Abstract
Autophagy, a conserved cellular process in eukaryotes, has evolved to a sophisticated process to dispose of intracellular constituents and plays important roles in plant development, metabolism, and efficient nutrients remobilization under suboptimal nutrients conditions. Here, we show that OsATG8b, an AUTOPHAGY-RELATED8 (ATG8) gene in rice, was highly induced by nitrogen (N) starvation. Elevated expression of OsATG8b significantly increased ATG8 lipidation, autophagic flux, and grain yield in rice under both sufficient and deficient N conditions. Overexpressing of OsATG8b could greatly increase the activities of enzymes related to N metabolism. Intriguingly, the 15N-labeling assay further revealed that more N was remobilized to seeds in OsATG8b-overexpressing rice, which significantly increased the N remobilization efficiency (NRE), N harvest index, N utilization efficiency (NUE), and N uptake efficiency (NUpE). Conversely, the osatg8b knock-out mutants had the opposite results on these characters. The substantial transcriptional changes of the overexpressed transgenic lines indicated the presence of complex signaling to developmental, metabolic process, and hormone, etc. Excitingly, the transgenic rice under different backgrounds all similarly be boosted in yield and NUE with OsATG8b overexpression. This work provides an excellent candidate gene for improving N remobilization, utilization, and yield in crops simultaneously.
Introduction
Autophagy (self-eating) is an ancient and universal process for degrading and removing cellular debris, which consists of five stages: initiation, the formation of autophagosomes, expansion, engulfment of cargoes, and delivery of the cargo for degradation [1]. It plays an essential role in the maintenance of cellular homeostasis [2]. The AUTOPHAGY-RELATED (ATG) gene was originally identified by Yoshinori Ohsumi in yeast [3]. Subsequently, a large number of plant autophagy genes are characterized from the orthologs of yeast ATG, such as Arabidopsis (Arabidopsis thaliana), rice (Oryza sativa), maize (Zea mays), tobacco (Nicotiana tabacum), foxtail millet (Setaria italica) and the alga (Chlamydomonas reinhardtii) [4–9]. It was shown that a variety mutants of Arabidopsis, maize, and rice displayed growth inhibition, accelerated leaf senescence, hypersensitivity to nutrition starvation, and reduced fecundity and yield [10–15]. Typically, these phenotypes are more pronounced when mutants are grown under nutrient-deficient conditions.
N is one of the main determinants for crop yield formation, breeding cultivars with high NUE has been an urgent requirement for sustainable crop productivity [16]. However, plant NUE is complex and regulated by multiple interacting genetic and environmental factors [17, 18]. Autophagy is essential for N remobilization and reuse, which affects the yield and quality of grain in cereal crops, especially under N deficiency stress [19]. A study of the Arabidopsis atg5, atg9, and atg18a mutants first evidenced that the autophagy regulated N remobilization from leaves to seeds [20]. It was subsequently found that the NUE in atg5 mutant seeds sharply decreased to about 50% of that in the wild-type seeds under N restriction [20]. Similar results were found in studies of crops such as rice and maize, the biomass and NUE of rice osatg7-1 mutant were strongly affected during the vegetative growth stage [14]. 15N partitioning studies revealed that the amount of recycled N exported in maize atg12 mutant was substantially decreased by two-fold [5]. Using a multi-omics strategy, McLoughlin showed that autophagy also significantly affects the remodeling of the maize proteome and lipids [21]. A relationship between autophagy and the deficiency of other nutrients (P, S, and Zn) has also been found [22–24], but the role of autophagy under these conditions has not been clearly defined.
Among the many plant ATG proteins, ATG8 is a key protein and central player involved in the trafficking and vacuolar fusion of autophagosomes because it provides a docking site for ATG8 interaction motif (AIM)-containing autophagic receptors that selectively recruit cargo [25]. The interaction of ATG8 and autophagy receptors leads to the formation of autophagosomes [26, 27]. However, there is also evidence that ATG8-independent autophagy occurs in maize aleurone cells, delivering storage proteins to novel vacuoles [28]. Jia et al. identified an unrecognized conserved senescence regulatory pathway controlled by ATG8-ABS3-mediated proteostasis, which is not dependent on autophagy in wheat [29].
There is a single ATG8 in yeast and other fungi, but there are several ATG8 isoforms with redundant functions in higher eukaryotes [30–32]. Intriguingly, several studies found that overexpression of ATG8 homologs in transgenic plants not only led to better resistance to stresses and starvations, but also accelerated growth under optimal growth conditions, such as overexpression of AtAtg8f in Arabidopsis could promote the growth, increased rosettes size and conferred tolerance to N and C limitation [33]. Overexpression of apple MdATG8i in Arabidopsis could promote plant growth, advanced bolting, accelerated leaf senescence, and enhanced tolerance to N and C starvation, while in apple callus cells could accelerate growth and enhanced N starvation tolerance [34]. Overexpression of soybean GmATG8c in Arabidopsis could promote plant growth, advanced bolting, increased yield and tolerance to N and C starvation, while in soybean callus could enhance N starvation tolerance and accelerate the growth of the calli [35]. Foxtail millet SiATG8a overexpressing in Arabidopsis could increase resistance to both drought stress and N starvation [36], while in rice could confer tolerance to N starvation [6]. The recent study also showed that overexpression of AtATG8a, AtATG8e, AtATG8f, or AtATG8g in Arabidopsis could improve seed N% and reduce N waste in dry remains under full nitrate conditions, though there was little difference in plant phenotype [37]. Seven ATG8 isoforms (OsATGa to OsATGg) have been identified in the rice genome. The first cloned rice autophagy associated gene was OsATG8a, which interacted with OsATG4 [38]. OsATG8a to OsATG8c share high levels of amino acid sequence identity, and OsATG8d is highly homologous to AtATG8i [8, 9]. The mRFP-OsATG8a and mRFP-OsATG8d fusions were used as autophagy markers to monitor autophagy directly [39]. OsATG8e has a gene locus but lacks supporting expressed sequence tag (EST) data, and OsATG8f has no corresponding full-length cDNA, while OsATG8i has not been mapped to the rice genome [8]. Although these research reported the OsATG8s of rice, whose physiological and molecular functions are still not clear in rice, except for our own research showed that overexpression of OsATG8a or OsATG8c caused higher NUE and yield in rice [40, 41].
In this study, we found that constitutive overexpression of OsATG8b in rice enhanced the autophagic flux and grain yield, leading to the strong stimulation of growth and N stress resistance. N partitioning studies showed that overexpression of OsATG8b significantly enhanced N harvest index (NHI) and NUE, and in a 15N isotope tracer experiment the 15N allocation to seeds was significantly higher than that in the control group. In contrast, osatg8b mutants showed opposite effects to those of transgenic overexpression lines. The global gene expression analysis suggested key transcriptional changes associated with OsATG8b-mediated up-regulated autophagy. Our findings reveal that accelerating nutrient mobilization via OsATG8b-mediated autophagy could have agronomic benefits and suggest that OsATG8b is an important candidate gene for synergistically enhancing grain yield and NUE in rice.
Results
N-depletion induced OsATG8b expression and autophagosomes formation
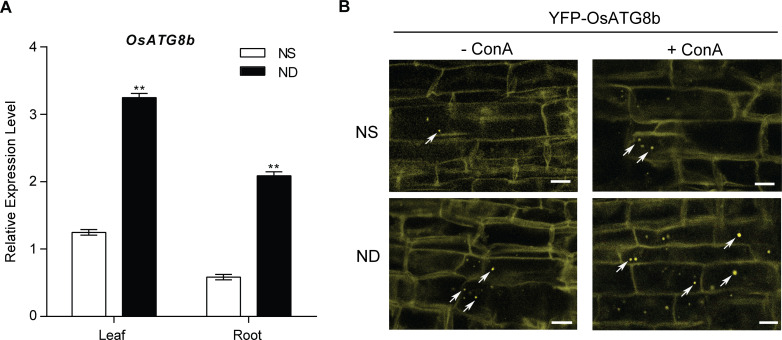
To confirm whether OsATG8b is expressed in response to N starvation, Shennong9816 (SN9816) rice seedlings were placed under no N (ND) condition for 24 h, the expression levels of OsATG8b in leaves and roots were significantly induced (Fig 1A). We produced transgenic rice expressing the YFP-OsATG8b fusion protein to visualize the formation of autophagosomes in response to N-depletion. Concanamycin A (ConA) is a vacuolar acidification inhibitor that blocks the last stages of autophagy by blocking the acidification of lysosomes to facilitate observation of autophagosomes [42, 43]. Compared with N sufficient (NS, 2.88 mM N) condition, we found a significantly increased number of YFP-OsATG8b labeled autophagosomal structures in root cells with or without ConA under the ND treatment (Fig 1B). The fluorescence ratio (positive signal/area) of YFP-OsATG8b labeled autophagosomal structures in roots under ND condition was 3.25 times more than that under NS condition, while 2.42 or 5.49 times more under NS or ND conditions with application of ConA than that under NS condition. These results indicated that N-depletion induced OsATG8b expression and autophagosomes formation.
Fig 1. Nitrogen-induced expression pattern of OsATG8b gene and confocal analysis of YFP-OsATG8b in rice.
(A) 14-day-old rice seedlings of SN9816 cultured with NS solution transferred to the same NS (2.88 mM N) solution and the ND (0 mM N) solution for 24h (n = 10), the expression of OsATG8b gene in leaf and root. OsActin1 was used as an internal control. Values are means ± SD (n = 3), **P < 0.01 (t-test). (B) Seedlings were grown under NS (2.88 mM N) or ND (0 mM N) conditions with or without 0.5 μM ConA for 14h. Representative confocal microscopy images of the epidermal root cells expressing YFP-OsATG8b in SN9816. The white arrowheads indicate the YFP-OsATG8b labeled autophagosomes. Scale bars, 10 μm.
Overexpression of OsATG8b increased autophagic flux in rice
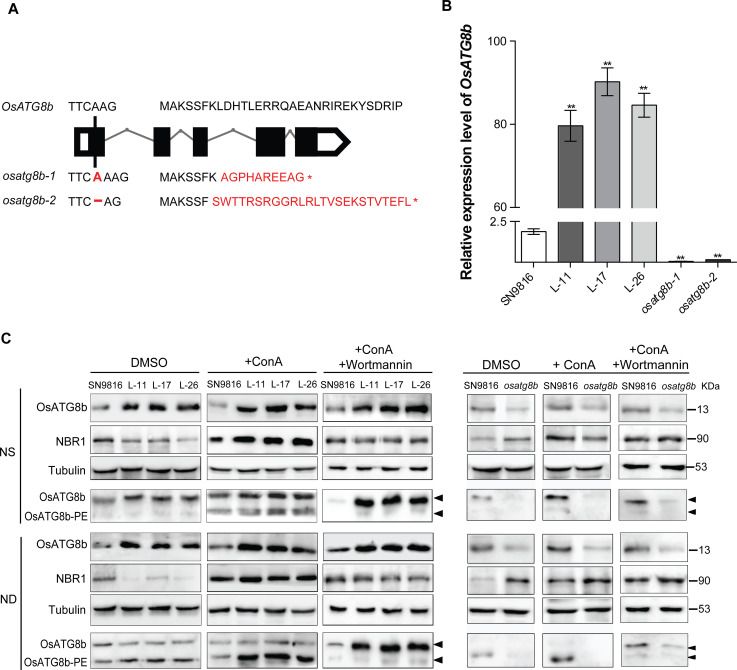
To investigate the function of OsATG8b in rice, we obtained more than 10 OsATG8b-overexpressing transgenic lines in SN9816 background, lines 11, 17, and 26 (L-11, L-17, and L-26) were selected as representative overexpressed lines. Additionally, we knocked out the OsATG8b sequence in SN9816 using the CRISPR/Cas9 system and obtained two independent mutants with different targets. Sequencing of genomic DNA in mutants osatg8b-1 or osatg8b-2 showed that there was a single base insertion or deletion in the first exon (18th bp) of OsATG8b to make a termination codon (Fig 2A). The expression of OsATG8b was up-regulated in OsATG8b-overexpressing lines, but dramatically down-regulated in osatg8b mutants compared with the control plant SN9816 (Fig 2B). To eliminate the influence of altered OsATG8b expression on other OsATG8 genes, we also detected the expression of other OsATG8s. It was shown that the transcript levels of the other OsATG8s were not affected in OsATG8b-overexpressing lines, but down-regulated in osatg8b mutants (S1 Fig). The OsATG8b protein level was significantly increased in OsATG8b-overexpressing lines, while reduced in osatg8b mutants than in SN9816 under both NS and ND conditions using anti-OsATG8b (Fig 2C).
Fig 2. Altered expression of OsATG8b regulated autophagy levels in rice.
(A) Targets of gene knock-out for OsATG8b in SN9816 with CRISPR/Cas9 system and identification of the osatg8b mutants by sequencing of the target sites. (B) The expression level of OsATG8b in leaves of SN9816, OsATG8b-overexpressing lines, and osatg8b mutant. OsActin1 was used as an internal control. Values are means ± SD (n = 3), **P < 0.01 (t-test). (C) 14-day-old rice seedlings of SN9816, OsATG8b-overexpressing lines, and osatg8b mutants cultured with NS or ND solution with DMSO or 0.5 μM ConA with or without 5 μM wortmannin for 24h (n = 10), immunoblot analysis the accumulation of OsATG8b and NBR1 with anti-OsATG8b and anti-AtNBR1, near-equal protein loads were confirmed by immunoblot analysis with an α-tubulin antibody. The membrane fraction was used to detect the level of lipidated (OsATG8b-PE) and free OsATG8b in the leaves.
Since the Arabidopsis autophagy receptor Neighbor of BRCA1 (NBR1) is an autophagy substrate degraded in the vacuole, the amount of NBR1 protein degradation can be used as a measure of selective autophagic flux in plants [42, 44]. As shown in Fig 2C, NBR1 protein accumulation was lower in OsATG8b-overexpressing lines, but increased in osatg8b, especially under the ND treatment. Wortmannin is an early autophagic inhibitor that dampens autophagosome formation in plants [45]. However, the accumulation of NBR1 protein was greatly increased in OsATG8b-overexpressing lines when treatment with ConA, (Fig 2C, +ConA), and this process could be suppressed with the addition of wortmannin (Fig 2C, +ConA+Wortmannin), but there was no significant effect on the protein levels in osatg8b. It was shown that the accumulation of OsATG8b-PE was significantly increased in of OsATG8b-overexpressing lines, thus the OsATG8b lipidation was enhanced, especially under ND condition (Fig 2C, DMSO), and increased to a greater extent after ConA treatment (Fig 2C, +ConA), but suppressed by the wortmannin (Fig 2C, +ConA+Wortmannin). However, the accumulation of OsATG8b-PE was scarcely in the osatg8b mutants both under two N conditions with or without ConA or wortmannin. Although the anti-OsATG8b antibody could not distinguish the OsATG8a, OsATG8b, and OsATG8c (S2 Fig), the transcriptional expression levels of OsATG8a and OsATG8c were not affected in OsATG8b-overexpressing lines, and the overall autophagy flux was greatly enhanced, so we speculated that these results still related to the overexpression of OsATG8b. These results revealed that overexpression of OsATG8b significantly increases autophagic flux in transgenic rice, especially under N starvation.
Enhanced autophagy promoted photosynthesis and N metabolism in rice
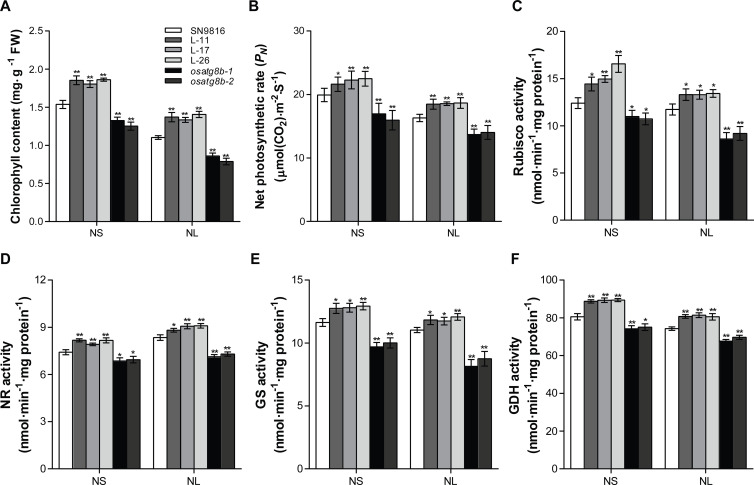
We further investigated the physiological function of OsATG8b in the flag leaves at the grain-filling stage. It was shown that the chlorophyll content, net photosynthetic rate (PN) and Rubisco activity in OsATG8b-overexpressing lines were significantly higher than that of SN9816 under NS (225 kg·ha-1) (increased by an average of 20.13%, 8.51%, and 23.47%, respectively) and NL (75 kg·ha-1) (increased by an average of 24.5%, 13.87%, and 13.82%, respectively) conditions, but dramatically decreased in osatg8b mutants under both two N conditions (Fig 3A–3C). Given that OsATG8b-overexpressing lines performed better under both NS and NL conditions, we speculated that OsATG8b may affect N metabolism. Compared with SN9816, the activities of nitrate reductase (NR), glutamine synthetase (GS) and glutamate dehydrogenase synthesis (GDH) were about 9 to 12% and 7 to 9% higher in the OsATG8b-overexpressing lines under NS and NL conditions, respectively. However, the osatg8b mutants showed significant decreases in activities of these N metabolism-related enzymes (decreased by about 7–15% in NS and 8–23% in NL for both traits) (Fig 3D–3F). Consequently, we concluded that OsATG8b plays a positive role in both photosynthesis and N metabolism.
Fig 3. OsATG8b gene affected the chlorophyll content, photosynthetic character, and enzyme activity involved in photosynthesis and N metabolism.
(A) The chlorophyll content, (B) Net photosynthetic rate (PN), (C) Rubisco activity, (D) Nitrate reductase (NR) activity, (E) Glutamine synthetase (GS) activity, and (F) Glutamate dehydrogenase synthesis (GDH) in flag leaves of SN9816, OsATG8b-overexpressing lines and osatg8b mutants under NS (225 kg·ha-1) and NL (75 kg·ha-1) conditions at the grain-filling stage. Values are means ± SD (n = 12), *P < 0.05, **P < 0.01 (t-test).
Enhanced autophagy significantly improved grain yield
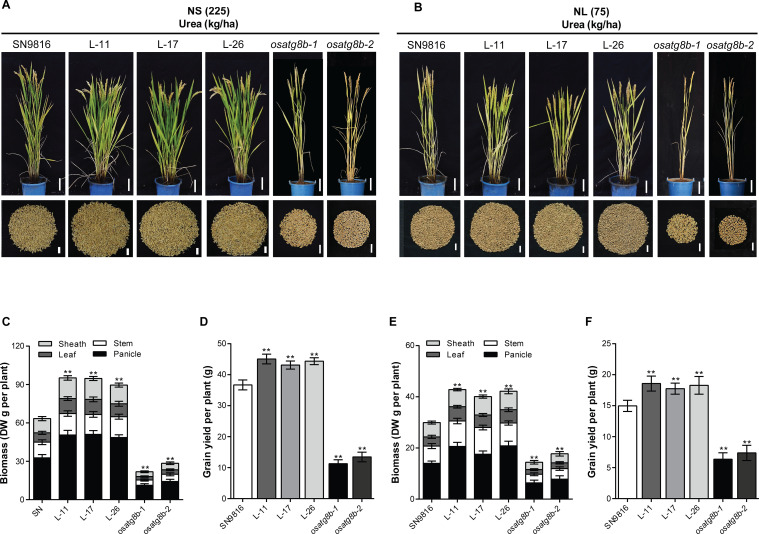
As expected, lines overexpressing OsATG8b exhibited more robust and vigorous growth continued until the ripening stage both under two N conditions (Fig 4A and 4B). Besides, the OsATG8b-overexpressing lines had a significantly higher dry weight of panicle, sheath, stem, and leaf under both NS and NL conditions, resulting in about 46.98% and 39.32% increases in biomass, respectively (Fig 4C and 4E). On the contrary, the osatg8b mutants displayed inhibitory growth and the biomass accumulation decreased by more than 50% than that of SN9816 under both two N conditions. The potential effect of OsATG8b on rice grain yield was further analyzed under both two N conditions. Compared with SN9816, plants overexpressing OsATG8b showed significantly increased total grains number, whereas down-regulation of OsATG8b induced the opposite effects (Fig 4A and 4B). Further analyzed the major agronomic traits showed that the 1000-grain weight, the number of grains, and second branches per panicle were significantly higher in OsATG8b-overexpressing lines under both NS and NL conditions (S2 Table). Although the seed setting rate in the OsATG8b-overexpressing lines was decreased relative to SN9816, the actual grain number per plant was still increased by 39.59% and 30.69% under NS and NL conditions, respectively (S2 Table). Thus, the grain yield per plant in the OsATG8b-overexpressing lines increased by an average of 20.41% and 21.55% under NS and NL conditions. However, the osatg8b mutants displayed dramatically decreases in grain number per panicle and panicles number per plant, resulting in 60.96% and 54.14% reduction in grain yield per plant under NS and NL conditions (Fig 4D and 4F; S2 Table). Taken together, these results suggested that the expression level of OsATG8b was directly and positively correlated to the biomass and grain yield.
Fig 4. Phenotypes, biomass, and grain yield of rice with altered expression of OsATG8b.
(A) and (B) Phenotypes of the whole rice plants and total grains per plant in SN9816, OsATG8b-overexpressing lines, and osatg8b mutants under NS (225 kg·ha-1) and NL (75 kg·ha-1) at ripening stage. Scale bars, 10 cm and 2 cm. (C) and (D) Biomass and grain yield under NS (225 kg·ha-1) condition. (E) and (F) Biomass and grain yield under NL (75 kg·ha-1) condition. Values are means ± SD (n = 12), **P < 0.01 (t-test).
Elevated OsATG8b gene expression improved N utilization and remobilization efficiency in rice
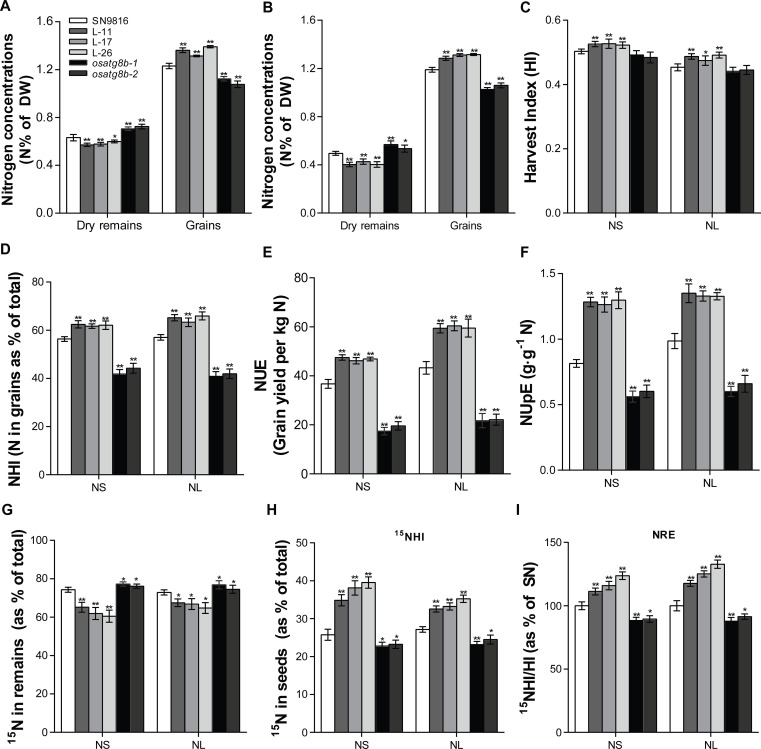
Given the increased yield and biomass in OsATG8b-overexpressing lines, we next assessed whether altered the expression level OsATG8b affected N utilization. Compared with SN9816, the lines overexpressing OsATG8b accumulated more N in seeds but had lower N in dry remains, which reduced the waste of N under both NS (225 kg·ha-1) and NL (75 kg·ha-1) conditions. By contrast, N accumulation in osatg8b mutants was significantly decreased in seeds but increased in dry remains (Fig 5A and 5B). In accordance with the higher yield and N content of seeds from OsATG8b-overexpressing lines, the HI and NHI were significantly higher in the transgenic lines than in SN9816 both under NS (increased by 4.38% and 10.16%) and NL (increased by 6.79% and 13.64%) conditions (Fig 5C and 5D). However, osatg8b mutants showed a little but not significant decrease in HI and a significant decrease in NHI (decreased by 21.87% in NS and 28.11% in NL). As expected, the NUE of OsATG8b-overexpressing lines was significantly increased by 27.51% relative to SN9816 under the NS condition but decreased by 52.55% in osatg8b mutants (Fig 5E). More excitingly, the NUE of OsATG8b-overexpressing lines were 38.16% higher compared with SN9816 under the NL condition, while the osatg8b mutants exhibited a 49.76% reduction in NUE (Fig 5E). Consistent with NUE, NUpE was also significantly increased in the OsATG8b-overexpressing lines, but greatly repressed in osatg8b mutants both under two N conditions (Fig 5F). These results suggested that OsATG8b confers a substantial improvement in NUE and NUpE, which was particularly useful for the maintenance of plant fitness and adaptation to nutrient limitation.
Fig 5. Evaluation of N Utilization Efficiency (NUE) and N Remobilization Efficiency (NRE) in transgenic rice.
(A) and (B) N concentration of dry remains (N %Dr) and seeds (N %SEEDS) under NS (225 kg·ha-1) and NL (75 kg·ha-1) conditions in SN9816, OsATG8b-overexpressing lines and osatg8b mutant. (C) Harvest index (HI), (D) Nitrogen harvest index (NHI), (E) Nitrogen use efficiency (NUE), (F) Nitrogen uptake efficiency (NUpE), (G) Partitioning of 15N in remains, (H) Partitioning of 15N in seeds (15NHI) and (I) 15NHI/HI ration was calculated as a percentage of SN9816 and used to estimate nitrogen remobilization efficiency (NRE) in SN9816 and transgenic rice under NS (225 kg·ha-1) and NL (75 kg·ha-1) conditions. Values are means ± SD (n = 12), *P < 0.05, **P < 0.01 (t-test).
To accurately monitor the movement of N, the partitioning of 15N in SN9816 and transgenic lines was tracked. This analysis showed that overexpression of OsATG8b accumulated more 15N in seeds (increased by 45.6% in NS and 24.01% in NL), and reduced the partitioning of 15N into the remains relative to SN9816 under both N nutrient solutions (decreased by 15.81% in NS and 8.95% in NL). Whereas the opposite results were found in osatg8b mutants (Fig 5G and 5H). The ratio of 15NHI to HI was next used to compare the NRE as described previously [5, 21]. Compared to SN9816, the 15NHI/HI ratio was 16.96% and 25.19% higher in OsATG8b-overexpressing lines than in SN9816 under NS and NL conditions, while decreased by 11.71% and 12.22% in osatg8b mutants (Fig 5I). These results suggested that overexpression of OsATG8b significantly improved N remobilization into seeds, which resulted in favorably increased yield and NUE under varying N conditions.
Transcriptional profiling of rice with enhanced autophagy
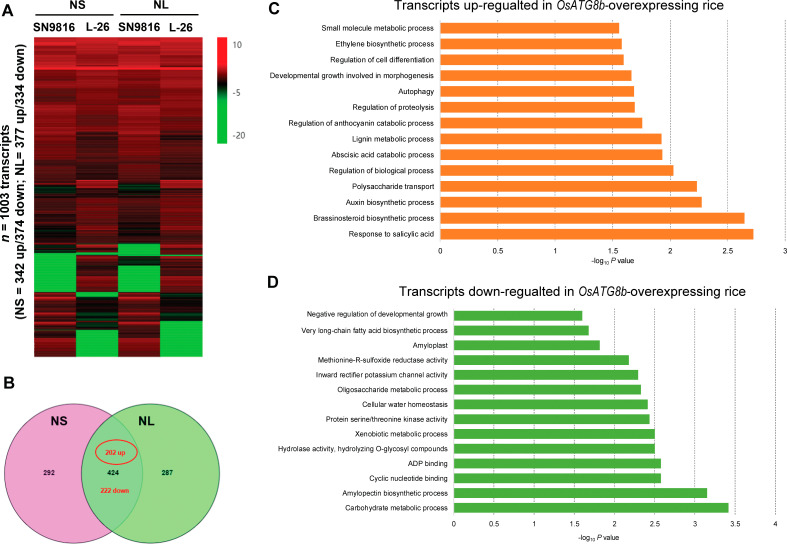
To investigate the possible molecular mechanism by which OsATG8b overexpression affects rice growth and yield, differences between the global gene expression of SN9816 and OsATG8b-overexpressing rice were determined by RNA-sequencing. We identified 1,003 DEGs by comparing the gene expression levels in OsATG8b-overexpressing rice to those in SN9816 under NS or NL conditions (Fig 6A). Of a core set of 424 genes that were significantly differentially expressed, 202 were up-regulated and 222 were down-regulated (Fig 6B). GO enrichment analysis (P < 0.05) showed that genes involved in processes such as biological process regulation, cell differentiation regulation, and plant hormones were up-regulated in OsATG8b-overexpressing plants (Fig 6C). Among these processes, salicylic acid response, abscisic acid, anthocyanin catabolic process, ethylene biosynthetic process, and polysaccharide transport, were previously identified as being impacted by autophagy [46–48]. Conversely, Gene Ontology (GO) enrichment analysis of DEGs down-regulated in OsATG8b-overexpressing plants under NS and NL revealed that these genes were related to negative regulation of developmental growth, carbohydrate and oligosaccharide metabolic processes, biosynthesis of amylopectin, cyclic nucleotide and ADP binding, long-chain fatty acid, and hydrolase activity (Fig 6D). Although N metabolism was not identified as a significant GO term, real-time RT-PCR revealed that the transcript levels of several genes known to be involved in N metabolism [17, 49–52] were up-regulated under both NS and NL conditions (S3 Fig). As shown in S3 Table, the impact of OsATG8b overexpression on transcript abundance was confirmed for several selected DEGs. These results indicate that OsATG8b profoundly influences the rice transcriptome and has great potential for improving growth and development.
Fig 6. Transcriptional trends characteristic for OsATG8b-overexpressing rice under NS (225 kg·ha-1) and NL (75 kg·ha-1) conditions at the grain-filling stage.
(A) Heat maps showing the transcripts differentially expressed in OsATG8b-overexpressing rice versus SN9816 under NS and NL conditions. The color legend indicates normalized gene expression values. (B) Venn diagram showing the overlap in the numbers of transcripts that were consistently up-or down-regulated in OsATG8b-overexpressing rice versus SN9816 grown under NS and NL conditions. (C) and (D) Gene ontology enrichment of DEGs “up-regulated” and “down-regulated” in OsATG8b-overexpressing rice versus SN9816 both under NS and NL conditions.
OsATG8b overexpression conferred high yield and NUE to rice under different genetic backgrounds
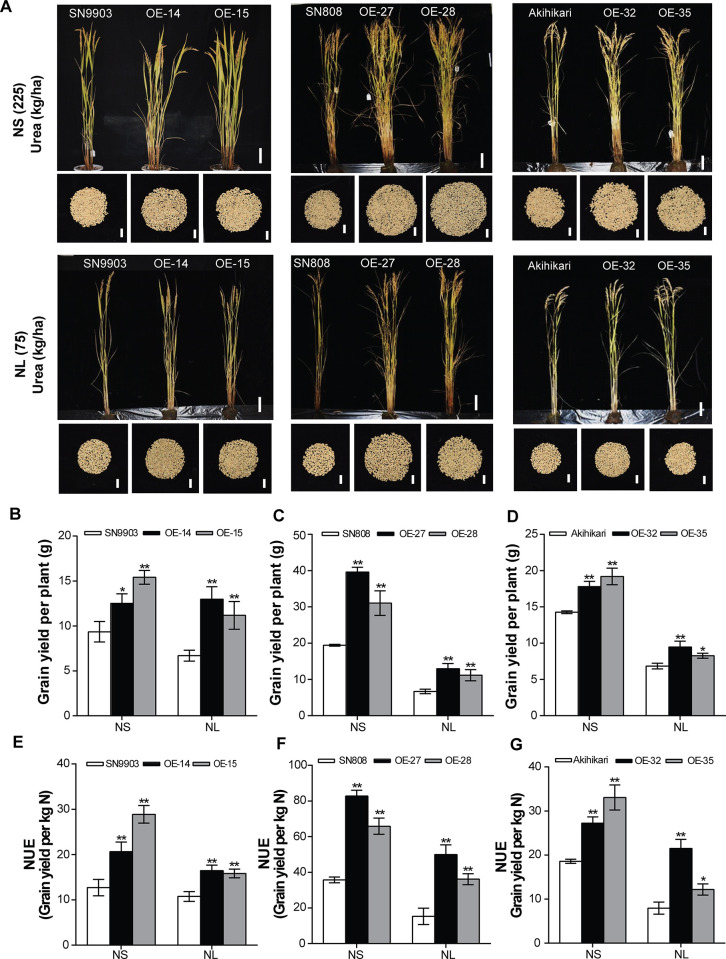
To further verify whether overexpression of OsATG8b in other rice cultivars would similarly be boosted in yield and NUE, we generated OsATG8b-overexpressing transgenic rice in other japonica cultivars, including Shennong9903 (SN9903), Shennong808 (SN808) and AKihiRari. All the transgenic plants displayed similar phenotypes with the SN9816 background's OsATG8b-overexpressing lines, which produced more grains compared to their wild types both under NS and NL conditions (Fig 7A). In accordance with the improved growth phenotypes, the grain yield in these overexpressing lines was also significantly increased both under NS and NL conditions (Fig 7B–7D). Besides, the NUE of these transgenic lines in different backgrounds were also greatly increased under two N conditions (Fig 7E–7G). Taken together, the high yield and NUE of OsATG8b-overexpressing transgenic rice under different backgrounds, indicating the functions of OsATG8b in rice are stable and heritable.
Fig 7. Overexpression of OsATG8b increased grain yield and NUE in rice under different genetic backgrounds.
(A) Phenotypes of the whole rice plants and total grains per plant in SN9903, SN808, Akihikari, and their OsATG8b-overexpressing lines under NS (225 kg·ha-1) and NL (75 kg·ha-1) at ripening stage. Scale bars, 10 cm and 2 cm. (B)-(D) Grain yield of OsATG8b-overexpressing lines in different genetic backgrounds under NS (225 kg·ha-1) and NL (75 kg·ha-1) conditions. (E)-(G) Nitrogen use efficiency (NUE) of OsATG8b-overexpressing lines in different genetic backgrounds under NS (225 kg·ha-1) and NL (75 kg·ha-1) conditions. Values are means ± SD (n = 12), *P < 0.05, **P < 0.01 (t-test).
Discussion
In plants, autophagy is essential for proper nutrient allocation, although it operates at a low constitutive level [42, 53, 54]. Autophagy has two opposite roles during leaf senescence and nutrients starvation: one is to maintain longevity through the circulation of nutrients; the other is to participate in the systematic degradation of tissues and export of recyclable nutrients to areas of growth and storage [19]. Because of these roles, autophagy can be used to regulate important agricultural traits, tolerance to abiotic and biotic stress, and mitigate the effects of nutrient depletion. Consistent with the high autophagy activity, the transcription of many plant ATG genes is significantly up-regulated under nutrient-limited conditions [4, 8, 13, 55]. In agreement with previous studies, we confirmed that the transcript level of rice OsATG8b and the number of YFP-OsATG8b labeled autophagosomes were significantly increased under N deficiency (Fig 1B), indicating that OsATG8b-mediated autophagy may confer tolerance to N stress in rice. Minina et al. mentioned that overexpression of ATG5 or ATG7 in Arabidopsis increased autophagic activity [43]. Here, we confirmed that elevated autophagic activity due to overexpression of OsATG8b could significantly increase the tolerance to N starvation, which was presumably associated with the increased formation and triggering of autophagosomes to degrade the insoluble proteins.
N availability strongly influences grain yield because N utilization is tightly associated with the plant’s life cycle, and there are sophisticated mechanisms for N uptake, translocation, assimilation, and remobilization [17, 56]. Autophagy plays a vital role in N management in plants when N starvation. When autophagy is defective, redundant N in the cells cannot be effectively recovered or utilized, leading to nutrient accumulation, wastage, cell death, and lower amino acid content, all of which inhibit plant growth and reduce NUE [57]. Thus, modifying the autophagy process in crops could improve NUE and increase crop yield [58]. Our results support this conclusion and indicate that OsATG8b plays a critical role in N uptake and utilization. Functional characterization of the OsATG8b loss-of-function mutants and overexpressed transgenic lines demonstrated that overexpression of OsATG8b could increase the activity and expression of key enzymes and genes related to N metabolism (Fig 3D–3F and S3 Fig), which promoted the N uptake and assimilation in rice. Thus, the OsATG8b-overexpressing lines had significantly higher NHI and NUpE, especially NUE, and the opposite results in osatg8b mutant under both NS and NL conditions (Fig 5D–5F). Since lipidation of OsATG8b was greatly increased in OsATG8b-overexpressing lines but decreased in the osatg8b mutant, we considered that all of these presumably because of the increased autophagy flux.
Another significant finding of this study was that overexpression of OsATG8b could enhance the reuse and remobilization of N from the vegetative tissues, increase the transfer of 15N to seeds, which causes increased NRE in rice (Fig 5G–5I). As 75% to 80% of N in the leaf is present in the chloroplast, and the main forms are Rubisco and chlorophyll [59], the higher chlorophyll content and Rubisco activity during the grain-filling stage conferred a higher capacity to convert light energy into photosynthetic products. Besides, the increased autophagic activity in the OsATG8b-overexpressing lines resulted in an accelerated overall of the N metabolic cycle, so more N (nutrients) could be mobilized to chloroplasts to produce organic materials. Consequently, the OsATG8b-overexpressing lines showed obviously increased biomass and grain yield. N partitioning studies in maize atg12 mutants implied that the reduced seed yield presumably because of impaired N recycling by autophagy [5]. Our studies also indicated that the greatly increased grain yield in OsATG8b-overexpressing lines was at least partially caused by the enhanced autophagic recycling leading to efficient N cycling from the leaves to the reproductive organ (seeds), which reduced the waste of available N and promoted the utilization and remobilization of N. Additionally, the increased number of the effective panicles number and secondary branches leading to significant increases in total grains number per plant, as well as the higher 1000-grain weight (S2 Table), could also explain the high productivity in OsATG8b-overexpressing lines. Excitingly, the OsATG8b-overexpressing transgenic rice under different backgrounds consistently displayed markedly increased NUE and grain yield (Fig 7), further confirming the great potential of OsATG8b for yield improvement in rice.
Consistent with the obvious improvement of the phenotype in OsATG8b-overexpressing lines, elevated OsATG8b expression broadly impacted the transcriptomes of rice. Our RNA-seq analyses showed that the affected transcripts trend of SN9816 in vivo was similar in the heat map both under NS and NL conditions, so did the transgenic lines (Fig 6A), suggesting that the ability to resist low N stress in the transgenic rice was due to the overexpression of OsATG8b. Previous metabolomics studies reported that the autophagy-deficient mutants might have increased synthesis of the stress hormones salicylic acid and abscisic acid, which could be connected to the hyperaccumulation of flavonoid or anthocyanin in atg mutants [21, 46, 47]. Similarly, our results showed that overexpression of OsATG8b significantly up-regulated genes involved in the response to salicylic acid, synthesis of growth-promoting hormones (auxin and brassinosteroids), biological regulation, cell differentiation, and morphogenesis, etc. Brassinosteroids and auxin have a synergistic effect on plant development by regulating cell elongation, cell division, and cell differentiation [60, 61]. Moreover, recent research in tomato showed that brassinosteroids-induced autophagy involved in N remobilization and mediated the response to N starvation [62]. It might partially explain the remarkably increased biomass and seed production as well as the improved NRE and resistance to N starvation in OsATG8b-overexpressing rice. In addition, the up-regulation of genes involved in autophagy regulation, the proteasome, and the small molecule metabolic process (Fig 6C) further confirm that OsATG8b has a stimulatory effect on autophagic activity and that its overexpression increases the clearing and recycling of unwanted or dysfunctional complexes. Studies in Arabidopsis showed that the transport of sugars from rosette leaves to the inflorescence plays a vital role in sustaining seed development [63], and higher seed yield in ATG-overexpressing plants might be attributed to up-regulation of genes involved in sugar transport [43]. Our data showed that overexpression of OsATG8b significantly up-regulated genes involved in polysaccharide transport (Fig 6C), which could also partially explain the improved N remobilization and yield of transgenic rice. By contrast, genes involved in carbohydrate and oligosaccharide metabolic processes, biosynthesis of amylopectin and long-chain fatty acids, and hydrolase activity were significantly down-regulated in transgenic rice (Fig 6D), possibly indicating that overexpression of OsATG8b could effectively promote the autophagy recycling pathway during plant growth and grain formation and consequently reduce the demand for several biosynthesis and metabolic pathways. Besides, overexpression of OsATG8b broadly and directly impacted the expression of plant metabolism genes. This result suggested that the cellular metabolic status in transgenic rice might be altered by regulating enzyme activity in several processes, which made a benefit to push the development in an irreversible direction and to recycle the nutrient sequentially.
The OsATG8a, OsATG8b, and OsATG8c belong to the same family and they maybe have redundant functions in rice. In our previous studies, it was found that the expressions of other OsATG8s were up-regulated during N-limited conditions, and overexpressed OsATG8a or OsATG8c in transgenic rice could increase autophagy activity and confer tolerance to N limitation [40, 41]. Besides, our group was the first to show that overexpression of OsATG8b could increase the autophagy activity and NRE in transgenic Arabidopsis, leading to significantly higher NUE and yield [64]. This research further confirmed that overexpression of OsATG8b has enormous potential as a strategy to increase yields and NUE in both monocot and eudicot crops. A recent research by Fan et al. showed that OsATG8b-mediated autophagy is involved in N recycling to grains and contributes to the grain quality in rice under normal growth conditions [65]. We two did completely independent research and got similar conclusions during the same period, which should be the verification and support for each other. However, there were also clear differences between us. In Fan’s study, the OsATG8b-RNAi and OsATG8b-OE lines showed a relatively normal phenotype under both low N and high N levels at the vegetative stages, and they considered that OsATG8s function redundantly in response to nutrient stress [65]. In our research, the OsATG8b-overexpressing lines performed better than the control group at all growth stages, especially under the NL condition, while the osatg8b mutants showed severe growth retardation under both two N conditions. Another point worth mentioned was that the NL treatment that we used (one-third of the N in the NS treatment) was already a very harsh condition. Even under this harsh condition, the OsATG8b-overexpressing lines could still use the restricted amount of N transferred to the seeds more effectively, enhance internal N remobilization, and improved yield and NUE more efficiently. In addition, compared the observed phenotypes and autophagic activity of three OsATG8s-related overexpression transgenic rice and the mutants, OsATG8b was the most significant and obvious, followed by OsATG8a, while OsATG8c was the weakest. Intriguingly, it was found that osatg8b knockout mutants caused downregulation of the OsATG8a and OsATG8c although their coding sequences were not changed. Furthermore, there were no such remarkable phenotypes as osatg8b mutants had been observed in the osatg8a and osatg8c mutants. We considered that this might be due to the high levels of amino acid sequence identity between these 3 genes, and OsATG8b was the dominant gene of ATG8s in rice. The severe reduction of NUE, NRE, and yield in osatg8b mutants further supports this conclusion.
Collectively, our results show that elevated OsATG8b expression can increase autophagy activity, photosynthetic products, NUE, and grain yield. Additionally, OsATG8b-mediated increased autophagy can effectively regulate N remobilization and plays a positive role in the maintenance of plant fitness and adaptation to nutrient limitation. Our results revealed a new mechanism by which autophagy increases NUE and yield in rice, it will greatly help in exploring the application value of autophagy and provide a beneficial strategy to increase NUE and crop yield through manipulation of autophagy and N recycling, especially under suboptimal growth conditions. Impressively, the effect of OsATG8b was not only verified in different rice backgrounds but also in Arabidopsis, indicating the great potential for the use of this gene in crop improvement programs for multiple crop species.
Materials and methods
Plant materials and growth conditions
Rice (Oryza sativa, japonica) varieties SN9816, SN9903, SN808, and Akihikari were used as the wild type and recipient for genetic transformation in this study. For N-limitation hydroponic culture experiment, rice seedlings were cultured with modified International Rice Research Institute (IRRI) nutrient solution (pH = 5.7) as described previously [66] with different concentrations of N in an artificial climate growth chamber as described. The sufficient-N nutrient solution contained the following macronutrients (mM): (NH4)2SO4 (0.72), Ca(NO3)2·4H2O (0.72), CaCl2 (0.28), K2SO4 (0.36), KH2PO4 (0.32), MgSO4·7H2O (1.67), Na2SiO3·9H2O (1.60) and micronutrients (μM): MnCl2·4H2O (9.11), (NH4)6Mo7O24·4H2O (0.074), H3BO3 (18.52), ZnSO4·7H2O (0.15), CuSO4·5H2O (0.16), Fe(II)-EDTA (35.81). The modified IRRI nutrient solution with 0.24 mM (NH4)2SO4 and 0.24 mM Ca(NO3)2·4H2O was used for low-N (NL), and the N-free solution for deficient-N (ND), the lack of Ca2+ was replaced with CaCl2.
Binary vector construction and rice transformation
The OsATG8b gene has that encodes 120 amino acids. To construct OsATG8b-overexpressing lines, a 360-bp coding sequence (CDS) of OsATG8b (LOC_Os04g53240; Os04g0624000) was amplified by PCR from cDNA of SN9816 and ligated into binary vector pCAMBIA1301. The 35S-YFP-OsATG8b fusion construct was produced by inserting the complete coding region of OsATG8b into the pCAMBIA1300-35S-YFP vector. The OsATG8b CRISPR vector construct was prepared using a plant CRISPR/Cas9-based genome editing system as described [67]. We designed sgRNA to target specific sites in the first exon of OsATG8b by using E-CRISPR (http://www.e-crisp.org/E-CRISP/index.html), and a pair of DNA oligonucleotides with appropriate cloning linkers were synthesized for the target site, then annealed and ligated into Bsa I-digested pRGE32 vectors. All the constructions were confirmed by DNA sequencing. The rice transformation was conducted as described previously [68]. The OsATG8b-overexpressing and 35S-YFP-OsATG8b transgenic lines were screened by PCR amplification with the hygromycin gene, the osatg8b mutants were screened by DNA sequencing with specific primers, and the T3 homozygous transgenic plants were taken for the experiments. The corresponding primers used in this study were listed in S1 Table.
Total RNA extraction and gene expression analysis
Total RNA extraction was conducted using the Eastep Super Total RNA Extraction Kit (Promega) and the first-strand cDNA was synthesized with the PrimeScript RT Master Mix (TaKaRa). Real-time RT-PCR was performed as described previously [40] by using SYBR Premix Ex TaqII (TaKaRa) on an Applied Biosystems 7500 Real-Time PCR System. The following standard thermal profile was used for all PCRs: 95°C for 30 s, 40 cycles of 95°C for 5 s, and 60°C for 34 s. All reactions were done at least in triplicates. OsActin1 was used as an internal control. The primers used for RT-PCR analysis were listed in S1 Table. Three independent biological replicates were conducted.
Protein isolation and western blot analysis
14-day-old seedlings of SN9816, OsATG8b-overexpressing lines and osatg8b mutants cultured hydroponically with NS solution were transferred to NS and ND solution with DMSO or 0.5 μM ConA (Sigma-Aldrich) dissolved in DMSO with or without 5 μM wortmannin (Sigma-Aldrich) for 24h. The full-length OsATG8b was used to raise a polyclonal antibody (GenScript, Nanjing China). Anti-AtNBR1 and Anti-Tubulin (Agrisera) were used as primary antibodies (1: 1000), goat anti-rabbit (IgG) (Sigma-Aldrich) was used as the secondary antibody (1: 10000). Total protein extraction and membrane protein extraction were conducted using the Minute™ Total Protein Extraction Kit and Membrane Protein Isolation Kit for plants (Invent Biotechnologies, Inc), then the protein concentration was determined with the BCA Protein Assay Kit (TaKaRa) referred to the instructions. Immunoblotting was done as described [40]. The obtained proteins were loaded into a 12–15% SDS-PAGE with 6 M urea gel. After electrophoresis separation, the proteins were transferred to a PVDF membrane for protein profiles analysis or immunoblot analysis. Three independent biological replicates were conducted.
Confocal microscopy for detecting autophagosome activity
14-day-old seedlings of 35-YFP-OsATG8b transgenic rice seedlings were transferred to NS (2.88 mM N) or ND (0 mM N) liquid medium with or without 0.5 μM ConA for 14h, and root epidermal cells of the elongation zone were observed by confocal microscope (Zeiss LSM 780) as described [39]. The YFP-OsATG8b labeled autophagosomal structures were monitored by a 514 nm excitation laser. The average number of puncta for three cells belonging to the same root was considered as a single measurement, at least 10 seedlings per genotype per treatment were imaged and then counted by Image J.
Field trials of rice
Field tests were performed under natural growth conditions from 2016 to 2019 during the rice-growing season at the experimental farm of Shenyang Agricultural University (Shenyang, China; Longitude: 123.34°E Latitude: 41.49°N). The brown loam soil used in our experiment contained organic matter of 16.3 g·kg-1, total N of 0.88 g·kg-1, available P of 28.64 mg·kg-1, available K of 114.04 mg·kg-1, and pH = 7.11. Rice plants were cultivated in pots, each pot containing four plants and ten pots as replicates were planted for each N condition or genotype. There were three N levels in this experiment NS (225 kg·ha-1), NL (75 kg·ha-1), and ND (without N fertilizer) as blank control. Urea was used as the only N source for NS and NL conditions and applied for three times as base, tillering and panicle fertilization, with 36%, 24%, and 40% of total applied urea respectively. 112.5 kg·ha-1 P2O5 was supplied as basic fertilizer, and 112.5 kg·ha-1 K2O was supplied as basic fertilizer and panicle fertilizer twice. Superphosphate (12% P2O5) and potassium chloride (60% K2O) were used as phosphate and potassium fertilizers.
Measurement of total chlorophyll, leaf enzyme activity, and photosynthetic characters
Flag leaves at the grain-filling stage (18 days after heading) were sampled, and 12 plants for each genotype under NS (225 kg·ha-1) or NL (75 kg·ha-1) conditions were randomly selected as replicates to measure. Total chlorophyll content was measured as described [69], about 0.5 g flag leaves were collected from the main stem in 10 mL 96% ethanol, and total chlorophyll content was calculated spectrophotometrically based on the absorbance of the supernatant at 649 and 665 nm. The activities of nitrate reductase (NR, EC 1.6.6.2), glutamine synthetase (GS, EC 6.3.1.2), and glutamate dehydrogenase synthesis (GDH, EC 1.4.1.2) were determined using the enzyme activity assay kits (Shanghai Enzyme-linked Biotechnology Co., Ltd., China), about 0.5 g flag leaves were collected from the main stem and frozen in liquid N, and then stored at -80°C until measurements. The procedures were conducted following the instructions provided by the manufacturer. A portable infrared gas analyzer (CIRAS 3, PP Systems, Amesbury, MA, USA) was used to measure net photosynthetic rate (PN) at the heading stage on flag leaves during 9:00 am-12:00 pm on sunny days. The approximate sunrise time for the period in which the photosynthetic measurements was 4:45 am. Photosynthetic photon flux densities of 1200 μmol m-2s-1) were provided by a LED source, CO2 concentration was provided by the CO2 injector system and maintained at 390 ppm in the leaf chamber.
Biomass, total N content, NUpE, NUE calculations, and agronomic traits analysis
Rice plants grown under NS (225 kg·ha-1), NL (75 kg·ha-1) and ND (without N fertilizer) conditions were sampled at the ripening stage by separation into the stem, leaf sheath, leaf blade and panicle at harvest, then all samples were kept in a dry oven at 80°C for 7 days, and dry mass was weighted as DWDr (dry weight of stem, leaf sheath, and leaf blade) and DWseeds (dry weight of total seeds). Then the dry mass of all these tissue organs was ground to powder, total N concentrations (N%) were measured using an Elemental Analyzer (Elementa, Vario MAX C, Germany), which presented as N% = mg N / 100 mg DW. Biomass was calculated as DWDr + DWSeeds. The harvest index (HI) was calculated as DWSeeds / (DWSeeds + DWDr) ratio and was used as an important indicator for yield. The NHI was calculated as (N%Seeds × DWSeeds) / (N%Seeds × DWSeeds + N%Dr × DWDr). The NUpE was calculated as (total aboveground N accumulation in NS or NL pot)–(total aboveground N accumulation in ND pot) / N supply, and the NUE was calculated as (grain yield in NS or NL pot)–(grain yield in ND pot) / N supply. The agronomic traits including the seed-setting rate, thousand-grain weight, panicles number, and grain yield were analyzed at the ripening stage according to the methods for measurement described previously [49]. The panicle branches are lateral organs at the reproductive stage of rice, which are composed of primary branches and secondary branches. All the above traits were measured on a single-plant basis, and 12 plants under each N conditions were randomly selected as replicates to measure.
15N-Labeling and NRE calculations
SN9816, OsATG8b-overexpressing lines, and osatg8b mutants were grown hydroponically with NS or NL nutrient solution as above. The 15N-Labeling experiment was conducted as previous studies described [5, 14] at the booting stage (70 days after sowing) when most of the 11th leaves on the main stem just emerged from the sheath of the 10th leaves, the nutrient solution was replaced with the 15N-labeled (15NH4)2SO4 and Ca(15NO3)2·4H2O (10.6 atom% excess) as 15NS or 15NL nutrient solution for 3 d. Afterward, the pots were applied with NS or NL solution every 3 days until plant maturity, then plants were separated into seed, stem, leaf sheath, and leaf blade. The 15N abundance, 15NHI, and NRE were estimated as described [20]. All the above traits were measured on a single-plant basis, and 12 plants under each N conditions were randomly selected as replicates to measure.
RNA-sequencing analysis
Flag leaves of SN9816 and OsATG8b-overexpressing rice under NS and NL growth conditions were sampled at the grain-filling stage. For each N treatment, three independent biological replicates were conducted. Sequencing libraries were generated using NEBNext UltraTM RNA Library Prep Kit for Illumina (NEB, USA) following the manufacturer’s recommendations and index codes were added to attribute sequences to each sample. RNA quality was determined using Agilent 2100 Bioanalyzer (Agilent Technologies). RNA libraries were constructed from 1 μg of total RNA and subjected to deep sequencing at an Illumina Hiseq 2500 platform (BioMarker Technologies). Differentially expressed genes (DEGs) were identified by DEseq2 with the criteria of absolute log2 (fold change) ≥1 and false discovery rate (FDR) ≤ 0.05. GO enrichment analysis of the differentially expressed genes (DEGs) was implemented by the GOseq R packages based Wallenius non-central hypergeometric distribution [70].
Statistical analysis
The statistically significant differences of all data were analyzed based on Student's t-test with the comparisons between two groups of data separately (OsATG8b-overexpressing lines vs SN9816 and mutants vs SN9816) at significance levels of *P < 0.05 and **P < 0.01.
Supporting information
OsActin1 was used as an internal control. Values are means ± SD (n = 3), **P < 0.01 (t-test).
(DOCX)
Immunoblot analysis of the protein level of The OsATG8a, OsATG8b, or OsATG8c expressed in E. coli with the anti-OsATG8b.
(DOCX)
qRT-PCR validation of several known genes related to N metabolism in flag leaves of SN9816 and OsATG8b-overexpressing rice under NS (225 kg·ha-1) and NL (75 kg·ha-1) conditions at grain-filling stage. OsActin1 was used as an internal control. Values are means ± SD (n = 3), *P < 0.05, **P < 0.01 (t-test).
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(PDF)
Data Availability
The RNA-sequencing data are available at the NCBI Sequence Read Archive database under the BioProject number PRJNA532827.
Funding Statement
This work was supported by grants from the National Key Research and Development Program of China (grant number 2018YFD0200200), the National Natural Science Foundation of China (grant number 31401298) and the National Key Research and Development Program of China (grant number 2018YFD0300300).
References
- 1.Liu Y, Bassham DC. Autophagy: pathways for self-eating in plant cells. Annual Review of Plant Biology. 2012; 63: 215–237. 10.1146/annurev-arplant-042811-105441 [DOI] [PubMed] [Google Scholar]
- 2.Marshall RS, Vierstra RD. Autophagy: the master of bulk and selective recycling. Annual Review of Plant Biology. 2018; 69: 173–208. 10.1146/annurev-arplant-042817-040606 [DOI] [PubMed] [Google Scholar]
- 3.Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. Febs Letters. 1993; 333: 169–174. 10.1016/0014-5793(93)80398-e [DOI] [PubMed] [Google Scholar]
- 4.Chung T, Suttangkakul A, Vierstra RD. The ATG autophagic conjugation system in maize: ATG transcripts and abundance of the ATG8-lipid adduct are regulated by development and nutrient availability. Plant Physiology. 2009; 149: 220–234. 10.1104/pp.108.126714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li F, Chung T, Pennington JG, Federico ML, Kaeppler HF, Kaeppler SM, et al. Autophagic recycling plays a central role in maize nitrogen remobilization. The Plant Cell. 2015; 27: 1389–1408. 10.1105/tpc.15.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li WW, Chen M, Wang E, Hu L, Hawkesford MJ, Zhong L, et al. Genome-wide analysis of autophagy-associated genes in foxtail millet (Setaria italica L.) and characterization of the function of SiATG8a in conferring tolerance to nitrogen starvation in rice. BMC Genomics. 2016; 17: 797 10.1186/s12864-016-3113-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.María Esther PP, Florencio FJ, Crespo JL. Inhibition of target of rapamycin signaling and stress activate autophagy in Chlamydomonas reinhardtii. Plant Physiology. 2010; 152: 1874–1888. 10.1104/pp.109.152520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia K, Liu T, Ouyang J, Wang R, Fan T, Zhang M. Genome-Wide Identification, Classification, and Expression Analysis of Autophagy-Associated Gene Homologues in Rice (Oryza sativa L.). DNA Research. 2011; 18: 363–377. 10.1093/dnares/dsr024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou X, Zhao P, Wang W, Zou J, Cheng T, Peng X, et al. A comprehensive, genome-wide analysis of autophagy-related genes identified in tobacco suggests a central role of autophagy in plant response to various environmental cues. DNA Research. 2015; 22: 245–257. 10.1093/dnares/dsv012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anongpat S, Faqiang L, Taijoon C, Vierstra RD. The ATG1/ATG13 protein kinase complex is both a regulator and a target of autophagic recycling in Arabidopsis. The Plant Cell. 2011; 23: 3761–3779. 10.1105/tpc.111.090993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doelling JH, Walker JM, Friedman EM, Thompson AR, Vierstra RD. The APG8/12-activating Enzyme APG7 Is Required for Proper Nutrient Recycling and Senescence in Arabidopsis thaliana. Journal of Biological Chemistry. 2002; 277: 33105–33114. 10.1074/jbc.M204630200 [DOI] [PubMed] [Google Scholar]
- 12.Hanaoka H, Noda T, Shirano Y, Kato T, Hayashi H, Shibata D, et al. Leaf senescence and starvation-induced chlorosis are accelerated by the disruption of an Arabidopsis autophagy gene. Plant Physiology. 2002; 129: 1181–1193. 10.1104/pp.011024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson AR, Doelling JH, Suttangkakul A, Vierstra RD. Autophagic nutrient recycling in Arabidopsis directed by the ATG8 and ATG12 conjugation pathways. Plant Physiology. 2005; 138: 2097–2110. 10.1104/pp.105.060673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wada S, Hayashida Y, Izumi M, Kurusu T, Hanamata S, Kanno K, et al. Autophagy supports biomass production and nitrogen use efficiency at the vegetative stage in rice. Plant Physiology. 2015; 168: 60–73. 10.1104/pp.15.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong Y, Contento AL, Bassham DC. AtATG18a is required for the formation of autophagosomes during nutrient stress and senescence in Arabidopsis thaliana. The Plant Journal. 2005; 42: 535–546. 10.1111/j.1365-313X.2005.02397.x [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Cui Z, Fan M, Vitousek P, Zhao M, Ma W, et al. Producing more grain with lower environmental costs. Nature. 2014; 514: 486–489. 10.1038/nature13609 [DOI] [PubMed] [Google Scholar]
- 17.Xu G, Fan X, Miller AJ. Plant nitrogen assimilation and use efficiency. Annual Review of Plant Biology. 2012; 63: 153–182. 10.1146/annurev-arplant-042811-105532 [DOI] [PubMed] [Google Scholar]
- 18.Krapp A. Plant nitrogen assimilation and its regulation: a complex puzzle with missing pieces. Current Opinion in Plant Biology. 2015; 25: 115–122. 10.1016/j.pbi.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 19.Masclaux-Daubresse C, Chen Q, Havé M. Regulation of nutrient recycling via autophagy. Current Opinion in Plant Biology. 2017; 39: 8–17. 10.1016/j.pbi.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 20.Guiboileau A, Yoshimoto K, Soulay F, Bataillé MP, Avice JC, Masclaux-Daubresse C. Autophagy machinery controls nitrogen remobilization at the whole-plant level under both limiting and ample nitrate conditions in Arabidopsis. New Phytologist. 2012; 194: 732–740. 10.1111/j.1469-8137.2012.04084.x [DOI] [PubMed] [Google Scholar]
- 21.McLoughlin F, Augustine RC, Marshall RS, Li F, Kirkpatrick LD, Otegui MS, et al. Maize multi-omics reveal roles for autophagic recycling in proteome remodelling and lipid turnover. Nature Plants. 2018; 4: 1056–1070. 10.1038/s41477-018-0299-2 [DOI] [PubMed] [Google Scholar]
- 22.Eguchi M, Kimura K, Makino A, Ishida H. Autophagy is induced under Zn limitation and contributes to Zn-limited stress tolerance in Arabidopsis (Arabidopsis thaliana) Soil Science & Plant Nutrition. 2017; 63: 1–9. [Google Scholar]
- 23.Zientara-Rytter K, Łukomska J, Moniuszko G, Gwozdecki R, Surowiecki P, Lewandowska M, et al. Identification and functional analysis of Joka2, a tobacco member of the family of selective autophagy cargo receptors. Autophagy. 2011; 7: 1145–1158. 10.4161/auto.7.10.16617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tasaki M, Asatsuma S, Matsuoka K. Monitoring protein turnover during phosphate starvation-dependent autophagic degradation using a photoconvertible fluorescent protein aggregate in tobacco BY-2 cells. Frontiers in Plant Science. 2014; 5: 172 10.3389/fpls.2014.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellner R, Jc DLC, Maqbool A, Kamoun S, Dagdas YF. ATG8 expansion: a driver of selective autophagy diversification? Trends in Plant Science. 2017; 22: 204–214. 10.1016/j.tplants.2016.11.015 [DOI] [PubMed] [Google Scholar]
- 26.Wurzer B, Zaffagnini G, Fracchiolla D, Turco E, Abert C, Romanov J, et al. Oligomerization of p62 allows for selection of ubiquitinated cargo and isolation membrane during selective autophagy. Elife. 2015; 4: e08941 10.7554/eLife.08941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertipaglia C, Schneider S, Jakobi AJ, Tarafder AK, Bykov YS, Picco A, et al. Higher-order assemblies of oligomeric cargo receptor complexes form the membrane scaffold of the Cvt vesicle. EMBO Reports. 2016; 17: 1044–1060. 10.15252/embr.201541960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reyes FC, Taijoon C, David H, Rudolf J, Richard V, Otegui MS. Delivery of prolamins to the protein storage vacuole in maize aleurone cells. The Plant Cell. 2011; 23: 769–784. 10.1105/tpc.110.082156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia M, Liu X, Xue H, Wu Y, Shi L, Wang R, et al. Noncanonical ATG8-ABS3 interaction controls senescence in plants. Nature Plants. 2019; 5: 212–224. 10.1038/s41477-018-0348-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popelka H, Klionsky DJ. Post-translationally-modified structures in the autophagy machinery: an integrative perspective. FEBS Journal. 2015; 282: 3474–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo E, Woo J, Park E, Bertolani SJ, Siegel JB, Choi D, et al. Comparative analyses of ubiquitin-like ATG8 and cysteine protease ATG4 autophagy genes in the plant lineage and cross-kingdom processing of ATG8 by ATG4. Autophagy. 2016; 12: 2054–2068. 10.1080/15548627.2016.1217373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shpilka T, Weidberg H, Pietrokovski S, Elazar Z. Atg8: an autophagy-related ubiquitin-like protein family. Genome Biology. 2011; 12: 226 10.1186/gb-2011-12-7-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slavikova S, Ufaz S, Avinwittenberg T, Levanony H, Galili G. An autophagy-associated Atg8 protein is involved in the responses of Arabidopsis seedlings to hormonal controls and abiotic stresses. Journal of Experimental Botany. 200859: 4029–4043. 10.1093/jxb/ern244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang P, Sun X, Jia X, Wang N, Gong X, Ma F. Characterization of an autophagy-related gene MdATG8i from apple. Frontiers in Plant Science. 2016; 7: 720 10.3389/fpls.2016.00720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia T, Xiao D, Liu D, Chai W, Gong Q, Wang N. Heterologous expression of ATG8c from soybean confers tolerance to nitrogen deficiency and increases yield in Arabidopsis. PLoS ONE. 2012; 7: e37217 10.1371/journal.pone.0037217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li WW, Chen M, Zhong L, Liu JM, Xu ZS, Li LC, et al. Overexpression of the autophagy-related gene SiATG8a from foxtail millet (Setaria italica L.) confers tolerance to both nitrogen starvation and drought stress in Arabidopsis. Biochemical & Biophysical Research Communications. 2015; 468: 800–806. 10.1016/j.bbrc.2015.11.035 [DOI] [PubMed] [Google Scholar]
- 37.Chen Q, Soulay F, Saudemont B, Elmayan T, Marmagne A, Masclaux-Daubresse C. Overexpression of ATG8 in Arabidopsis stimulates autophagic activity and increases nitrogen remobilization efficiency and grain filling. Plant and Cell Physiology. 2019; 60: 343–352. 10.1093/pcp/pcy214 [DOI] [PubMed] [Google Scholar]
- 38.Su W, Ma H, Liu C, Wu J, Yang J. Identification and characterization of two rice autophagy associated genes, OsAtg8 and OsAtg4. Molecular Biology Reports. 2006; 33: 273–278. 10.1007/s11033-006-9011-0 [DOI] [PubMed] [Google Scholar]
- 39.Izumi M, Hidema J, Wada S, Kondo E, Kurusu T, Kuchitsu K, et al. Establishment of monitoring methods for autophagy in rice reveals autophagic recycling of chloroplasts and root plastids during energy limitation. Plant Physiology. 2015; 167: 1307–1320. 10.1104/pp.114.254078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu J, Zhen X, Li X, Li N, Xu F. Increased Autophagy of Rice Can Increase Yield and Nitrogen Use Efficiency (NUE) Frontiers in Plant Science. 2019; 10: 584 10.3389/fpls.2019.00584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhen X, Li X, Yu J, Xu F. OsATG8c-Mediated Increased Autophagy Regulates the Yield and Nitrogen Use Efficiency in Rice. International journal of molecular sciences. 2019; 20: 4956 10.3390/ijms20194956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hafrén A, Macia JL, Love AJ, Milner JJ, Drucker M, Hofius D. Selective autophagy limits cauliflower mosaic virus infection by NBR1-mediated targeting of viral capsid protein and particles. Proceedings of the National Academy of Sciences. 2017; 114: 2026–2035. 10.1073/pnas.1610687114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minina EA, Moschou PN, Vetukuri RR, Sanchez-Vera V, Cardoso C, Liu Q, et al. Transcriptional stimulation of rate-limiting components of the autophagic pathway improves plant fitness. Journal of Experimental Botany. 2018; 69: 1415–1432. 10.1093/jxb/ery010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Svenning S, Lamark T, Krause K, Johansen T. Plant NBR1 is a selective autophagy substrate and a functional hybrid of the mammalian autophagic adapters NBR1 and p62/SQSTM1. Autophagy. 2011; 7: 993–1010. 10.4161/auto.7.9.16389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merkulova EA, Guiboileau A, Naya L, Masclaux-Daubresse C, Yoshimoto K. Assessment and optimization of autophagy monitoring methods in Arabidopsis roots indicate direct fusion of autophagosomes with vacuoles. Plant Cell Physiol. 2014; 55: 715–726. 10.1093/pcp/pcu041 [DOI] [PubMed] [Google Scholar]
- 46.Avin-Wittenberg T, Bajdzienko K, Wittenberg G, Alseekh S, Tohge T, Bock R, et al. Global analysis of the role of autophagy in cellular metabolism and energy homeostasis in Arabidopsis seedlings under carbon starvation. The Plant Cell. 2015; 27: 306–322. 10.1105/tpc.114.134205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masclaux-Daubresse C, Clément G, Anne P, Routaboul JM, Guiboileau A, Soulay F, et al. Stitching together the Multiple Dimensions of Autophagy Using Metabolomics and Transcriptomics Reveals Impacts on Metabolism, Development, and Plant Responses to the Environment in Arabidopsis. The Plant Cell. 2014; 26: 1857–1877. 10.1105/tpc.114.124677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshimoto K, Jikumaru Y, Kamiya Y, Kusano M, Consonni C, Panstruga R, et al. Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. The Plant Cell. 2009; 21: 2914–2927. 10.1105/tpc.109.068635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu B, Wang W, Ou S, Tang J, Li H, Che R, et al. Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nature Genetics. 2015; 47: 834–838. 10.1038/ng.3337 [DOI] [PubMed] [Google Scholar]
- 50.Cai H, Zhou Y, Xiao J, Li X, Zhang Q, Lian X. Overexpressed glutamine synthetase gene modifies nitrogen metabolism and abiotic stress responses in rice. Plant Cell Reports. 2009; 28: 527–537. 10.1007/s00299-008-0665-z [DOI] [PubMed] [Google Scholar]
- 51.Fan X, Tang Z, Tan Y, Zhang Y, Luo B, Yang M, et al. Overexpression of a pH-sensitive nitrate transporter in rice increases crop yields. Proceedings of the National Academy of Sciences. 2016; 113: 7118–7123. 10.1073/pnas.1525184113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li H, Hu B, Chu C. Nitrogen use efficiency in crops: lessons from Arabidopsis and rice. Journal of Experimental Botany. 2017; 68: 2477–2488. 10.1093/jxb/erx101 [DOI] [PubMed] [Google Scholar]
- 53.Guiboileau A, Avila-Ospina L, Yoshimoto K, Soulay F, Azzopardi M, Marmagne A, et al. Physiological and metabolic consequences of autophagy deficiency for the management of nitrogen and protein resources in Arabidopsis leaves depending on nitrate availability. New Phytologist. 2013; 199: 683–694. 10.1111/nph.12307 [DOI] [PubMed] [Google Scholar]
- 54.Li F, Vierstra RD. Autophagy: a multifaceted intracellular system for bulk and selective recycling. Trends in Plant Science. 2012; 17: 526–537. 10.1016/j.tplants.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Yu B, Zhao J, Guo J, Li Y, Han S, et al. Autophagy contributes to leaf starch degradation. The Plant Cell. 2013; 25: 1383–1399. 10.1105/tpc.112.108993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chardon F, Noël V, Masclaux-Daubresse C. Exploring NUE in crops and in Arabidopsis ideotypes to improve yield and seed quality. Journal of Experimental Botany. 2012; 63: 3401–3412. 10.1093/jxb/err353 [DOI] [PubMed] [Google Scholar]
- 57.Ren C, Liu J, Gong Q. Functions of autophagy in plant carbon and nitrogen metabolism. Frontiers in Plant Science. 2014; 5: 301 10.3389/fpls.2014.00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Avin-Wittenberg T, Baluška F, Bozhkov PV, Elander PH, Fernie AR, Galili G, et al. Autophagy-related approaches for improving nutrient use efficiency and crop yield protection. Journal of Experimental Botany. 2018; 69: 1335–1353. 10.1093/jxb/ery069 [DOI] [PubMed] [Google Scholar]
- 59.Masclaux-Daubresse C, Danielvedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A. Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Annals of Botany. 2010; 105: 1141–1157. 10.1093/aob/mcq028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gallavotti A. The role of auxin in shaping shoot architecture. Journal of Experimental Botany. 2013; 64: 2593–2608. 10.1093/jxb/ert141 [DOI] [PubMed] [Google Scholar]
- 61.Tong H, Chu C. Functional Specificities of Brassinosteroid and Potential Utilization for Crop Improvement. Trends in Plant Science. 2018; 23: 1016–1028. 10.1016/j.tplants.2018.08.007 [DOI] [PubMed] [Google Scholar]
- 62.Wang Y, Cao J, Wang K, Xia X, Shi K, Zhou Y, et al. BZR1 mediates brassinosteroid-induced autophagy and nitrogen starvation in tomato. Plant Physiology. 2019; 179: 671–685. 10.1104/pp.18.01028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wingenter K, Schulz A, Wormit A, Wic S, Trentmann O, Hoermiller II, et al. Increased activity of the vacuolar monosaccharide transporter TMT1 alters cellular sugar partitioning, sugar signaling, and seed yield in Arabidopsis. Plant Physiology. 2010; 154: 665–677. 10.1104/pp.110.162040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhen X, Xu F, Zhang W, Li N, Li X. Overexpression of rice gene OsATG8b confers tolerance to nitrogen starvation and increases yield and nitrogen use efficiency (NUE) in Arabidopsis. PLoS ONE. 2019; 14: e0223011 10.1371/journal.pone.0223011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fan T, Yang W, Zeng X, Xu X, Xu Y, Fan X, et al. A Rice Autophagy Gene OsATG8b Is Involved in Nitrogen Remobilization and Control of Grain Quality. Frontiers in Plant Science. 2020; 11: 588 10.3389/fpls.2020.00588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cock J, Yoshida S, Forno DA. Laboratory manual for physiological studies of rice. Int. Rice Res. Inst. 1976. [Google Scholar]
- 67.Xie K, Yang Y. RNA-guided genome editing in plants using a CRISPR–Cas system. Molecular Plant. 2013; 6: 1975–1983. 10.1093/mp/sst119 [DOI] [PubMed] [Google Scholar]
- 68.Hiei Y, Ohta S, Komari T, Kumashiro T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. The Plant Journal. 1994; 6: 271–282. 10.1046/j.1365-313x.1994.6020271.x [DOI] [PubMed] [Google Scholar]
- 69.Arnon D. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiology. 1949; 24: 1–15. 10.1104/pp.24.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biology. 2010; 11: R14–R14. 10.1186/gb-2010-11-2-r14 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
OsActin1 was used as an internal control. Values are means ± SD (n = 3), **P < 0.01 (t-test).
(DOCX)
Immunoblot analysis of the protein level of The OsATG8a, OsATG8b, or OsATG8c expressed in E. coli with the anti-OsATG8b.
(DOCX)
qRT-PCR validation of several known genes related to N metabolism in flag leaves of SN9816 and OsATG8b-overexpressing rice under NS (225 kg·ha-1) and NL (75 kg·ha-1) conditions at grain-filling stage. OsActin1 was used as an internal control. Values are means ± SD (n = 3), *P < 0.05, **P < 0.01 (t-test).
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(PDF)
Data Availability Statement
The RNA-sequencing data are available at the NCBI Sequence Read Archive database under the BioProject number PRJNA532827.