Abstract
Our aim was to analyze characteristics of atrial fibrillation (AF) patients with chronic kidney disease (CKD) from the Croatian cohort of the ESH A Fib survey and to determine the association of estimated glomerular filtration rate (eGFR) with cardiovascular (CV) mortality after 24 months of follow-up.
Consecutive sample of 301 patients with AF were enrolled in the period 2014 to 2018. Hypertension was defined as BP > 140/90 mm Hg and/or antihypertensive drugs treatment, CKD was defined as eGFR (CKD Epi) < 60 ml/min/1.73 m2 which was confirmed after 3 months.
CKD was diagnosed in 45.2% of patients (13.3% in CKD stage > 3b). CKD patients were older than non-CKD and had significantly more frequent coronary heart disease, heart failure and valvular disease. CKD patients had significantly higher CHA2DS2-VASc score and more CKD than non-CKD patients had CHA2DS2-VASc > 2. Crude CV mortality rate per 1000 population at the end of the first year of the follow-up was significantly higher in CKD vs non-CKD group who had shorter mean survival time. CV mortality was independently associated with eGFR, male gender, CHA2DS2VASc and R2CHA2DS2VASc scores.
Prevalence of CKD, particularly more advanced stages of CKD, is very high in patients with AF. Observed higher CV mortality and shorter mean survival time in CKD patients could be explained with higher CHA2DS2VASc score which is a consequence of clustering of all score components in CKD patients. However, eGFR was independently associated with CV mortality. In our cohort, R2CHA2DS2VASc score was not associated significantly more with CV mortality than CHA2DS2VASc score.
Keywords: atrial fibrillation, cardiovascular mortality, chronic kidney disease, renal impairment
1. Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia in the general population and prevalence is even higher in patients with chronic kidney disease (CKD) occurring in 15% to 21% of non-dialysis dependent CKD patients and 15–40% in patients undergoing chronic dialysis what is an important global health burden keeping in mind that CKD is diagnosed in about 10% of adult population.[1–7] CKD is acknowledged as a major cardiovascular (CV) risk factor but as CKD and AF share many risk factors it is still a matter of debate whether CKD independently increases global and particularly stroke risk in patients with AF. It was reported that the adjusted risk ratios of stroke with AF varied considerably across CKD subpopulations.[8] Bansal et al have yielded almost 3-time more deaths per 1000 person-years in CKD patients with AF compared to CKD patients without AF, and after adjustment incident, AF was associated with 66% increase in the relative rate of death in CKD patients.[9] In ATRIA study it was found that estimated glomerular filtration rate (eGFR) of 15 − 59 ml/min/1.73 m2 was associated with a significantly increased risk of venous thromboembolism after adjustment for major risk factors.[10] Decreased eGFR was associated with an increased risk of ischaemic stroke which gradually increased as eGFR decreased.[10] However, some studies with end-stage-renal-disease patients have observed conflicting data on the impact of AF on outcomes.[11,12] Thus, even though that impaired kidney function in patients with AF is associated with an increased risk of stroke, CKD has not been included in current risk scores like CHA2DS2-VASc.[13,14] CKD patients often need anticoagulation due to frequent development of deep venous thrombosis and AF but on the other hand the risk of anticoagulation-related complications like major bleeding is increased which often complicates the decision for anticoagulation initiation.[15,16]
CHADS2 and CHA2DS2-VASc stroke prediction scores are validated in patients undergoing chronic hemodialysis[5,17–19] but interestingly it remains unresolved are they valid in CKD patients. As CKD was recognized as an independent risk factor for thromboembolism it was aimed to improve risk scores and stroke prediction by adding 2 points for creatinine clearance < 60 ml/min to CHADS2 score (so-called R2CHADS2) but obtained results were not promising.[13,20,21] Neither ATRIA score fulfilled expectations.[22] While lacking further evidence, the pragmatic recommendation of KDIGO was to use CHA2DS2VASc for risk stratification and treatment decision in CKD patients.[8]
Our aim was to determine characteristics of non-dialyzed CKD patients with valvular and non-valvular AF and the association of eGFR on mortality after 2 years of follow-up.
2. Methods
Consecutive sample of 301 patients with AF (176 men, 125 women; average age 70.6) was enrolled in the ESH Excellence Center Zagreb in period 2014 to 2017 representing the Croatian cohort of the European Society of Hypertension Atrial Fibrillation Research Project (ESH A Fib project). The main aim of the ESH A Fib project was to analyze the characteristics of hypertensive patients with AF treated in the ESH Excellence Centers. This was a multicentric, international, retrospective, observational, longitudinal follow-up study. The protocol was approved by the hospital ethics committee (UHC Zagreb, Croatia) in accordance with the Helsinki Declaration and all participants gave written informed consent. Inclusion criteria were: age older than 18 years, hospitalization of patients who were admitted with AF. Exclusion criteria was: not signed informed consent.
The detailed medical history of the patients was entered into a specified ESH questionnaire and a complete physical examination was conducted. In this cohort, office blood pressure was measured using a calibrated mercury sphygmomanometer and proper cuffs according to the ESH/ESC guidelines.[21] In all patients following laboratory data were collected: complete blood count, international normalized ratio, fasting blood glucose, total cholesterol, HDL-cholesterol, triglycerides, serum sodium, potassium and creatinine.
AF was categorized into 4 types: first diagnosed, paroxysmal, permanent and persistent.
CKD was defined as eGFR < 60 mL/min/1.73 m2 using CKD Epi equation[22] and diagnosis of CKD was confirmed 3 months later. The underlying disease responsible for CKD was diabetes in 37 (27.2%) patients, hypertension in 57 (41.9%) patients and heart failure (cardiorenal syndrome) in 42 (30.8%) patients. There were no patients with end-stage-renal-disease on hemodialysis. Hypertension was defined as blood pressure ≥140/90 mmHg and/or antihypertensive drugs treatment.[23] CHA2DS2VASc, R2CHADS2 and R2CHA2DS2VASc scores were calculated for all enrolled patients. The calculation of CHA2DS2VASc score awarded 1 point each for the presence of congestive heart failure, hypertension, vascular diseases, diabetes and female sex; 2 points for prior stroke or TIA and 0, 1 or 2 points depending on age. The calculation of R2CHADS2 and R2CHA2DS2VASc scores awarded an additional 2 points for CrCl < 60 mL/min and GFR < 60 mL/min/1.73 m2. Follow-up was performed by routine clinic visits and lasted until the last enrolled patient reached the 24-months’ time point or till the time of death. CV mortality was defined as death from fatal CV events: heart failure, stroke, myocardial infarction or from valvular disease. Mortality data were obtained from the Croatian National Public Health Institute records.
Statistical analysis was performed using SPSS version 23.0 (IBM Corp.).[24] Normality of data distribution was tested using Kolmogorov-Smirnov test. Preliminary analyses were performed to ensure no violation of the assumptions of normality, linearity and homoscedasticity. Categorical data were expressed as numbers and frequencies. Correlations were obtained using Pearson's test for normally distributed variables and Spearman rank correlation for non-normally distributed variables. Normally distributed variables were presented as means ± standard deviations and Student's t test for independent samples was used for comparisons between 2 groups. Non-normally distributed data were presented as median and interquartile range and Mann-Whitney U-test was used in comparison between 2 groups. Categorical variables were compared using χ2- test. Survival analysis was done with Kaplan-Meier curves which were tested with log-rank test while hazard ratios were estimated with Cox proportional hazards regression. Multiple linear regression was used to explore the influence of different variables on eGFR and CHA2DS2VASc, R2CHA2DS2VASc and R2CHADS2 score, while logistic regression was used for categorical dependent variables. For assessing the influence of different variables on CHA2DS2VASc score we have excluded variables which are components of CHA2DS2VASc. We constructed 5 linear regression models to assess independent associations of multiple independent variables which included variables known to be associated with increased mortality. Model 1: age, gender, eGFR; Model 2: diabetes, hypertension and history of stroke; Model 3: prior myocardial infarction and peripheral arterial disease; Model 4: smokers and BMI; Model 5: CHA2DS2VASc, R2CHADS2 and R2CHA2DS2VASc score. Crude mortality rate was calculated by formula in which number of patients died from CV event in 1 year was divided by the number of enrolled patients at midyear and multiplied by 1000. Blant-Altman analysis with inter-rater agreement analysis was performed for comparing predictive values of CHA2DS2VASc and R2CHA2DS2VASc scores for future cardiovascular mortality. A P value < .05 (2-sided tests) was considered significant.
3. Results
The prevalence of CKD in our group of hypertensive patients with AF was 45.4% (men 61%, women 39%) and 13.4% of patients were in CKD stages 4 or 5. Demographic, clinical and laboratory data of enrolled patients are demonstrated in Table 1. There were no differences in gender, smoking status and BMI between CKD and non-CKD groups (Table 2). Patients with CKD were older than non-CKD patients. Significantly more CKD patients had prior hypertension, diabetes, coronary heart disease, heart failure, peripheral arterial disease (PAD) and valvular disease when compared to non-CKD patients (41.1% vs 20.0%; 25.7% vs 11.5%; 91.9% vs 20.0%; 16.2% vs 8.5% and 45.6% vs 20.0%, all P< .05 respectively) while there were no differences in thyroid disease and previous stroke. At baseline, the prevalence of hypertension was higher in CKD patients (99.3% vs 93.9%, P< .05), but there were no statistically significant differences in control of hypertension between CKD and non-CKD patients (25.9% vs 29.0%, P> .05) as well as in a number of hypertensive drugs per patient (2.93 vs. 2.81; P > .05). We have not found differences in anti-hypertensive drug classes used in CKD and non-CKD patients except beta-blockers and diuretics which were prescribed more often in CKD patients (beta-blockers 104 vs 98; diuretics 103 vs 84; all P < .05). CKD patients had significantly more frequent CHA2DS2VASc ≥2 than non-CKD patients (93.7% vs 75.4%; P = .01).
Table 1.
Demographic, clinical and laboratory data of enrolled patients.
| Demographic parameters | |
| Age (yr) | 70.6 ± 6.05 |
| Gender - men N (%) | 176 (58.5) |
| BMI (m/kg2) | 27.3 ± 4.30 |
| Smoker -yes N (%) | 39 (12.9) |
| Diabetes -yes N (%) | 89 (29.6) |
| Hypertension -yes N (%) | 290 (96.3) |
| Coronary heart disease -yes N (%) | 54 (17.9) |
| Stroke -yes N (%) | 54 (17.9) |
| Heart failure -yes N (%) | 158 (52.5) |
| Valvular disease -yes N (%) | 95 (31.6) |
| Peripheral arterial disease -yes N (%) | 36 (11.9) |
| Thyroid disease -yes N (%) | 43 (14.3) |
| Clinical parameters | |
| Type of AF -yes N (%) | |
| first diagnosed | 18 (6.0) |
| paroxysmal | 117 (38.9) |
| permanent | 135 (44.8) |
| persistent | 31 (10.3) |
| CHA2DS2VASc score | 3.69 ± 0.9 |
| R2CHA2DS2VASc score | 4.60 ± 1.1 |
| Systolic blood pressure (mm Hg) | 132.4 ± 23.0 |
| Diastolic blood pressure (mm Hg) | 79.9 ± 13.2 |
| Heart rate (b/min) | 72 (48-120) |
| EF (%) | 42 (20–65) |
| Therapy | |
| Anticoagulant therapy -yes N (%) | 207 (68.8) |
| Warfarin -yes N (%) | 131 (63.3) |
| NOAC -yes N (%) | 76 (36.7) |
| ACE-inhibitors -yes N (%) | 146 (48.5) |
| ARBs -yes N (%) | 46 (15.3) |
| Calcium channel blockers -yes N (%) | 60 (19.9) |
| Beta blockers -yes N (%) | 202 (67.1) |
| Diuretics -yes N (%) | 187 (62.1) |
| Number of antihypertensive drugs | 2.87 ± 0.4 |
| Antiplatelet drug -yes N (%) | 62 (20.6) |
| Statins -yes N (%) | 112 (37.2) |
| Renal function | |
| Serum creatinine (μmol/L) | 112 (44–402) |
| eGFR (ml/min/1.73m2) | 61.0 ± 12.5 |
| CKD stages N (%) | |
| eGFR > 60 ml/min/1.73m2 | 165 (54.8) |
| 3a | 54 (17.9) |
| 3b | 42 (13.9) |
| 4 | 29 (9.7) |
| 5 | 11 (3.7) |
| Outcome | |
| Survival (months) | 21.9 ± 5.4 |
| Death -yes N (%) | 45 (14.9) |
| Cardiovascular | 40 (88.8) |
| heart failure | 13 (32.5) |
| stroke | 12 (30.0) |
| myocardial infarction | 8 (20.0) |
| severe valvular disease | 7 (17.5) |
| Other | 5 (11.1) |
ACE = angiotensin-converting enzyme, AF = atrial fibrillation, ARB = angiotensin-II receptor blockers, BMI = body mass index, CKD = chronic kidney disease, EF = ejection fraction, GFR = glomerular filtration ratio, NOAC = new oral anticoagulants.
Table 2.
Demographic, clinical and laboratory data patients divided in CKD and non-CKD groups.
| CKD N = 136 | non-CKD N = 164 | P | |
| Demographic parameters | |||
| Age (yr) | 73.01 + 7.12 | 68.67 ± 5.34 | <.01 |
| Gender-men yes N (%) | 83 (61.0) | 93 (56.3) | .41 |
| BMI (m/kg2) | 27.4 ± 4.29 | 27.3 ± 4.28 | .94 |
| Smoker -yes N (%) | 17 (12.5) | 22 (13.3) | .83 |
| Diabetes -yes N (%) | 56 (41.1) | 33 (20.0) | <.001 |
| Hypertension -yes N (%) | 135 (99.3) | 155 (93.9) | <.05 |
| Coronary heart disease -yes N (%) | 35 (25.7) | 19 (11.5) | <.05 |
| Stroke -yes N (%) | 23 (16.9) | 31 (18.8) | .67 |
| Heart failure -yes N (%) | 125 (91.9) | 33 (20.0) | <.001 |
| Valvular disease -yes N (%) | 62 (45.6) | 33 (20.0) | <.01 |
| Peripheral arterial disease -yes N (%) | 22 (16.2) | 14 (8.5) | <.05 |
| Thyroid disease -yes N (%) | 18 (13.2) | 25 (15.1) | .64 |
| Clinical parameters | |||
| Type of AF -yes N (%) | |||
| first diagnosed | 7 (5.1) | 11 (6.7) | .58 |
| paroxysmal | 50 (36.8) | 67 (40.6) | .49 |
| permanent | 65 (47.8) | 70 (42.4) | .35 |
| persistent | 14 (10.3) | 17 (10.3) | .85 |
| CHA2DS2VASc score- average | 4.0 ± 1.1 | 3.4 ± 0.7 | <.01 |
| low (score = 0) | 2 (1.2) | 0 (0) | <.01 |
| intermedium (score = 1) | 17 (10.3) | 0 (0) | |
| high (score ≥ 2) | 145 (88.4) | 136 (100) | |
| Systolic blood pressure (mmHg) | 130.2 ± 22.1 | 134.2 ± 23.8 | .16 |
| Diastolic blood pressure (mmHg) | 78.3 ± 12.6 | 81.2 ± 15.1 | .08 |
| Heart rate (b/min) | 81 (52–124) | 83 (53–126) | .55 |
| EF (%) | 31 (15–56) | 49 (24–68) | <.01 |
| Therapy | |||
| Anticoagulant therapy -yes N (%) | 99 (72.8) | 108 (65.5) | .17 |
| warfarin | 65 (65.6) | 66 (61.1) | .28 |
| NOAC | 34 (34.4) | 42 (38.9) | |
| ACE-inhibitors -yes N (%) | 72 (52.9) | 74 (44.8) | .16 |
| ARBs -yes N (%) | 21 (15.4) | 25 (15.1) | .94 |
| Calcium channel blockers -yes N (%) | 23 (16.9) | 37 (22.4) | .23 |
| Beta blockers -yes N (%) | 104 (76.5) | 98 (59.4) | <.01 |
| Diuretics -yes N (%) | 103 (75.7) | 84 (50.9) | <.001 |
| Number of antihypertensive drugs | 2.93 ± 0.4 | 2.81 ± 0.4 | .86 |
| Antiplatelet drug -yes N (%) | 29 (21.3) | 33 (20.0) | .77 |
| Statins -yes N (%) | 50 (36.7) | 62 (37.6) | .88 |
| Outcome | |||
| Survival (months) | 20.2 ± 2.66 | 23.37 ± 3.42 | <.001 |
| Death -yes N (%) | 35 (25.7) | 10 (6.1) | <.001 |
| Cardiovascular | 33 (94.3) | 7 (40.0) | <.001 |
| heart failure | 11 (33.3) | 2 (28.6) | .81 |
| stroke | 10 (30.4) | 2 (28.6) | .93 |
| myocardial infarction | 7 (21.2) | 1 (14.2) | .68 |
| severe valvular disease | 5 (15.1) | 2 (28.6) | .39 |
| Other | 2 (5.7) | 3 (30.0) | <.001 |
NOAC = new oral anticoagulants, ACE = angiotensin-converting enzyme, AF = atrial fibrillation, ARB = angiotensin-II receptor blockers, BMI = body mass index, EF = ejection fraction, GFR = glomerular filtration ratio.
On univariate analysis, eGFR negatively correlated with age (r-0,211; P < .001), PAD (r-0,191; P < .01) and CHA2DS2VASc score (r-0,221; P < .001) while did not correlate with prior diabetes or hypertension. In the linear regression model, age, PAD (β= -0.144, P = .013) and higher CHA2DS2VASc score (β= -0.237, P = .013) were predictors for lower eGFR. On logistic regression, older patients, CHA2DS2VASc score ≥2 and PAD had increased OR for CKD (1.03 [CI 1.01, 1.05], 1.22 [CI 1.06, 1.41] and 0.44 [CI 0.21, 0.91], respectively).
We failed to find gender differences neither in the whole group nor in the CKD group in clinical and laboratory parameters except more men were smokers and had CHA2DS2VASc < 2 while more women had thyroid disease (all P < .05). On univariate analysis, CHA2DS2VASc score correlated negatively with eGFR (r-0,221; P < .001). In the linear regression analysis, higher CHA2DS2VASc score was independently negatively associated with eGFR (β= 0.466, P < .001). On logistic regression, lower eGFR had increased risk for higher CHA2DS2VASc score (OR 1.15 [CI 1.06, 1.25] and OR 0.98 [CI 0.89, 1.07], respectively).
At the end of the follow-up period of 24 months, 45 (14.9%) deaths occurred. Forty patients died from fatal CV events (88%): 13 from heart failure, 12 from stroke, 8 from myocardial infarction and 7 from valvular disease, while 5 patients died from other causes. Significantly more CKD patients died from CV events compared to non-CKD patients (Table 2). Crude CV mortality rate per 1000 population at the end of the first year of the follow-up was significantly higher in CKD vs- non-CKD group (66.4 vs.36.6). Mean survival time was longer in non-CKD than CKD patients (23.37 (95% CI 22.9, 23.8) vs. 20.2 (95% CI 18.9, 21.3) months; p < 0.001) (Fig. 1). The significant difference in mean survival time was observed between CKD stages 3b and 4 and CKD stages 1–3a (16.67 (95% CI 15.9, 17.4) vs. 23.52 (95% CI 23.1, 32.9) months, p < 0.001). No difference in mean survival time observed between CKD stage 5 compared to CKD stages 1–3a was probably due to the small number of patients in CKD stage 5 subgroup (Fig. 2). In the whole group, in models of the linear regression analysis CV mortality was independently associated with eGFR (β= 0.169, P = .04), male gender (β= 0.156, P = .03), CHA2DS2VASc (β= 0.467, P = .02) and R2CHA2DS2VASc scores (β= 0.391, P = .04) but not with R2CHADS2 score (Table 3). Both higher CHA2DS2VASc and R2CHA2DS2VASc score were associated with higher CV mortality in the whole group (HR 0.48 [0.34, 0.62] and HR 1.89 [1.55, 2.23], respectively) as well as in non-CKD group (HR 1.24 [0.92, 1.56] and HR 0.94 [0.60, 1.30], respectively. Inter-rater agreement analysis (Kappa 0.474) showed a moderately convincing statistical agreement between R2CHA2DS2VASc and CHA2DS2VASc score. When analyzing different CKD stages, stage 3b and 4 were associated with higher CV mortality in the whole group (HR 2.65 [1.00, 4.23] and HR 3.70 [1.20, 5.20], respectively) (Fig. 3).
Figure 1.
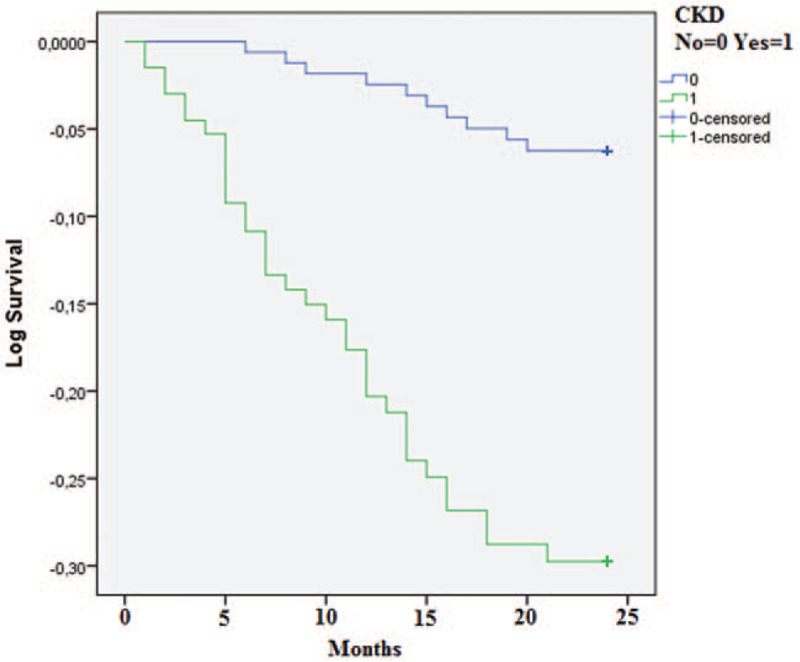
Cardiovascular mortality in CKD and non-CKD patients at the end of follow-up CKD-chronic kidney disease.
Figure 2.
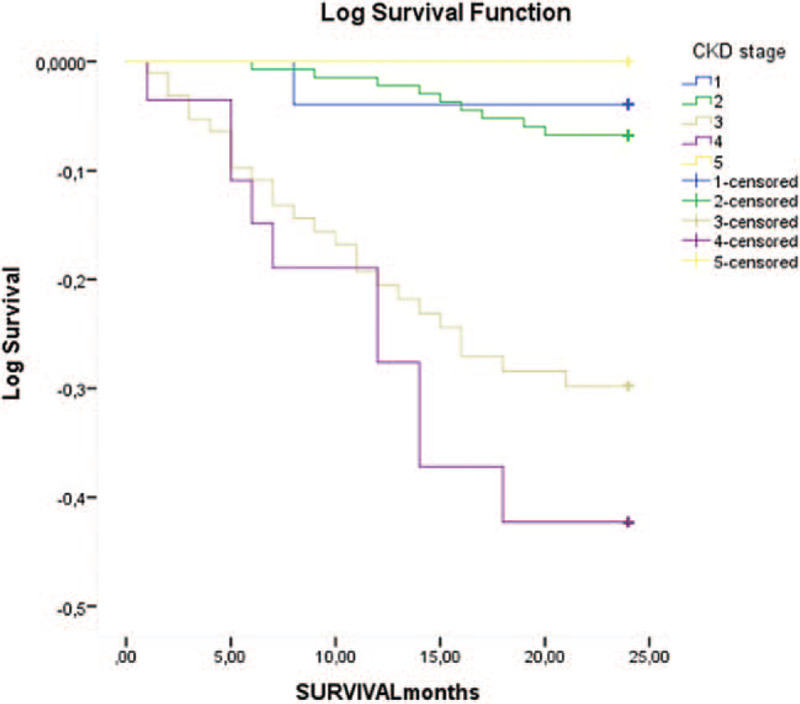
Cardiovascular mortality in patients with different CKD stages at the end of follow-up CKD-chronic kidney disease.
Table 3.
Linear regression analysis – cardiovascular mortality.
| Unstandardized Coefficients | Standardized Coefficients | |||||
| BetaModel | B | Std.Error | Beta | T | Sig. | |
| (Constant) | –,098 | 1,076 | –,091 | ,927 | ||
| MODEL 1 | Age | ,783 | ,228 | ,266 | 1,135 | ,145 |
| Sex (males) | ,851 | ,202 | ,156 | 1,920 | ,003 | |
| eGFR | ,009 | ,004 | ,169 | 1,975 | ,040 | |
| MODEL 2 | Diabetes | ,082 | ,045 | ,105 | 1,836 | ,067 |
| Hypertension | –,041 | ,109 | –,022 | –,376 | ,707 | |
| History of Stroke | ,006 | ,031 | ,012 | ,207 | ,836 | |
| MODEL 3 | Prior Myocardial Infarction | ,024 | ,052 | 0,36 | ,285 | ,802 |
| PAD | ,007 | ,008 | ,074 | 1,224 | ,312 | |
| MODEL 4 | Smokers | ,672 | ,178 | ,188 | 1,014 | ,266 |
| BMI | –,004 | ,020 | –,011 | –,193 | ,847 | |
| MODEL 5 | CHA2DS2VASc score | ,924 | ,346 | ,467 | 6,273 | ,002 |
| R2CHADS2 score | ,546 | ,144 | ,192 | ,893 | ,112 | |
| R2CHA2DS2VASc score | ,892 | ,308 | ,391 | 5,114 | ,004 | |
BMI = body mass index, DBP = diastolic blood pressure, GFR = estimated glomerular filtration ratio, HR = heart rate, PAD = peripheral arterial disease, SBP = systolic blood pressure.
Figure 3.
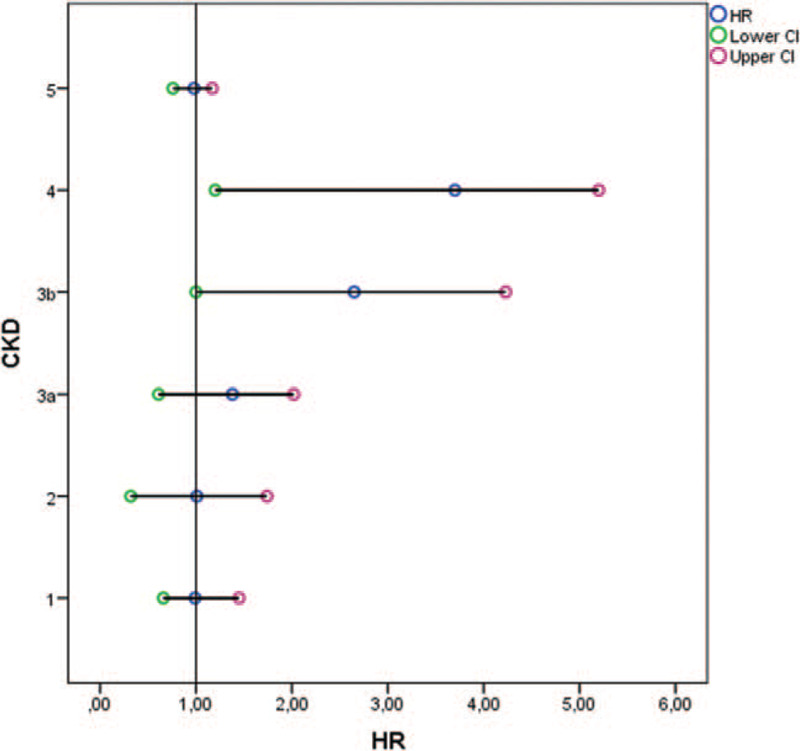
Cox proportional hazards regression for cardiovascular mortality in patients with different CKD stages at the end of follow-up A forest plot showing the hazard ratio and 95% confidence intervals associated with different CKD stages considered in the univariable analyses with time to the primary endpoint (cardiovascular mortality) as the dependent variable. Circles represent the hazard ratio and the horizontal bars extend from the lower limit to the upper limit of the 95% confidence interval of the estimate of the hazard ratio. CKD-chronic kidney disease; CI-confidence interval; HR-hazard ratio.
4. Discussion
The prevalence of CKD in our patients with AF was 45.4% what is in agreement with results obtained by the majority of authors but higher than the prevalence found in the Loire Valley Atrial Fibrillation Project where only patients with non- valvular AF were enrolled.[14,23,25–27] The majority of our CKD patients were in CKD stage 3 what is again in line with other reports.[9,14,28–30] In all reports the prevalence of CKD stage 3 was higher compared to the prevalence in general population. Importantly, in our group of patients with AF the prevalence of CKD stages 4 and 5 were even higher than in the general population. This could be explained by more advanced age and the presence of various risk factors for CKD in AF patients. The average age of 70.6 years and the proportion of men in our cohort were similar to other reports.[31,32] As reported by others, we also found CKD patients to be significantly older than non-CKD patients. While many authors detected a higher proportion of men in AF patients with CKD, Prioetti et al and Reinecke et al reported a higher proportion of women than men in AF patients with CKD.[26,33] We failed to find a difference in gender proportion between CKD and non-CKD patients. Reinecke et al speculated whether a higher proportion of women could be a reflection of previous higher mortality in men. One could argue whether this might indicate that women with AF are more prone to CKD.[33] Patients with CKD and AF in our cohort had a higher prevalence of hypertension, diabetes, heart failure and previous stroke than reported by other authors.[14,25,33] This difference could be explained by the fact that our patients were in the tertiary center where are the most difficult patients from this region while other studies included patients from several centers probably mirroring „real-life“ situations. Our results are in agreement with data collected by Ananthapanyasut et al.[28] In our cohort, CKD patients had more diabetes, coronary heart disease, heart failure and PAD than non-CKD patients what is in line with other reports.[14,25,33] Prevalence of hypertension was slightly higher in CKD than in non-CKD patients which were also observed by others.[14,25,33] In large German cohort, Reinecke et al observed that the prevalence of hypertension, diabetes, coronary heart disease, heart failure, stroke and thyroid disease increased as kidney function deteriorated.[33] Interestingly, in our cohort of patients there was no difference in the number of previous strokes between CKD and non- CKD patients. This finding was also reported by others reflecting probably higher previous stroke mortality rate in CKD patients.[14,25,33] The prevalence of paroxysmal, permanent and persistent AF in our whole group was 38.9%, 44.8%, and 10.3%, respectively. We failed to find statistically significant differences in the proportion of various AF types between CKD and non-CKD patients. Permanent AF was the most frequent AF type in our and Italian group of CKD patients (42.4% and 59.7%, respectively) while other authors reported paroxysmal AF to be the most common form of AF in CKD patients.[14,25,28] Reinecke et al observed that the prevalence of paroxysmal AF decreased (41.5%-34.7%-35.6%) and the prevalence of permanent AF increased (22.1%-35.5%-37.3%) from CKD stage 2 to 3 and 4/5 what is in line with French results.[14,33] Prevalence of persistent AF was among various studied groups reported in wide range from 5.8% to 26.5% which could be explained by the differences in AF etiology and/or disease severity and global risk.[14,25,28,33] In our whole group, 68.8% patients were treated with anticoagulant therapy what is in concordance with data observed in the German Competence NETwork on Atrial Fibrillation (68.6%) and Italian AntiThrombotic Agents Atrial Fibrillation study (68.6%)[25,33] but higher than reported from France (51.8%)[14] indicating that anticoagulant therapy is underused.
Interestingly, neither we nor other authors found differences in anticoagulant therapy prescription between CKD and non-CKD patients.[14,25,29] It was observed that not only oral anticoagulation but also antiarrhythmic drugs and catheter ablations were used significantly less often in advanced CKD despite those patients had significantly higher CHADS2 scores.[29,33]
In our group approximately only one-third of anticoagulated patients were treated with NOACs without differences between CKD and non-CKD patients. Lower usage of NOACs in our cohort could be explained by advanced CKD, the reluctance of some physicians to use NOACs in patients with native valve disease and with high patients reimbursement rate. As expected, CHA2D2SVasc score was higher in our CKD patients than in non CKD patients with similar results observed by many other.[21,29,33,34] However, in the French cohort significantly more patients had low and intermediate CHA2D2SVasc score and fewer patients had CHA2D2SVasc ≥ 2 compared to our group (49.6%vs.88.4%) which could be explained with differences in demographic (younger age, fewer women) and clinical characteristics (less hypertension, stroke, diabetes and vascular disease).[14] Patients’ characteristics also could be the reason why the analysis of data from the Danish registry failed to find differences in CHA2D2SVasc score between CKD and non CKD patients.[35] On the contrary, Reinecke et al and Wu et al observed an increasing prevalence of higher CHADS2 scores in more advanced CKD stages what is in line with our data.[29,33]
At the end of follow-up, 45 deaths (14.9%) were registered and most of them (88%) were cardiovascular. The most frequent cause of death was heart failure followed by stroke and myocardial infarction. Significantly more CKD patients died from CV events and stroke than non-CKD patients (12.8 %/year vs 3.1 %/year). Accordingly, survival time was significantly shorter in CKD than in non-CKD patients. In the linear regression analysis, CV mortality was independently associated with eGFR (β= 0.169, P = .04) what is in line with the results of Parsons et al[31] who found that lower GFR significantly correlated with mortality and following data of Proietti et al where eGFR < 60 ml/min/1.73 m2 was associated with higher CV mortality rate.[26] It was observed in J Rhythm and ATA-AF registries that even moderately impaired GFR was independently associated with CV mortality and worse prognosis among patients with AF while Guo et al reported that renal dysfunction carries a greater risk of stroke and death in women.[32,36,37]
The CV risk is independently associated with impaired GFR and CKD through various mechanisms.[38,39] Coronary microvascular abnormalities are a consequence of structural and functional changes like arteriolar remodeling, capillary rarefaction, endothelial and smooth muscle cell dysfunction which is associated with CKD.[40–42] With the presence of left ventricle hypertrophy, the development of coronary epicardial and microcirculatory dysfunction increases the risk for myocardial ischemia and fibrosis and therefore the incidence of sudden cardiac death and heart failure is a common occurrence in CKD patients.[38–43] Bajaj et al reported the transition from physiological to pathological left ventricular remodeling caused by severe microvascular dysfunction which is associated with severely impaired GFR.[44] These changes increase the risk of heart failure and death in patients with CKD.
As already mentioned, there is conflicting evidence whether kidney function should be included into various prediction models and risk scores.[8,31,45] Nakagawa et al reported that long-term mortality, cardiac events and stroke were more than 8 times higher when eGFR < 60 mL/min/1.73 m2 was associated with CHADS2 score≥ 2.[34] Lin et al found that patients with low CHA2DS2 Vasc score between 1 and 2 and with eGFR < 60 mL/min/1.73 m2 have a higher risk for CV mortality while Parsons et al concluded that adding renal impairment to CHA2DS2 Vasc score mildly improves the score's prediction for thromboembolism and mortality.[31,46] In ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) Piccine et al found that a model that included creatinine clearance (R2CHADS2) improved net classification of CHADS2 or CHA2DS2 Vasc score and concluded that stroke risk stratification in patients with AF should include renal function.[20] On the contrary, Banerjee et al found that eGFR as a categorical variable lost its predictability for CV events after adjustment for other confounding variables and concluded that adding renal impairment to CHADS2 or CHA2DS2 Vasc score did not independently improve the predictive value of these scores. This is in line with results from Amadeus trial and results published by Roldan et al who concluded that adding CKD to the CHADS2 and CHA2DS2-VASc stroke risk scores did not independently improve predictive value.[13,14,46] Reports from the literature on the association of R2CHA2DS2VASc with CV mortality are inconclusive.[13,14,20,31,34,45,46]
It is indisputable that the presence of renal impairment and CKD in AF patients increases the risk of CV events and CV mortality but obviously the addition of eGFR to the risk scores acknowledged in the general population has no additional prognostic value in this group of patients. This is very probably, as Soliman et al reported in the CRIC study, since risk factors for AF patients with CKD do not mirror those reported in the general population.[47] In our study, the linear regression analysis models have not shown the association of CV mortality with diabetes or hypertension. Other authors also failed to find a positive association of diabetes and/or hypertension with AF in CKD patients.[10,33,45,47,48] Baber et al concluded in the REGARDS study that the high prevalence of hypertension limited the ability to detect an association between hypertension and AF.[28,48] Reinecke et al nicely concluded that in fact, patients with AF and CKD represent a negative selection of patients with a high prevalence of comorbidities and risk factors.[33] This is why risk prediction models developed in the general AF population could not be applied in CKD patients and an explanation why simple adding any biomarker of renal impairment could not improve the predictive value of currently used scores. Further investigation is needed to establish an appropriate risk predictive model where kidney function will be included.
Our study has several limitations. First, this is a report from only 1 ESH Excellence center included in the ESH A Fib study with a relatively small number of patients. Nevertheless, some authors had the same or even smaller number of patients.[27,34,36] Second, the follow-up period was just 2 years. However, other authors had a similar or even shorter period of follow-up.[13,26,32]
Third, we included patients with non-valvular and valvular AF so our results could not be completely comparable to other studies where only non-valvular AF patients were enrolled.
Fourth, we did not analyze the impact of proteinuria on the clinical course. Proteinuria/albuminuria is an established CV risk factor. Ohayama et al in a group of more than 20.000 subjects found a significant association of proteinuria and lower eGFR with AF.[49] Alonso et al in the ARIC study observed that albuminuria was strongly associated with AF[50] while Go et al suggested that proteinuria may improve risk stratification and included it into the ATRIA risk score.[10] Our study has also several strengths. First, eGFR was calculated using CKD Epi equation which is recommended by all relevant international guidelines while other authors used MDRD equation or even creatinine clearance. Second, CKD was confirmed after 3 months, so we have selected a group of patients with truly CKD what was done very seldom by other authors. Third, we did not lose any of the patient during the follow-up period.
5. Conclusion
Prevalence of CKD, particularly more advanced stages of CKD, is very high in patients with AF. Observed higher CV mortality and shorter mean survival time in CKD patients could be explained with higher CHA2DS2VASc score which is a consequence of clustering of all score components in CKD patients. However, eGFR was independently associated with CV mortality. In our cohort, R2CHA2DS2VASc score was not associated with CV mortality more than CHA2DS2VASc score. Further research is needed to determine the appropriate risk score for AF patients with CKD.
Author contributions
Conceptualization: Vedran Premuzic, Ranko Stevanović, Massimo Salvetti, Martina Lovrić-Benčić, Davor Miličić, Enrico Agabiti-Rosei, Bojan Jelaković.
Data curation: Vedran Premuzic, Petra Radić, Ana Jelaković.
Formal analysis: Vedran Premuzic, Petra Radić, Ana Jelaković, Krunoslav Capak.
Investigation: Ranko Stevanović, Petra Radić, Ana Jelaković, Krunoslav Capak.
Methodology: Vedran Premuzic, Ranko Stevanović, Martina Lovrić-Benčić, Ana Jelaković, Davor Miličić, Krunoslav Capak, Enrico Agabiti-Rosei, Bojan Jelaković.
Project administration: Davor Miličić, Krunoslav Capak, Bojan Jelaković.
Software: Petra Radić, Martina Lovrić-Benčić, Krunoslav Capak.
Supervision: Massimo Salvetti, Martina Lovrić-Benčić, Davor Miličić, Enrico Agabiti-Rosei, Bojan Jelaković.
Visualization: Bojan Jelaković.
Writing – original draft: Vedran Premuzic, Ranko Stevanović, Massimo Salvetti, Bojan Jelaković.
Writing – review & editing: Vedran Premuzic.
Glossary
Abbreviations: AF = atrial fibrillation, CKD = chronic kidney disease, CV = cardiovascular, eGFR = estimated glomerular filtration rate, PAD = peripheral arterial disease.
References
- [1].Chugh S, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zimmerman D, Sood M, Rigatto C, et al. Systematic review and meta-analysis of incidence, prevalence and outcomes of atrial fibrillation in patients on dialysis. Nephrol Dial Transplant 2012;27:3816–22. [DOI] [PubMed] [Google Scholar]
- [3].Wetmore J, Mahnken JD, Rigler S, et al. The prevalence of and factors associated with chronic atrial fibrillation in Medicare/Medicaid-eligible dialysis patients. Kidney Int 2012;81:469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Genovesi S, Pogliani D, Faini A, et al. Prevalence of atrial fibrillation and associated factors in a population of longterm hemodialysis patients. Am J Kidney Dis 2005;46:897–902. [DOI] [PubMed] [Google Scholar]
- [5].Wizemann V, Tong L, Satayathum S, et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int 2010;77:1098–106. [DOI] [PubMed] [Google Scholar]
- [6].Roy-Chaudhury P, Williamson D, Tumlin J, et al. Arrhythmic risk in patients with type II diabetes on hemodialysis: preliminary results from the Monitoring in Dialysis (MiD) Clinical Study. J Am Soc Nephrol 2015;26:275A. [Google Scholar]
- [7].Konigsbrugge O, Posch F, Antlanger M, et al. Prevalence of atrial fibrillation and antithrombotic therapy in hemodialysis patients: cross-sectional results of the Vienna InVestigation of AtriaL Fibrillation and Thromboembolism in Patients on HemoDIalysis (VIVALDI). PLoS One 2017;12:e0169400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Turakhia M, Blankestijn P, Carrero JJ, et al. Conference Participants. Chronic kidney disease and arrhythmias: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J 2018;39:2314–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bansal N, Fan D, Hsu CY, et al. Incident atrial fibrillation and risk of death in adults with chronic kidney disease. J Am Heart Assoc 2014;3:e001303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Go A, Fang M, Udaltsova N, et al. ATRIA Study Investigators. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation 2009;119:1363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wiesholzer M, Harm F, Tomasec G, et al. Incidence of stroke among chronic hemodialysis patients with nonrheumatic atrial fibrillation. Am J Nephrol 2001;21:35–9. [DOI] [PubMed] [Google Scholar]
- [12].Vazquez E, Sanchez-Perales C, Borrego F, et al. Influence of atrial fibrillation on the morbido-mortality of patients on hemodialysis. Am Heart J 2000;140:886–90. [DOI] [PubMed] [Google Scholar]
- [13].Roldan V, Marin F, Manzano-Fernandez S, et al. Does chronic kidney disease improve the predictive value of the CHADS2 and CHA2DS2-VASc stroke stratification risk scores for atrial fibrillation? Thromb Haemost 2013;109:956–60. [DOI] [PubMed] [Google Scholar]
- [14].Banerjee A, Fauchier L, Vourc’h P, et al. Renal impairment and ischemic stroke risk assessment in patients with atrial fibrillation: the Loire Valley Atrial Fibrillation Project. J Am Coll Cardiol 2013;61:2079–87. [DOI] [PubMed] [Google Scholar]
- [15].Jun M, James MT, Manns BJ, et al. The association between kidney function and major bleeding in older adults with atrial fibrillation starting warfarin treatment: population based observational study. BMJ 2015;350:h246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dahal K, Kunwar S, Rijal J, et al. Stroke, major bleeding, and mortality outcomes in warfarin users with atrial fibrillation and chronic kidney disease: a meta-analysis of observational studies. Chest 2016;149:951–9. [DOI] [PubMed] [Google Scholar]
- [17].Wetmore J, Ellerbeck E, Mahnken J, et al. Atrial fibrillation and risk of stroke in dialysis patients. Ann Epidemiol 2013;23:112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chan P, Huang D, Yip P, et al. Ischaemic stroke in patients with atrial fibrillation with chronic kidney disease undergoing peritoneal dialysis. Europace 2016;18:665–71. [DOI] [PubMed] [Google Scholar]
- [19].Chao T, Liu C, Wang K, et al. Incidence and prediction of ischemic stroke among atrial fibrillation patients with end-stage renal disease requiring dialysis. Heart Rhythm 2014;11:1752–9. [DOI] [PubMed] [Google Scholar]
- [20].Piccini J, Stevens S, Chang Y, et al. ROCKET AF Steering Committee and Investigators. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation 2013;127:224–32. [DOI] [PubMed] [Google Scholar]
- [21].Friberg L, Benson L, Lip GY. Balancing stroke and bleeding risks in patients with atrial fibrillation and renal failure: the Swedish Atrial Fibrillation Cohort study. Eur Heart J 2015;36:297–306. [DOI] [PubMed] [Google Scholar]
- [22].Rule AD. The CKD-EPI equation for estimating GFR from serum creatinine: real improvement or more of the same? Clin J Am Soc Nephrol 2010;5:6951–3. [DOI] [PubMed] [Google Scholar]
- [23].ESH guidelines 2013ESH/ESC. Task Force for the Management of Arterial, Hypertension., Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC):, ESH/ESC., Task Force for the Management of Arterial, Hypertension. J Hypertens 2013;31:1925–38. [DOI] [PubMed] [Google Scholar]
- [24].George D, Mallery P. IBM SPSS statistics 23 step by step: a simple guide and reference. 2016. [Google Scholar]
- [25].Kerr K, Wang Z, Janes H, et al. Net reclassification indices for evaluating risk prediction instruments: a critical review. Epidemiology 2014;25:114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Proietti R, Gonzini L, Pizzimenti G, et al. ATAAF Investigators Glomerular filtration rate: A prognostic marker in atrial fibrillation—A subanalysis of the AntiThrombotic Agents Atrial Fibrillation Clin Cardiol 2018;41:1570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang D, Liu M, Hao Z, et al. Association between reduced kidney function and clinical outcomes after ischaemic stroke with atrial fibrillation. Eur J Neurol 2014;21:160–6. [DOI] [PubMed] [Google Scholar]
- [28].Ananthapanyasut W, Napan S, Rudolph E, et al. Prevalence of atrial fibrillation and its predictors in nondialysis patients with chronic kidney disease. Clin J Am Soc Nephrol 2010;5:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wu D, Mansoor G, Kempf C, et al. Renal function, attributes and coagulation treatment in atrial fibrillation (R-FACT Study): retrospective, observational, longitudinal cohort study of renal function and antithrombotic treatment patterns in atrial fibrillation patients with documented eGFR in real-world clinical practices in Germany. Int J Clin Pract 2014;68:714–24. [DOI] [PubMed] [Google Scholar]
- [30].Laiblea M, Horstmanna S, Rizosa T, et al. Prevalence of renal dysfunction in ischaemic stroke and transient ischaemic attack patients with or without atrial fibrillation. Eur J Neurol 2015;22:64–9. [DOI] [PubMed] [Google Scholar]
- [31].Parsons Ch, Cha S, Shen WK, et al. Usefulness of the addition of renal function to the CHA2DS2-VASc score as a predictor of thromboembolism and mortality in patients without atrial fibrillation. Am J Cardiol 2018;122:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Guo Y, Gao J, Ye P, et al. Comparison of atrial fibrillation in CKD and non-CKD populations: a cross-sectional analysis from the Kailuan study. Int J Cardiol 2019;277:125–9. [DOI] [PubMed] [Google Scholar]
- [33].Reinecke H, Nabaue M, Gerth A, et al. AFNET Study Group. Morbidity and treatment in patients with atrial fibrillation and chronic kidney disease. Kidney Int 2015;87:200–9. [DOI] [PubMed] [Google Scholar]
- [34].Nakagawa K, Hirai T, Takashima S, et al. Chronic kidney disease and CHADS(2) score independently predict cardiovascular events and mortality in patients with nonvalvular atrial fibrillation. Am J Cardiol 2011;15:912–6. [DOI] [PubMed] [Google Scholar]
- [35].Olesen JB, Lip GY, Kamper AL, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med 2012;367:625–35. [DOI] [PubMed] [Google Scholar]
- [36].Sauer EM, Sauer R, Kallmünzer B, et al. Impaired renal function in stroke patients with atrial fibrillation. J Stroke Cerebrovasc Dis 2014;23:1225–8. [DOI] [PubMed] [Google Scholar]
- [37].Kodani E, Atarashi H, Inoue H, et al. J-RHYTHM Registry Investigators. J-RHYTHM Registry Investigators. Impact of creatinine clearance on outcomes in patients with non-valvular atrial fibrillation: a subanalysis of the J-RHYTHM Registry. Eur Heart J Qual Care Clin Outcomes 2018;4:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Parfrey PS, Foley RN. The clinical epidemiology of cardiac disease in chronic renal failure. J Am Soc Nephrol 1999;10:1606–15. [DOI] [PubMed] [Google Scholar]
- [39].Murthy VL, Naya M, Foster CR, et al. Coronary vascular dysfunction and prognosis in patients with chronic kidney disease. JACC Cardiovasc Imaging 2012;5:1025–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tatematsu S, Wakino S, Kanda T, et al. Role of nitric oxide-producing and -degrading pathways in coronary endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol 2007;18:741–9. [DOI] [PubMed] [Google Scholar]
- [41].Stenvinkel P. Endothelial dysfunction and inflammation: is there a link? Nephrol Dial Transplant 2001;16:1968–71. [DOI] [PubMed] [Google Scholar]
- [42].Taqueti VR, Solomon SD, Shah AM, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 2018;39:840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hensen LCR, Goossens K, Delgado V, et al. Prognostic implications of left ventricular global longitudinal strain in predialysis and dialysis patients. Am J Cardiol 2017;120:500–4. [DOI] [PubMed] [Google Scholar]
- [44].Bajaj NS, Singh A, Zhou W, et al. Coronary microvascular dysfunction, left ventricular remodeling, and clinical outcomes in patients with chronic kidney impairment. Circulation 2020;141:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Apostolakis S, Guo Y, Lane DA, et al. Renal function and outcomes in anticoagulated patients with non-valvular atrial fibrillation: the AMADEUS trial. Eur Heart J 2013;34:3572–9. [DOI] [PubMed] [Google Scholar]
- [46].Lin WY, Lin YJ, Chung FP, et al. Impact of renal dysfunction on clinical outcome in patients with low risk of atrial fibrillation. Circ J 2014;78:853–8. [DOI] [PubMed] [Google Scholar]
- [47].Soliman E, Prineas R, Go A, et al. Chronic renal insufficiency cohort (CRIC) study group. chronic kidney disease and prevalent atrial fibrillation. Am Heart J 2010;159:1102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Baber U, Howard VJ, Halperin JL, et al. Association of chronic kidney disease with atrial fibrillation among adults in the United States: Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circ Arrhythm Electrophysiol 2011;4:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ohyama Y, Imai M, Kurabayashi M. Estimated Glomerular Filtration Rate and Proteinuria Are Separately and Independently Associated with the Prevalence of Atrial Fibrillation in General Population. PLoS ONE. 8(11):e79717. doi:10.1371/journal.pone.0079717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Alonso A, Lopez F, Matsushita K, et al. Chronic kidney disease is associated with the incidence of atrial fibrillation: the atherosclerosis risk in communities (ARIC) study. Circulation 2011;123:2946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]


