Abstract
Acute respiratory distress syndrome secondary to severe acute respiratory syndrome coronavirus-2 pneumonia or coronavirus disease 2019-related acute respiratory distress syndrome is the primary cause of mortality in coronavirus disease 2019. Some studies have described the concept of “high and low” elastance coronavirus disease 2019-related acute respiratory distress syndrome and proposed individualized management for the acute respiratory distress syndrome, deviating from low tidal volume ventilation. We report simultaneously measured respiratory parameters (static lung compliance, alveolar dead space ventilation, and shunt fraction) in 14 patients with advanced coronavirus disease 2019-related acute respiratory distress syndrome. The results were consistent with typical acute respiratory distress syndrome and did not support the concept of high-type coronavirus disease 2019-related acute respiratory distress syndrome and low-type coronavirus disease 2019-related acute respiratory distress syndrome.
Keywords: acute respiratory distress syndrome, coronavirus disease 2019, dead space, lung compliance, shunt fraction
To the Editor:
Acute respiratory distress syndrome (ARDS) secondary to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pneumonia is the primary cause of mortality in coronavirus disease 2019 (COVID-19). The reported mortality of mechanically patients with COVID-19-related ARDS or CARDS has varied from 30.9% to 97% (1, 2).
A few studies have suggested that ARDS in the setting of COVID-19 infection is not typical ARDS as seen in non-COVID-19 infection. During early phases of pandemic, Gattinoni et al (3) proposed two different phenotypes of CARDS; “high (H) and low (L)” types based on high and low elastances, respectively. Gattinoni et al (4) reported high shunt fraction in some patients with L phenotype and he postulated that these findings may be due to the loss of hypoxemic vasoconstriction. However, there is no report describing simultaneous respiratory shunt fraction, dead space ventilation, and static compliance in patients with CARDS. Here, we report the comprehensive physiologic parameters such as static lung compliance, alveolar dead space fraction, and shunt fraction in mechanically ventilated patients with ARDS to elucidate the physiologic concepts.
MATERIALS AND METHODS
We conducted a retrospective study of prospectively collected data. The study was reviewed and approved by the Institutional Review Board at Albany Medical Center (5825). All consecutive mechanically patients with COVID-19-related ARDS who were admitted to a tertiary-care hospital between March 17, 2020, and April 21, 2020, were enrolled. Respiratory parameters such as static lung compliance, Pao2/Fio2, shunt fraction, and alveolar dead space fraction were recorded in real time. Arterial blood gas samples were obtained with patients receiving 100% oxygen for 20 minutes. The shunt fraction was calculated by using previously validated formula used for noninvasive calculation of shunt fraction.
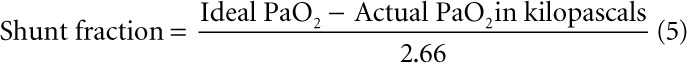 |
Dead space ventilation was calculated by the following formula:
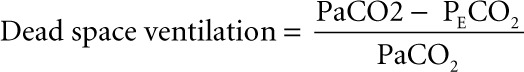 |
where Paco2 is the partial pressure of CO2 in the artery and PEco2 represents the end-tidal CO2.
The Paco2 and PEco2 values were obtained from arterial blood gas and simultaneous real-time measurement of partial pressure of carbon dioxide by end-tidal capnography using Masimo Nomoline side stream capnographers (Irvine, CA). Static lung compliance was calculated using the following equation:
Static lung compliance, Cs = Tidal volume/(Plateau pressure (Pplat) – Positive end-expiratory pressure [PEEP]) mL/cm H2O
The Pplat was measured while the patient was paralyzed using the inspiratory pause maneuver during volume control ventilation with square-flow waveform. Additional data such as age, sex, ethnicity, disease severity, and outcomes (inhospital mortality, 28-d ventilator, and 30-d hospital-free days) were obtained by chart review. The physiologic parameter measurements were repeated in for six patients who underwent prone positioning.
RESULTS
We reported a cohort of 14 patients who have respiratory parameters measured. The majority (10/14, 71%) of the patients were male with the median age of 56 years (Table 1). The mean Pao2/Fio2 was 166 with a mean Sequential Organ Failure Assessment score of 7. The physiologic data were collected after a median of 10 days following intubation, indicating advanced disease. The median static compliance of the respiratory system was low, 28.4 mL/cm H2O, (interquartile range [IQR], 22–35.3). The median alveolar dead space fraction was 20% and median shunt fraction was 30%. There was no significant correlation found between the static lung compliance and shunt fraction (r = –0.092; p = 0.680) (Fig. 1); however, there was a weak negative correlation noted between static lung compliance and alveolar dead space fraction (r = –0.692; p = 0.02) (Fig. 2). There were only two patients with relatively higher static lung compliance. There was no difference in the dead space ventilation or shunt fraction between the patients with low or high lung compliance. Similarly, there were no significant changes in the static lung compliance, dead space ventilation, or shunt fraction following proning.
Table 1.
Demographic Data, Severity of Illness, Respiratory Parameters, and Outcome Data
| Variables | Intubated Coronavirus Disease 2019, n = 14 |
|---|---|
| Sex, n (%) | |
| Male | 10 (71) |
| Female | 4 (29) |
| Age, yr | 57.5, 56.0 (49.5–65.8) |
| Ethnicities, n (%) | |
| White | 5 (36) |
| Black | 3 (21) |
| Hispanic | 3 (21) |
| Other | 3 (21) |
| Charlson score | 2.5, 2.0 (1.0–3.0) |
| Severity indexes | |
| Acute Physiologic Assessment and Chronic Health Evaluation II | 24.3, 23.0 (18.8–27.3) |
| Simplified Acute Physiology II | 54.4, 57.0 (43.3–65.0) |
| Sequential Organ Failure Assessment | 8.6, 7.0 (6.0–11.3) |
| Respiratory parameters at ICU admission | |
| Pao2/Fio2 ratio | 166.3, 136.5 (89.5–178.5) |
| Positive end-expiratory pressure | 12.3, 12.0 (10.0–13.5) |
| Plateau pressure supine | 28.7, 27.0 (22.0–35.3) |
| Interval after postintubation before shunt calculation, d | 10.3, 9.5 (5.3–17) |
| Shunt fraction supine | 0.3, 0.3 (0.2–0.3) |
| Dead space supine | 0.2, 0.2 (0.0–0.4) |
| Static compliance supine | 28.4, 27 (17.8–36.0) |
| Outcomes | |
| Inhospital mortality, n (%) | 5 (33.3) |
| 28-d ventilator-free day | 11.73, 8 (0–26) |
| 30-d hospital-free day | 7.73, 5 (0–18) |
All data were reported as mean, median, and interquartile range unless otherwise specified.
Figure 1.
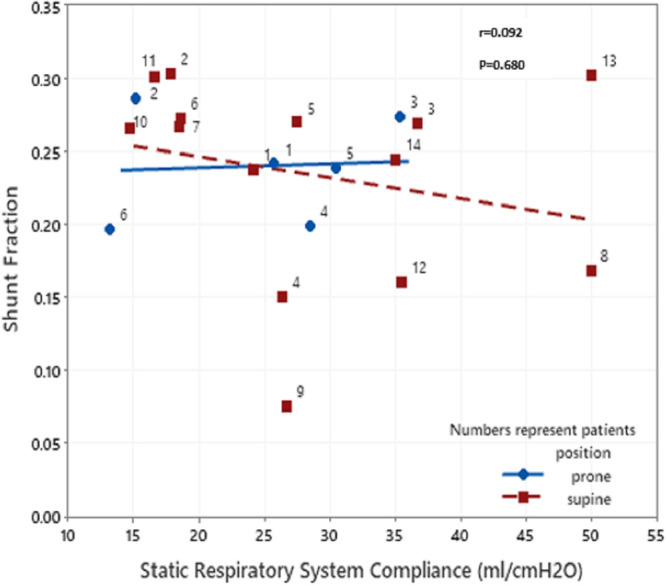
Scatterplot showing correlation between static lung compliance and shunt fraction. No correlation was seen between static lung compliance and shunt fraction.
Figure 2.
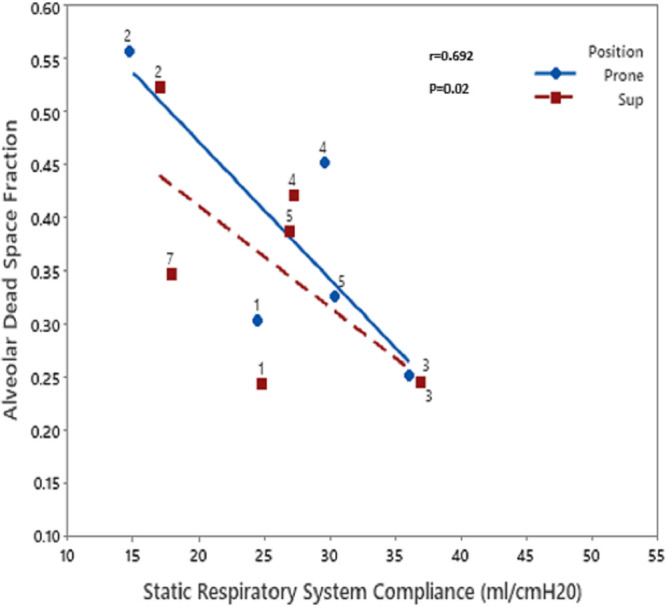
Scatterplot showing correlation between static lung compliance and dead space ventilation. A weak negative correlation was seen between static lung compliance and dead space ventilation.
DISCUSSION
In our study, we find that majority of patients have low lung compliance. There was weakly negative correlation noted between static lung compliance and dead space ventilation; however, there was no correlation noted between static lung compliance and shunt fraction. This study shows that the physiologic parameters in CARDS are comparable with previously reported ARDS patients.
Gattinoni et al (3) proposed the concept of “high and low elastance” pneumonia with COVID-19. In their early report, patients with low elastance had a relatively preserved static compliance (greater than 50 mL/cm H2O) despite fulfilling Berlin criteria for ARDS. In our study, majority of patients demonstrated high lung elastance and low compliance (< 30 mL/cm H2O). No patients had a compliance greater than 50 mL/cm H2O. We believe that these observed differences in static lung compliance were due to the reporting of patients with later stages of ARDS in our cohort compared with patients reported by Gattinoni et a (3, 4) l. These discrepancies can be explained by the pathologic evolution of CARDS in a stepwise fashion that has also been observed in patients with non-COVID ARDS (6). CARDS is a heterogeneous disease, and the proposed evolution of L to H phenotype may be more theoretical than factual (4).
Gattinoni et al (3) also reported significantly elevated shunt fraction in CARDS. They suggested that the loss of hypoxic vasoconstriction was likely responsible for this disproportionately high shunt fraction and refractory hypoxia in early COVID-19 pneumonia (3). In addition, the authors proposed further worsening of right to left shunt with the conversion of “L type” to “H type” disease (4). Contrasting this hypothesis, a very high shunt fraction was not observed in our patients. The shunt fraction was moderately elevated, with a median of 30%. No correlation was seen between the respiratory system compliance and shunt fraction (r = 0.092; p = 0.680) (8). Poor correlation between the respiratory compliance and shunt fraction may be due to the multiple factors: 1) physiologic parameters were measured at variable times (median = 9.5; IQR, 5–17 d after intubation) in the disease course while patients were on mechanical ventilation, 2) variable amount of PEEP was used to obtain the optimal compliance, and 3) previous studies have shown that shunt fraction varies widely in typical ARDS and functional shunt (as measured by blood gas) poorly correlates with the anatomical shunt (as measured by the whole lung CT scan) (7).
COVID-19 infection is associated with the development of endothelialitis, microangiopathy, and thrombophilia. Autopsy studies have consistently revealed pulmonary vascular thrombosis. Pulmonary microvascular occlusion causes an increase in the physiologic dead space, which can be manifested by progressive and refractory hypercapnia. Increasing dead space ventilation is associated with higher mortality in ARDS (8). Our patients showed a mean alveolar dead space ventilation fraction of 20%, which was only modestly increased. It is possible that the observed trend was due to therapeutic anticoagulation, which is being widely used in patients with COVID-19 infection based on recent studies showing a possible mortality benefit in mechanically ventilated patients (9).
There were concerns at the beginning of the pandemic that the ARDS from SARS-CoV-2 was different from typical ARDS (low elastance with high dead space), causing clinicians to deviate from proven ventilator management strategies (4, 10). However, our study clearly shows that the physiologic parameters in CARDS are comparable with previously reported ARDS patients (7).
Our study has several limitations. One of the major limitations was that respiratory parameters were measured only once at variable times during the disease course of ARDS. Respiratory parameters were measured at a median of 9.5 days (IQR, 5–17 d) after intubation. Therefore, we cannot determine the exact pathophysiological state of the cohort. Additionally, this is a retrospective study with a small number of patients. However, the respiratory parameters were recorded in real time. Finally, the shunt fraction was calculated noninvasively rather than invasively obtaining mixed venous oxygen content, which was not feasible in COVID-19 ARDS patients.
CONCLUSIONS
Patients with CARDS demonstrate respiratory physiologic parameters that are comparable to patients with ARDS of non-COVID-19 origin. These patients should be managed by strictly following standard ventilator strategies, known to improve survival in patients with ARDS.
Footnotes
Drs. Ghalib, Chieng, Pezzano, Lydon, and Chopra were involved in direct patient care and collection of data. Dr. Chopra conceptualized the study. Dr. Saha analyzed and prepared the initial article. Dr. Feustel was involved in the statistical analysis of the study. Drs. Saha, Smith, and Chopra finalized the article.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Twigg HL, III, Khan SH, Perkins AJ, et al. Mortality rates in a diverse cohort of mechanically ventilated patients with novel coronavirus in the urban Midwest. Crit Care Explor. 2020; 2:e0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson S, Hirsch JS, Narasimhan M, et al. the Northwell COVID-19 Research Consortium Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020; 323:2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gattinoni L, Coppola S, Cressoni M, et al. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020; 201:1299–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020; 46:1099–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ming DK, Patel MS, Hopkinson NS, et al. The ‘anatomic shunt test’ in clinical practice; contemporary description of test and in-service evaluation. Thorax. 2014; 69:773–775 [DOI] [PubMed] [Google Scholar]
- 6.Cardinal-Fernández P, Lorente JA, Ballén-Barragán A, et al. Acute respiratory distress syndrome and diffuse alveolar damage. New insights on a complex relationship. Ann Am Thorac Soc. 2017; 14:844–850 [DOI] [PubMed] [Google Scholar]
- 7.Cressoni M, Caironi P, Polli F, et al. Anatomical and functional intrapulmonary shunt in acute respiratory distress syndrome. Crit Care Med. 2008; 36:669–675 [DOI] [PubMed] [Google Scholar]
- 8.Nuckton TJ, Alonso JA, Kallet RH, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002; 346:1281–1286 [DOI] [PubMed] [Google Scholar]
- 9.Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020; 76:122–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020; 323:2329–2330 [DOI] [PubMed] [Google Scholar]


