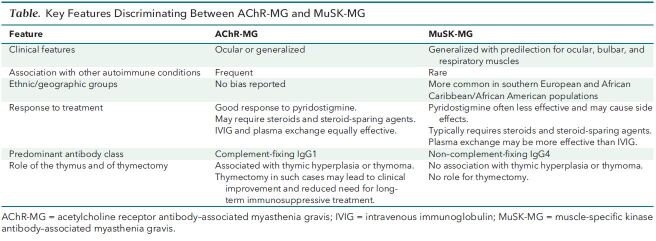
Background: Reports about the neurologic consequences of coronavirus disease 2019 (COVID-19) describe more cases of central than peripheral nervous system involvement. Myasthenia gravis is an autoimmune neuromuscular disorder that affects the peripheral nervous system. Other observers recently described 3 patients who developed myasthenia gravis with antibodies against the acetylcholine receptor (AChR) after infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1). Acetylcholine receptor antibodies are found in more than 80% of patients with generalized myasthenia gravis, whereas muscle-specific kinase (MuSK) antibodies are found in approximately 8% of cases. The pathogenic mechanisms of AChR antibody myasthenia gravis (AChR-MG) are different from those of MuSK antibody myasthenia gravis (MuSK-MG). In addition, the 2 types of myasthenia gravis have important epidemiologic, clinical, therapeutic, immunologic, and pathologic features (2–4), and they should be considered distinct entities (Table).
Table. Key Features Discriminating Between AChR-MG and MuSK-MG.
Objective: To describe what we believe is the first reported case of MuSK-MG after COVID-19.
Case Report: A 24-year-old previously healthy woman of Pakistani origin presented to our emergency department in June 2020 with a flu-like illness consistent with COVID-19. She was discharged without a SARS-CoV-2 nasopharyngeal polymerase chain reaction swab test, which was in accordance with United Kingdom guidelines at the time. She self-isolated at home and recovered completely.
Four weeks later, the patient developed diplopia, slurred speech, dysphagia, and global limb weakness. Examination revealed bilateral fatigable ptosis, complex ophthalmoplegia, symmetrical lower motor neuron facial weakness, and dysarthria. She had weakness (Medical Research Council [MRC] grade 3 to 4) and fatigability in all 4 limbs. Neck flexion and extension were also weak. Reflexes were brisk and symmetrical throughout. Her FVC was 0.97 L, so she was transferred to intensive care for monitoring without ventilator support.
The patient had blood tests for creatinine kinase and thyroid function, which were normal. Testing for antinuclear, antineutrophil cytoplasmic, and antiganglioside antibodies yielded negative results, but SARS-CoV-2 antibodies were detected. Magnetic resonance imaging of the brain and spine and routine cerebrospinal fluid analysis were unremarkable. Results of single-fiber electromyography of the left orbicularis oculi were abnormal, and repetitive nerve stimulation of the left abductor digiti minimi muscle showed abnormal decrementing responses, consistent with myasthenia gravis. Computed tomography of the chest revealed no thymoma.
Diagnostic radioimmunoprecipitation assay results were negative for AChR antibodies but positive for MuSK antibodies, confirming the diagnosis of generalized MuSK-MG. Two serum samples, collected 4 weeks apart, were tested for MuSK antibodies with a cell-based assay. Both samples had a higher level of IgG4 than IgG1–3 antibodies, with a proportional reduction in the second sample across all IgG subclasses. No IgM MuSK antibodies were detected in either sample (Figure).
Figure. Relevant clinical and laboratory findings, including the MuSK ab titers in serum samples tested by RIPA and by CBA.

One pair of symbols shows the MuSK ab of IgG4, and the other shows the MuSK ab of IgG1–3. ab = antibody; CBA = cell-based assay; IVIG = intravenous immunoglobulin; MG = myasthenia gravis, MuSK = muscle-specific kinase; RIPA = radioimmunoprecipitation assay; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
We administered intravenous immunoglobulin, pyridostigmine, and prednisolone to the patient, and her symptoms improved. However, the effect of intravenous immunoglobulin was relatively short-lived. The patient developed side effects with higher doses of pyridostigmine, so we reduced the dose and added salbutamol. She currently is 20 weeks from symptom onset, is receiving 50 mg of prednisolone every other day, and has mild to moderate dysarthria and mild limb weakness (MRC grade 4+). If her condition relapses as the steroid dosage is reduced, we will consider treatment with rituximab.
Discussion: Other authors have reported that MuSK-MG may develop after viral infection (5), and we note that our patient's clinical syndrome combined with subsequent positive results on SARS-CoV-2 antibody testing means that she probably was infected with SARS-CoV-2 at least 4 weeks before she developed MuSK-MG. Although our findings demonstrate a temporal association between COVID-19 infection and MuSK-MG, we recognize that we cannot definitively conclude causality. Nevertheless, we believe that this report of MuSK-MG associated with COVID-19, along with reports of AChR-MG associated with COVID-19 (1), provide clues to possible mechanisms for the association. For example, cross-reactivation of SARS-CoV-2 antibodies with both AChR and MuSK proteins is highly unlikely given their molecular differences; therefore, the development of myasthenia gravis after COVID-19 more likely represents a breakdown in self-tolerance mechanisms than cross-reactivation. In addition, it is too soon after the diagnosis to determine whether this case of MuSK-MG is an acute, monophasic, postinfectious phenomenon or a chronic autoimmune disorder requiring long-term immunosuppressive treatment, which can be determined only by long-term follow-up of our patient as well as any future patients.
Footnotes
This article was published at Annals.org on 12 January 2021.
References
- 1. Restivo DA , Centonze D , Alesina A , et al. Myasthenia gravis associated with SARS-CoV-2 infection [Letter]. Ann Intern Med. 2020;173:1027-8. doi: 10.7326/L20-0845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koneczny I , Cossins J , Vincent A . The role of muscle-specific tyrosine kinase (MuSK) and mystery of MuSK myasthenia gravis. J Anat. 2014;224:29-35. [PMID: ] doi: 10.1111/joa.12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leite MI , Jacob S , Viegas S , et al. IgG1 antibodies to acetylcholine receptors in ‘seronegative' myasthenia gravis. Brain. 2008;131:1940-52. [PMID: ] doi: 10.1093/brain/awn092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Evoli A , Tonali PA , Padua L , et al. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain. 2003;126:2304-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 5. Belbezier A , Deroux A , Sarrot-Reynauld F , et al. Myasthenia gravis associated with acute hepatitis E infection in immunocompetent woman [Letter]. Emerg Infect Dis. 2014;20:908-10. [PMID: ] doi: 10.3201/eid2005.131551 [DOI] [PMC free article] [PubMed] [Google Scholar]