Abstract
Background and aims
Pulmonary edema is one of the most common acute respiratory disorders that diagnosis and treatment of the disease still remain as a health problem. The aim of this study was to compare the efficacy of intravenous furosemide and nebulized furosemide in control of the symptoms of the patients with pulmonary edem.
Methods
In this clinical trial, 80 patients were enrolled with pulmonary edema. Patients were randomly divided into two groups. In the intervention group the patients received nebulized furosemide at a dose of 1 mg furosemide for 20 minutes in 2 mL of sodium chloride 0.9% and in the control group the patients received intravenous furosemide at a dose of 1 mg/kg. Then, hemodynamic parameters and estimation of the clinical severity of the pulmonary edema in both groups was performed for 2 hours.
Results
According to our results, we can say that nebulized furosemide is not superior to intravenous furosemide in reducing dyspnea and crackles in patients with acute pulmonary edema, but significantly improved respiratory rate and arterial blood oxygen and has less hemodynamic changes than the intravenous furosemide.
Conclusions
The results of this study showed the beneficial effects of nebulized furosemide in the treatment of pulmonary edema, which can be prescribed as a treatment in addition to standard treatment and significantly lead in better control of pulmonary edema in the short term.
Keywords: furosemide, intravenous, nebulizer, pulmonary edema
1. INTRODUCTION
Pulmonary edema (PE) is defined as pathological accumulation of fluid in the extravascular space. 1 PE is one of the most emergencies that physicians are faced with it in the emergency department. 2 The most important manifestation of PE is fluid from pulmonary capillaries into the alveolar cavity through the alveolar–capillary membrane. 3
PE will ultimately lead to defects in gas exchange and may cause respiratory failure. Given the underlying cause of its creation, PE may be divided into two main sub‐groups, cardiogenic and noncardiogenic. In addition to these two main groups, also rare sub‐groups called neurogenic pulmonary and re‐expansion of pulmonary edema have been described. 4 Also, patients show generally pulmonary congestion, tachypnea, tachycardia, and elevated systolic blood pressure. 5
Pharmaceutical treatment in PE with the aim of increasing hydrostatic pressure in the pulmonary circulation is carried out mainly by reducing filling pressure (preload) and decreased peripheral arterial pressure (afterload), by venous and arterial dilatation. In addition, traditionally, sometimes, their focus has also been on removal of excess fluid by diuresis. 6 , 7
Medications commonly used in the treatment of PE include, nitroglycerin, as one of the most effective fast‐acting drugs for lowering preload, morphine sulfate, as a drug with vasodilator properties that decreases the preload and also loop diuretics. 8
Furosemide with the lowest toxicity is one of the most powerful diuretics. By binding to the potassium sodium cotransporter, the drug applies its diuretic effect on the thick ascending limb of the loop of Henle. 9 Furosemide has a short half‐life (1.3 ± 8.0 hours) and low bioavailability.
The drug creates problems, including the loss of drug effect before the next dose. This increases the use of drug intravenously. 10 Clinically, finding a safe and effective technique that can be alternative to the injection method is very important. This need has led to studies on a comparison between other methods of drug use with furosemide injection. Due to the lack of an inhaled form of many drugs, nebulization of these drugs through a mechanical ventilator is growing. The main advantage of nebulized drugs is that high concentrations of such drugs can be used in bronchus without any systemic side effects. The drug nebulization may remove the first‐pass effect of rapid absorption from the respiratory tract. 11 , 12
Based on these findings, the present study aimed to compare the shape and type of nebulized furosemide with injectable furosemide in reducing the complications of patients with PE.
2. METHODS
2.1. Trial design
This study was design according Consolidated Standards of Reporting Trials (CONSORT) checklist. In this randomized clinical trial study, patients with pulmonary edema, referred to the Golestan and Imam Khomeini hospitals, were evaluated. At first, patients were visited by a doctor and underwent the clinical assessment and physical examination. This study was approved by the ethics committee of Ahvaz Jundishapur University of Medical Sciences, trial registration (IRCT2017030332853N1), http://www.irct.ir, and patients signed the written informed consent and were enrolled.
2.2. Participants
2.2.1. Inclusion criteria
Patients with shortness of breath during the past 6 hours, patients with clinical signs of pulmonary edema (tachypnea, increased lung function, use of accessory muscles, crackles, wheezes, and gallop rhythm), and radiographically diagnosis of pulmonary edema.
2.3. Exclusion criteria
Cardiogenic shock and blood pressure less than 90 mm Hg, noncardiogenic pulmonary edema, myocardial infarction, severe heart valve disease, history of pulmonary obstructive disease, patients requiring intubation, cardiac arrhythmias, liver failure, cancer sensitivity to furosemide, and patient's dissatisfaction with the disease and pregnancy. Vital signs (PP, RR, PR, BP, O2sat), crackles score (no, one third of the base, 1 second of the base, and two third of the base), dyspnea score, and sweating score (no sweating, sweating on the forehead, excessive sweating of forehead, extensive sweating) of the patients were recorded in their file, and also before any clinical trial, resuscitation equipment was ready on the patients' bedside. In this study, using echocardiography, all pulmonary edema patients with cardiac and noncardiac origin were differentiated, and, if the patients did not have echocardiography, echocardiography was used.
2.4. Interventions
Before the intervention and based on history, the physical examination of the patient was done by talking in a sentence or phrase or word, by the patient; and consciousness state, use of accessory muscles of breathing, crackles, wheezing, and parameters of PP, RR, PR, BP O2sats, as well as triplet scores and clinical status were assessed.
In all patients before the intervention, 10.6 L of 100% oxygen, 10 μg/min of nitrate, and morphine at a dose of 5 to 3 mg were administered. Then, in a group of patients, 1 mg per kg intravenous furosemide with oxygen was used, and another group, the nebulized furosemide (1 mg furosemide is diluted in 2 mL of sodium chloride 0.9%) was used.
2.5. Outcomes
The patients' lungs at minutes of 15, 30,60, and 120 were listened by an emergency medicine specialist ear, and the differences between the two groups were recorded. The RR, PR, BP, and SPO2 at 15, 120, 60, and 30 were recorded, and according to the Dyspnea Scale criteria, the degree of difficulty in breathing was estimated from 0 to 3 (0 = no shortness of breath while lying, 1 = shortness of breath while lying, 2 = shortness of breath when while semi‐bending, 3 = shortness of breath when sitting). Then, the results were statistically analyzed. Other lines of treatment or repeated intervention was used if both groups of patients did not give an appropriate response to therapeutic intervention and suffered deterioration and dyspnea escalation; if there was so before the 20 minutes, the patient was excluded.
2.6. Randomization
Using the permuted block technique, patients were randomly divided into two groups of 40. Patient and doctor were not informed of what treatment every person has received person, who measured answers, had no information on each patient is in which group.
2.7. Sample size calculation
According to the literature review, 40 patients were significant for inclusion in this study.
2.8. Data analysis
At first, the data obtained were examined in terms of descriptive indicators and, then, to compare quantities between the two groups; according to normality of data, t‐test and Mann–Whitney test were used. Normality of data was evaluated by the Kolmogorov–Simonov normality. Statistical analyses were performed using the SPSS version 20. A P‐value less than 0.05 was considered as significance level.
3. RESULTS
Overall, 85 people were evaluated during the study. Of these, five were eliminated, and the other 80 were randomly divided into two groups (Figure 1). Of the 80 patients, 57 patients (71.3%) were male and 23 (28.8%) were women. The mean age of the subjects was 64.85 ± 11.02 years. Demographic variables of patients are shown in Table 1. According to these findings, demographic information for patients and also the time length of shortness of breath and left ventricular ejection fraction (LEVF) in both groups did not show significant differences.
FIGURE 1.
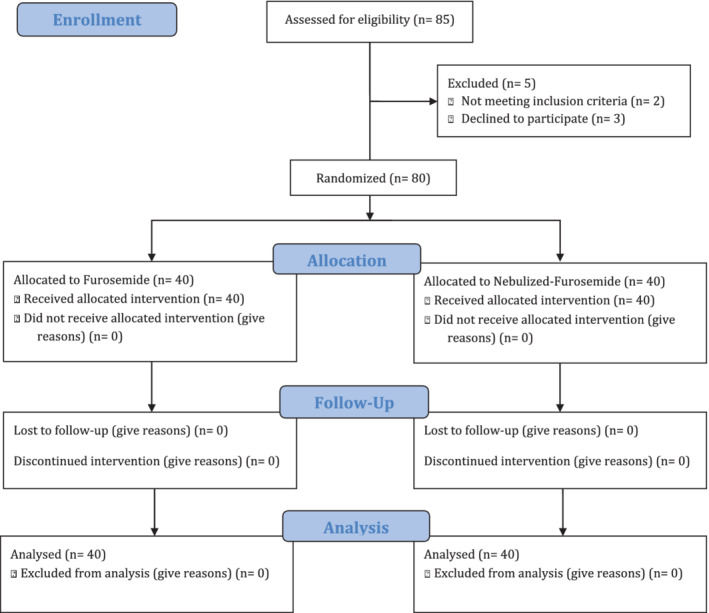
CONSORT flow diagram
TABLE 1.
Characteristic of patients
| Variables | Intravenous Furosemide (N = 40) | Nebulized Furosemide (N = 40) | P‐value |
|---|---|---|---|
| Age, year (Mean ± SD) | 66.66±11.99 | 63.1±10.9 | .157 |
| Gender, Male N (%) | 27 (75) | 30 (67.5) | .005 |
| LVEF (Mean ± SD) | 27.25 ± 9.93 | 26.11 ± 0.27 | .6 |
| Onset of shortness of breath, min (Mean ± SD) | 8.05 ± 0.96 | 11.05 ± 9.54 | .058 |
Abbreviation: LVEF, left ventricular ejection fraction.
Results showed that there was no significant difference between the two groups in the mean arterial blood oxygen until 30 minutes (P = .637). But in 60 minutes, the nebulizer significantly increased the arterial blood oxygen (95.05% vs 94.1%; P = .005), but at 120 minutes, arterial blood oxygen increased more in the intravenous group compared to the nebulizer group (96.4% vs 95.8%; P = .012; Table 2). In addition, the mean number of breaths per minute 60 and 120 in the nebulizer group was significantly lower than the intravenous group, and the difference was statistically significant (Table 2). The severity of dyspnea during the study was faced with a strict decrease, this is while, there was no statistically significant difference in the mean severity of shortness of breath in any of the studied time points between the two groups (Table 2). In addition, there was no a statistically significant difference in the mean severity of sweating in any of the studied time points between the two groups (Table 2). In addition, the mean severity of crackles did not show a statistically significant difference in any of the studied time points between the two groups (Table 2).
TABLE 2.
Comparison of study objectives in different times
| Variables | Before intervention | 15 minutes | 30 minutes | 60 minutes | 120 minute | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intravenous | Nebulized | Intravenous | Nebulized | Intravenous | Nebulized | Intravenous | Nebulized | Intravenous | Nebulized | |
| Mean arterial blood oxygen (mm Hg) | 89.85 ± 4.12 | 90.3 ± 4.29 | 91.25 ± 3.46 | 91.35 ± 3.87 | 92.3 ± 3.20 | 92.6 ± 2.40 | 94.1 ± 1.72 | 95.05 ± 1.13 | 96.4 ± 1.33 | 95.8 ± 0.60 |
| P‐Value | .634 | .903 | .637 | .005 | .012 | |||||
| Mean number of breaths per minute | 30.7 ± 3.63 | 30.2 ± 6.07 | 29.45 ± 3.99 | 26.9 ± 5.30 | 27.2 ± 3.70 | 25.75 ± 4.84 | 24.35 ± 2.66 | 21.3 ± 2.95 | 20.55 ± 2.89 | 17.2 ± 1.48 |
| P‐Value | .656 | .017 | .137 | <.000 | <.000 | |||||
| Mean severity of dyspnea | 2.55 ± 0.50 | 2.45 ± 0.74 | 2.5 ± 0.59 | 2.45 ± 0.74 | 1.9 ± 0.84 | 1.75 ± 0.70 | 1.1 ± 0.63 | 1 ± 0.55 | 0.36 ± 0.48 | 0.35 ± 0.48 |
| P‐Value | .486 | .743 | .391 | .454 | .868 | |||||
| Mean severity of sweating | 1.75 ± 0.63 | 1.55 ± 0.67 | 1.45 ± 0.87 | 1.05 ± 0.98 | 0.7 ± 0.64 | 0.55 ± 0.67 | 0.25 ± 0.43 | 0.1 ± 0.30 | 0 | 0 |
| P‐Value | .176 | .059 | .315 | .08 | ‐ | |||||
| Mean severity of crackles | 2.2 ± 0.68 | 2.25 ± 0.43 | 2.2 ± 0.68 | 2.25 ± 0.43 | 2 ± 0.55 | 1.95 ± 0.50 | 1.3 ± 0.85 | 1.25 ± 0.43 | 0.95 ± 0.81 | 0.85 ± 0.36 |
| P‐Value | .699 | .699 | .674 | .743 | .481 | |||||
| Mean heart rate (bpm) | 97.45 ± 11.41 | 93.5 ± 19.46 | 97.8 ± 9.17 | 91.9 ± 16.28 | 93.45 ± 8.11 | 89.7 ± 15.07 | 87.8 ± 6.36 | 84.2 ± 12.64 | 84.85 ± 3.93 | 83.8 ± 10.79 |
| P‐Value | .272 | .049 | .17 | .113 | .566 | |||||
| Mean systolic blood pressure (mm Hg) | 158.05 ± 30.18 | 144.55 ± 28.89 | 155.6 ± 26.39 | 144 ± 24.56 | 151.05 ± 23.21 | 139.6 ± 22.53 | 142.3 ± 17.84 | 135.5 ± 22.38 | 135.35 ± 21.47 | 131.95 ± 22.04 |
| P‐Value | .044 | .045 | .028 | .137 | .487 | |||||
| Mean diastolic blood pressure (mm Hg) | 94.15 ± 12.99 | 97.15 ± 18.20 | 91.55 ± 14.94 | 90.85 ± 24.05 | 91.55 ± 11.17 | 92.2 ± 21.10 | 86.85 ± 9.89 | 85.6 ± 18.65 | 83.2 ± 9.86 | 80.75 ± 15.61 |
| P‐Value | .399 | .876 | .864 | .71 | .404 | |||||
The mean heart rate for 15 minutes in the nebulizer group was significantly lower than the IV group (91.9 vs 97.8) and the difference was statistically significant (P = .272), but at other times, it was not significant (Table 2). The mean systolic blood pressure before treatment in minutes of 15 and 30 in the nebulizer group was significantly lower than the intravenous group and the difference was statistically significant (Table 2). In other words, the administration of intravenous nebulizer decreased 23 mm Hg by the end of 120 minutes, but in the nebulizer group, 13 reduction units occurred in systolic blood pressure.
4. DISCUSSION
Pathologic accumulation of fluid in the intracellular tissue of the lung that causes disruption in the functioning of the pulmonary system can be defined as pulmonary edema that is one of the most emerging departments. Currently, in addition to the oxygen therapy, the management of these patients is prescription of nitroglycerin, morphine, and diuretics. The most important drug used in patients is furosemide. Furosemide, orally, due to low first‐pass effect and the low half‐life has low bioavailability. Thus, some studies have sought to increase its bioavailability through changes in its use route. Now, the IV furosemide has the most bioavailability. In clinical practice, it is important to find ways other than intravenous injection. Therefore, this study aimed to compare the nebulized furosemide with IV furosemide in answer to the question whether the two have similar effects and safety.
The present study shows that the amount of arterial oxygen in both groups showed no significant difference before intervention; however, at the point of time of 60 minutes, the rate in the group receiving nebulized furosemide and at time point of 120 minutes was higher in the group receiving intravenous furosemide. The difference in both points was statistically significant. In addition, respiratory rate in the group receiving nebulized furosemide from 60 minutes onward was significantly lower in the group receiving intravenous furosemide. So, it seems that the difference between the amount of arterial oxygen from 60 minutes to the next is due to a further decline in respiratory rate in patients receiving nebulized furosemide. Pendino et al.'s study in line with the present study showed that use of furosemide reduces the respiratory rate. 13 The findings also show that other symptoms of PE in both groups declined, so that the severity of dyspnea during the study was faced with a strict reduction while there was no statistically significant difference in mean severity of dyspnea in any of the time points of the study between the two groups. The mean severity of sweating in none of the studied time points of the study showed statistically significant difference between the two groups. In addition, the mean severity of the crackles in none of the studied time points of the study showed statistically significant difference between the two groups. So nebulized furosemide, also alike IV nebulizer, causes the improvement of respiratory function in patients with PE. Vahedi et al. have shown that the furosemide nebulizer causes significant improvement in respiratory factors such as FEV1, dyspnea, and the pH. 14 Also, Jensen et al. and Alshehri showed similar results of improvement of respiratory symptoms after taking furosemide. 15 , 16 Biddle et al. have shown that furosemide improves the symptoms of patients with PE. They showed that furosemide reduces excess fluid into the lungs in some patients while all patients, including those who have not shown the reduced liquid, show improvement of pulmonary symptoms. 17 So, it seems that furosemide, in addition to reducing the fluid within the lungs, improves the symptoms of these patients by other mechanisms.
It has been shown that furosemide caused a significant decrease in blood pressure, while no significant effect on heart rate. 18 , 19 In this regard, the present study also shows that there was no significant difference in heart rates before the intervention and at the end of follow‐up (120). In addition, our findings indicate that intravenous administration of nebulizer decreased 23 mmHg until the end of 120 minutes, but in the nebulizer group, 13 reduction units in systolic blood pressure occurred.
5. CONCLUSION
Overall, the findings of this study show that nebulized furosemide has an effect similar with intravenous furosemide and may be an alternative to it. Use of nebulizer as a less invasive treatment by facilitating the use of these drugs in patients with PE can improve the management of these patients in emergency departments. A strength of this study was to compare intravenous furosemide and nebulizer furosemide for the first time. While the limitations of the study were the low sample size and lack of long follow‐up of the patients as well as the lack of a comparison of drug side effects in both groups.
FUNDING
The authors wish to acknowledge the support of the deputy of research affairs of the Ahvaz Jundishapur University of Medical Sciences as part of Somayeh Shaabani's thesis under the research and financial code U‐96010.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: Hasan Barzegari, Ali Delirrooyfard
Formal analysis: Ali Khavanin
Funding acquisition: Ali Delirrooyfard
Writing—review and editing: Ali Delirrooyfard, Hasan Barzegari
Writing—original draft: Somayeh Shaabani
Ali Delirrooyfard had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
All authors have read and approved the final version of the manuscript.
TRANSPARENCY STATEMENT
The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Barzegari H, Khavanin A, Delirrooyfard A, Shaabani S. Intravenous furosemide vs nebulized furosemide in patients with pulmonary edema: A randomized controlled trial. Health Sci Rep. 2021;4:e235 10.1002/hsr2.235
Funding information Ahvaz Jundishapur University of Medical Sciences, Grant/Award Number: U‐96010
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article.
REFERENCES
- 1. Bachmann M, Waldrop JE. Noncardiogenic pulmonary edema. Compendium. 2012;34(11):E1. [PubMed] [Google Scholar]
- 2. Patrício C, da Silva FP, Brotas V. Pulmonary oedema in the emergency room: what is hidden beyond an apparently common presentation. BMJ Case Rep. 2014;1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tilman V. Pulmonary edema: a unifying pathophysiological formula. J Cardiovasc Dis Diagn. 2015;2015. [Google Scholar]
- 4. Birukov KG, Zebda N, Birukova AA. Barrier enhancing signals in pulmonary edema. Comprehensive Physiology. 2013;3(1):429‐484. [DOI] [PubMed] [Google Scholar]
- 5. Chioncel O, Ambrosy AP, Bubenek S, et al. Epidemiology, pathophysiology, and in‐hospital management of pulmonary edema: data from the Romanian acute heart failure syndromes registry. J Cardiovasc Med. 2016;17(2):92‐104. [DOI] [PubMed] [Google Scholar]
- 6. Bellone A, Barbieri A, Bursi F, Vettorello M. Management of acute pulmonary edema in the emergency department. Curr Heart Fail Rep. 2006;3(3):129‐135. [DOI] [PubMed] [Google Scholar]
- 7. Ellingsrud C, Agewall S. Morphine in the treatment of acute pulmonary oedema—why? Int J Cardiol. 2016;202:870‐873. [DOI] [PubMed] [Google Scholar]
- 8. Mattu A, Martinez JP, Kelly BS. Modern management of cardiogenic pulmonary edema. Emerg Med Clin North Am. 2005;23(4):1105‐1125. [DOI] [PubMed] [Google Scholar]
- 9. Prandota J. Furosemide: progress in understanding its diuretic, anti‐inflammatory, and bronchodilating mechanism of action, and use in the treatment of respiratory tract diseases. Am J Ther. 2002;9(4):317‐328. [DOI] [PubMed] [Google Scholar]
- 10. Klausner EA, Lavy E, Stepensky D, et al. Furosemide pharmacokinetics and pharmacodynamics following gastroretentive dosage form administration to healthy volunteers. J Clin Pharmacol. 2003;43(7):711‐720. [PubMed] [Google Scholar]
- 11. Berry B, Altman P, Rowe J, Vaisman J. Comparison of pharmacokinetics of Vardenafil administered using an ultrasonic nebulizer for inhalation vs. a single 10‐mg oral tablet. J Sex Med. 2009;13:1‐9. [DOI] [PubMed] [Google Scholar]
- 12. Cryan SA. Carrier‐based strategies for targeting protein and peptide drugs to the lungs. AAPS J. 2005;7(1):E20‐E41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pendino JC, Nannini LJ, Chapman KR, Slutsky A, Molfino NA. Effect of inhaled furosemide in acute asthma. J Asthma. 1998;35(1):89‐93. [DOI] [PubMed] [Google Scholar]
- 14. Vahedi HS, Mahshidfar B, Rabiee H, et al. The adjunctive effect of nebulized furosemide in COPD exacerbation: a randomized controlled clinical trial. Respir Care. 2013;58(11):1873‐1877. [DOI] [PubMed] [Google Scholar]
- 15. Alshehri M, Almegamesi T, Alfrayh A. Efficacy of nebulized furosemide in children with moderate attack of asthma. West Afr J Med. 2005;24(3):246‐251. [DOI] [PubMed] [Google Scholar]
- 16. Jensen D, Amjadi K, Harris‐McAllister V, Webb KA, O'Donnell DE. Mechanisms of dyspnea relief and improved exercise endurance after furosemide inhalation in COPD. Thorax. 2008;63(7):606‐613. [DOI] [PubMed] [Google Scholar]
- 17. Biddle TL, Paul NY. Effect of furosemide on hemodynamics and lung water in acute pulmonary edema secondary to myocardial infarction. Am J Cardiol. 1979;43(1):86‐90. [DOI] [PubMed] [Google Scholar]
- 18. Reverentia S, Suryadharma AK, Hidayati A, et al. Effect of short‐acting loop diuretics on 24‐hour heart rate variability and blood pressure in patients with acute decompensated heart failure. J Hypertension. 2015;33:e29. [Google Scholar]
- 19. Haegeli L, Rocca BL, Peter H, et al. Sublingual administration of furosemide: new application of an old drug. Br J Clin Pharmacol. 2007;64(6):804‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.


