Supplemental Digital Content is Available in the Text.
Posttraumatic stress disorder encompasses latent subgroups of individuals with qualitative differences in pain perception.
Keywords: Posttraumatic stress, PTSD, Somatosensory testing, QST, Systematic review, Meta-analysis, Combat, Trauma, Stress, Pain threshold, Pain tolerance, Pain sensitivity, Conditioned pain modulation, Wind-up
Abstract
Posttraumatic stress disorder (PTSD) is a known risk factor for the development of chronic pain conditions, and almost 1 in 5 individuals with chronic pain fulfills the criteria for PTSD. However, the relationship between PTSD and pain is poorly understood and studies on pain perception in patients with PTSD show inconsistent results suggesting that different sensory profiles exist among individuals with PTSD. Here, we (1) systematically summarize the current literature on experimentally evoked pain perception in patients with PTSD compared to subjects without PTSD, and (2) assess whether the nature of the traumatic event is associated with different patterns in pain perception. The main outcome measures were pain threshold, pain tolerance, and pain intensity ratings as well as measures of temporal summation of pain and conditioned pain modulation. A systematic search of MEDLINE, EMBASE, Web of Science, PsycINFO, and CINAHL identified 21 studies for the meta-analysis, including 422 individuals with PTSD and 496 PTSD-free controls. No main effect of PTSD on any outcome measure was found. However, stratification according to the nature of trauma revealed significant differences of small to medium effect sizes. Combat-related PTSD was associated with increased pain thresholds, whereas accident-related PTSD was associated with decreased pain thresholds. No clear relationship between PTSD and experimentally evoked pain perception exists. The type of trauma may affect pain thresholds differently indicating the presence of different subgroups with qualitative differences in pain processing.
1. Introduction
Posttraumatic stress disorder (PTSD) presents as a complex and protracted clinical picture with often severe social and health consequences for those affected. The symptoms of PTSD are very heterogeneous and varied, and in addition to the classic symptom triad of intrusion, avoidance, and hyperarousal, PTSD is characterized by a high comorbidity with chronic pain. Prevalence rates of chronic pain vary depending on how PTSD is assessed and defined, but self-reported pooled prevalence of PTSD is above 20% in individuals with chronic pain.46 Moreover, individuals with pain and comorbid PTSD report greater pain severity5 and pain-related impairment.12 Although there is strong evidence that PTSD is a risk factor for the development of chronic pain conditions, the underlying mechanisms are not well understood and may vary depending on trauma type.
The findings of previous studies examining pain perception in individuals with the presence of PTSD are inconsistent and partly contradictory, with results showing increased,9,11,53 decreased,5,7,15 as well as unaltered pain perception.24,31 On the one hand, these inconsistent observations could indicate that PTSD has no coherent influence on somatosensory pain measures. On the other hand, this ambiguity may be due to PTSD being a very heterogeneous, highly comorbid disease.26,27,35,38,40,54 Indeed, it has been suggested that PTSD may consist of latent subgroups of individuals with qualitative differences in the clinical manifestations.4,17,23,30 This seems to be especially true for the connection between trauma and pain because 2 important PTSD-related symptom clusters, anxiety and dissociation, are associated with opposing effects (eg, hyperalgesic vs hypoalgesic) on pain perception.7 Furthermore, there is evidence to suggest that interpersonal traumas are associated with higher PTSD risk and more complex trauma reactions compared to accident-related traumas, and may affect the sensory profiles in different ways.25
The differentiation and characterization of these subgroups is crucial for a better understanding towards the process of pain chronicity in individuals exposed to trauma.
A promising approach to explain contradictory data and to understand heterogeneity is the use of meta-analysis. Therefore, this article aims to (1) systematically review and meta-analyse current data on pain perception of experimentally evoked pain in individuals with PTSD compared to individuals without PTSD, and (2) compare whether the nature of the traumatic event is associated with different sensory profiles.
2. Methods
2.1. Procedures
This review was performed according to the recommendations of the Cochrane Collaboration21 when appropriate and is reported after the PRISMA statement.36 All steps and methods of the review were specified in advance in a predetermined review protocol registered with PROSPERO (CRD42018083779).
We searched Medline, EMBASE, Web of Science, PsycINFO, and CINAHL. The search strategy was adapted for each database if necessary (see the web appendix for complete search strategy, available at http://links.lww.com/PR9/A78). In addition, reference lists from identified articles and reviews were screened for published and unpublished data, and all promising references were scrutinized. For promising abstracts, complete publications were retrieved. In addition, a citation search on the included articles was performed. Searches were performed independently by 2 reviewers (J.T. and H.B.V.). Only peer-reviewed studies that were published in English, German, Danish, Swedish, or Norwegian were included. The 2 reviewers independently scanned the titles and abstracts of eligible studies. Both reviewers independently scanned the full-text articles to determine whether the articles met the selection criteria. Disagreements between the 2 reviewers were resolved by discussion, and if agreements between the 2 reviewers could not be achieved, a third reviewer was consulted (T.E.A.).
2.2. Eligibility criteria
We selected all studies that investigated measures of pain thresholds, pain tolerance, pain intensity ratings, temporal summation of pain, and conditioned pain modulation (CPM) in human adults older than 18 years of age with PTSD compared with a PTSD-free control group. In studies with more than one control group, the control group clinically closest to the PTSD group was chosen (eg, trauma-exposed controls without PTSD before healthy controls). The presence of possible PTSD had to have been qualified either (1) by interview according to either Diagnostic and Statistical Manual of Mental Disorders/DSM1 or International Classification of Diseases/ICD55 system or (2) according to a preestablished cutoff criteria for one of the existing validated PTSD questionnaires.
2.3. Outcomes
The primary outcomes were the more static measures of pain perception in experimentally evoked pain, namely pain thresholds, pain tolerance, and pain intensity ratings as well as the more dynamic measures of pain processing, namely temporal summation of pain and CPM. For this purpose, the following definitions were used.
2.3.1. Pain threshold
Pain threshold was defined as the minimum intensity of a stimulus that was perceived as painful.33
2.3.2. Pain tolerance
Pain tolerance was defined as the length of time an individual was willing to endure a noxious stimulus (eg, cold-pressor task) or by the maximum stimulus intensity that one was willing to endure in a given situation.33
2.3.3. Pain intensity rating
Pain intensity rating was defined as the individual pain intensity rating (eg, on a 0–10 numerical rating scale) an individual perceived based on a given noxious stimulus (eg, pressure).
2.3.4. Temporal summation of pain
Temporal summation of pain was defined as the amplification of pain intensity ratings after repeated or continuous administration of constant noxious stimuli (eg, pressure or heat). The presence of temporal summation of pain or wind-up indicates the involvement of sensitization processes at spinal neurons rather than damage or inflammation of peripheral structures.32
2.3.5. Conditioned pain modulation
Conditioned pain modulation captures the phenomenon when the perception of a test-pain stimulus given together with (or directly after) a second painful conditioning stimulus is perceived differently than when the test stimulus is given alone. The extent of perceived pain reduction represents the value of CPM efficiency and has in the case of a perceived pain reduction usually a sign of “minus”; in the case when no pain decrease can be observed, the value of CPM is zero or positive and it is considered as less-efficient CPM.56
2.4. Assessment of risk of bias
A modification of the Newcastle–Ottawa Scale19 was used for judging the risk of bias of the included studies. The Newcastle–Ottawa Scale is a priori standardized checklist of predefined criteria for assessing the quality of nonrandomized cohort studies in meta-analyses. It assesses the quality of nonrandomized studies with its design, content, and ease of use directed to the task of incorporating the quality assessments in the interpretation of meta-analytic results. According to the Newcastle–Ottawa Scale, the quality scoring was divided into 3 sections: (1) Selection (including 3 items: representativeness of the cohort with PTSD, selection of the PTSD-free control cohort, and ascertainment of exposure/PTSD diagnosis); (2) Comparability (including 2 items: Comparability of cohorts by controlling for age and sex, and comparability of cohorts by controlling for pain at the test side); and (3) Outcome (including 2 items: Blinded assessment of outcomes, and assessment of outcomes in the same body area for both samples). An additional item considered obvious methodological flaws (eg, high dropout rate). Its content validity and interrater reliability have been established.19 Its criterion validity with comparisons to more comprehensive but cumbersome scales and its intrarater reliability are currently being examined. The checklist was slightly adapted to the specific needs of this systematic review. Before the registration of the final protocol, 3 articles were assessed and data extraction conducted by J. Tesarz and H.B. Vaegter to establish the consistency in the procedure and to adapt the data extraction form and the scoring system to the needs of this review.
2.5. Statistical analysis
Standardized mean differences (SMDs) were calculated as Hedges g for each measure of pain perception, comparing the individuals with PTSD with PTSD-free controls for each study. To compare and combine the different studies, we used a DerSimonian–Laird random-effects model8 to calculate pooled estimates with 95% confidence intervals (CI95). A random-effects model was chosen because studies differed in the nature of trauma and kind of pain induction methods.8 Heterogeneity among the studies was described using the I2 statistic, and I2 values over 50% indicated strong heterogeneity.22
Potential small study bias (ie, the association of publication probability with the statistical significance of study results) was investigated using Begg and Egger tests. All calculations were performed with the metan and metabias packages for STATA.
3. Results
3.1. Characteristics of included studies
Overall, 21 studies including 422 individuals with PTSD (201 women and 221 men) and 496 PTSD-free controls (267 women and 229 men) provided data for our meta-analysis (Figure S1 of the supplemental material, available at http://links.lww.com/PR9/A78). Seven of these 21 studies were conducted in the United States,31,34,37,42,43,50,51 2 studies were conducted in Australia,11,49 3 studies were performed in Israel,5–7 and 9 studies were performed in Europe.9,13,15,20,24,28,29,45,53 Veterans/victims of war/torture were assessed in 9 studies5,6,13,29,31,42,43,50; PTSDs that were closely linked to traffic accidents were assessed in 4 studies,11,20,47,48,53 and 4 studies assessed samples with “mixed” trauma types. In 4 studies, the type of trauma was not sufficiently described (see also Table S1 of the supplemental material, available at http://links.lww.com/PR9/A78). For the majority of studies, a low risk of bias was found (see Table S2 of the supplemental material, available at http://links.lww.com/PR9/A78).
3.2. Meta-analysis
3.2.1. Pain threshold
Thirteen studies reported data on pain threshold in individuals with PTSD (n = 299) and without PTSD (n = 385). Random-effect analysis showed no significant difference between the 2 groups (Fig. 1). The pooled standardized mean difference was −0.10 with a CI95 −0.39 to 0.19 (I2 = 97.8%). Compared to controls, 7 studies showed lower pain thresholds in individuals with PTSD,9,11,20,34,45,47,53 3 studies showed higher pain thresholds in individuals with PTSD,5,7,15 and 3 studies found no significant differences between the 2 groups.24,31,37 The funnel plot was symmetrical with no evidence of relevant small study bias (Egger test: P = 0.465).
Figure 1.
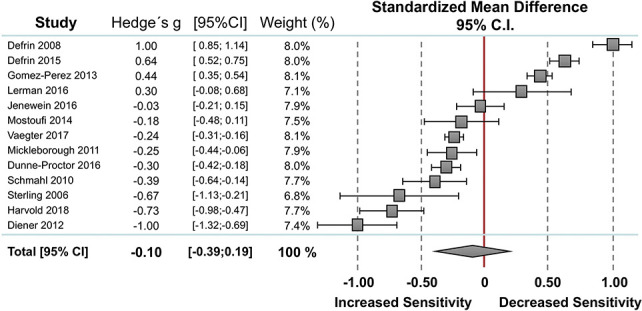
Overall effect on pain threshold. Standardized mean differences (SMDs) were calculated as Hedges g. A DerSimonian–Laird random-effects model was used to calculate pooled estimates with 95% confidence intervals (95% CI). Heterogeneity among the studies was I2 = 97.8%, P = 0.000.
3.2.2. Pain tolerance
Seven studies reported measures on pain tolerance including 167 individuals with PTSD and 180 PTSD-free controls (Fig. 2). Random-effect analysis showed no significant difference between the 2 groups. The pooled standardized mean difference was 0.03 with a CI95 −0.34 to 0.39 (I2 = 97.2%). Compared to controls, 2 studies showed lower pain tolerances in individuals with PTSD,9,53 3 studies showed higher pain threshold in PTSD,15,37,42 and 2 studies found no significant differences between the 2 group.6,24 The funnel plot was symmetrical with no evidence of relevant small study bias (Egger test: P = 0.708).
Figure 2.
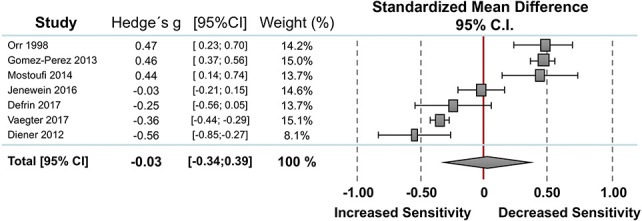
Overall effect on pain tolerance. Standardized mean differences (SMDs) were calculated as Hedges g. A DerSimonian–Laird random-effects model was used to calculate pooled estimates with 95% confidence intervals (95% CI). Heterogeneity among the studies was I2 = 97.2%, P = 0.000.
3.2.3. Pain intensity ratings
Thirteen studies reported measures of pain intensity ratings including 257 individuals with PTSD and 264 PTSD-free controls (Fig. 3). Random-effect analysis showed no significant difference between the 2 groups. The pooled standardized mean difference was −0.01 with a CI95 −0.28 to 0.27 (I2 = 96.1%). Compared to controls, 4 studies showed lower pain intensity ratings in individuals with PTSD,13,29,34,43 5 studies showed higher pain intensity ratings in individuals with PTSD,5,7,9,15,28 and 4 studies revealed no significant differences between the 2 groups.24,31,50,51 The funnel plot was symmetrical with no evidence of relevant small study bias (Egger test: P = 0.094).
Figure 3.
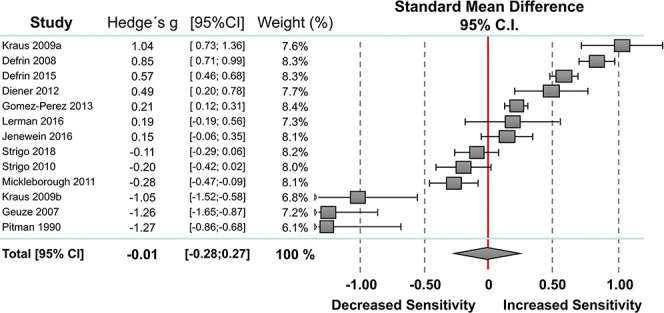
Overall effect on pain intensity. Standard mean differences (SMDs) were calculated as Hedges g. A DerSimonian–Laird random-effects model was used to calculate pooled estimates with 95% confidence intervals (95% CI). Heterogeneity among the studies was I2 = 96.1%, P = 0.000.
3.2.4. Temporal summation
Three studies reported data on temporal summation of pain between individuals with PTSD (n = 86) and without PTSD (n = 91). Random-effect analysis showed no significant difference between the 2 groups (Fig. 4). The pooled standardized mean difference was 0.01 with a CI95 −0.06 to 0.07 (I2 = 0%, P = 0.886). The funnel plot was symmetrical with no evidence of relevant small study bias (Egger test: P = 0.873).
Figure 4.
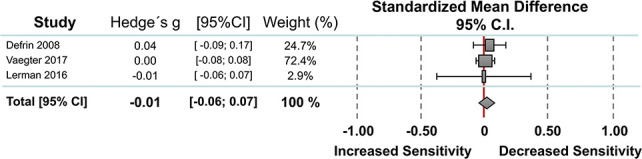
Overall effect on temporal summation of pain. Standardized mean differences (SMDs) were calculated as Hedges g. A DerSimonian–Laird random-effects model was used to calculate pooled estimates with 95% confidence intervals (95% CI). Heterogeneity among the studies was I2 = 0%, P = 0.000.
3.2.5. Conditioned pain modulation
Two studies reported data on CPM between individuals with PTSD (n = 56) and without PTSD (n = 78). No meta-analysis was possible due to the small number of included studies. Vaegter et al.53 observed no differences in CPM comparing patients with accident-related back pain with PTSD to those without PTSD (Hedges g = −0.13 ± 0.04), whereas Defrin et al.6 observed reduced CPM in individuals with chronic combat-/torture-related PTSD and delayed-onset PTSD compared (Hedges g = −1.19 ± 0.18) to 2 PTSD-free groups (resilient and healthy controls).
3.2.6. Subgroup analyses
Stratifying pain threshold measures for the kind of trauma, subgroup analyses showed significant increased pain thresholds in subjects with PTSD with combat-related trauma in terms of a “hypoalgesic effect” of medium effect size (Hedges g = 0.68, CI95 0.36–1.01; I2 = 90.4%; n = 3), and significant decreased pain thresholds in terms of a “hyperalgesic effect” of small effect size in subjects with PTSD with accident-related trauma (Hedges g = −0.41, CI95 −0.60 to −0.23; I2 = 81.1%; n = 4) (Table S3, available at http://links.lww.com/PR9/A78, Fig. 5). No significant difference was seen for the mixed-trauma group (Hedges g = −0.09, CI95 −0.56 to 0.38; I2 = 95.9%; n = 4). Due to the limited number of studies included, subgroup stratification was only possible for mixed trauma sample regarding the pain tolerance measures and for combat-related trauma regarding the pain intensity measures, indicating no significant differences within these subgroups. No subgroup analyses were possible due to the small number of included studies for the measures of temporal summation and CPM.
Figure 5.
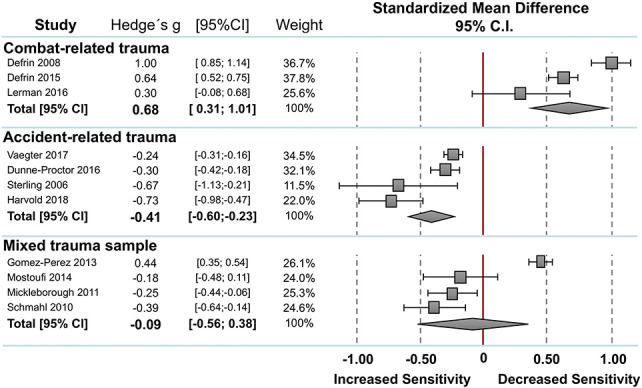
Stratification for kind of trauma for pain threshold measures. Standard mean differences (SMDs) were calculated as Hedges g. A DerSimonian–Laird random-effects model was used to calculate pooled estimates with 95% confidence intervals (95% CI). Heterogeneity among the studies was I2 = 90.4%, P = 0.000 for the combat-related trauma sample, I2 = 81.1%, P = 0.000 for the accident-related trauma sample, I2 = 89.8%, P = 0.000 for the sample without comorbid pain conditions, and I2 = 81.1%, P = 0.001 for the sample with comorbid pain conditions.
4. Discussion
4.1. Summary of findings
Based on a systematical summary of the current evidence on pain perception of experimentally evoked pain in individuals with PTSD compared with individuals without PTSD, no clear relationship between PTSD and pain perception exists. The type of trauma revealed some significant differences of small to medium effect sizes between individuals with PTSD and without PTSD, possibly suggesting that groups of individuals with PTSD consists of subgroups with qualitative pain perception differences. However, the available data are extremely heterogeneous, from small sample sizes, and limited to inclusion of only a few trauma types.
4.2. Subgroups stratified according to the nature of traumatization
With regard to the nature of traumatization, the analyses suggest that combat-related PTSD is associated with increased pain thresholds, whereas accident-related PTSD is associated with decreased pain thresholds. Alterations in pain thresholds seem to be related to the underlying trauma rather than to the presence of PTSD symptoms per se, and may indicate differential underlying mechanisms and studies comparing accident-related trauma with combat-related trauma are warranted.
One possible hypothesis behind a difference in pain threshold between trauma types is that complex PTSD symptomatology is more common in combat-related trauma compared to accident-related trauma and whiplash-associated disorder. This may affect coping mechanisms. For example, the dissociative subtype of PTSD is rarely seen in whiplash-associated disorder,18 whereas pronounced levels of dissociation have been seen in complex PTSD.3 Subjects with accident-related or whiplash-associated PTSD conditions may show more pronounced levels of anxiety and pain catastrophizing, and lower levels of dissociation.18 It has been shown that experimental pain ratings are negatively related to dissociation levels,7 and positively related to anxiety associated with fearful appraisals of pain.41 It has therefore been suggested that reduced conscious attention allocated toward incoming stimuli, resulting from dissociation, causes delayed response in pain threshold measurement, whereas biases toward threatening and higher intensity stimuli are responsible for the intensification of experimental and chronic pain.7 Posttraumatic stress disorder may thus lead to both habituation and sensitization according to this dual process theory—depending on whether one or the other process predominates, a hypoalgetic or a hyperalgetic effect occurs.
A similar phenomenon of psychophysiologically higher pain threshold on the one hand and increased risk for chronic pain on the other is known from studies with patients with borderline personality disorder, which is closely associated with early childhood trauma. This phenomenon was explained by a lower body perception14 and suppression of emotional experiences10 in those affected. It was postulated that this leads to an increased sympathetic activity and an increase in allostatic load, which in turn is a risk factor for the development of chronic pain in many ways.10 Others have suggested that decreased acute pain sensitivity is a result of stress-induced analgesia or dissociation,2 and alterations in the brain's regulation of the affective motivational component of pain.39 Increased chronic pain sensitivity has been conceptualized as a failure to self-regulate44 or has been linked to depression.52
An alternative hypothesis is related to the willingness to report pain. As pain endurance is a part of military training and practice, veterans might lack motivation to report pain (“stoicism”). In this regard, “stoic veterans” should feel as much pain as others but express their experience less. In the included studies, pain reports relied on subjective pain ratings and may therefore have given the appearance of increased pain thresholds. This idea is supported by the study of Kraus et al.,29 which reported similar pain thresholds between veterans with and without PTSD, but significantly higher pain thresholds in veterans compared to healthy controls. Yet, the data provided by Defrin et al.5,7 suggest paradoxical effects of both hyperalgesia and hypoalgesia in combat veterans, underlining that any conclusions about the latent causes of the apparent effect remain highly speculative and should be considered with caution.
A possible additional interference effect must also be taken into account here: the studies on accident-related trauma predominantly included patients with concomitant comorbid pain conditions (eg, whiplash), whereas studies on veterans or other traumas included heterogeneous or even pain-free patient samples. Moreover, there is relevant heterogeneity in pain induction methods used by the included studies. There are often differences in pain threshold and tolerance as a function of method used.16 Accordingly, the subgroup analyses should be interpreted with caution because it cannot be concluded from the data available here that veterans in principle have higher pain thresholds than nonveterans, but more research is needed to address this question.
4.3. Limitations
Several limitations of our review should be noted. First, there were a low number of studies for some of the pain perception measures. This applies in particular to CPM. A further limitation is that research on pain perception is primarily based on experimental pain tests, which somewhat lack ecological validity. Therefore, the transfer of our results to “real life” must be considered with caution because the available data were generally collected under controlled conditions.
Another important critique is the assessment of PTSD. It might be the case that those with accident-related trauma are only qualifying for subsyndromic PTSD dominated by hyperarousal syndromes, whereas victims of combat-related trauma can be more often characterized by the full symptom cluster of PTSD. In particular, the definition of the stressor criterion for PTSD (“Criterion A1”) can be discussed critically in accident-related trauma, but are clearer cut fulfilled in combat-related PTSD. Further research is warranted to investigate the relationship between different types of trauma and pain perception and to identify possible underlying mechanisms. At the same time, these results provide important information for the planning and implementation of future studies. Posttraumatic stress disorder is a very heterogeneous condition. As long as the individual studies conflate heterogeneous samples or limit themselves to individual subsamples, the relationship between PTSD and pain perception is likely to be inconsistent and the literature will be cluttered with findings in opposing directions. A greater focus on larger orchestrated studies could produce interesting findings that account more for complex relationship between PTSD and pain perception.
5. Conclusions
In conclusion, our data indicate that there is no clear relationship between PTSD and pain perception. However, the nature of trauma may affect pain perception differently because differential effects of PTSD on pain perception were observed. This has a central consequence for further research in this area: If alterations in pain processing not only depends of the presence of PTSD symptomatology itself, but also on the nature of the traumatic event, this would suggest that future pain treatments for patients with trauma exposure should be approached in a subgroup-specific manner.
Disclosures
The authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A78.
Acknowledgments
The submitted article does not contain information about medical device(s)/drug(s).
This work was supported by German Research Foundation (DFG) within the Collaborative Research Center (SFB) 1158 and by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung; PerPAIN consortium, FKZ:01 EC1904A). No benefits in any form have been or will be received from a commercial party directly or indirectly related to the subject of this article. Especially, the authors thank Ruth Defrin, Slawomira Diener, Michele Sterling, Christian Schmahl, Lydia Gómez-Pérez, JosefJenewein, Emanuel Lerman, Maria Densmore, Scott Orr, and Irina Strigo, who took the trouble to look into data records, some of them dating back years, to add the missing information.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
References
- [1].APA. Diagnostic and statistical manual of mental disorders. Washington: American Psychiatric Association, 2015. [Google Scholar]
- [2].Bohus M, Limberger M, Ebner U, Glocker FX, Schwarz B, Wernz M, Lieb K. Pain perception during self-reported distress and calmness in patients with borderline personality disorder and self-mutilating behavior. Psychiatry Res 2000;95:251–60. [DOI] [PubMed] [Google Scholar]
- [3].Boyd J, Protopopescu A, O'Connor C, Neufeld R, Jetly R, Hood H, Lanius R, McKinnon M. Dissociative symptoms mediate the relation between PTSD symptoms and functional impairment in a sample of military members, veterans, and first responders with PTSD. Eur J Psychotraumatol 2018;17:1463794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Breslau N, Reboussin B, Anthony J, Storr C. The structure of posttraumatic stress disorder: latent class analysis in 2 community samples. Arch Gen Psychiatry 2005;62:1343–51. [DOI] [PubMed] [Google Scholar]
- [5].Defrin R, Ginzburg K, Solomon Z, Polad E, Bloch M, Govezensky M, Schreiber S. Quantitative testing of pain perception in subjects with PTSD—implications for the mechanism of the coexistence between PTSD and chronic pain. PAIN 2008;138:450–9. [DOI] [PubMed] [Google Scholar]
- [6].Defrin R, Lahav Y, Solomon Z. Dysfunctional pain modulation in torture survivors: the mediating effect of PTSD. J Pain 2017;18:1–10. [DOI] [PubMed] [Google Scholar]
- [7].Defrin R, Schreiber S, Ginzburg K. Paradoxical pain perception in posttraumatic stress disorder: the unique role of anxiety and dissociation. J Pain 2015;16:961–70. [DOI] [PubMed] [Google Scholar]
- [8].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [9].Diener SJ, Wessa M, Ridder S, Lang S, Diers M, Steil R, Flor H. Enhanced stress analgesia to a cognitively demanding task in patients with posttraumatic stress disorder. J Affect Disord 2012;136:1247–51. [DOI] [PubMed] [Google Scholar]
- [10].Dixon-Gordon K, Berghoff C, McDermott M. Borderline personality disorder symptoms and pain in college students: the role of emotional suppression. J Pers Disord 2018;32:277–88. [DOI] [PubMed] [Google Scholar]
- [11].Dunne-Proctor RL, Kenardy J, Sterling M. The impact of posttraumatic stress disorder on physiological arousal, disability, and sensory pain thresholds in patients with chronic whiplash. Clin J Pain 2016;32:645–53. [DOI] [PubMed] [Google Scholar]
- [12].Geisser M, Roth R, Bachman J, Eckert T. The realtionship between symptoms of post-traumatic stress disorder and pain, affective disturbance and disability among patients with accident and non-accident related pain. PAIN 1996;66:207–14. [DOI] [PubMed] [Google Scholar]
- [13].Geuze E, Westenberg HG, Jochims A, de Kloet CS, Bohus M, Vermetten E, Schmahl C. Altered pain processing in veterans with posttraumatic stress disorder. Arch Gen Psychiatry 2007;64:76–85. [DOI] [PubMed] [Google Scholar]
- [14].Ginzburg K, Biran I, Aryeh IG, Tsur N, Defrin R. Pain perception and body awareness among individuals with borderline personality disorder. J Pers Disord 2018;32:618–35. [DOI] [PubMed] [Google Scholar]
- [15].Gomez-Perez L, Lopez-Martinez AE. Association of trauma, posttraumatic stress disorder, and experimental pain response in healthy young women. Clin J Pain 2013;29:425–34. [DOI] [PubMed] [Google Scholar]
- [16].Graven-Nielsen T, Vaegter H, Finocchietti S, Handberg G, Arendt-Nielsen L. Assessment of musculoskeletal pain sensitivity and temporal summation by cuff pressure algometry: a reliability study. PAIN 2015;156:2193–2202. [DOI] [PubMed] [Google Scholar]
- [17].Green B, Krupnick J, Chung J, Siddique J, Krause E, Revicki D, Frank L, Miranda J. Impact of PTSD comorbidity on one-year outcomes in a depression trial. J Clin Psychol 2006;62:815–35. [DOI] [PubMed] [Google Scholar]
- [18].Hansen M, Hyland P, Armour C, Andersen T. Assessing the existence of dissociative PTSD in sub-acute patients of whiplash. J Trauma Dissociation 2018;16:1–16. [DOI] [PubMed] [Google Scholar]
- [19].Hartling L, Hamm M, Milne A. Validity and inter-rater reliability testing of quality assessment instruments. Rockville: Agency for Healthcare Research and Quality (US), 2012. [PubMed] [Google Scholar]
- [20].Harvold M, MacLeod C, Vaegter HB. Attentional avoidance is associated with increased pain sensitivity in patients with chronic posttraumatic pain and comorbid posttraumatic stress. Clin J Pain 2018;34:22–9. [DOI] [PubMed] [Google Scholar]
- [21].Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration, 2009. [Google Scholar]
- [22].Higgins J, Thompson S. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [23].Jaramillo C, Cooper D, Wang C, Tate D, Eapen B, York G, Pugh M. Subgroups of US Iraq and Afghanistan veterans: associations with traumatic brain injury and mental health conditions. Brain Imaging Behav 2015;9:445–55. [DOI] [PubMed] [Google Scholar]
- [24].Jenewein J, Erni J, Moergeli H, Grillon C, Schumacher S, Mueller-Pfeiffer C, Hassanpour K, Seiler A, Wittmann L, Schnyder U, Hasler G. Altered pain perception and fear-learning deficits in subjects with posttraumatic stress disorder. J Pain 2016;17:1325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kessler R, Aguilar-Gaxiola S, Alonso J, Benjet C, Bromet E, Cardoso G, Degenhardt L, de Girolamo G, Dinolova R, Ferry F, Florescu S, Gureje O, Haro J, Huang Y, Karam E, Kawakami N, Lee S, Lepine J, Levinson D, Navarro-Mateu F, Pennell B, Piazza M, Posada-Villa J, Scott K, Stein D, Ten Have M, Torres Y, Viana M, Petukhova M, Sampson N, Zaslavsky A, Koenen K. Trauma and PTSD in the WHO world mental health surveys. Eur J Psychotraumatol 2017;8(suppl 5):1353383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kessler R, Sonnega A, Bromet E, Hughes M, Nelson C. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Surv 1995;52:1048–60. [DOI] [PubMed] [Google Scholar]
- [27].Kilpatrick D, Ruggiero K, Acierno R, Saunders B, Resnick H, Best C. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: results from the National Survey of Adolescents. J Consult Clin Psychol 2003;71:692–700. [DOI] [PubMed] [Google Scholar]
- [28].Kraus A, Esposito F, Seifritz E, Di Salle F, Ruf M, Valerius G, Ludaescher P, Bohus M, Schmahl C. Amygdala deactivation as a neural correlate of pain processing in patients with borderline personality disorder and co-occurrent posttraumatic stress disorder. Biol Psychiatry 2009;65:819–22. [DOI] [PubMed] [Google Scholar]
- [29].Kraus A, Geuze E, Schmahl C, Greffrath W, Treede R-D, Bohus M, Vermetten E. Differentiation of pain ratings in combat-related posttraumatic stress disorder. PAIN 2009;143:179–85. [DOI] [PubMed] [Google Scholar]
- [30].Lanius R, Bluhm R, Lanius U, Pain C. A review of neuroimaging studies in PTSD: heterogeneity of response to symptom provocation. J Psychiatr Res 2006;40:709–29. [DOI] [PubMed] [Google Scholar]
- [31].Lerman I, Davis BA, Bertram TM, Proudfoot J, Hauger RL, Coe CL, Patel PM, Baker DG. Posttraumatic stress disorder influences the nociceptive and intrathecal cytokine response to a painful stimulus in combat veterans. Psychoneuroendocrinology 2016;73:99–108. [DOI] [PubMed] [Google Scholar]
- [32].Magerl W, Wilk S, Treede R-D. Secondary hyperalgesia and perceptual wind-up following intradermal injection of capsaicin in humans. PAIN 1998;74:257–68. [DOI] [PubMed] [Google Scholar]
- [33].Merskey H, Bogduk N. IASP task force on taxonomy. Classification of chronic pain. Seattle: IASP press, 1994. pp. 209–14. [Google Scholar]
- [34].Mickleborough MJ, Daniels JK, Coupland NJ, Kao R, Williamson PC, Lanius UF, Hegadoren K, Schore A, Densmore M, Stevens T, Lanius RA. Effects of trauma-related cues on pain processing in posttraumatic stress disorder: an fMRI investigation. J Psychiatry Neurosci 2011;36:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Miller M, Kaloupek D, Dillon A, Keane T. Externalizing and internalizing subtypes of combat-related PTSD: a replication and extension using the Psy-5 scales. J Abnormal Psychol 2004;113:636–45. [DOI] [PubMed] [Google Scholar]
- [36].Moher D, Liberati A, Tetzlaff J, Altman D; PRISMA-Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Int Med 2009;151:1–7. [PMC free article] [PubMed] [Google Scholar]
- [37].Mostoufi S, Godfrey KM, Ahumada SM, Hossain N, Song T, Wright LJ, Lohr JB, Afari N. Pain sensitivity in posttraumatic stress disorder and other anxiety disorders: a preliminary case control study. Ann Gen Psychiatry 2014;13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nandi A, Beard J, Galea S. Epidemiologic heterogeneity of common mood and anxiety disorders over the lifecourse in the general population: a systematic review. BMC Psychiatry 2009;9:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Niedtfeld I, Schulze L, Kirsch P, Herpertz S, Bohus M, Schmahl C. Affect regulation and pain in borderline personality disorder: a possible link to the understanding of self-injury. Biol Psychiatry 2010;68:383–91. [DOI] [PubMed] [Google Scholar]
- [40].Nugent N, Koenen K, Bradley B. Heterogenity of posttraumatic stress symptoms in a highly traumatized low income, urban, African American sample. J Psychiatr Res 2012;46:1576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ocañez K, McHugh R, Otto M. A meta-analytic review of the association between anxiety sensitivity and pain. Depress Anxiety 2010;27:760–7. [DOI] [PubMed] [Google Scholar]
- [42].Orr SP, Meyerhoff JL, Edwards JV, Pitman RK. Heart rate and blood pressure resting levels and responses to generic stressors in Vietnam veterans with posttraumatic stress disorder. J Trauma Stress 1998;11:155–64. [DOI] [PubMed] [Google Scholar]
- [43].Pitman RK, Orr SP, van der Kolk BA, Greenberg MS, Meyerhoff JL, Mougey EH. Analgesia: a new dependent variable for the biological study of posttraumatic stress disorder. In: Wolf ME, Mosnaim AD, Wolf ME, Mosnaim AD, editors. Posttraumatic stress disorder: Etiology, phenomenology, and treatment. Arlington: American Psychiatric Association, 1990. pp. 141–7. [Google Scholar]
- [44].Sansone R, Sansone L. Borderline personality and the pain paradox. Psychiatry 2007;4:40–6. [PMC free article] [PubMed] [Google Scholar]
- [45].Schmahl C, Meinzer M, Zeuch A, Fichter M, Cebulla M, Kleindienst N, Ludascher P, Steil R, Bohus M. Pain sensitivity is reduced in borderline personality disorder, but not in posttraumatic stress disorder and bulimia nervosa. World J Biol Psychiatry 2010;11:364–71. [DOI] [PubMed] [Google Scholar]
- [46].Siqveland J, Hussain A, Lindstrøm J, Ruud T, Hauff E. Prevalence of posttraumatic stress disorder in persons with chronic pain: a meta-analysis. Front Psychiatry 2017;8:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sterling M, Jull G, Kenardy J. Physical and psychological factors maintain long-term predictive capacity post-whiplash injury. PAIN 2006;122:102–8. [DOI] [PubMed] [Google Scholar]
- [48].Sterling M, Jull G, Vicenzino B, Kenardy J, Darnell R. Physical and psychological factors predict outcome following whiplash injury. PAIN 2005;114:141–8. [DOI] [PubMed] [Google Scholar]
- [49].Sterling M, Kenardy J. The relationship between sensory and sympathetic nervous system changes and posttraumatic stress reaction following whiplash injury—a prospective study. J Psychosomat Res 2006;60:387–93. [DOI] [PubMed] [Google Scholar]
- [50].Strigo I, Spadoni A, Inslicht S, Simmons A. Repeated exposure to experimental pain differentiates combat TBI with and without PTSD. Neuropsychopharmacology 2017;43:S334–5. [Google Scholar]
- [51].Strigo IA, Simmons AN, Matthews SC, Grimes EM, Allard CB, Reinhardt LE, Paulus MP, Stein MB. Neural correlates of altered pain response in women with posttraumatic stress disorder from intimate partner violence. Biol Psychiatry 2010;68:442–50. [DOI] [PubMed] [Google Scholar]
- [52].Tragesser S, Bruns D, Disorbio J. Borderline personality disorder features and pain: the mediating role of negative affect in a pain patient sample. Clin J Pain 2010;26:348–53. [DOI] [PubMed] [Google Scholar]
- [53].Vaegter HB, Andersen TE, Harvold M, Andersen PG, Graven-Nielsen T. Increased pain sensitivity in accident-related chronic pain patients with comorbid posttraumatic stress. Clin J Pain 2018;34:313–21. [DOI] [PubMed] [Google Scholar]
- [54].Waelde L, Silvern L, Fairbank J. A taxometric investigation of dissociation in Vietnam veterans. J Trauma Stress 2005;18:359–69. [DOI] [PubMed] [Google Scholar]
- [55].WHO. The IDC-10 classification of mental and behavioural disorders. Diagnostic criteria for research. Geneva: World Health Organisation, 1993. [Google Scholar]
- [56].Yarnitsky D, Bouhassira D, Drewes A, Fillingim R, Granot M, Hansson P, Landau R, Marchand S, Matre D, Nilsen K, Stubhaug A, Treede R, Wilder-Smith O. Recommendations on practice of conditioned pain modulation (CPM) testing. Eur J Pain 2015;19:805–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A78.


