Abstract
BACKGROUND:
Cisplatin-induced hearing loss (CIHL) is a common and debilitating toxicity for childhood cancer survivors. Understandingprovider perspectives is crucial to developing otoprotectionstudies that are both informative and feasible. Two international trials (ACCL0431, SIOPEL6) investigated the drug sodium thiosulfate (STS) as an otoprotectant, but definitive interpretation of the findings of these trials has been challenging. Adoption of STS has therefore been uneven and provider perspectives on its role unknown.
PROCEDURE:
TheChildren’s Oncology Group (COG) Cancer Control and Supportive Care Neurotoxicity Subcommittee therefore conducted asurvey of providers at COG institutionsto determine perspectives on pediatric otoprotectionpractices and researchsurrounding three major themes: (1) prevalence of routine use of STS with cisplatin-based regimens, (2) application of audiometry to cisplatin therapy, and (3) preferred modalities for otoprotection research.
RESULTS:
Survey respondents (45%, 44/98surveyed institutions) were of diverse institutional sizes, practice settings, and geographical locations primarily in United States and Canada. Overall, respondents considered CIHL an important toxicity and indicated strong enthusiasm for future studies (98%, 40/41). Results indicated that while STS was the current or planned standard of care in a minority of responding institutions (36%, 16/44), most sites were receptive to its inclusion in appropriate study designs. Application of audiometry for ototoxicity monitoring varied widely across sites. For otoprotection research, systemic agents were preferred (68%, 28/41) as compared to intratympanic approaches.
CONCLUSION:
These results suggest that pediatric otoprotection trials remain of interest to providers; the emphasis of these trials should remain on systemic and not intratympanic therapy.
Keywords: Cisplatin, hearing loss, ototoxicity, otoprotection, sodium thiosulfate
INTRODUCTION
The chemotherapy agent cisplatin is the therapeutic backbone for nearly 40% of newly diagnosed non-hematologic tumors in children and adolescents.[1]More than 5,000 pediatric patients are treated with cisplatin each year.[2] While effective for cure, cisplatin damagesthe auditory sensory structures of the cochleaand causes permanent and progressive cisplatin-induced hearing loss (CIHL) in up to 80% of children receiving platinum-intensive therapy.[3–6]CIHL impacts the quality of life in survivors due to neurocognitive deficits, difficulty with socialization, and poorer long-term academic and work achievement.[7–11]
Recently, sodium thiosulfate (STS) was evaluated for potential to prevent CIHL in two international, contemporaneous, Phase III randomized trials, the Children’s Oncology Group (COG) ACCL0431[12]and the International Childhood Liver Tumors Strategy Group (SIOPEL) SIOPEL-6.[13] In comparing the randomized groups, both trialsdocumented a significant, approximately 50% reduction in the proportional incidence of hearing loss among those who received STS. Results concerning potential impact of STS on cisplatin efficacy differ somewhat by study. Briefly, SIOPEL-6 indicated no impactof STS on event free survival (EFS) or overall survival (OS) in young children treated for standard-risk hepatoblastoma using one protocol-specified cisplatin regimen. In contrast, ACCL0431 enrolled a heterogeneousmix of patients with multiple cancer types and stages receiving variouscisplatin regimens; in a post hocstratified survival analysis by extent of disease, lower EFS and OSwere found in those receiving STS and classified as having disseminated disease, but not in those with localized disease.Definitive interpretation of these combined results has proven challenging due to key design differences between the two studies.[14]Consequently, theresults of ACCL0431 and SIOPEL-6 are considered complementary andhave been used in combination to informthoughtful discussion regarding the efficacy, safety, and role of STS in pediatric cancer care and in otoprotection research.[13,15–18]However, to our knowledge, provider perspectiveson these issues have not been assessed in light of thesestudies and are currently unknown.
To determine an approach tootoprotection research that is both scientifically appropriate and feasible, it is necessary to have an informed understanding of provider practices and viewpoints regarding STS and other potential otoprotectants.Within the COG, the Cancer Control and Supportive Care (CCL) Committeeconducts research focused on the “reduction of acute and delayed treatment-related toxicities in children with cancer.”[19]To investigate issues most pressing to thosetreating patients across the spectrum of academic and community-based sites, the CCL Committee created a “Responsible Individual Network” (RI Network) to obtain input from clinical oncology providers.[19–21]Using this representative network, the CCL Neurotoxicity Subcommittee conducted a survey to understand provider perspectives within three domains relevant to otoprotectionresearch in the cooperative group setting: (1) prevalence of routine use of STS with cisplatin-based regimens, (2) application of audiometry to cisplatin therapy, and (3) preferred modalities for otoprotection research.
METHODS
Survey design
The COG CCL Committee developed an anonymous, electronic survey to explore views on otoprotection use, ototoxicity monitoring, and potentialresearch. The survey was piloted with COG CCL Committee leadership and revised according to feedback prior to wider dissemination. The survey (see Supplemental Data) included answer formatswith dichotomized yes/no responses as well as multiple-choice matrices and optional free-text fields for additional comment.The survey introduction referenced both aforementionedSTStrials and the goal to understand the implications of the results for standard of care (SOC) and otoprotectionresearch. Questions regarding STS were prefaced with references to both trials. Questions regarding physiological and behavioral audiometry techniques were prefaced with lay-language descriptions of each technique.The electronic survey was distributed to the CCL RI network via emailbetween February and August 2019 with three email reminders to maximize response rate. Respondents with incomplete surveys (skipped questions) were included and results were therefore presented for each question inclusive of the denominator to indicate the answer pool. Statistical analyses incorporated tests of proportions and non-parametric comparisons (Wilcoxon) using STATA SE version 15 (StataCorp LP, College Station, TX).
RESULTS
Characteristics of responding institutions
Survey responses were received from 44of 98 CCL RI sites (45%). These included institutions located in the United States (n= 23 states), Canada (n=8 sites), and New Zealand (n=1 site). Estimated patient volume varied by institution with a median of 17 pediatric patients treated with cisplatin-containing regimens per year (range 3–50);25% (11/44) treated <10 patients/year, 39% (17/44) treated 10–19 patients/year, and 36% (16/44) treated ≥20 patients/year.
Prevalence of routine use of STS
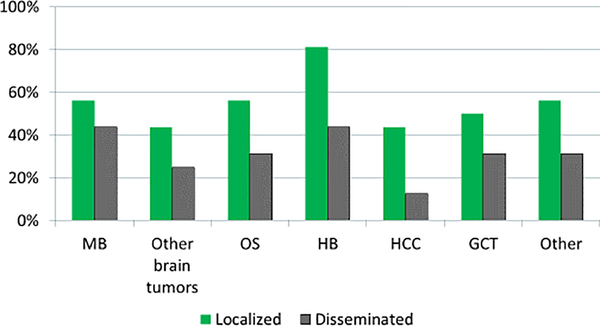
A minority of respondents (36%, 16/44) indicated their institutions had current or future plans to routinely incorporate STS into any cisplatin-based therapy as SOC. Among these sites, criteria for use of STS varied (Table 1). STS was reported as used variably across different tumor types and in patients with localized and disseminated tumors (Figure 1). The broadest support for routine use of STS was for patients with localized hepatoblastoma(81%, 13/16), and to a lesser extent in treatment ofmedulloblastoma and osteosarcoma (56% for both, 9/16). Of institutions indicating they had no plans to use STS as SOC (64%, 28/44), 82% (23/28) provided free-text responses of their views on current barriers to routine usage of STS (Table 1). There was no difference in use of STS by institutional patient volume (median of 13 versus 12 patients/year, p=0.685).
Table 1:
Descriptive responses of barriers and criteria for routine incorporation of STS for otoprotection
| Barriers to routine use of STS | Criteria used to determine routine use of STS |
|---|---|
| • Need for additional efficacy and/or safety data for STS | • Tumor stage at diagnosis • Biologic risk of tumor recurrence |
| • Competing efforts to investigate reduction of therapy for low-risk tumors (and if/how to incorporate STS into them) | • Patient age • Pre-existing hearing loss • Risk of ototoxicity from the regimen |
| • Lack of regulatory approval for the indication of otoprotection | |
| • Absence of consensus guidelines recommending its use |
Figure 1: Proportion of institutions using sodium thiosulfate by tumor type and stage.
Planned usage of sodium thiosulfate (STS) for routine otoprotection across tumor types and by disease stage at presentation. MB = medulloblastoma. OS = osteosarcoma. HB= hepatoblastoma. HCC = hepatocellular carcinoma. GCT = germ cell tumor. Other = other cisplatin-treated tumors.
Application of audiometry to cisplatin therapy
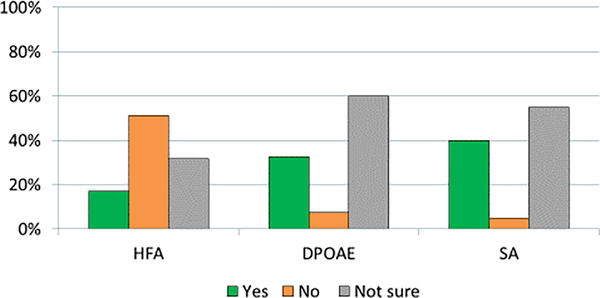
Allinstitutions (100%, 41/41) routinely assessCIHL at baseline, during treatment, and/or after completion of therapy. However, audiometry assessment schedules and modes of testing vary widely among sites. A minority of sites assess hearing before every dose of cisplatin (27%, 11/41) while most sites evaluate hearing prior to autologous hematopoietic cell transplantation (66%, 27/41%). Sites were asked whether they would factor results of extended behavioral and physiological audiometry into clinical decision-making changes; the minority replied they would do so for high-frequency audiometry (HFA)at frequencies >8,000 Hz (17%, 7/41), distortion-product otoacoustic emission (DPOAE)at “speech frequencies” (33%, 13/40), and/or speech audiometry to complement conventional audiograms (40%, 16/40). Most sites reported they were “not sure” how to use these audiometry results to guide clinical decision making (Figure 2). Patient age did not influence frequency of audiometry testing protocols for most institutions (78%, 32/41). Enrollment in a research trial did not alter planned audiometry for nearly all institutions (90%, 37/41).
Figure 2: Proportion of institutions incorporating expanded audiometry into clinical decision making.
Depiction of how responding sites currently factor into clinical decision making for cisplatin dosing the results from expanded audiometric testing such as high frequency audiometry (HFA, >8,000 Hz), distortion-product otoacoustic emissions (DPOAE), and speech audiometry (SA).
Preferred modalities for otoprotection research
All but oneinstitution (98%, 40/41) supported continued otoprotectionresearchinto systemic agents for localized tumors. The primary concern from the single opposing site was the limitations of otoprotective agents currently available for study. Sites were asked about a potential control arm for a future randomized trial in localized tumors. Among sites not routinely using STS, most were supportive of usingSTS as the control arm (96% [26/27]). Among sites routinely using STS for at least some patient subsets, fewer were amenable toan observation-only arm (67% [10/15), p=0.009). When asked about the preferred modality of a prospective investigative agent, 68% (28/41) preferred systemic otoprotectionwhile 32% (13/41) favored an intratympanic approach. Centers open to investigating anintratympanicapproach treated more patients per year than those favoring systemic agents (median 25 versus 10 patients/year, p=0.025). Reasonsin favor of pursuing intra-tympanicotoprotectionincluded eliminating concerns over tumor protection and the challenging side effect profile of systemic agents. Conversely, barriers to intratympanic regimens included concerns for sedation, procedural invasiveness, the availability of otolaryngologists, and logistical challenges of coordinating the procedure.
DISCUSSION
This survey of pediatric oncology providers in the COG CCL RI network was undertaken to determine current clinical practice patterns for use of STS, application of audiometry among children and adolescents receiving cisplatin, and how providers are prioritizing engagement in otoprotection research. These three themes are essential to understand how findings from the recent otoprotection trials are being applied to clinical practice in order to guide the focus and implementation of future trials. Though the survey was conducted within the COG, respondents included a wide range of program sizes and geographic locations. The cohort was thus largely representative of pediatric oncology practice settings in the United States and Canada.
Two international randomized clinical trials, ACCL0431 and SIOPEL-6, have demonstrated efficacy of STS in preventing CIHL in children with cancer. Bothsignificantly reduced the prevalence of hearing loss from approximately 55–60% among children in the control arm to approximately 29–33% in those whoreceived STS [12, 13]. Despite this,survey data showedthat a majority of responding institutions currently do not use, and have no plans to use,STS with cisplatin-containing regimens as standard of care.Respondents described a perceived need foradditional data validatingthe efficacy and safety of STS. Specifically, respondents questioned whether current trial results can be generalized across all cisplatin-based treatment regimens, including among different tumor types and stages. These concerns likely stem from the differing results of ACCL0431 and SIOPEL-6regardingsurvival by randomized group (STS versus control). The primary endpointof the ACCL0431 study was hearing loss; EFS and OS were therefore monitoredonly to detect unanticipated large differences. As such, the study enrolled multiple tumor types,comprised oftumors of any disease extent and staging, and included a wide variety of cisplatin-containing regimens. At completion of the STS trial, an unplanned post hoc analysis examined EFS and OS stratified by extent of disease (localized versus disseminated).Participants classifiedas having localized disease demonstrated no survival difference by randomized group, whereas those with disseminated disease demonstrated no difference in EFS but a significant four-fold increase in risk for lower OS if they had received STS (log-rank p=0.0090).[12]However, as a post hoc analysis, it is unknown if this difference in OS reflects an imbalance of unmeasured biological risk factors between the randomized groups. In contrast, SIOPEL-6 enrolled only children with localized/standard-risk hepatoblastomareceiving the same regimen and found no difference in tumor necrosis, EFS, and OS by randomized arm.[13] While caution must be used in interpreting the post hoc analysis of ACCL0431,particularly in the context of the reassuring findings from SIOPEL-6, these study results nonetheless continue to influence current usage of STS as evidenced by this survey.Even among sites currently using STS, criteria for its use varyacross tumor types, with greatest consensus surrounding use only for treatment of localized hepatoblastoma. This pattern of use is consistent with a recently published clinical practice guideline for STS.[22] This new guideline may alleviate concerns at those sites which identified the need for consensus input intouse of STS. However, absent new clinical datafor STS within different tumor types, and especiallyin metastatic disease, these questions will likely persist for many providers. Thus, new otoprotection trials are warranted to expand and/or refine routine use of STS and other systemic otoprotectants.
The survey also identified a knowledge gap for incorporating contemporary audiometry into therapeutic decisions. Current monitoring recommendations focus on conventional audiometry[23,24]as data for HFA, DPOAE, and speech audiometry are lacking. The majority of respondents were simply unsure how to apply these results to clinical practice. As ototoxicity monitoring during cisplatin therapy remains heterogeneous, trials must clearly specify required audiometry. However, even when audiometry is consistently employed, differences among ototoxicity grading systems in use internationally[25] complicate comparison of findings between trials.[12,13] Earlyinternational input into a consensus ototoxicity endpoint will improve inter-trial comparisons and create a foundation for advances in otoprotection. Planned research must address not only agent and route, but also the application of audiometry to cisplatin therapy.Opportunity exists within intervention trials to better understand clinical applications of expanded audiometry and “pre-clinical” physiologic changes, such as found with DPOAE.
It is notable that enthusiasm in otoprotection trials within the COGcooperative group setting is high. Nearly all surveyed institutions (98%) were supportive of continued research into pediatric otoprotection. In this survey, providers expressed two clear preferences regarding such research. First,and as exemplified by the SIOPEL-6 study, it was important to respondents that the design ofotoprotection trials involving systemic agents with potential for chemotherapy interferencemustinclude the ability to assess tumor response and survival as definitively as possible. Second, there was a lack of enthusiasm for theintratympanic approach of administering otoprotectantsdesigned to abrogate the risk of tumor protection posed by systemic modalities.[26]Pending demonstration of feasibility in a cooperative group setting, research in the immediate future should continue to focus on appropriately designed studies of systemic otoprotection.
This study has several strengths and some limitations. Strengths include use of the diverse and well-established CCL RI network, which afforded input from a range of academic and community practice institutions. Results are thus likely representative of real-world practice settings in the United States and Canada. Additionally, this survey assessed usage of STS, an agent with demonstrated efficacy that is currently the subject of new drug applications under review by both the Food and Drug Administration and European Medicines Agency for otoprotection in patients receiving cisplatin for localized tumors[27]. Limitations of this study include a somewhat low response rate despite multiple reminders which may increase the response bias inherent to survey-based research. However, the diverse representation of institutional types, practice settings, and geographical regions among the responses we did receive mitigates these concerns.It will also be important to reevaluate changing international perspectives and practice patterns following the regulatory determinations. With increasing emphasis on quality of life in cancer research in the United States and Europe,[28,29] this survey provides a strong indicator of the continued significance of CIHL as an important long-term toxicity to be addressed by the COG and other cooperative groups.
For children treated with cisplatin, STS is being adopted primarily for use in certain, mostly localized, tumors, albeit with considerable variation among sites. Otoprotectionresearch in cooperative group settings should include consensus audiometry guidance and endpoints; pending feasibility data for intratympanic approaches, trials should currently focus on systemic agents.CIHL remains an important toxicity to address within the pediatric oncology community with strong enthusiasm for continued otoprotection research.
Supplementary Material
Acknowledgments
Funding source: This work was supported by research funding from the National Institute of Deafness and other Communication Disorders at the National Institutes of Health (1K23DC014291, Orgel) and the Children’s Oncology Group, the National Cancer Institute at the National Institutes of Health via U10 CA180886 and U10 CA180899 and NCORP grants U10 CA095861 and UG1CA 189955, and the St. Baldrick’s Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviation
- CCL
Cancer Control and Supportive Care Committee
- CIHL
Cisplatin-induced hearing loss
- COG
Children’s Oncology Group
- DPOAE
Distortion-product otoacoustic emissions
- HFA
High-frequency audiometry
- RI
Responsible individual (liaison)
- SIOPEL
International Childhood Liver Tumors Strategy Group
- SOC
Standard of care
- STS
Sodium thiosulfate
Footnotes
Conflicts of Interest Statement: E Orgel served on an advisory board for Servier Pharmaceuticals outside the scope of this work. No other authors report any disclosures.
Data Sharing Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.American Cancer Society. Cancer Facts & Figures 2014: Special Section on Children and Adolescents. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 2.The voice of the patient: childhood cancer hearing loss. Food and Drug Administration; (externally-led patient focused drug development meeting, September 13, 2018):https://www.fda.gov/media/132522/download (Last accessed March 25, 2020). [Google Scholar]
- 3.Rybak LP, Whitworth CA, Mukherjea D, et al. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res 2007:226(1–2):157–167. [DOI] [PubMed] [Google Scholar]
- 4.Brock PR, Knight KR, Freyer DR, et al. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J Clin Oncol 2012:30(19):2408–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clemens E, de Vries AC, Pluijm SF, et al. Determinants of ototoxicity in 451 platinum-treated Dutch survivors of childhood cancer: A DCOG late-effects study. Eur J Cancer 2016:69:77–85. [DOI] [PubMed] [Google Scholar]
- 6.Landier W Ototoxicity and cancer therapy. Cancer 2016:122(11):1647–1658. [DOI] [PubMed] [Google Scholar]
- 7.Schreiber JE, Gurney JG, Palmer SL, et al. Examination of risk factors for intellectual and academic outcomes following treatment for pediatric medulloblastoma. Neuro Oncol 2014:16(8):1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orgel E, O’Neil SH, Kayser K, et al. Effect of Sensorineural Hearing Loss on Neurocognitive Functioning in Pediatric Brain Tumor Survivors. Pediatr Blood Cancer 2016:63(3):527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the Childhood Cancer Survivor Study. Neuropsychology 2009:23(6):705–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinkman TM, Bass JK, Li Z, et al. Treatment-induced hearing loss and adult social outcomes in survivors of childhood CNS and non-CNS solid tumors: Results from the St. Jude Lifetime Cohort Study. Cancer 2015:121(22):4053–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurney JG, Tersak JM, Ness KK, et al. Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: a report from the Children’s Oncology Group. Pediatrics 2007:120(5):e1229–1236. [DOI] [PubMed] [Google Scholar]
- 12.Freyer DR, Chen L, Krailo MD, et al. Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2017:18(1):63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brock PR, Maibach R, Childs M, et al. Sodium Thiosulfate for Protection from Cisplatin-Induced Hearing Loss. N Engl J Med 2018:378(25):2376–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minasian LM, Frazier AL, Sung L, et al. Prevention of cisplatin-induced hearing loss in children: Informing the design of future clinical trials. Cancer Medicine 2018:7(7):2951–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurell G Pharmacological intervention in the field of ototoxicity. HNO 2019:67(6):434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouffet E Reducing cisplatin ototoxicity in children: some hope and many questions. Lancet Oncol 2017:18(1):6–7. [DOI] [PubMed] [Google Scholar]
- 17.Freyer DR, Frazier AL, Sung L. Sodium Thiosulfate and Cisplatin-Induced Hearing Loss. New England Journal of Medicine 2018:379(12):1180–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Killock D Sodium thiosulfate halves the risk of cisplatin-induced hearing loss. Nature Reviews Clinical Oncology 2018:15(9):533–533. [DOI] [PubMed] [Google Scholar]
- 19.Sung L, Zaoutis T, Ullrich NJ, et al. Children’s Oncology Group’s 2013 blueprint for research: cancer control and supportive care. Pediatr Blood Cancer 2013:60(6):1027–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VanHoff D, Hesser T, Kelly KP, et al. Facilitating accrual to cancer control and supportive care trials: the clinical research associate perspective. BMC Medical Research Methodology 2013:13(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haugen M, Kelly KP, Leonard M, et al. Nurse-Led Programs to Facilitate Enrollment to Children’s Oncology Group Cancer Control Trials. Journal of pediatric oncology nursing : official journal of the Association of Pediatric Oncology Nurses 2016:33(5):387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freyer DR, Brock PR, Chang KW, et al. Prevention of cisplatin-induced ototoxicity in children and adolescents with cancer: a clinical practice guideline. The Lancet Child & Adolescent Health 2020:4(2):141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bass JK, White ST, Jones SE. Monitoring Ototoxicity in the Pediatric Oncology Population. American Speech-Language-Hearing Association; (Last Accessed July 1, 2015) <http://www.asha.org/aud/Articles/Monitoring-Ototoxicity-in-the-Pediatric-Oncology-Population/>. [Google Scholar]
- 24.Durrant JD, Campbell K, Fausti S, et al. American Academy of Audiology Position Statement and Clinical Practice Guidelines: Ototoxicity Monitoring. American Academy of Audiology; October 2009. <www.audiology.org, Last Accessed 4/1/2013>. [Google Scholar]
- 25.Clemens E, Brooks B, de Vries ACH, et al. A comparison of the Muenster, SIOP Boston, Brock, Chang and CTCAEv4.03 ototoxicity grading scales applied to 3,799 audiograms of childhood cancer patients treated with platinum-based chemotherapy. PLOS ONE 2019:14(2):e0210646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freyer DR, Brock P, Knight K, et al. Interventions for cisplatin-induced hearing loss in children and adolescents with cancer. The Lancet Child & Adolescent Health 2019:3(8):578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27..FennecPharmaceuticals, Press Releases (http://investors.fennecpharma.com/events-and-presentations/news-releases, Last Accessed, April 26, 2020).
- 28.Sloan JA, Berk L, Roscoe J, et al. Integrating patient-reported outcomes into cancer symptom management clinical trials supported by the National Cancer Institute-sponsored clinical trials networks. J Clin Oncol 2007:25(32):5070–5077. [DOI] [PubMed] [Google Scholar]
- 29.van Leeuwen M, Husson O, Alberti P, et al. Understanding the quality of life (QOL) issues in survivors of cancer: towards the development of an EORTC QOL cancer survivorship questionnaire. Health and Quality of Life Outcomes 2018:16(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.