to the effects directly relevant for our hypothesis, that is, Group × Time interactions.
Table 2.
Mean or median values (BCa 95% CI) of self-reported; sensory, autonomic, and motor; and neuropsychological secondary outcome measures at each time point (intention-to-treat analysis).
| Measure | Group | Time point | |||||
|---|---|---|---|---|---|---|---|
| RS1 | RS2 | RS3 | RS4 | LTFU1 | LTFU2 | ||
| Self-report questionnaires | |||||||
| Pain | |||||||
| Pain severity (Brief Pain Inventory[/10]) M | PA | 5.91 (5.17 to 6.58) | 6.02 (5.28 to 6.71) | 5.41 (4.50 to 6.26) | 5.43 (4.55 to 6.24) | 5.62 (4.69 to 6.48) | 5.59 (4.69 to 6.41) |
| Sham | 5.81 (5.02 to 6.50) | 5.95 (5.12 to 6.78) | 5.85 (5.04 to 6.65) | 5.84 (4.82 to 6.74) | 6.04 (5.12 to 6.80) | 5.95 (5.07 to 6.73) | |
| Pain interference (Brief Pain Inventory[/10]) Mdn | PA | 6.71 (6.29 to 6.71) | 6.43 (5.00 to 7.08) | 5.29 (3.57 to 6.43) | 5.57 (4.71 to 6.29) | 6.00 (5.22 to 6.14) | 5.86 (4.57 to 6.86) |
| Sham | 5.79 (5.00 to 7.14) | 5.86 (5.72 to 5.86) | 5.57 (5.43 to 5.57) | 5.64 (4.00 to 6.14) | 5.50 (3.71 to 6.57) | 5.72 (4.14 to 6.57) | |
| Neuropathic features of pain (Pain Detect Questionnaire[/38]) Mdn | PA | 26.00 (26.00 to 26.00) | 25.00 (20.00 to 26.00) | 24.00 (21.00 to 27.00) | 24.00 (20.00 to 26.00) | 26.00 (25.00 to 26.00) | 26.00 (21.46 to 28.00) |
| Sham | 23.50 (21.50 to 27.00) | 24.00 (23.00 to 24.00) | 23.50 (20.00 to 26.00) | 22.50 (17.06 to 26.00) | 23.00 (20.00 to 25.00) | 22.50 (18.00 to 26.00) | |
| Body representation | |||||||
| Bath CRPS Body Perception Disturbance Scale (/57) M | PA | 27.65 (22.83 to 32.34) | 27.78 (24.00 to 31.22) | 22.13 (17.88 to 26.44) | 24.39 (20.48 to 28.57) | 25.52 (21.78 to 29.30) | 24.57 (20.91 to 28.44) |
| Sham | 28.96 (23.96 to 33.76) | 27.73 (21.98 to 33.92) | 29.00 (23.00 to 35.36) | 26.81 (20.92 to 33.61) | 26.77 (21.48 to 32.68) | 27.65 (22.53 to 33.28) | |
| Emotional functioning | |||||||
| Fear of movement (Tampa Scale for Kinesiophobia[/68]) M | PA | 38.79 (35.45 to 41.95) | 38.52 (35.02 to 41.73) | 37.43 (34.26 to 40.50) | 37.91 (34.70 to 41.17) | 38.74 (35.33 to 41.95) | 40.05 (36.22 to 43.71) |
| Sham | 40.38 (37.17 to 43.35) | 39.73 (36.43 to 42.81) | 38.27 (34.90 to 41.45) | 37.42 (34.00 to 40.71) | 38.24 (34.97 to 41.56) | 37.27 (33.45 to 40.79) | |
| Mood disturbance (Profile of Mood States[/229]) M | PA | 94.81 (79.96 to 109.93) | 98.25 (82.66 to 113.93) | 86.52 (71.16 to 100.10) | 86.21 (73.85 to 99.00) | 88.80 (74.42 to 103.51) | 95.54 (73.56 to 117.95) |
| Sham | 84.22 (70.94 to 98.08) | 91.27 (76.05 to 106.01) | 83.21 (68.95 to 96.96) | 83.35 (68.76 to 97.56) | 82.42 (68.05 to 96.53) | 89.13 (70.81 to 106.31) | |
| Perceived improvement due to treatment | |||||||
| Patients' Global Impression of Change (/7) Mdn | PA | — | — | 2.00 (2.00 to 4.00) | 3.00 (3.00 to 3.00) | 2.00 (2.00 to 4.00) | 3.00 (2.00 to 3.00) |
| Sham | — | — | 3.00 (1.00 to 4.00) | 4.00 (3.00 to 4.00) | 2.00 (2.00 to 3.00) | 2.00 (1.00 to 4.00) | |
| Clinical assessments | |||||||
| Sensory functions | |||||||
| Mechanical detection threshold ratio Mdn | PA | −0.04 (−0.43 to 0.38) | −0.35 (−1.12 to 0.17) | −0.44 (−0.84 to −0.06) | −0.54 (−1.51 to −0.10) | — | — |
| Sham | −0.30 (−1.37 to 0.24) | −0.05 (−0.25 to 0.17) | −0.14 (−0.91 to 0.30) | −0.22 (−0.76 to 0.28) | — | — | |
| Mechanical pain threshold ratio Mdn | PA | 0.62 (0.06 to 0.69) | 0.50 (0.43 to 0.56) | 0.07 (−0.32 to 0.66) | 0.50 (0.06 to 0.69) | — | — |
| Sham | 0.57 (0.24 to 0.67) | 0.56 (0.38 to 0.73) | 0.50 (0.32 to 0.71) | 0.43 (0.24 to 0.78) | — | — | |
| Allodynia (/100) Mdn | PA | 14.00 (5.76 to 26.67) | 18.87 (4.67 to 30.89) | 16.90 (6.00 to 26.17) | 10.73 (2.87 to 18.26) | — | — |
| Sham | 20.50 (9.00 to 33.83) | 14.37 (6.47 to 25.03) | 13.87 (6.47 to 46.47) | 18.03 (7.33 to 33.33) | — | — | |
| Two-point discrimination threshold ratio Mdn | PA | −0.06 (−0.16 to 0.11) | 0.00 (−0.08 to 0.13) | −0.08 (−0.20 to 0.00) | −0.04 (−0.21 to 0.03) | — | — |
| Sham | 0.15 (−0.07 to 0.31) | −0.13 (−0.25 to 0.10) | −0.09 (−0.17 to 0.00) | 0.05 (−0.30 to 0.22) | — | — | |
| Autonomic functions | |||||||
| Absolute temperature difference (°C) Mdn | PA | 0.47 (0.27 to 1.40) | 0.30 (0.14 to 0.68) | 0.35 (0.20 to 0.73) | 0.50 (0.17 to 1.17) | — | — |
| Sham | 0.47 (0.30 to 0.78) | 0.82 (0.53 to 1.07) | 0.77 (0.43 to 1.05) | 0.67 (0.40 to 1.00) | — | — | |
| Oedema difference (cm) M | PA | −0.01 (−0.42 to 0.43) | −0.04 (−0.36 to 0.28) | −0.19 (−0.58 to 0.21) | −0.23 (−0.64 to 0.20) | — | — |
| Sham | −0.11 (−0.51 to 0.34) | −0.02 (−0.40 to 0.38) | −0.12 (−0.52 to 0.30) | 0.04 (−0.33 to 0.43) | — | — | |
| Motor functions | |||||||
| Grip strength ratio Mdn | PA | 0.35 (0.17 to 0.39) | 0.31 (0.25 to 0.44) | 0.35 (0.30 to 0.46) | 0.39 (0.30 to 0.46) | — | — |
| Sham | 0.32 (0.20 to 0.65) | 0.33 (0.18 to 0.58) | 0.44 (0.26 to 0.60) | 0.42 (0.23 to 0.60) | — | — | |
| Delta finger-to-palm distance ratio Mdn | PA | 0.70 (0.60 to 0.88) | 0.67 (0.61 to 0.87) | 0.73 (0.63 to 0.84) | 0.79 (0.70 to 0.82) | — | — |
| Sham | 0.69 (0.55 to 0.90) | 0.72 (0.53 to 0.88) | 0.79 (0.53 to 0.89) | 0.77 (0.60 to 0.93) | — | — | |
| Experimental tests of neuropsychological functions | |||||||
| Visuospatial attention | |||||||
| Temporal Order Judgement task (Point of Subjective Simultaneity [ms]) Mdn | PA | 0.16 (−13.82 to 9.02) | −3.26 (−14.51 to 8.35) | −1.00 (−8.65 to 9.71) | 5.18 (−1.74 to 10.87) | — | — |
| Sham | −0.05 (−7.40 to 7.06) | −0.75 (−8.55 to 6.65) | 1.17 (−6.16 to 7.33) | −2.12 (−10.48 to 6.07) | — | — | |
| Landmark task (Point of Subjective Equality[°]) Mdn | PA | 0.04 (−0.20 to 0.28) | 0.09 (−0.01 to 0.19) | 0.03 (−0.09 to 0.40) | −0.02 (−0.13 to 0.19) | — | — |
| Sham | 0.06 (−0.07 to 0.21) | 0.06 (−0.12 to 0.17) | −0.05 (−0.09 to 0.10) | 0.05 (−0.04 to 0.10) | — | — | |
| Grayscales task M | PA | 0.17 (−0.07 to 0.41) | 0.12 (−0.11 to 0.34) | 0.08 (−0.13 to 0.30) | 0.11 (−0.12 to 0.34) | — | — |
| Sham | 0.09 (−0.08 to 0.26) | 0.12 (−0.08 to 0.32) | 0.07 (−0.10 to 0.25) | 0.14 (−0.06 to 0.31) | — | — | |
| Mental representation of space | |||||||
| Mental Number Line Bisection task M | PA | −0.06 (−0.76 to 0.67) | −0.10 (−0.73 to 0.54) | 0.04 (−0.58 to 0.63) | −0.06 (−0.55 to 0.42) | — | — |
| Sham | 0.12 (−0.51 to 0.77) | 0.24 (−0.50 to 0.99) | 0.39 (−0.36 to 1.21) | 0.31 (−0.34 to 0.99) | — | — | |
| Spatially defined motor function | |||||||
| Directional hypokinesia, affected hand, index A (ms) Mdn | PA | −4.88 (−41.02 to 29.55) | −2.23 (−40.87 to 16.76) | −15.41 (−58.35 to −9.44) | −21.93 (−40.85 to −9.44) | — | — |
| Sham | −15.65 (−79.21 to 26.94) | 21.51 (−21.38 to 56.06) | −24.31 (−61.88 to −12.51) | −12.26 (−47.44 to 16.73) | — | — | |
| Directional hypokinesia, affected hand, index B (ms) Mdn | PA | −37.53 (−90.19 to 16.61) | −25.46 (−84.04 to 13.63) | −48.49 (−80.33 to −22.88) | 4.10 (−40.19 to 10.67) | — | — |
| Sham | −40.43 (−48.52 to −21.96) | −0.40 (−61.60 to 15.61) | −8.19 (−48.87 to 13.72) | 3.32 (−43.06 to 20.37) | — | — | |
| Directional hypokinesia, unaffected hand, index A (ms) Mdn | PA | 0.14 (−15.93 to 19.88) | 10.28 (1.15 to 22.22) | −6.76 (−19.29 to 13.43) | −2.78 (−45.45 to 13.43) | — | — |
| Sham | 5.57 (−24.54 to 26.03) | −7.88 (−20.59 to 14.27) | 6.88 (−15.63 to 18.92) | 2.51 (−13.48 to 23.38) | — | — | |
| Directional hypokinesia, unaffected hand, index B (ms) Mdn | PA | 4.84 (−6.43 to 11.89) | 9.41 (−17.73 to 25.19) | −3.40 (−21.35 to 33.52) | 7.43 (−26.43 to 38.21) | — | — |
| Sham | −23.63 (−48.34 to 12.93) | 9.18 (−12.92 to 28.54) | 11.01 (−10.84 to 28.46) | 16.47 (−2.91 to 26.35) | — | — | |
| Directional bradykinesia, affected hand, index A (ms) Mdn | PA | 97.95 (23.29 to 216.69) | 64.71 (22.36 to 123.85) | 46.11 (14.19 to 72.77) | 52.74 (22.18 to 66.01) | — | — |
| Sham | 3.73 (−32.67 to 67.35) | 50.72 (−5.50 to 64.21) | 31.79 (3.63 to 87.48) | 41.09 (11.92 to 64.21) | — | — | |
| Directional bradykinesia, affected hand, index B (ms) Mdn | PA | −49.68 (−125.50 to −8.16) | −180.86 (−235.79 to −16.96) | −124.70 (−129.09 to −122.32) | −78.67 (−115.85 to −42.31) | — | — |
| Sham | −63.53 (−149.00 to −57.28) | −103.18 (−170.48 to −54.09) | −77.46 (−97.96 to −17.51) | −75.60 (−99.62 to −48.41) | — | — | |
| Directional bradykinesia, unaffected hand, index A (ms) Mdn | PA | 48.80 (35.53 to 64.67) | 69.36 (35.74 to 103.71) | 79.01 (45.24 to 99.85) | 79.78 (59.27 to 116.99) | — | — |
| Sham | 86.46 (54.45 to 127.39) | 69.84 (24.68 to 113.96) | 84.79 (76.26 to 86.26) | 48.80 (35.53 to 64.67) | — | — | |
| Directional bradykinesia, unaffected hand, index B (ms) Mdn | PA | 31.39 (−13.35 to 64.92) | 69.35 (25.05 to 98.88) | 36.70 (21.07 to 63.50) | 20.37 (−13.72 to 66.72) | — | — |
| Sham | −28.35 (−71.98 to 41.21) | 3.34 (−39.16 to 44.61) | 28.60 (6.71 to 53.45) | 3.38 (−22.98 to 12.57) | — | — | |
| Body representation | |||||||
| Hand laterality recognition, accuracy index (%) M | PA | −1.65 (−5.66 to 2.34) | −2.26 (−5.68 to 1.43) | 1.30 (−2.23 to 4.37) | 1.57 (−2.70 to 6.00) | — | — |
| Sham | 2.77 (−1.32 to 7.37) | −1.77 (−5.83 to 2.21) | 3.92 (0.19 to 7.72) | 2.54 (−2.00 to 6.83) | — | — | |
| Hand laterality recognition, reaction time index (ms) M | PA | −97.74 (−268.99 to 70.43) | −57.20 (−187.91 to 70.02) | −37.44 (−155.06 to 78.98) | −130.05 (−240.98 to −27.84) | — | — |
| Sham | −236.04 (−448.24 to −65.14) | −129.37 (−263.61 to 21.19) | −28.65 (−173.14 to 95.70) | 19.77 (−112.95 to 161.67) | — | — | |
BCa 95% CI, bootstrapped bias-corrected and accelerated 95% confidence interval; CRPS, complex regional pain syndrome; LTFU1 and LTFU2, long-term follow-ups 1 and 2; M, mean; Mdn, median; PA, prism adaptation treatment; RS1, RS2, RS3, and RS4, research sessions 1, 2, 3, and 4; Sham, sham treatment.
Results of 2 × 6 ANOVAs on the self-reported pain-related, body representation, and emotional functioning outcomes and 2 × 4 ANOVAs on the sensory, motor, autonomic, and neuropsychological functions are reported in Table 3. Among these outcomes, the mechanical detection threshold, mechanical pain threshold, two-point discrimination threshold, grip strength, and delta finger-to-palm distance ratio data, the Landmark task, and spatially defined motor function data were analysed using linear mixed models regression because of severe violations of normality, homogeneity of variance, and/or sphericity assumptions. The results are reported in Table 4.
Table 3.
Analysis of variance results for secondary outcome measures (intention-to-treat analysis).
| Measure | Effect | df* | F | P | |
|---|---|---|---|---|---|
| Self-report questionnaires | |||||
| Pain severity (Brief Pain Inventory) | Time | 4.12, 193.81 | 1.24 | 0.295 | 0.03 |
| Group | 1, 47 | 0.19 | 0.664 | < 0.01 | |
| Time × Group | 4.12, 193.81 | 1.06 | 0.379 | 0.02 | |
| Pain interference (Brief Pain Inventory) | Time† | 2.88, 135.32 | 2.84 | 0.043 | 0.06 |
| Group | 1, 47 | 0.04 | 0.838 | < 0.01 | |
| Time × Group | 2.88, 135.32 | 0.74 | 0.526 | 0.02 | |
| Neuropathic features of pain (Pain Detect Questionnaire) | Time† | 3.29, 154.50 | 3.32 | 0.018 | 0.07 |
| Group | 1, 47 | 0.32 | 0.574 | 0.01 | |
| Time × Group | 3.29, 154.50 | 0.61 | 0.625 | 0.01 | |
| Bath CRPS Body Perception Disturbance Scale | Time | 3.41, 160.11 | 2.43 | 0.059 | 0.05 |
| Group | 1, 47 | 0.57 | 0.455 | 0.01 | |
| Time × Group† | 3.41, 160.11 | 2.60 | 0.047 | 0.05 | |
| Fear of movement (Tampa Scale for Kinesiophobia) | Time | 3.86, 181.61 | 2.41 | 0.053 | 0.05 |
| Group | 1, 47 | <0.01 | 0.993 | <0.01 | |
| Time × Group† | 3.86, 181.61 | 2.89 | 0.025 | 0.06 | |
| Mood disturbance (Profile of Mood States) | Time | 3.60, 169.21 | 2.29 | 0.069 | 0.05 |
| Group | 1, 47 | 0.36 | 0.554 | 0.01 | |
| Time × Group | 3.60, 169.21 | 0.25 | 0.894 | 0.01 | |
| Patients' Global Impression of Change | Time | 3, 120 | 0.96 | 0.414 | 0.02 |
| Group | 1, 40 | 0.02 | 0.890 | <0.01 | |
| Time × Group | 3, 120 | 0.56 | 0.644 | 0.01 | |
| Clinical assessments | |||||
| Allodynia (affected limb) | Time | 2.23, 104.67 | 1.03 | 0.367 | 0.02 |
| Group | 1, 47 | 0.25 | 0.616 | 0.01 | |
| Time × Group | 2.23, 104.67 | 0.35 | 0.730 | 0.01 | |
| Absolute temperature difference | Time | 3, 141 | 0.43 | 0.731 | 0.01 |
| Group | 1, 47 | 0.16 | 0.695 | <0.01 | |
| Time × Group | 3, 141 | 0.63 | 0.595 | 0.01 | |
| Oedema difference | Time | 2.41, 113.08 | 0.99 | 0.387 | 0.02 |
| Group | 1, 47 | 0.06 | 0.805 | <0.01 | |
| Time × Group | 2.41, 113.08 | 1.86 | 0.153 | 0.04 | |
| Experimental tests of neuropsychological functions | |||||
| Temporal-Order Judgement task (Point of Subjective Simultaneity) | Time | 1.70, 79.69 | 1.08 | 0.335 | 0.02 |
| Group | 1, 47 | 0.16 | 0.692 | <0.01 | |
| Time × Group | 1.70, 79.69 | 0.63 | 0.512 | 0.01 | |
| Grayscales task | Time | 2.17, 101.82 | 0.57 | 0.581 | 0.01 |
| Group | 1, 47 | 0.02 | 0.899 | <0.01 | |
| Time × Group | 2.17, 101.82 | 0.52 | 0.609 | 0.01 | |
| Mental Number Line Bisection task | Time | 2.39, 112.17 | 0.48 | 0.656 | 0.01 |
| Group | 1, 47 | 0.50 | 0.481 | 0.01 | |
| Time × Group | 2.39, 112.17 | 0.14 | 0.899 | <0.01 | |
| Hand laterality recognition, accuracy index | Time | 3, 141 | 2.39 | 0.072 | 0.05 |
| Group | 1, 47 | 1.54 | 0.221 | 0.03 | |
| Time × Group | 3, 141 | 0.44 | 0.723 | 0.01 | |
| Hand laterality recognition, reaction time index | Time | 3, 141 | 1.32 | 0.269 | 0.03 |
| Group | 1, 47 | 0.05 | 0.826 | <0.01 | |
| Time × Group | 3, 141 | 1.48 | 0.224 | 0.03 |
Greenhouse–Geisser adjusted degrees of freedom are reported where sphericity assumption was violated.
Statistically significant effect (P < 0.05).
Table 4.
Results of the bootstrapped linear mixed models regressions of scores for the tests of sensory and motor function, visuospatial attention, and spatially defined motor function (intention-to-treat analysis).
| Model term | Coefficient estimate (95% CI) | |||||
|---|---|---|---|---|---|---|
| Sensory functions | Motor functions | Visuospatial attention | ||||
| Mechanical detection threshold ratio | Mechanical pain threshold ratio | Two-point discrimination threshold ratio | Grip ratio | Delta finger-to-palm distance ratio | Landmark task (Point of Subjective Equality) | |
| Intercept | −1.27 (−1.96 to −0.64)* | 0.17 (−0.12 to 0.45) | −0.04 (−0.16 to 0.07) | 0.39 (0.35 to 0.43)* | 0.71 (0.65 to 0.76)* | 0.13 (0.02 to 0.24)* |
| Time (RS2 = 0) | ||||||
| RS1 | −0.40 (−1.99 to 1.05) | −0.44 (−0.99 to 0.07) | −0.02 (−0.21 to 0.15) | −0.03 (−0.08 to 0.01) | −0.04 (−0.12 to 0.03) | 0.09 (−0.18 to 0.38) |
| RS3 | −0.26 (−1.29 to 0.69) | −0.49 (−1.00 to −0.07)* | −0.14 (−0.31 to 0.03) | 0.01 (−0.05 to 0.06) | −0.02 (−0.11 to 0.06) | 0.06 (−0.09 to 0.21) |
| RS4 | −0.58 (−1.83 to 0.48) | −0.14 (−0.51 to 0.24) | −0.04 (−0.21 to 0.13) | 0.03 (−0.03 to 0.09) | 0.03 (−0.04 to 0.10) | −0.02 (−0.16 to 0.14) |
| Group (PA = 0) | ||||||
| Sham | 0.78 (−0.09 to 1.71) | 0.12 (−0.30 to 0.52) | −0.16 (−0.37 to 0.05) | 0.02 (−0.04 to 0.07) | −0.05 (−0.13 to 0.02) | −0.11 (−0.24 to 0.03) |
| Time × Group (RS2, PA = 0) | ||||||
| RS1, sham | −0.03 (−1.74 to 1.72) | 0.34 (−0.32 to 1.05) | 0.27 (−0.08 to 0.64) | 0.01 (−0.05 to 0.08) | 0.03 (−0.07 to 0.13) | 0.04 (−0.30 to 0.34) |
| RS3, sham | −0.72 (−2.51 to 1.03) | 0.65 (0.08 to 1.31)* | 0.15 (−0.12 to 0.44) | 0.05 (−0.03 to 0.12) | 0.05 (−0.05 to 0.16) | −0.05 (−0.24 to 0.15) |
| RS4, sham | 0.47 (−1.10 to 1.92) | 0.15 (−0.43 to 0.72) | −0.08 (−0.54 to 0.30) | 0.01 (−0.09 to 0.09) | 0.00 (−0.09 to 0.10) | 0.01 (−0.18 to 0.21) |
| Model term | Coefficient estimate (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| Directional hypokinesia (movement initiation time) | Directional bradykinesia (movement execution time) | |||||||
| Affected hand | Unaffected hand | Affected hand | Unaffected hand | |||||
| Index A | Index B | Index A | Index B | Index A | Index B | Index A | Index B | |
| Intercept | −10.05 (−39.20 to 21.45) | −24.15 (−53.82 to 6.72) | 10.02 (−4.94 to 25.30) | 3.88 (−17.06 to 24.22) | 15.22 (−56.77 to 78.91) | −121.77 (−189.03 to −56.16)* | 79.38 (58.21 to 97.75)* | 62.23 (32.32 to 92.68)* |
| Time (RS2 = 0) | ||||||||
| RS1 | 6.89 (−36.08 to 49.02) | −21.12 (−68.19 to 25.55) | −5.25 (−35.93 to 29.34) | 2.34 (−26.63 to 32.30) | 102.01 (−0.40 to 210.27) | 44.71 (−56.29 to 153.07) | 14.99 (−20.31 to 48.61) | −25.34 (−76.29 to 25.35) |
| RS3 | −33.02 (−84.48 to 9.86) | −45.53 (−111.05 to 7.54) | −10.09 (−38.16 to 15.04) | 0.89 (−26.55 to 29.32) | 8.63 (−77.98 to 94.43) | −39.71 (−138.47 to 49.83) | 5.36 (−22.59 to 36.65) | −22.48 (−59.69 to 12.28) |
| RS4 | −16.09 (−68.73 to 34.27) | 25.03 (−25.51 to 74.29) | −24.41 (−53.09 to 2.65) | −5.74 (−49.46 to 35.70) | 20.89 (−59.88 to 111.76) | 52.43 (−32.35 to 141.20) | −6.33 (−34.75 to 21.80) | −32.80 (−73.29 to 7.13) |
| Group (PA = 0) | ||||||||
| Sham | 32.66 (−19.54 to 90.94) | 20.19 (−21.86 to 65.35) | −6.51 (−28.25 to 18.48) | 7.61 (−21.91 to 38.77) | 44.89 (−27.15 to 124.77) | −18.92 (−118.56 to 80.89) | 5.20 (−29.93 to 38.56) | −59.74 (−100.47 to −22.16)* |
| Time × Group (RS2, PA = 0) | ||||||||
| RS1, sham | −42.92 (−123.52 to 34.03) | 18.95 (−62.13 to 98.40) | 2.46 (−43.43 to 44.09) | −21.64 (−65.60 to 21.48) | −111.16 (−233.33 to 14.22) | −30.20 (−171.51 to 100.54) | −7.59 (−58.92 to 43.47) | 14.50 (−57.28 to 82.84) |
| RS3, sham | −27.60 (−100.88 to 40.87) | 13.76 (−71.15 to 108.58) | 10.89 (−25.75 to 45.98) | −1.53 (−41.93 to 39.18) | −1.62 (−112.01 to 102.07) | 82.47 (−55.81 to 211.05) | −10.65 (−55.35 to 31.62) | 44.51 (−2.63 to 93.84) |
| RS4, sham | −32.05 (−116.58 to 53.89) | −12.08 (−99.58 to 74.10) | 25.45 (−9.96 to 61.39) | 19.69 (−33.43 to 74.47) | −15.01 (−111.56 to 78.71) | 0.90 (−126.26 to 127.46) | −18.42 (−61.34 to 27.17) | 27.25 (−23.77 to 76.93) |
The reference condition for dummy variable coding is indicated within parentheses for each term.
Significant effect (95% CI around the coefficient estimate does not include 0).
CI, confidence interval; PA, prism adaptation treatment; RS1, RS2, RS3, and RS4, research sessions 1, 2, 3, and 4; Sham, sham treatment.
Contrary to our hypothesis, we found no evidence of significantly greater reductions in self-reported CRPS-related and psychological disturbances or sensory, autonomic, and motor impairments after PA compared with sham treatment. We also did not find any significantly greater reductions in biases in spatial cognition, motor control, and body representation after PA compared with sham treatment. That is, most interactions between treatment group and time on these outcomes were not significant, and any effects revealed by the planned contrasts following the few significant interactions did not withstand correction for multiple comparisons. These effects are further elaborated below. A significant interaction on the Bath CRPS Body Perception Disturbance scores appeared to be due to reductions in body perception disturbance after the PA treatment, ts ≤ 2.86, Psadj ≥ 0.336, ds ≤ 0.54. A significant interaction on the Tampa Scale for Kinesiophobia scores appeared to be driven by reductions in fear of movement after the sham treatment, ts ≤ 2.63, Psadj ≥ 0.312, ds ≤ 0.26. A significant interaction on the mechanical pain threshold ratios seemed to be due to a reduction in hyperalgesia over the treatment period in the PA group, Zs ≤ 1.68, Psadj ≥ 0.440, ds ≤ 0.51. All these effects were no longer significant after Holm–Bonferroni correction, and there were no other significant interactions. On average, participants in both treatment groups perceived their symptoms to be either “almost the same” or “a little better” (2-3 of 7 on the Patients' Global Impression of Change) at each posttreatment time point.
The PA and sham treatment groups also did not differ on their average daily logbook ratings of pain intensity, symptom interference, and range of movement at any time point [pain intensity: ts(45) ≤ 1.75, Ps ≥ 0.093, ds ≤ 0.51; symptom interference: ts(45) ≤ 1.24, Ps ≥ 0.240, ds ≤ 0.36; and range of movement: ts(45) ≤ 1.81, Ps ≥ 0.062, ds ≤ 0.53]. The planned analyses of the number of days to reach peak improvement and from peak improvement to return to baseline on each of these measures34 are not reported because they would not be informative in the absence of treatment effects, but logbook ratings for each group are illustrated in Supplemental Figure S2 (available at http://links.lww.com/PAIN/B162).
Overall, our analyses did not reveal any significant effects of PA compared with sham treatment on any of the secondary outcome measures.
3.5. Predictors of complex regional pain syndrome progression over time
Because there was no effect of treatment on the primary outcomes, we did not explore potential predictors of the response to PA treatment as proposed in the trial protocol.34 However, to explore whether the absence of the PA effect could be explained by the lack of group-level neuropsychological deficits in our sample, we visualised individual relationships between the changes on the primary outcomes over the treatment period and baseline spatial bias and body representation distortion in Supplemental Figs. S4 and S5, respectively (available at http://links.lww.com/PAIN/B162). Overall, there were no apparent clusters of participants or relationships between these factors. Subgroup analyses of whether response to treatment depended on clinical phenotypes of CRPS16 or baseline neuropsychological differences are also reported in Supplemental Text 3 (available at http://links.lww.com/PAIN/B162), showing results consistent with the primary analyses.
To address research question 4 about the predictors of CRPS progression over time, we explored which baseline factors (RS1) could predict overall change in pain intensity (across RS1-RS4 and LTFU1-LTFU2) and CRPS severity (across RS1-RS4). Table 5 summarises the models identified through best subsets regression analyses and their respective values of our model selection criteria (the lowest Akaike Information Criteria and cross-validation errors indicating the best models). Greater reduction in pain intensity was best predicted by smaller change in hand preference since CRPS onset (absolute change on the Edinburgh Handedness Inventory; t = 2.34, P = 0.024, ß = 0.33) in a 1-factor model, F(1, 46) = 5.46, P = 0.024. Greater reduction in CRPS severity was best predicted by lower pain intensity (t = 3.69, P < 0.001, ß = 0.52), less swelling of the affected limb (t = 2.52, P = 0.015, ß = 0.37), and more accurate recognition of images of the affected hand (ie, smaller Hand Laterality Recognition Accuracy Index; t = 2.43, P = 0.019, ß = 0.32), as measured at baseline (RS1), in a 3-factor model, F(3, 45) = 6.23, P = 0.001.
Table 5.
Best subsets of factors (as measured in RS1) for predicting overall change in pain intensity and CRPS severity throughout the study period (intention-to-treat analysis).
| Best subsets models | Adj. R2 | AIC | CV |
|---|---|---|---|
| Change in pain intensity† | |||
| Model 1: (+) absolute change in handedness* | 0.09 | −132.24 | 0.28 |
| Model 2: (+) absolute change in handedness,* (+) index A of directional bradykinesia for the unaffected hand | 0.13 | −119.08 | 0.29 |
| Model 3: (+) oedema difference,** (−) index B of directional hypokinesia for the affected hand,** (−) delta finger-to-palm distance ratio* | 0.25 | −105.79 | 0.30 |
| Model 4: (+) oedema difference,** (−) index B of directional hypokinesia for the affected hand,* (−) delta finger-to-palm distance ratio,* (+) mood disturbance | 0.27 | −105.68 | 0.30 |
| Model 5: (+) oedema difference,** (−) index B of directional hypokinesia for the affected hand,** (+) Mental Number Line Bisection score,* (+) absolute change in handedness, (−) delta finger-to-palm distance ratio | 0.32 | −107.60 | 0.35 |
| Change in CRPS severity† | |||
| Model 1: (+) current pain intensity** | 0.13 | −50.21 | 0.58 |
| Model 2: (+) current pain intensity,** (−) index B of directional bradykinesia for the unaffected hand* | 0.23 | −48.39 | 0.60 |
| Model 3: (+) current pain intensity,*** (+) oedema difference,* (+) hand laterality recognition accuracy index* | 0.25 | −55.52 | 0.54 |
| Model 4: (+) allodynia on the affected limb,* (−) index B of directional bradykinesia for the unaffected hand,* (−) index B of directional hypokinesia for the unaffected limb,* (+) disease duration | 0.21 | −45.66 | 0.62 |
| Model 5: (−) index B of directional bradykinesia for the unaffected hand,* (−) index B of directional hypokinesia for the unaffected limb, (+) allodynia on the affected limb, (+) disease duration, (+) body perception disturbance score | 0.21 | −44.84 | 0.62 |
Figures in bold indicate the lowest AIC and CV.
* P < 0.05, ** P < 0.01, and *** P < 0.001 indicate significant predictors; (+), positive predictor; (−), negative predictor.
Predicted outcomes were quantified as individual regression slopes based on pain intensity ratings throughout RS1-RS4 and LTFU1-LTFU2 and CRPS severity scores throughout RS1-RS4 (negative slopes indicate reductions in pain/CRPS severity).
Adj. R2, adjusted R-squared; AIC, Akaike information criterion; CRPS, complex regional pain syndrome; CV, cross-validation error; LTFU1 and LTFU2, long-term follow-ups 1 and 2; RS, RS1, RS2, RS3, and RS4, research sessions 1, 2, 3, and 4.
4. Discussion
The results from this double-blind, randomized, sham-controlled trial do not support the effectiveness of PA treatment for upper-limb CRPS-I. First, we found no evidence that 2 weeks of twice-daily PA treatment performed with the affected arm reduced the primary outcomes of current pain intensity or symptom severity more than sham treatment in long-standing CRPS. Second, we found no evidence that PA affected the secondary outcomes of self-reported CRPS-related and psychological functioning; sensory, motor, and autonomic signs; or spatial cognition, motor function, and body representation.
Our findings contradict the conclusions of previous studies that PA could relieve pain and other CRPS symptoms. In the first of these, 2 weeks of once-daily PA training resulted in 50% pain relief and reduced oedema and skin discoloration in 5 people with CRPS.100 In the second study, 3 weeks of daily PA effectively resolved 1 patient's pain, reduced autonomic symptoms, and improved motor function.9 In the third study, 4 days of twice-daily PA resulted in 36% pain relief in 7 people with CRPS.12 In the 2 latter studies, its effects on pain were maintained for up to 2 weeks after discontinuing the treatment. While addressing the limitations of these preliminary small-sample, uncontrolled, unblinded studies, our robust trial showed no evidence of any benefits of PA for CRPS beyond those of a control treatment. A small reduction in pain intensity immediately after PA (13% reduction) was not significantly greater than after sham treatment (3%). Similarly, there was an overall reduction in CRPS severity immediately after treatment that persisted for 4 weeks but was present in both PA (7%) and sham (8%) treatment groups. Although a lack of evidence for superiority of PA relative to sham treatment does not prove their equivalence, the effect sizes of any differences were negligible to small. Consistent across per-protocol and intention-to-treat analyses, there is no evidence that PA is any more effective than sham treatment for CRPS.
The decrease in CRPS severity across both treatment groups could be explained by a placebo effect and/or general benefits of moving the affected limb. Meta-analyses of clinical trials found that placebo response can correspond to an 1.84-point immediate posttreatment reduction in CRPS pain60 or a 0.65-point reduction in chronic pain generally (on a 0-10 scale).39 This effect might also be responsible for the reduction in CRPS severity in our trial. Increased movement of the affected limb is a likely alternative explanation because all participants performed the pointing task with their affected hand, regardless of the treatment condition. Physical exercise is a core pillar of CRPS management,28 and this additional daily activity might have been sufficient to reduce CRPS severity. It is unlikely that the observed changes were due to natural recovery, which might occur within the first year from diagnosis,1 as participants were on average diagnosed with CRPS for 5 years. Disease duration was also unrelated to changes in pain intensity or CRPS severity (Supplemental Fig. S3, available at http://links.lww.com/PAIN/B162). Regression to the mean cannot fully account for the decrease in CRPS severity, as no changes occurred over the baseline period. Overall, our findings reinforce the importance of including control treatment arms in pain rehabilitation studies and the role of active movement in managing long-standing CRPS.
We address 3 potential reasons why we did not find the hypothesised effects of PA on clinical outcomes: (1) noncentral pathophysiology of CRPS, (2) absence of neuropsychological symptoms, and (3) trial limitations. First, because PA targets neuropsychological deficits, it would possibly be most appropriate for a subset of individuals who predominately show signs of central neuroplasticity (compared with peripheral inflammation).5,16 However, post hoc classification of participants into central or peripheral phenotypes and follow-up exploratory subgroup analysis did not reveal different responses to PA vs sham treatment (Supplemental Table S1 and Text S3, available at http://links.lww.com/PAIN/B162).
Second, it is possible that we found no effect of PA on participants' spatial cognition or body representation because, in contrast to previous findings,11,21,25,27,76,84,85,94,99 they did not have any systematic deficits on baseline experimental measures of spatial cognition and body representation. One hypothesised mechanism of the apparent benefits of PA in previous CRPS studies is that it reduces pain by correcting the “neglect-like” bias away from the affected side. A potential second mechanism is based on the proposal that distorted body representation gives rise to discrepancies between anticipated and actual consequences of movement, which cause or exacerbate pain in conditions such as CRPS.8,38,61,62 The transient sensorimotor incongruence introduced by wearing prisms is thought to provide an error signal that triggers normalisation of body representation and sensorimotor integration.9,100 On average, our participants showed balanced distributions of spatial attention and spatial representations, no systematic slowing of movements directed towards the affected side, and unimpaired laterality recognition of images of affected hands at baseline (Table 2 and Ref. 32). Cognitive after-effects of PA have been shown to depend on baseline spatial bias.14,29,44 Therefore, if altering spatial cognition and/or body representation were integral mechanisms through which PA reduces CRPS symptoms, the absence of preexisting neuropsychological deficits could preclude any effects of PA on the primary clinical outcomes. However, we dismiss this explanation, based on the following reasons. In the follow-up exploratory analyses, we found no relationships between the extent of baseline spatial or body representation deficits and changes in the primary outcomes over the treatment period (Supplemental Figs. S4 and S5, available at http://links.lww.com/PAIN/B162). We also found no evidence that PA benefitted subgroups of individuals who did present with “neglect-like” symptoms or distorted representation of the affected limb (Supplemental Text S3, available at http://links.lww.com/PAIN/B162). Furthermore, Christophe et al.12 reported reduced CRPS pain after PA in the absence of any baseline spatial deficits and without any effect on spatial cognition or motor control. Finally, Sumitani et al.100 found a significant reduction in pain after treatment and a simultaneous shift in the coding of external spatial information relative to the body away from the affected side (ie, direction opposite to the expected PA spatial after-effects). Because these previous studies12,100 had no control treatment arms, the apparent benefits of PA could be due to other nonspecific factors. Nonetheless, overall, it seems that response to PA treatment is unrelated to “neglect-like” spatial bias or body representation distortion.
Third, we considered several limitations of our study that might explain why we did not find the hypothesised effects of PA. Because we tested a protocol of PA that could realistically be integrated into CRPS management as a self-administered, home-based treatment, we cannot rule out compliance violations. We relied solely on participants' self-reported adherence, and therefore, the lack of apparent difference between the effects of PA and sham treatment could be due to deviations from the instructed treatment protocol. However, previous CRPS studies reported symptom improvement after less frequent,100 fewer,12 and home-based9 PA sessions. Prism adaptation protocols similar to ours using sufficiently strong prisms (10-20°) and 10 or more treatment sessions also found generalizable, long-lasting effects on hemispatial neglect.24,95 Our use of home-based treatments meant that it was not feasible to confirm adaptation by measuring pointing after-affects. Yet, previous studies using 50 pointing movements have shown that this is sufficient to create after-effects.9,90,100 Overall, we do not consider that these limitations provide reason to doubt our findings that PA is not an effective treatment for long-standing CRPS. Nonetheless, greater confidence could be gained from a trial of supervised PA with a greater number of sessions and confirmed adaptation. Similarly, acute patients, in whom symptoms are less established, might yet benefit from PA.
This longitudinal study allowed us to explore potential baseline predictors of CRPS progression over 10 to 30 weeks, regardless of treatment. Smaller change in hand preference since CRPS onset predicted greater reduction in pain intensity. Consistent with the learned nonuse hypothesis,80 underutilization of the CRPS-affected limb and compensatory use of the unaffected extremity might maintain CRPS symptoms and hinder recovery. Overall reduction in CRPS severity was predicted by smaller pain intensity and oedema of the affected limb, suggesting that people with milder symptoms are likely to improve more. Individuals who were better at recognising images of affected relative to unaffected hands also achieved greater reduction in CRPS severity. Body perception disturbance was previously linked to longer CRPS duration and more severe sensory and motor signs of CRPS.46,50,105 Our findings that less distorted representation and maintained use of the affected limb predict greater symptom improvement support multidisciplinary pain management approaches, which aim to normalise body representation and foster active movement.28,73,74 These interpretations are, however, tentative, as the analyses were exploratory, and the abovementioned factors explained only 9% and 25% of variance in the overall changes in pain intensity and CRPS severity, respectively.
We conclude that there is no evidence that PA reduces pain and other symptoms more than sham treatment in long-standing CRPS. The benefits of PA reported in previous studies are likely due to the placebo effect, greater movement of the affected limb, regression to the mean, and/or natural recovery.
Conflict of interest statement
M. Halicka and A.D. Vittersø were supported by studentships from the University of Bath and the GW4 BioMed Medical Research Council Doctoral Training Partnership (ref. 1793344), respectively. H. McCullough and A. Goebel have received support from the Pain Relief Foundation, Liverpool. A. Goebel, L. Heelas, and J.H. Bultitude are committee members of the CRPS-UK Research Network. L. Heelas is a committee member of the Physiotherapy Pain Association and the British Pain Society. The authors have no other competing interests to declare.
Oral presentations of the preliminary results were delivered at the UK Sensorimotor Conference in London, UK (2019), and the Pain Research Meeting in Brussels, Belgium (2019).
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B162.
Supplemental video content
A video abstract associated with this article can be found at http://links.lww.com/PAIN/B186.
Acknowledgements
The study was supported by a grant from the Reflex Sympathetic Dystrophy Syndrome Association (RSDSA) awarded to J.H. Bultitude and M.J. Proulx. The RSDSA approved the design of the study. The funders had no role in data collection and analysis, decision to publish, or preparation of the manuscript. The authors thank the individuals with CRPS for taking part in the trial and the CRPS-UK Research Network, Dr Nicholas Shenker, Professor Candida McCabe, and other health professionals for their assistance with participant recruitment. The authors further thank their research assistants (Sophie Brown, Louise Quaife, Phoebe Carlisle, Matthew Haywood, Elizabeth Nikitopoulou, Sanya Manglani, Abdulaziz Aldaughaither, Danesh Paul, Rajavee Arora, Josephine Pedder, and Olivia Cousins) for their help with data entry.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Bean DJ, Johnson MH, Kydd RR. The outcome of complex regional pain syndrome type 1: a systematic review. J Pain 2014;15:677–90. [DOI] [PubMed] [Google Scholar]
- [2].Beerthuizen A, Stronks DL, van't Spijker A, Yaksh A, Hanraets BM, Klein J, Huygen FJPM. Demographic and medical parameters in the development of complex regional pain syndrome type 1 (CRPS1): prospective study on 596 patients with a fracture. PAIN 2012;153:1187–92. [DOI] [PubMed] [Google Scholar]
- [3].Berberovic N, Pisella L, Morris AP, Mattingley JB. Prismatic adaptation reduces biased temporal order judgements in spatial neglect. Neuroreport 2004;15:1199–204. [DOI] [PubMed] [Google Scholar]
- [4].Bingel U, Wanigasekera V, Wiech K, Mhuircheartaigh RN, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med 2011;3:70ra14. [DOI] [PubMed] [Google Scholar]
- [5].Birklein F, Schlereth T. Complex regional pain syndrome—significant progress in understanding. PAIN 2015;156:S94–S103. [DOI] [PubMed] [Google Scholar]
- [6].Blanca MJ, Alarcón R, Arnau J. Non-normal data: is ANOVA still a valid option? Psicothema 2017:552–7. [DOI] [PubMed] [Google Scholar]
- [7].Blanca MJ, Alarcón R, Arnau J, Bono R, Bendayan R. Effect of variance ratio on ANOVA robustness: might 1.5 be the limit? Behav Res Methods 2018;50:937–62. [DOI] [PubMed] [Google Scholar]
- [8].Brun C, Mercier C, Grieve S, Palmer S, Bailey J, McCabe CS. Sensory disturbances induced by sensorimotor conflicts are higher in complex regional pain syndrome and fibromyalgia compared to arthritis and healthy people, and positively relate to pain intensity. Eur J Pain 2019;23:483–94. [DOI] [PubMed] [Google Scholar]
- [9].Bultitude JH, Rafal RD. Derangement of body representation in complex regional pain syndrome: report of a case treated with mirror and prisms. Exp Brain Res 2010;204:409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bultitude JH, Rafal RD, Tinker C. Moving forward with prisms: sensory-motor adaptation improves gait initiation in Parkinson's disease. Front Neurol 2012;3:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bultitude JH, Walker I, Spence C. Space-based bias of covert visual attention in complex regional pain syndrome. Brain 2017;140:2306–21. [DOI] [PubMed] [Google Scholar]
- [12].Christophe L, Chabanat E, Delporte L, Revol P, Volckmann P, Jacquin-Courtois S, Rossetti Y. Prisms to shift pain away: pathophysiological and therapeutic exploration of CRPS with prism adaptation. Neural Plast 2016;2016:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cleeland CS. The Brief Pain Inventory. In: McDowell I, Newell C, editors. Measuring health outcomes. New York: Oxford University Press, 1996. p. 352–7. [Google Scholar]
- [14].Colent C, Pisella L, Bernieri C, Rode G, Rossetti Y. Cognitive bias induced by visuo-motor adaptation to prisms: a simulation of unilateral neglect in normal individuals? Neuroreport 2000;11:1899–902. [DOI] [PubMed] [Google Scholar]
- [15].Dehaene S, Bossini S, Giraux P. The mental representation of parity and number magnitude. J Exp Psychol Gen 1993;122:371–96. [Google Scholar]
- [16].Dimova V, Herrnberger MS, Escolano-Lozano F, Rittner HL, Vlckova E, Sommer C, Maihöfner C, Birklein F. Clinical phenotypes and classification algorithm for complex regional pain syndrome. Neurology 2020;94:e357–67. [DOI] [PubMed] [Google Scholar]
- [17].Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. PAIN 2005;113:9–19. [DOI] [PubMed] [Google Scholar]
- [18].Estimating Sample Size for Comparison of Two Means—Plant Breeding and Genomics. Available at: https://plant-breeding-genomics.extension.org/estimating-sample-size-for-comparison-of-two-means/. Accessed March 14, 2020. [Google Scholar]
- [19].Facchin A, Daini R, Toraldo A. Prismatic adaptation in the rehabilitation of neglect patients: does the specific procedure matter? Front Hum Neurosci 2013;7:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Farrar JT, Young JP, La Moreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. PAIN 2001;94:149–58. [DOI] [PubMed] [Google Scholar]
- [21].Filbrich L, Alamia A, Verfaille C, Berquin A, Barbier O, Libouton X, Fraselle V, Mouraux D, Legrain V. Biased visuospatial perception in complex regional pain syndrome. Sci Rep 2017;7:9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Filbrich L, Torta DM, Vanderclausen C, Azañón E, Legrain V. Using temporal order judgments to investigate attention bias toward pain and threat-related information. Methodological and theoretical issues. Conscious Cogn 2016;41:135–8. [DOI] [PubMed] [Google Scholar]
- [23].Fortis P, Maravita A, Gallucci M, Ronchi R, Grassi E, Senna I, Olgiati E, Perucca L, Banco E, Posteraro L. Rehabilitating patients with left spatial neglect by prism exposure during a visuomotor activity. Neuropsychology 2010;24:681. [DOI] [PubMed] [Google Scholar]
- [24].Frassinetti F, Angeli V, Meneghello F, Avanzi S, Làdavas E. Long-lasting amelioration of visuospatial neglect by prism adaptation. Brain 2002;125:608–23. [DOI] [PubMed] [Google Scholar]
- [25].Frettlöh J, Hüppe M, Maier C. Severity and specificity of neglect-like symptoms in patients with complex regional pain syndrome (CRPS) compared to chronic limb pain of other origins. PAIN 2006;124:184–9. [DOI] [PubMed] [Google Scholar]
- [26].Freynhagen R, Baron R, Gockel U, Tölle TR. Pain DETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911–20. [DOI] [PubMed] [Google Scholar]
- [27].Galer BS, Butler S, Jensen MP. Case reports and hypothesis: a neglect-like syndrome may be responsible for the motor disturbance in reflex sympathetic dystrophy (complex regional pain syndrome-1). J Pain Symptom Manage 1995;10:385–91. [DOI] [PubMed] [Google Scholar]
- [28].Goebel A, Barker CH, Turner-Stokes L. Complex regional pain syndrome in adults: UK guidelines for diagnosis, referral and management in primary and secondary care. 2nd edition. London, United Kingdom: RCP, 2018. [Google Scholar]
- [29].Goedert KM, Leblanc A, Tsai S-W, Barrett AM. Asymmetrical effects of adaptation to left- and right-shifting prisms depends on pre-existing attentional biases. J Int Neuropsychol Soc 2010;16:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Grieve S, Jones L, Walsh N, McCabe CS. What outcome measures are commonly used for complex regional pain syndrome clinical trials? A systematic review of the literature. Eur J Pain 2016;20:331–40. [DOI] [PubMed] [Google Scholar]
- [31].Grieve S, Perez RS, Birklein F, Brunner F, Bruehl S, Harden RN, Packham T, Gobeil F, Haigh R, Holly J, Terkelsen A, Davies L, Lewis J, Thomassen I, Connett R, Worth T, Vatine J-J, McCabe CS. Recommendations for a first Core Outcome Measurement set for complex regional PAin syndrome Clinical sTudies (COMPACT). PAIN 2017;158:1083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Halicka M, Vittersø AD, McCullough H, Goebel A, Heelas L, Proulx MJ, Bultitude JH. Disputing space-based biases in unilateral complex regional pain syndrome. Cortex 2020;127:248–68. [DOI] [PubMed] [Google Scholar]
- [33].Halicka M, Vittersø AD, Proulx MJ, Bultitude JH. Neuropsychological changes in complex regional pain syndrome (CRPS). Behav Neurol 2020;2020:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Halicka M, Vittersø AD, Proulx MJ, Bultitude JH. Pain reduction by inducing sensory-motor adaptation in complex regional pain syndrome (CRPS PRISMA): protocol for a double-blind randomized controlled trial. BMC Neurol 2020;20:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Harden RN, Bruehl S, Perez RSGM, Birklein F, Marinus J, Maihofner C, Lubenow T, Buvanendran A, Mackey S, Graciosa J, Mogilevski M, Ramsden C, Chont M, Vatine J-J. Validation of proposed diagnostic criteria (the “Budapest criteria”) for complex regional pain syndrome. PAIN 2010;150:268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Harden RN, Bruehl S, Perez RSGM, Birklein F, Marinus J, Maihofner C, Lubenow T, Buvanendran A, Mackey S, Graciosa J, Mogilevski M, Ramsden C, Schlereth T, Chont M, Vatine J-J. Development of a severity score for CRPS. PAIN 2010;151:870–6. [DOI] [PubMed] [Google Scholar]
- [37].Harden RN, Maihofner C, Abousaad E, Vatine J-J, Kirsling A, Perez RSGM, Kuroda M, Brunner F, Stanton-Hicks M, Marinus J, van Hilten JJ, Mackey S, Birklein F, Schlereth T, Mailis-Gagnon A, Graciosa J, Connoly SB, Dayanim D, Massey M, Frank H, Livshitz A, Bruehl S. A prospective, multisite, international validation of the complex regional pain syndrome severity score. PAIN 2017;158:1430–6. [DOI] [PubMed] [Google Scholar]
- [38].Harris AJ. Cortical origin of pathological pain. Lancet 1999;354:1464–6. [DOI] [PubMed] [Google Scholar]
- [39].Hróbjartsson A, Gøtzsche PC. Is the placebo powerless? N Engl J Med 2001;344:1594–602. [DOI] [PubMed] [Google Scholar]
- [40].Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulative Physiol Ther 2004;27:26–35. [DOI] [PubMed] [Google Scholar]
- [41].IBM SPSS Statistics. SPSS 23.0 for windows. Armonk, NY: IBM Corp, 2015. [Google Scholar]
- [42].ISRCTN—ISRCTN46828292: treatment of complex regional pain syndrome (CRPS) with sensory-motor adaptation. Available at: 10.1186/ISRCTN46828292. Published 2017. Accessed July 14 2020. [DOI] [Google Scholar]
- [43].Jacquin-Courtois S, O'Shea J, Luauté J, Pisella L, Revol P, Mizuno K, Rode G, Rossetti Y. Rehabilitation of spatial neglect by prism adaptation. Neurosci Biobehav Rev 2013;37:594–609. [DOI] [PubMed] [Google Scholar]
- [44].Jewell G, McCourt ME. Pseudoneglect: a review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia 2000;38:93–110. [DOI] [PubMed] [Google Scholar]
- [45].Johnson S, Hall J, Barnett S, Draper M, Derbyshire G, Haynes L, Rooney C, Cameron H, Moseley GL, C Williams AC, McCabe CS, Goebel A. Using graded motor imagery for complex regional pain syndrome in clinical practice: failure to improve pain: GMI in CRPS. Eur J Pain 2012;16:550–61. [DOI] [PubMed] [Google Scholar]
- [46].Kolb L, Lang C, Seifert F, Maihöfner C. Cognitive correlates of “neglect-like syndrome” in patients with complex regional pain syndrome. PAIN 2012;153:1063–73. [DOI] [PubMed] [Google Scholar]
- [47].Làdavas E, Bonifazi S, Catena L, Serino A. Neglect rehabilitation by prism adaptation: different procedures have different impacts. Neuropsychologia 2011;49:1136–45. [DOI] [PubMed] [Google Scholar]
- [48].Lever J, Krzywinski M, Altman N. Model selection and overfitting. Nat Methods 2016:703–4. [Google Scholar]
- [49].Lewis J, McCabe CS. Body perception disturbance (BPD) in CRPS. Pract Pain Manag 2010;10:60–6. [Google Scholar]
- [50].Lewis J, Schweinhardt P. Perceptions of the painful body: the relationship between body perception disturbance, pain and tactile discrimination in complex regional pain syndrome: perceptions of the painful body in complex regional pain syndrome. Eur J Pain 2012;16:1320–30. [DOI] [PubMed] [Google Scholar]
- [51].Linde K, Witt CM, Streng A, Weidenhammer W, Wagenpfeil S, Brinkhaus B, Willich SN, Melchart D. The impact of patient expectations on outcomes in four randomized controlled trials of acupuncture in patients with chronic pain. PAIN 2007;128:264–71. [DOI] [PubMed] [Google Scholar]
- [52].Lipman MD, Hess DE, Werner BC, Deal DN. Fibromyalgia as a predictor of complex regional pain syndrome after distal radius fracture. Hand (N Y) 2019;14:516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Loftus AM, Nicholls MER, Mattingley JB, Bradshaw JL. Left to right: representational biases for numbers and the effect of visuomotor adaptation. Cognition 2008;107:1048–58. [DOI] [PubMed] [Google Scholar]
- [54].Loftus AM, Vijayakumar N, Nicholls MER. Prism adaptation overcomes pseudoneglect for the greyscales task. Cortex 2009;45:537–43. [DOI] [PubMed] [Google Scholar]
- [55].Luauté J, Halligan P, Rode G, Jacquin-Courtois S, Boisson D. Prism adaptation first among equals in alleviating left neglect: a review. Restor Neurol Neurosci 2006;24:409–18. [PubMed] [Google Scholar]
- [56].Lumley T. leaps: Regression subset selection. R package version 3.0 (based on Fortran code by Alan Miller). 2017. Available at: https://cran.r-project.org/package=leaps. [Google Scholar]
- [57].Makin TR, Wilf M, Schwartz I, Zohary E. Amputees “neglect” the space near their missing hand. Psychol Sci 2010;21:55–7. [DOI] [PubMed] [Google Scholar]
- [58].MATLAB and Statistics Toolbox R2018b.Natick: The MathWorks, Inc; 2018. [Google Scholar]
- [59].Mattingley JB, Bradshaw JL, Phillips JG. Impairments of movement initiation and execution in unilateral neglect: directional hypokinesia and bradykinesia. Brain 1992;115:1849–74. [DOI] [PubMed] [Google Scholar]
- [60].Mbizvo GK, Nolan SJ, Nurmikko TJ, Goebel A. Placebo responses in long-standing complex regional pain syndrome: a systematic review and meta-analysis. J Pain 2015;16:99–115. [DOI] [PubMed] [Google Scholar]
- [61].McCabe CS. A controlled pilot study of the utility of mirror visual feedback in the treatment of complex regional pain syndrome (type 1). Rheumatology 2002;42:97–101. [DOI] [PubMed] [Google Scholar]
- [62].McCabe CS, Blake DR. An embarrassment of pain perceptions? Towards an understanding of and explanation for the clinical presentation of CRPS type 1. Rheumatology 2008;47:1612–16. [DOI] [PubMed] [Google Scholar]
- [63].McCabe CS, Blake DR. Evidence for a mismatch between the brain's movement control system and sensory system as an explanation for some pain-related disorders. Curr Pain Headache Rep 2007;11:104–8. [DOI] [PubMed] [Google Scholar]
- [64].McNair DM, Lorr M, Droppleman LF. Manual for the POMS: San Diego: Educational and Industrial Testing Services, 1971. [Google Scholar]
- [65].Michel C. Beyond the sensorimotor plasticity: cognitive expansion of prism adaptation in healthy individuals. Front Psychol 2016;6:1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Michel C, Pisella L, Halligan PW, Luauté J, Rode G, Boisson D, Rossetti Y. Simulating unilateral neglect in normals using prism adaptation: implications for theory. Neuropsychologia 2003;41:25–39. [DOI] [PubMed] [Google Scholar]
- [67].Miller RP, Kori SH, Todd DD. The Tampa Scale: a measure of kinisophobia. Clin J Pain 1991;7:51. [Google Scholar]
- [68].Minim: allocation by minimisation in clinical trials. Available at: https://www-users.york.ac.uk/∼mb55/guide/minim.htm. Accessed July 26, 2017. [Google Scholar]
- [69].Mizuno K, Tsuji T, Takebayashi T, Fujiwara T, Hase K, Liu M. Prism adaptation therapy enhances rehabilitation of stroke patients with unilateral spatial neglect: a randomized, controlled trial. Neurorehabil Neural Repair 2011;25:711–20. [DOI] [PubMed] [Google Scholar]
- [70].de Mos M, de Bruijn AGJ, Huygen FJPM, Dieleman JP, Stricker ChBH, Sturkenboom MCJM. The incidence of complex regional pain syndrome: a population-based study. PAIN 2007;129:12–20. [DOI] [PubMed] [Google Scholar]
- [71].de Mos M, Huygen FJPM, Dieleman JP, Koopman JSHA, Stricker ChBH, Sturkenboom MCJM. Medical history and the onset of complex regional pain syndrome (CRPS). PAIN 2008;139:458–66. [DOI] [PubMed] [Google Scholar]
- [72].Moseley GL. Distorted body image in complex regional pain syndrome. Neurology 2005;65:773. [DOI] [PubMed] [Google Scholar]
- [73].Moseley GL. Graded motor imagery is effective for long-standing complex regional pain syndrome: a randomised controlled trial. PAIN 2004;108:192–8. [DOI] [PubMed] [Google Scholar]
- [74].Moseley GL. Is successful rehabilitation of complex regional pain syndrome due to sustained attention to the affected limb? A randomised clinical trial. PAIN 2005;114:54–61. [DOI] [PubMed] [Google Scholar]
- [75].Moseley GL, Gallace A, Iannetti GD. Spatially defined modulation of skin temperature and hand ownership of both hands in patients with unilateral complex regional pain syndrome. Brain 2012;135:3676–86. [DOI] [PubMed] [Google Scholar]
- [76].Moseley GL, Gallace A, Spence C. Space-based, but not arm-based, shift in tactile processing in complex regional pain syndrome and its relationship to cooling of the affected limb. Brain 2009;132:3142–51. [DOI] [PubMed] [Google Scholar]
- [77].Nicholls ME, Bradshaw JL, Mattingley JB. Free-viewing perceptual asymmetries for the judgement of brightness, numerosity and size. Neuropsychologia 1999;37:307–14. [DOI] [PubMed] [Google Scholar]
- [78].Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- [79].Peirce JW. PsychoPy—psychophysics software in Python. J Neurosci Methods 2007;162:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Punt DT, Cooper L, Hey M, Johnson MI. Neglect-like symptoms in complex regional pain syndrome: learned nonuse by another name? PAIN 2013;154:200–3. [DOI] [PubMed] [Google Scholar]
- [81].R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2015. [Google Scholar]
- [82].Redding GM, Rossetti Y, Wallace B. Applications of prism adaptation: a tutorial in theory and method. Neurosci Biobehav Rev 2005;29:431–44. [DOI] [PubMed] [Google Scholar]
- [83].Redding GM, Wallace B. Adaptive spatial alignment and strategic perceptual-motor control. J Exp Psychol Hum Percept Perform 1996;22:379. [DOI] [PubMed] [Google Scholar]
- [84].Reid E, Wallwork SB, Harvie D, Chalmers KJ, Braithwaite FA, Spence C, Gallace A, Moseley GL. Spatially-defined motor deficits in people with unilateral complex regional pain syndrome. Cortex 2018;104:154–62. [DOI] [PubMed] [Google Scholar]
- [85].Reid E, Wallwork SB, Harvie D, Chalmers KJ, Gallace A, Spence C, Moseley GL. A new kind of spatial inattention associated with chronic limb pain?: somatospatial inattention in pain. Ann Neurol 2016;79:701–4. [DOI] [PubMed] [Google Scholar]
- [86].Reinersmann A, Maier C, Schwenkreis P, Lenz M. Complex regional pain syndrome: more than a peripheral disease. Pain Manag 2013;3:495–502. [DOI] [PubMed] [Google Scholar]
- [87].Robinson ME, Brown JL, George SZ, Edwards PS, Atchison JW, Hirsh AT, Waxenberg LB, Wittmer V, Fillingim RB. Multidimensional success criteria and expectations for treatment of chronic pain: the patient perspective. Pain Med 2005;6:336–45. [DOI] [PubMed] [Google Scholar]
- [88].Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, Treede R-D. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain 2006;10:77. [DOI] [PubMed] [Google Scholar]
- [89].Rossetti Y, Jacquin-Courtois S, Rode G, Ota H, Michel C, Boisson D. Does action make the link between number and space representation?: visuo-manual adaptation improves number bisection in unilateral neglect. Psychol Sci 2004;15:426–30. [DOI] [PubMed] [Google Scholar]
- [90].Rossetti Y, Rode G, Pisella L, Farné A, Li L, Boisson D, Perenin M-T. Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature 1998;395:166–9. [DOI] [PubMed] [Google Scholar]
- [91].Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. PAIN 2003;103:199–207. [DOI] [PubMed] [Google Scholar]
- [92].Sapir A, Kaplan JB, He BJ, Corbetta M. Anatomical correlates of directional hypokinesia in patients with hemispatial neglect. J Neurosci 2007;27:4045–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol 1994;67:1063–78. [DOI] [PubMed] [Google Scholar]
- [94].Schwoebel J, Friedman R, Duda N, Coslett HB. Pain and the body schema. Brain 2001;124:2098–104. [DOI] [PubMed] [Google Scholar]
- [95].Serino A, Barbiani M, Rinaldesi ML, Làdavas E. Effectiveness of prism adaptation in neglect rehabilitation. Stroke 2009;40:1392–8. [DOI] [PubMed] [Google Scholar]
- [96].Serino A, Bonifazi S, Pierfederici L, Làdavas E. Neglect treatment by prism adaptation: what recovers and for how long. Neuropsychol Rehabil 2007;17:657–87. [DOI] [PubMed] [Google Scholar]
- [97].Spence C, Parise C. Prior-entry: a review. Conscious Cogn 2010;19:364–79. [DOI] [PubMed] [Google Scholar]
- [98].Striemer CL, Borza CA. Prism adaptation speeds reach initiation in the direction of the prism after-effect. Exp Brain Res 2017;235:3193–206. [DOI] [PubMed] [Google Scholar]
- [99].Sumitani M, Misaki M, Kumagaya S, Ogata T, Yamada Y, Miyauchi S. Dissociation in accessing space and number representations in pathologic pain patients. Brain Cogn 2014;90:151–6. [DOI] [PubMed] [Google Scholar]
- [100].Sumitani M, Rossetti Y, Shibata M, Matsuda Y, Sakaue G, Inoue T, Mashimo T, Miyauchi S. Prism adaptation to optical deviation alleviates pathologic pain. Neurology 2007;68:128–33. [DOI] [PubMed] [Google Scholar]
- [101].Torta DM, Legrain V, Rossetti Y, Mouraux A. Prisms for pain. Can visuo-motor rehabilitation strategies alleviate chronic pain? Eur J Pain 2016;20:64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Tseng H-H, Bossong MG, Modinos G, Chen K-M, McGuire P, Allen P. A systematic review of multisensory cognitive–affective integration in schizophrenia. Neurosci Biobehav Rev 2015;55:444–52. [DOI] [PubMed] [Google Scholar]
- [103].Turner JA, Deyo RA, Loeser JD, Korff MV, Fordyce WE. The importance of placebo effects in pain treatment and research. JAMA 1994;271:1609–14. [PubMed] [Google Scholar]
- [104].Vangkilde S, Habekost T. Finding Wally: prism adaptation improves visual search in chronic neglect. Neuropsychologia 2010;48:1994–2004. [DOI] [PubMed] [Google Scholar]
- [105].Wittayer M, Dimova V, Birklein F, Schlereth T. Correlates and importance of neglect-like symptoms in complex regional pain syndrome. PAIN 2018;159:978–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B162.
A video abstract associated with this article can be found at http://links.lww.com/PAIN/B186.