Abstract
Background
SARS-CoV-2 infection (COVID-19 disease) can induce systemic vascular involvement contributing to morbidity and mortality. SARS-CoV-2 targets epithelial and endothelial cells through the ACE2 receptor. The anatomical involvement of the coronary tree is not explored yet.
Methods
Cardiac autopsy tissue of the entire coronary tree (main coronary arteries, epicardial arterioles/venules, epicardial capillaries) and epicardial nerves were analyzed in COVID-19 patients (n = 6). All anatomical regions were immunohistochemically tested for ACE2, TMPRSS2, CD147, CD45, CD3, CD4, CD8, CD68 and IL-6. COVID-19 negative patients with cardiovascular disease (n = 3) and influenza A (n = 6) served as controls.
Findings
COVID-19 positive patients showed strong ACE2 / TMPRSS2 expression in capillaries and less in arterioles/venules. The main coronary arteries were virtually devoid of ACE2 receptor and had only mild intimal inflammation. Epicardial capillaries had a prominent lympho-monocytic endotheliitis, which was less pronounced in arterioles/venules. The lymphocytic-monocytic infiltrate strongly expressed CD4, CD45, CD68. Peri/epicardial nerves had strong ACE2 expression and lympho-monocytic inflammation. COVID-19 negative patients showed minimal vascular ACE2 expression and lacked endotheliitis or inflammatory reaction.
Interpretation
ACE2 / TMPRSS2 expression and lymphomonocytic inflammation in COVID-19 disease increases crescentically towards the small vessels suggesting that COVID-19-induced endotheliitis is a small vessel vasculitis not involving the main coronaries. The inflammatory neuropathy of epicardial nerves in COVID-19 disease provides further evidence of an angio- and neurotrophic affinity of SARS-COV2 and might potentially contribute to the understanding of the high prevalence of cardiac complications such as myocardial injury and arrhythmias in COVID-19.
Funding
No external funding was necessary for this study.
Keywords: COVID-19, Endothelial dysfunction, Epicardial capillaries, Epicardial nerves, Coronary arteries, ACE2-receptor, Microangiopathy
Research in Context.
Evidence before this study
The currently accepted concept of the pathogenesis in COVID-19 involves a primarily virus-associated tissue injury followed by an inflammatory host immune response, which drives hypercytokinemia and aggressive inflammation, resulting in endotheliitis, increased apoptotic activity, thrombotic events and intravascular coagulation. The excessive inflammatory response against SARS-CoV-2 is thought to define disease severity and death in patients with COVID-19 and is associated with profound lymphopenia and substantial mononuclear cell infiltration in the lungs, heart, spleen, lymph nodes, intestine and kidney, as was observed in postmortem analyses.
Added value of this study
The results of this study provide evidence that endotheliitis of small epicardial and intramyocardial vessels poses an important pathogenetic mechanism of myocardial injury in COVID-19 disease. Cardiac inflammatory neuropathy, which has not been explored yet in the literature, with identical immunophenotype as detected in cardiac vessels may contribute to arrhythmias in COVID-19-patients. The crescentic expansion of inflammatory reaction towards the small cardiac vessels could well explain the increased troponin levels with normal epicardial coronary arteries in the absence of acute coronary syndrome in patients with COVID-19.
We show in this autopsy cohort, that SARS-CoV-2 infection results in a small vessel cardiac endotheliitis and cardial neuritis with high level ACE2 and TMPRSS2 expression, which might explain arrhythmias in COVID-19. Furthermore this study provides data that COVID-19 disease presents with a different immunomodulation observed in other RNA viral infections, such as Influenza A infections as well as in COVID-19 negative cardiovascular disease.
Implications of all the available evidence
In light of these considerations, the aims of this study were: 1) to assess the occurrence of endotheliitis in the entire coronary tree including all anatomical structures from the smallest epicardial vessels through the main coronary artery and to detect possible involvement of epicardial nerves: 2) to characterize morphologically and immunohistochemically the inflammatory infiltrates. 3) to compare inflammatory changes in the coronary tree with COVID-19-negative control autopsies and 4) to correlate the above findings with ACE2, TMPRSS2, CD147 and interleukin-6 expression in the different anatomical structures including the cardiac nerve conduction system.
Alt-text: Unlabelled box
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by the beta coronavirus SARS-CoV-2, has been spreading dramatically worldwide since first being reported in Wuhan, Hubei province, China, in December 2019 [1]. While hypoxic respiratory failure due to acute respiratory distress syndrome (ARDS) is the leading cause of mortality in patients with COVID-19 infection, hypoxic respiratory failure due to acute respiratory distress syndrome (ARDS) [2,3], cardiovascular complications, including myocardial injury with increased troponin levels, ventricular arrhythmias and heart failure have been reported in 12 to 33% of infected patients 4, 5, 6. COVID-19 is considered a systemic vascular disease affecting multiple organs due to endothelial damage leading to endotheliitis of the small vessels [7]. To enter cells, SARS-CoV-2 binds the angiotensin-converting enzyme 2 (ACE2) receptor, activating the cascade of inflammation [8,9] and potentially causing severe disease course and eventually fatalities in patients with associated co-morbidities [10]. An inflammatory olfactory neuropathy and general neurotropism have been described, frequently resulting in anosmia (74.8%; range 5.1–85.6%) and ageusia (81.6%; range 5.6%−88%) [11,12]. However, the involvement of epicardial nerves in COVID-19 has not been yet studied.
Recently, ACE2 was unequivocally established as the functional host receptor for SARSCoV-2 [13,14]. Its expression varies between different organs/tissues, also in association with individual factors such as age, gender, genetic background, life style, medications and comorbidities [14]. Zou et al identified organ specific differential vulnerability in a large spectrum of organs depending on the ACE2 expression varying from low (such as liver, stomach) and high (such as heart, respiratory tract) [14]. It has been hypothesized that such differences could explain the clinical presentation of the disease as well as individual differences among infected patients [14]. ACE2 is highly expressed in lung alveolar cells (principally on type II alveolar cells) and plays a role in lung protection. SARS-CoV-2 binding to ACE2 receptor deregulates the lung protective pathways, contributing to viral pathogenicity [15]. Similarly, the ACE2 receptor is significantly expressed in endothelial cells of blood vessels, the heart, pericytes, adipocytes, and neural cells [16]. The expression of ACE2 receptor in small epicardial vessels and peripheral epicardial nerves however remains elusive.
Transmembrane protease serine type2 (TMPRSS2), belonging to the type II transmembrane serine protease family, can cleave the coronavirus spike (S) protein and was demonstrated together with ACE2 to be crucial for the entry of SARS-CoV-2 into the host cells [17,18]. Recently, it has been found that ACE2 and TMPRSS2 are expressed not only in lung tissues, but also in extrapulmonary organs including heart, kidney, liver, colon, esophagus, brain, gallbladder and testis, suggesting that SARS-CoV-2 may also affect extrapulmonary organs [19].
The currently accepted concept of the pathogenesis in COVID-19 involves a primarily virus-associated tissue injury followed by an inflammatory host immune response, which drives hypercytokinemia and aggressive inflammation, resulting in endotheliitis, increased apoptotic activity, thrombotic events and intravascular coagulation [5,7,9,20,21]. The excessive inflammatory response against SARS-CoV-2 is thought to define disease severity and death in patients with COVID-19 [22] and is associated with profound lymphopenia and substantial mononuclear cell infiltration in the lungs, heart [23], spleen, lymph nodes, intestine and kidney [24,25] as was observed in postmortem analyses.
While the lung is the most severely injured organ by SARS-CoV-2 infection in most patients, many other organs are affected as well, including the heart, liver, kidney, brain, and intestines, resulting in acute respiratory disease syndrome (71%), acute kidney injury (20%), cardiac injury (33%), and liver dysfunction (15%), and a need for vasopressor support in up to 67% of patients [2,7,11,26,27]. A recent multicenter study on a large international cohort of 639 critically ill patients with confirmed SARS-CoV-2 infection reported that troponin levels are significantly higher among non survivors than in survivors (p<0.001), highlighting the importance of myocardial involvement in COVID-19 [5].
In addition, a meta-analysis of 18 studies and 2984 COVID-19 patients found a significant increase of interleukin-6 (IL-6) in survivor vs. non-survivors, representing then a marker for potential progression to critical illness. Indeed, the exaggerated elevation of Interleukin-6 can lead to the so-called “cytokines storm” and may be a driver behind acute lung injury and ARDS and lead to other tissue damage progressing to multiorgan failure (MOF) [28,29].
In light of these considerations, the aims of this study were: 1) to assess the occurrence of endotheliitis in the entire coronary tree including all anatomical structures from the smallest epicardial vessels through the main coronary artery and to detect possible involvement of epicardial nerves, 2) to characterize morphologically and immunohistochemically the inflammatory infiltrates, 3) to compare inflammatory changes in the coronary tree with COVID-19-negative control autopsies and 4) to correlate the above findings with ACE2, TMPRSS2, CD147 and interleukin-6 expression in the different anatomical structures including the cardiac nerve conduction system.
2. Methods
2.1. Patients cohort
The entire coronary arterial tree and the surrounding epicardial soft tissue, including fat and epicardial nerves were examined in autopsies from six COVID-19 patients at the Department of Pathology and Molecular Pathology of the University Hospital Zurich, Switzerland, during the COVID-19-pandemic between March and April 2020. Five of six patients of the COVID-19- series (83.3%) had a SARS-CoV-2 infection confirmed by two positive pharyngeal swabs (RT-PCR SARS-CoV-2). One patient had a false negative pharyngeal swab, but the RNA-qRT-PCR examination on paraffin embedded postmortem material from the lung because of strong clinical suspect, was positive for SARS-CoV-2. Four patients were male (4/6, 66.7%) and two female (2/6, 33.3%) (Range 45–81, mean age 68.3 years). All patients had several comorbidities (Table 1). The cause of death in all cases was multi-organ failure and complications of ARDS. One patient had signs of an acute myocardial infarction three days before death (Patient 4), but no occlusion of a large vessel could by visualized by coronary angiography. At the time of admission to the hospital, the electrocardiographic monitoring showed sinus tachycardia (heart rate >100/min) in three of six patients (3/6, 50%) and in one among these also paroxysmal atrial fibrillation and non-specific repolarization abnormalities, in one of these six (1/6, 16.7%), non-specific repolarization abnormalities, in one these six (1/6, 16.7%) isolated ventricular extrasystoles (Table 1).
Table 1.
Clinico-pathological findings in COVID-19 positive patients at autopsy.
| PATIENT | AGE (years) | GENDER | MAIN CLINICAL COMORBIDITIES | CARDIAC INVOLVMENT (CLINICAL AND/OR postmortem diagnosis) | CAUSE OF DEATH |
|---|---|---|---|---|---|
| 1 | 70 | Male | Prostate adenocarcinoma, arterial hypertension, kidney transplantation due to Alport syndrome. | Moderately increased T-Troponin, Histologically focal peracute terminal myocardial ischemia. |
DAD/ARDS and pneumonia. |
| 2 | 77 | Female | Cutaneous melanoma (stage IIB), arterial hypertension. | Clinically not known, histologically focal peracute terminal myocardial ischemia. | DAD/ARDS, pneumonia |
| 3 | 79 | Male | Waldenström's macroglobulinemia, pulmonary hypertension, obesity (BMI 32.9 kg/m2). | Moderately increased T-Troponin, histologically fibrin-thrombi in myocardial capillaries. | DAD/ARDS, pneumonia |
| 4 | 58 | Female | Diabetes mellitus type 2, arterial hypertension, obesity (BMI 37.8 kg/m2) | Clinically acute myocardial infarction, strongly increased T-Troponin. At autopsy no thrombi in coronaries, but histologically peracute myocardial ischemia. | DAD/ARDS, pneumonia, intestinal ischemia |
| 5 | 81 | Male | COPD, arterial hypertension, prostate cancer. | Moderately increased T-Troponin, intraventricular thrombosis. Focal peracute myocardial ischemia. | DAD/ARDS, pneumonia |
| 6 | 45 | Male | Diabetes mellitus type I, diabetic nephropathy, kidney transplantation due to diabetic nephropathy, post-transplant, PTLD | Moderately increased T-Troponin, focal peracute myocardial ischemia.intraventricular thrombosis. Histologically focal peracute terminal myocardial ischemia | DAD/ARDS, pneumonia |
Abbreviations. COPD: chronic obstructive lung disease, PTLD: post transplant lymphoproliferative disease, ARDS: adult respiratory distress syndrome, DAD: diffuse alveolar damage.
Consent to perform the autopsy was given in all cases and the institutional review board (Department of Pathology and Molecular Pathology of the University Hospital Zurich, Switzerland) approved the study. Ethical aspects on research on autopsy tissue on deceased patients, postmortem diagnostic and molecular analyses were completed in accordance with the Swiss Federal Research Regulations (BASEC Nr. 2020.1316 and 2018.482).
2.2. Control cohorts
Two different control cohorts were selected.
-
1)
Tissues of same anatomical sites from three control autopsies (patients A-C) were also investigated, which were performed at our institution about one year before the COVID-19-pandemic (between May and June 2019) and which showed the same profile of risk factor observed in the COVID-19-positive cases (arterial hypertension, diabetes mellitus and obesity). All of them were middle-aged or elderly patients (range 62–82, mean age 73.7 years) and all had at least one comorbidity condition (Table 2). Two were male (2/3, 66.7%) and one was female (1/3, 33.3%). Two of these patients died due to an acute myocardial infarction (2/3, 66.7%) and one from aspiration-pneumonia (1/3, 33.3%). None of the control cases had a clinical or laboratory history of an ongoing viral infection (clinical data of the control cohort are summarized in Table 2a).
-
2)
A second control autopsy cohort consisted of six patients (patients d-I) died between 2016 and 2019 from respiratory insufficiency due to influenza A pneumonia or viral pneumonia. They were young, middle-aged or elderly patients (range 22–85, mean age 63.5 years). Two were male (2/6, 33.3%) and four female (4/6, 66.7%). Five of six (5/6, 83.3%) had a laboratory confirmed influenza A infection (through nasopharyngeal swab and RT-PCR with viral RNA detection). At one patient (1/6, 16.7%) the viral influenza A etiology of the pneumonia was suspected clinically, although the tests of the most common respiratory viruses (Adenovirus, Influenza A, Coronaviruses 229E, HKU1, NL63, OC43, Human Metapneumovirus, Bocavirus, Rhinovirus, Parainfluenza viruses, respiratory syncytial viruses and Herpes-Simplex 1 und 2) were negative (clinical data of the control cohort are summarized in Table 2b).
Table 2a.
Summary of relevant clinical of the control cohort (COVID-19 negative with cardiovascular diseases).
| PATIENT | AGE (years) | GENDER | MAIN COMORBIDITIES | CAUSE OF DEATHS |
|---|---|---|---|---|
| A | 82 | Male | Diabetes mellitus type 2, arterial hypertension, dementia | Aspiration-pneumonia |
| B | 62 | Male | Hypertension, prostate hyperplasia | Acute myocardial infarction |
| C | 77 | Female | Arterial hypertension, diabetes mellitus type 2, obesity (BMI 31.5 kg/m2) | Acute myocardial infarction |
Table 2b.
Clinical characteristics of Influenza A-positive autopsy cohort.
| PATIENT | AGE (years) | GENDER | MAIN COMORBIDITIES | CAUSE OF DEATHS |
|---|---|---|---|---|
| Case D | 64 | Female | Trisomy 21, bilateral pneumonia by influenza A. | Respiratory insufficiency by severe pneumonia. |
| Case E | 53 | Male | Bilateral Pneumonia by influenza A infection and superinfection with Aspergillus, Diabetes mellitus type 2, DLBCL (16 years before death). | Respiratory insufficiency by DAD/ARDS. |
| Case F | 84 | Female | Pneumonia by influenza A, Diabetes mellitus type 2, arterial hypertension, chronic cerebrovascular disease. | Respiratory insufficiency by severe pneumonia. |
| Case G | 73 | Male | Bilateral pneumonia with ARDS, clinically highly suspicious for viral pneumonia (although tests of most common respiratory viruses were negative), arterial hypertension, coronary artery disease. | Respiratory insufficiency by DAD/ARDS. |
| Case H | 22 | Female | Bilateral necrotic pneumonia by influenza A, massive necrosis of the liver of ischemic genesis. | Multiorgan insufficiency. |
| Case I | 85 | Female | Pneumonia by influenza A, arterial hypertension, permanent atrial fibrillation. | Respiratory insufficiency by severe pneumonia. |
Abbreviations: DLBCL diffuse large B-cell lymphoma, ARDS acute respiratory distress syndrome, DAD diffuse alveolar damage.
Consent to perform the autopsy was given in all control cases.
2.3. Sample selection and immunohistochemistry
Two or more representative sites of the major coronary arteries as well as the surrounding epicardial soft tissue were embedded in paraffin after formalin fixation (FFPE) for further histology in all COVID-19 and all control patients and processed for routine histology such as hematoxylin-eosin (H&E), elastin van Gieson (EVG) and immunohistochemical stains.
All immunohistochemical stains were performed on FFPE tissues in the Section of Special Technics of the Institute of Pathology and Molecular Pathology, University Hospital Zurich, with following clones and pretreatment modalities using the automated Ventana Benchmark stain system.
Representative slides from each case of the COVID-19-series and of the control series were stained for CD3 (common T cell marker, dilution 1:500, Leica, Order Nr. NCL-l-CD3–565), CD20 (common B cell marker, prediluted. Ventana / Roche, Order Nr 760–2531), CD45 (Leucocytes Common Antigen, dilution, 1:250, DAKO, Order Nr M 0701), Myeloperoxidase (MPO, a marker for neutrophil granulocytes, dilution 1:200, NeoMarkers, Order Nr RB-373-A1), CD68 (macrophages marker, dilution 1:50, DAKO, Order Nr M0876P), CD4 (marker of T-helper cells and of macrophages, prediluted, Ventana / Roche, Order Nr 790–4423), CD8 (cytotoxic T cell marker, dilution 1:100, DAKO, Order Nr M7103), Cleaved Caspase-3 (apoptosis marker, prediluted, Cell Signaling, Order Nr #9661,) and ACE-2 receptor (Angiotensin Converting Enzyme 2, entry point of SARS-CoV-2, dilution 1:500, Abcam Limited, Order Nr ab239924), CD31 (vessel endothelial marker, dilution 1:10, DAKO, Order Nr M0823), TMPRSS2 (CLINE EPR3861 Abcam, dilution 1:2000), CD147/EMMPRIN (BSG/963, ScyTek Laboratories/RA0428-C, dilution 1:100), IL-6 (10C12, Leica/ NCL-L-IL6, dilution: 1:50).
Additional stainings for TMPRSS2 (transmembrane protease serine 2), CD147 (extracellular matrix metalloproteinase inducer, EMMPRIN) and IL-6 (interleukin-6) were performed on all cases.
Each immunohistochemistry stain was then evaluated as negative (-), mild focally positive (+), moderately positive (++) and strongly positive (+++) independently by two pathologists (U.M. and Z.V), using a semi-quantitative visual scoring method without knowing the status of infection (Table 3).
Table 3.
Semi-quantitative method of evaluation of the immunohistochemistry of inflammatory infiltrates in epicardial tissues.
| Negative (-) | After careful examination even at greatest magnification (40x) no or just minimally, focal evidence of inflammatory cells. |
| Mild focally positive (+) | Not immediately recognizable inflammatory infiltrates, need to actively search at great magnification (20–40x) or just focal infiltrates. |
| Moderately positive (++) | Almost immediately recognizable inflammatory infiltrates already at middle magnification (10–20x), not just focal or circumscribed but involving several contiguous fields. |
| Strongly positive (+++) | Immediately recognizable, clear and diffuse inflammatory infiltrates. |
Statistical analysis in the small case number of he cohorts was not applied.
2.4. Quantitative RT-PCR analysis on paraffin embedded tissue
FFPE tissue from lung (Patient Nr. 2) and from myocardial tissue (patient Nr 1) underwent PCR based detection of SARS-CoV-2 RNA testing (Pathology, Cantonal Hospital Liestal, Switzerland), using probes for the SARS-CoV-2 ORFab1, SARS-CoV-2 S Gene, SARS-CoV-2 N Gene and a control positive human RNaseP Gene (all reactions were positive with a value Ct<40).
Lung sample: PCR auf SARS-CoV-2 ORFab1: Positive, Ct: 35.127., PCR on SARS-CoV-2 S Gene: Positive, Ct: 32.878. PCR on SARS-CoV-2 N Gene: Positive, Ct: 33.594., Internal control (PCR on humane RNasePGene): Positive, Ct: 12.7.
Myocardial sample (PCR on SARS-COV-2 ORFab1: Positive. Ct: 35.5, PCR on SARS-COV-2 S Gene: Positive. Ct: 34.3, PCR on SARS-COV-2 N Gene: Positive. Ct: 35.1, Internal control (PCR on humane RNaseP Gene): Positive. Ct: 14.6.
2.5. RNA in-situ hybridization on paraffin embedded tissue
In situ hybridization (ISH) for SARS CoV-2 was carried out using a commercially available probe specific for the viral nucleocapsid gene according the manufacturers instructions. RNAscope 2.5 LS Probe-V-nCoV2019-S and RNAscope 2.5 LS Reagent Kit-BROWN; Advanced Cell Diagnostic [ACD]) on an automated BondRx platform (Leica Biosystems). In addition, a human RNA control was applied (in-situ hybridization assays were performed in the Pathology, Cantonal Hospital Liestal, Switzerland).
2.6. Detection of spike glycoprotein and neurocapsid protein of SARS-CoV-2 using immunofluorescence
Immunofluorescence (IF) labeling of the spike (S) glycoprotein and nucleocapsid protein (NP) of SARS-CoV-2 was performed on formalin-fixed paraffin embedded (FFPE) myocardial tissue (which was positive with qRT-PCR) using following clones and antibodies (all purchased from www.sinobiological.com). We adapted a previously described procedure for FFPE tissue [30] and used the following antibodies:
-
1)
Polyclonal rabbit anti-S antibody, Cat#40150-T62-COV2, Dilution at 1/2000, Pretreatment Ultra CC1 er, visualized with anti-FITC fluorescent labeling,
-
2)
Monoclonal rabbit anti-NP antibody (labelled with orange tape), Cat# 40143-R001, Dilution at 1/5000, Pretreatment Ultra CC1 er, visualized with anti-FITC fluorescent labeling,
-
3)
Monoclonal mouse anti-NP antibody (labelled with purple tape) Cat#40143-MM05. Dilution at 1/8000, Pretreatment Ultra CC1 mild, visualized with goat anti-mouse Alexa Fluor 488 fluorescent labeling.
2.7. Role of funding source
No external funding was necessary for this study. All experiments were covered by the Internal University Research Funds of the Department of Pathology and Molecular Pathology University Hospital Zurich Switzerland, which contributed to study design, data analyses, interpretation and paper drafting.
3. Results
3.1. Inflammatory infiltration in COVID-19-positive and COVID-19-negative patients
A total of six SARS-CoV-2-positive patients (four males and two females), three control patients with cardiovascular disease (two males and one female) and six influenza A/viral pneumonia patients (four females and two males) were analyzed. Characteristics are summarized in Table 4 (COVID-19-positive patients), Table 5 (COVID-19-negative patients with cardiovascular diseases), Table 6 (Influenza A cohort) and graphically illustrated in Fig. 1.
Table 4.
COVID-19 positive cohort: Immunohistological findings in endothelial cells (ACE2) and endothelial inflammatory cells (CD68, CD45, CD4, CD3, CD8, MPO, Caspase 3) (- negative, + mild positive, ++ moderate positive, +++ strong positive).
| CORONARY | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
|---|---|---|---|---|---|---|
| ACE2 | – | – | – | – | – | – |
| CD68 | +++ | + | ++ | ++ | ++ | ++ |
| CD45 | ++ | ++ | ++ | ++ | ++ | + |
| CD4 | ++ | ++ | ++ | ++ | ++ | ++ |
| CD3 | ++ | – | + | + | + | – |
| CD8 | + | – | + | + | + | – |
| MPO | – | – | – | – | – | – |
| Caspase 3 | – | – | – | – | – | – |
| ARTERIOLES/VENULES | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
| ACE2 | + | + | – | + | + | + |
| CD68 | + | + | + | + | + | + |
| CD45 | + | + | + | + | + | + |
| CD4 | + | + | + | ++ | ++ | + |
| CD3 | – | + | – | – | – | – |
| CD8 | – | + | – | – | – | – |
| MPO | – | – | – | – | – | – |
| Caspase 3 | – | – | – | – | – | – |
| CAPILLARIES | Case1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
| ACE2 | ++ | ++ | ++ | +++ | ++ | +++ |
| CD68 | +++ | ++ | ++ | +++ | ++ | +++ |
| CD45 | ++ | + | ++ | +++ | ++ | ++ |
| CD4 | ++ | + | + | +++ | +++ | ++ |
| CD3 | – | – | – | – | – | + |
| CD8 | – | – | – | – | – | – |
| MPO | – | – | – | – | – | – |
| Caspase 3 | – | – | – | – | – | – |
| EPICARDIAL NERVES | Case1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
| ACE2 | + | + | ++ | ++ | + | ++ |
| CD68 | ++ | ++ | + | ++ | + | ++ |
| CD45 | ++ | + | + | ++ | + | + |
| CD4 | + | + | + | +++ | ++ | + |
| CD3 | – | – | – | – | – | – |
| CD8 | – | – | – | – | – | – |
| MPO | – | – | – | – | – | – |
| Caspase 3 | – | – | – | – | – | – |
Table 5.
COVID19-negative cohort with cardiovascular diseases: Immunohistological findings in endothelial cells (ACE2) and endothelial inflammatory cells (CD68, CD45, CD4, CD3, CD8, MPO, Caspse 3) (- negative, + mild positive, ++ moderate positive).
| CORONARY | Case A | Case B | Case C |
|---|---|---|---|
| ACE2 | – | – | – |
| CD68 | ++ | ++ | + |
| CD45 | ++ | ++ | + |
| CD4 | ++ | ++ | + |
| CD3 | – | ++ | – |
| CD8 | – | ++ | – |
| MPO | – | – | – |
| Caspase 3 | – | – | – |
| ARTERIOLES/VENULES | Case A | Case B | Case C |
| ACE2 | – | + | – |
| CD68 | – | – | – |
| CD45 | – | – | – |
| CD4 | – | – | – |
| CD3 | – | – | – |
| CD8 | – | – | – |
| MPO | – | – | – |
| Caspase 3 | – | – | – |
| CAPILLARIES | Case A | Case B | Case C |
| ACE2 | + | ++ | + |
| CD68 | – | – | – |
| CD45 | – | – | – |
| CD4 | – | – | – |
| CD3 | – | – | – |
| CD8 | – | – | – |
| MPO | – | – | – |
| Caspase 3 | – | – | – |
| EPICARDIAL NERVES | Case A | Case B | Case C |
| ACE2 | + | + | + |
| CD68 | – | + | – |
| CD45 | – | + | – |
| CD4 | – | + | – |
| CD3 | – | – | – |
| CD8 | – | – | – |
| MPO | – | – | – |
| Caspase 3 | – | – | – |
Table 6.
Influenza A cohort and immunohistological findings in endothelial cells. Immunohistological findings in endothelial cells (ACE2) and endothelial inflammatory cells (CD68, CD45, CD4, CD3, CD8, MPO, Caspase 3) (- negative, + mild positive, ++ moderate positive, +++ strong positive).
| CORONARY | Case D | Case E | Case F | Case G | Case H | Case I |
|---|---|---|---|---|---|---|
| ACE2 | – | – | – | NA | – | – |
| CD68 | + | +++ | + | NA | – | – |
| CD45 | ++ | ++ | + | NA | – | – |
| CD4 | ++ | ++ | + | NA | + | – |
| CD3 | – | + | – | NA | – | – |
| CD8 | – | – | – | NA | – | – |
| MPO | – | – | – | NA | – | – |
| Caspase 3 | – | – | – | NA | – | – |
| ARTERIOLES/VENULES | Case D | Case E | Case F | Case G | Case H | Case I |
| ACE2 | + | + | + | – | – | – |
| CD68 | – | – | – | – | – | – |
| CD45 | + | – | + | – | – | – |
| CD4 | – | + | – | – | + | – |
| CD3 | – | – | – | – | – | – |
| CD8 | – | – | – | – | – | – |
| MPO | – | – | – | – | – | – |
| Caspase 3 | – | – | – | NA | – | – |
| CAPILLARIES | Case D | Case E | Case F | Case G | Case H | Case I |
| ACE2 | + | ++ | + | + | ++ | + |
| CD68 | – | + | – | – | – | + |
| CD45 | – | – | – | + | – | + |
| CD4 | + | + | + | + | + | + |
| CD3 | – | – | – | – | – | – |
| CD8 | – | – | – | – | – | – |
| MPO | – | – | – | – | – | – |
| Caspase 3 | – | – | – | NA | – | – |
| EPICARDIAL NERVES | Case D | Case E | Case F | Case G | Case H | Case I |
| ACE2 | – | + | + | + | – | + |
| CD68 | – | + | – | – | – | – |
| CD45 | – | – | – | – | – | – |
| CD4 | + | + | – | – | – | – |
| CD3 | – | – | – | – | – | – |
| CD8 | – | – | – | – | – | – |
| MPO | – | – | – | – | – | – |
| Caspase 3 | – | – | – | NA | – | – |
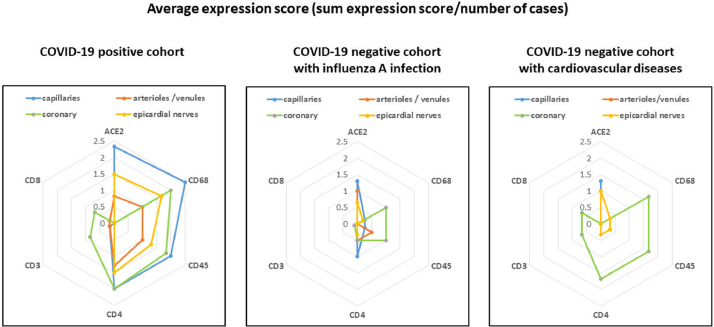
Fig. 1.
Summary of immunohistological findings (ACE2, CD68, CD45, CD4, CD3, CD8) in the coronary tree and epicardial nerves in COVID-19 positive and COVID-19 negative control patients. The COVID-19 negative control groups were selected as influenza A positive and a second cohort with cardiovascular diseases.
Major coronary arteries: All COVID-19 positive and negative patients showed atherosclerotic changes in different stages (fibrosis of intima, intimal xanthoma, fibroatheroma or calcified nodule) in the major coronary arteries. Both COVID-19 positive and negative patient tissue samples exhibited mild to moderate lympho-monocytic inflammatory infiltrates, demonstrated by immunohistochemical stains for CD45+, CD68+ and CD4+ cells. No relevant differences between COVID-19-positive and COVID-19-negative patients were found in the inflammation intensity and immunophenotype of inflammatory cells in the main coronary arteries (Fig. 2a, 2b, Fig. 3a, 3b, Fig. 4a, 4b). Of note, we did not find any typical Kawasaki-like vasculitis mediated histological changes such as fibrinoid necrosis and prominent lymphocytic inflammation. Moreover, no aneurysms were seen.
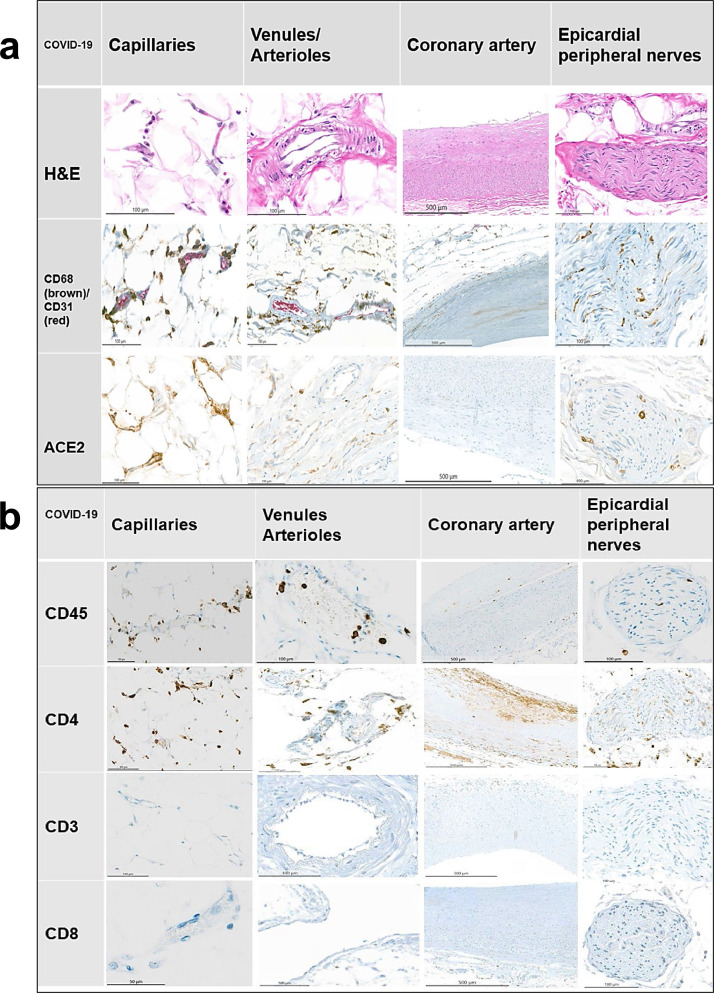
Fig. 2.
Fig. 2a and 2b: Representative immunostains and H&E sections from COVID-19-positive patients. An endotheliitis of small vessels and a neuritis with prevalence of CD4-positive and CD68-positive inflammatory cells are demonstrated along with a strong expression of ACE2 receptor. The red (CD31) stains show endothelial cell, the brown reaction products (all other stains) mark positively stained inflammatory cells. Relevant immunostains (CD68, CD45, CD68, ACE2) show a trend being the highest in the capillaries and venules and less pronounced in the main coronary arteries in a semi-quantitative analysis.
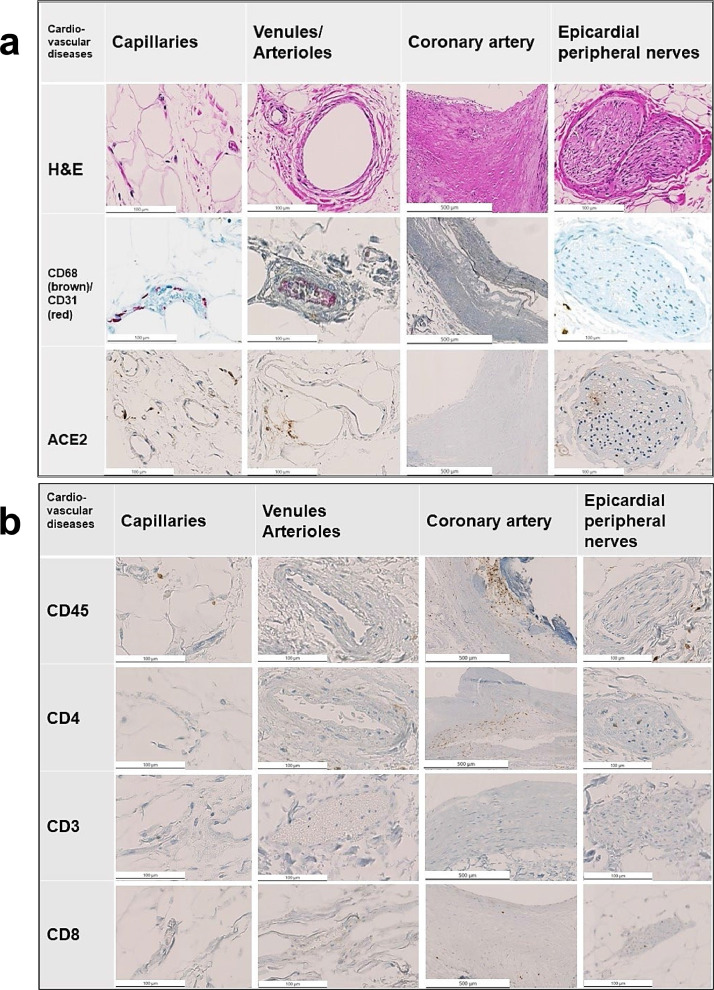
Fig. 3.
Fig. 3a and 3b: Representative immunostains and H&E sections from COVID-19-negative control patients with cardiovascular disease. Note the scattered presence of inflammatory cells in the control cohort.
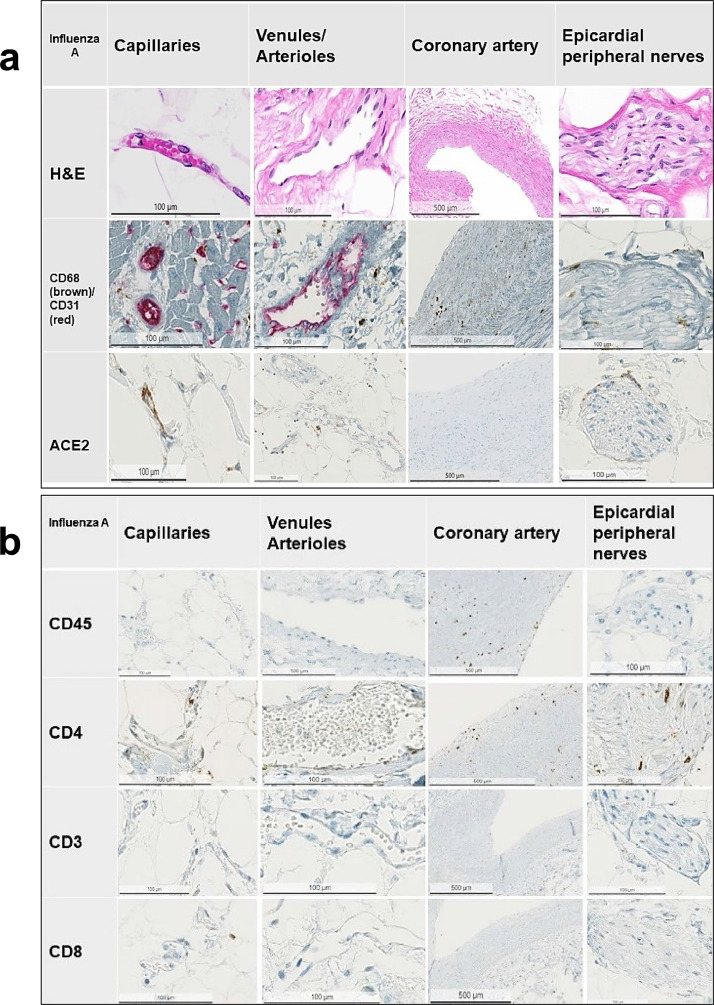
Fig. 4.
Fig. 4a and 4b: Representative immunostains and H&E sections from COVID-19-negative control patients with influenza A / viral pneumonia infection. Note the scattered presence of inflammatory cells in the control cohort.
Arterioles and venules: The arterioles and venules of the COVID-19-positive patients showed mild to moderate inflammatory infiltrates rich in lympho-monocytic cells (Fig. 2a, 2b). In contrast, COVID-19-negative patients (both patients with cardiovascular disease and influenza A-positive patients) exhibited no or only a minimal inflammatory reaction of lympho-monocytic cells (Fig. 3a, 3b, Fig 4a, 4b).
Epicardial capillaries: The epicardial capillaries of the COVID-19-positive patients displayed already on HE-stained sections a mild to moderate lympho-monocytic inflammation with a perivascular and/or a subendothelial distribution in five of six cases, while one of the cases showed presence of less inflammatory infiltrates (Fig. 2a, 2b and Fig 5). All COVID-19-negative patients (both patients with cardiovascular disease and influenza A-positive control groups) showed virtually no or only very few inflammatory cells in the epicardial capillaries (Fig. 3a, 3b, Fig 4a, 4b). These HE-findings were corroborated immunohistochemically: the arterioles/venules as well as the small capillaries in COVID-19-positive patients (Fig. 2a, 2b and Fig. 5) showed clearly moderate infiltrates, composed mainly of CD45+, CD68+ and CD4+ cells, whereas the control-cases showed only a very mild or none inflammatory change (Fig. 3a, 3b, 4a, 4b).
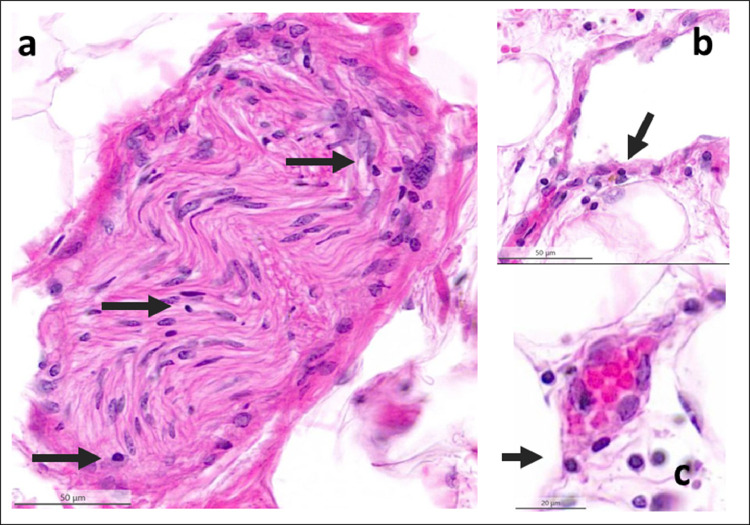
Fig. 5.
Lymphocytic inflammation (arrows) of epicardial nerve (a), venule (b) and capillary (c) from a COVID-19-positive patient. Lympho-monocytic cells infiltrate and damaged endothelia of small vessels (endotheliitis). Nerves are infiltrated too, not only in vasa nervorum but also in endonevrium and perineurium. H&E sections.
ACE2 receptors: The endothelium of the major coronary arteries of both COVID-19-positive and negative patients showed virtually negative ACE2 receptor staining. In contrast, five of the COVID-19-positive cases exhibited a mild ACE2 positivity in the venules and arterioles. Only four of nine COVID-19-negative control patients had mild ACE2 expression in the venules / arterioles.
ACE2 receptors were diffusely and strongly expressed in the capillaries and epicardial nerves of all COVID-19-positive cases and moderately in 3 of 9 COVID-19-negative control patients.
TMPRSS2: The endothelium of the major coronary arteries of both COVID-19-positive and negative patients showed a mild positive staining in five of six COVID-19 patients and negative in one patient. In COVID-19 negative patients with cardiovascular diseases just one was positive and two were negative, and in Influenza A patients we found a moderately positive staining in two patients, a mild positivity in one patient, whereas two were negative and of one no tissue from major coronary artery was available. In arterioles, venules, capillaries as well as in epicardial nerves, both COVID-19 positive patients and negative patients showed positive reaction ranging from mild to strong, without noteworthy differences among the three cohorts (Supplementary Table 1, and Supplementary Figures 1,2,3,4).
CD147: There were slightly more positive CD147 inflammatory cells in the capillaries of COVID-19 patients, however no relevant expression was find in the control the samples. (Supplementary Table 1, and Supplementary Figures 1,2,3,4).
Interleukin 6 (IL-6): COVID-19 positive patients had more IL-6 positive inflammatory cells especially in the capillaries than control influenza A and control cardiovascular patients (Supplementary Table 1, and Supplementary Figures 1,2,3,4).
COVID-19-positive and negative cases all had a very low respectively absent expression of CD3, CD8, CD20, myeloperoxidase and Caspase-3 (Supplementary Table 1, and Supplementary Figures 2,3,4).
Intramyocardial vessels: Intramyocardial small vessels of all COVID-19 autopsies showed some degree of endotheliitis too, ranging from mild to moderate intensity, which exhibited the same morphological and immunohistochemical pattern observed in small epicardial vessels. Here too, a moderate to strong positivity for ACE2 receptor and of TMPRSS2 in endothelial cells of small vessels as well as in myocardiocytes could be demonstrated in all cases (Fig. 6).
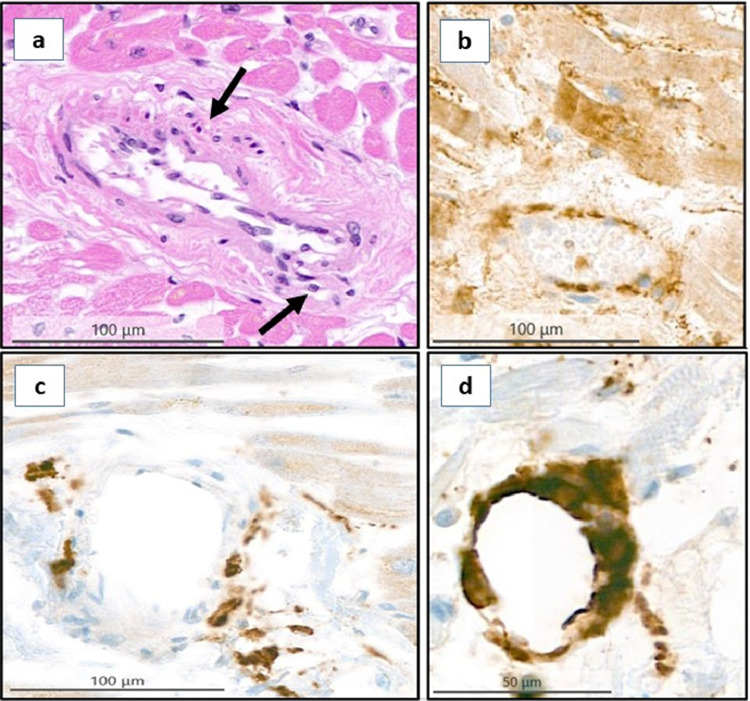
Fig. 6.
Intramyocardial small vessels in a COVID-19 patient. a) Small vessel endotheliitis with endothelial swelling, denudation, subendothelial lymphocytes and macrophages (arrows). b) ACE2 receptor immunohistochemistry. c) CD4 stain. d) CD68 stain.
3.2. RNA in-situ hybridization on paraffin embedded tissue
Myocardial sample: No signal was detected neither by antisense nor by sense probe. Cellular RNA control showed a positive signal.
3.3. Immunfluorescence labelled immunohistochemistry assays
Immunofluorescence labeled immunohistochemistry demonstrated scattered reaction products labeling the spikes (S) and the neurocapsid (NP) components of SARS-CoV-2 in endothelial cells of a myocardial sample, which was positive in qRT-PCR in FFPE tissue, corresponding to the indirect viral evidence within the endothelial cells (Supplementary Fig 5).
4. Discussion
In this study we could demonstrate a crescentic distribution of a lympho-monocytic inflammatory reaction within the different anatomical regions of the coronary tree in COVID-19 positive patients, which was strikingly different from the COVID-19 negative controls including Influenza A infections. Specifically we showed that the vascular inflammatory process in COVID-19 spared the major coronary arteries and exclusively induced a cardiac small vessel vasculitis affecting only the cardiac capillaries, arterioles and venules. Additionally we demonstrated a high endothelial ACE2 receptor expression only in small epicardial vessels/capillaries and in epicardial nerves of COVID-19 positive individuals, whereas there was no or only very weak ACE2 receptor expression in major coronary arteries. The endothelia of vasa nervorum and many neural cells and fibroblasts located in the epineurium were also positive for ACE2. Thus, our findings further confirm that COVID-19 disease presents with a different immunomodulation observed in other RNA viral infections, such as Influenza A infections as well as in COVID-19 negative cardiovascular disease [31].
We have recently demonstrated systemic endothelial inflammation in COVID-19 patients [7]. In vitro studies of cell lines as well as immunhistochemichal and electron microscopy analyses of human renal tissues suggested the presence of SARS-CoV-2 within endothelial cells [7,9,25,32]. Endothelial cell infection with consecutive inflammatory cell recruitment and endothelial dysfunction may explain impaired microcirculation observed across vascular beds with vasoconstriction, ischemia and a pro-coagulant state in COVID-19. Therefore, systemic endotheliitis has been suggested as the major cause of systemic impaired microcirculatory function observed in different vascular beds in patients with COVID-19 [7,9,25,32].
Here, we demonstrate high ACE2 expression in endothelial cells of capillaries and epicardial nerves, suggesting that these structures may be targeted by SARS-CoV-2 due to their enhanced expression of ACE2 receptor. As a consequence, mainly capillaries and epicardial nerves develop cardiac inflammatory microangiopathy and neuropathy [33,34]. In one of the myocardial samples, SARS-Cov-2 RNA was detected with qRT-PCR and we demonstrated most likely indirect viral effect in scattered endothelial cells on consecutive serial sections with immunofluorescence labelled immunohistochemistry, further supporting the presence of viral induced endothelial damage. Recently, ACE2 was established as the functional host receptor for SARSCoV-2 [35], but the role of ACE2 in SARS‐CoV‐2 cellular infection is complex [36] and some studies suggested down-regulation of ACE2-receptors after virus entry into cells [14, 15, 16,35, 36, 37, 38, 39]. Importantly, there was virtually no ACE2-receptor expression in major coronary arteries of both, COVID-19 positive and negative patients and only weak ACE2-receptor expression in arterioles/venules of COVID-19 positive patients. Based on our findings, one might hypothesize that SARS-CoV-2 induces a small vessel endotheliitis in the heart, because ACE2 receptors exhibit a heterogeneous distribution increasing toward the small cardiac vessels. Higher ACE expression in cardiac endothelial cells of small vessels combined with endothelial inflammation may support the central role of endothelial cells in COVID-19 disease with poor outcome [40] and could represent one mechanism of myocardial injury, for which a microangiopathy has already been postulated [41]. Caution is needed in interpreting differential vascular ACE expression between COVID-19-positive and COVID-19-negative cases. Pre-analytic factors such as the time between patients’ death, time to tissue sampling and to formalin fixation can impact the sensitivity for immunohistochemical stain for ACE2-receptor. In addition, some very common systemic disorders (such as diabetes mellitus and hypertension among others), drugs, age, gender, diet, smoking and ethnicity are known to influence ACE2 and consequently ACE2-receptor expression [37,38].
To our knowledge, we describe for the first time an inflammatory neuropathy of the epicardial nerves. This finding could explain reports of cardiac arrhythmic complications in COVID-19-positive patients [42]. A study on 138 hospitalized patients from Wuhan, China, reported an incidence of arrhythmic events, including both tachyarrhythmia and bradyarrhythmia, of 16.7% overall, of which 44.4% occurred in severe illness and 8.9% in mild cases In our series of COVID-19 patients, five of the six patients showed cardiac arrhythmias, such as sinus tachycardia, repolarisation abnormalities, ventricular extrasystoles and paroxysmal atrial fibrillation.
To date, no studies on the inflammatory changes and ACE2-receptor expression in epicardial nerves in COVID-19-infection have been published, although the involvement of central and peripheral nervous system [11,43] and the neuro‐invasive potential of coronaviruses (CoVs) has been well documented for most of the CoVs including SARS‐CoV-1 and MERS‐CoV [44]. It is currently unclear whether the observed cardiac inflammatory neuropathy is a result of direct viral damage or mediated by a „cytokine storm“. Thus, it is to hypothesize that SARS-CoV-2 targets epicardial nerves through their elevated ACE2-receptor expression, leading to a cardiac inflammatory neuropathy, which may result in arrhythmic complications.
Of note, the histology of the inflammation was not that of a lymphocytic or eosinophilic myocarditis, and cardiac myocytes were not the target of the inflammatory process. In major coronary arteries with atherosclerosis, we observed only mild inflammation [45] suggesting that the coronary arteries might not be initially targeted by the SARS-CoV-2 and the inflammation observed in the major coronary vessels are rather considered as minor reactive changes due to atherosclerosis. These results could at least partially explain why the hospitalization for acute coronary syndromes did not increase during the COVID-19 pandemic 46, 47, 48, 49. In our series, all COVID-19-positive patients had some form of myocardial damage ranging from moderately increased T-troponin without clinical signs to histologically demonstrable myocardial ischemia (Table 1). These findings are in concordance with a retrospective study on 112 COVID-19 patients from Wuhan, which reports increased level of troponin during hospitalization in 42 patients (37.5%) but did not demonstrate typical signs of myocarditis on echocardiography and electrocardiogram [50]. Moreover, our results support the findings of the above mentioned international prospective observational cohort study in 639 patients with severe SARS-CoV-2 infection admitted to intensive care units of whom 97 did not survive and among whom the troponin level was moderately increased, showing a statistical significant difference between the survivors group [5]. Myocardial damage is known to contribute to cardiac dysfunction and arrhythmia and associated with significantly increased mortality in COVID-19-positive patients [5,51,52]. However, the pathogenesis of myocardial damage in COVID-19 is not fully understood [53]. Some patients present with clinical signs of a myocardial infarction, despite coronary angiography visualized no occlusion of major coronary arteries. Heart MRI analyses rather suggest a peripheral capillary injury, which is consistent with our findings of a microangiopathy due to a small vessel vasculitis in COVID-19 [54]. In addition to endothelial damage, multiple mechanisms appear to contribute, individually or in conjunction with one another, and they include respiratory failure induced hypoxia, inflammatory cytokine storms, direct viral infiltration and subsequent myocyte death, and myocardial dysfunction from acute illness [55].
The pathogenetic mechanism in COVID-19 disease is complex and involves also the activation of several pro-inflammatory factors such as IL-1, Il-6 [13,56,57]. In addition to ACE2, further receptors such as TMPRSS2, cathepsins, furin and CD147 (EMMPRIN) play a role in the initiation and progression of SARS CoV2 infection [13,56]. CD147 has been shown to be closely involved in cardial inflammation [13,56]. Blocking CD147 can inhibit SARS-Cov-2 replication and thus invasion of host cells [13,56]. We tested our study cohort for these factors and could show an increased TMPRSS2 expression in COVID-19 small cardiac vessels, although the influenza A and cardiovascular control cases exhibited a similar tendency as well.
CD147 and IL-6 both had a tendency to be more expressed in inflammatory cells of COVID-19 patients especially in the capillaries, although the small proportion of positively stained inflammatory cells most likely do not justify any conclusions in terms of inflammasome activation with cardiac involvement as described recently [58].
Direct endothelial viral damage has been documented in several previous studies using in vivo or in vitro samples [59,60]. Vascular organoids infected with SARS-CoV-2 were described earlier this year and the recently published study by Meinhardt et al. demonstrates endotheliitis and SARS-CoV S protein in the endothelial cells of small CNS vessels [59,60].
Discrepant viral evidence results between qRT-PCR, ISH and IF/IHC technologies have been the subject of several recent studies 60, 61, 62, 63. The presence of current viral load, the phase of disease course as well as the severity of COVID-19 all have impact on the detection rate of viral RNA or viral protein in a given sample 60, 61, 62, 63.
We showed evidence of SARS-CoV-2 RNA using qRT-PCR in a FFPT myocardial tissue although the exact anatomical location of viral RNA evidence could not be proven with ISH methodology, implying an indirect endothelial damage possibly triggered and enhanced by the inflammatory cellular reaction rather than the virus itself. On the other hand, IF labelled reaction products similar as recently described in central nervous vascular endothelium using the same methodology with qRT-PCR and IF labeling on FFPE autopsy tissue as in our cohort, further support a direct endothelial damage though the SARS-CoV-2 virus [60,64].
Discrepant SARS-CoV-2 RNA and protein detection can be further influenced by low level of RNA as was described by Sekulic et al., with similar CT values at qRT-PCR as it was the case in our myocardial sample. [64]. Discrepancies between qRT-PCR and ISH RNA results (ISH negative and qRT-PCR positive pulmonary samples) were demonstrated on FFPE tissue in a large autopsy study by Massoth et al. [64]. Another recent study by Ko et al. demonstrated protein spikes fragments in endothelial cells of cutaneous lesions lacking RNA evidence in ISH suggesting that endothelial incorporated spikes elements rather than the presence of virions may play the pathogenic role in COVID-19 endotheliitis [65]
A multisystem inflammatory syndrome similar to Kawasaki disease has been increasingly reported in association with COVID-19 in children and young adults. This syndrome is rare in adults with just 81 cases reported between 2010 and 2015 [66], known to potentially affect the main coronary arteries [67], and has been recently reported in children in association with COVID-19-infection 68, 69, 70. One study from Bergamo, Italy, reported a 30-fold increased incidence in pediatric population during the pandemic [70,71]. We did not find typical changes of Kawasaki-disease in the main coronary arteries in our adult patients. To date, there are only two cases reported in adults in association with COVID-19-infection [72,73] and further studies are needed to clarify the association between COVID-19 and Kawasaki-disease.
Interestingly, the inflammatory infiltrates of the small vessel cardiac vasculitis in our cohort was composed of macrophages and dominant CD4+ (to less extent CD8+) T-cells, as was also described in a very recent case report [32]. This phenotype is very similar to those seen in a moderate antibody-mediated transplant rejection [75]. We identified endothelial damage in intramyocardial small vessels associated with inflammatory infiltrates of lymphocytes and macrophages without interstitial hemorrhage, capillary fragmentation, edema or pyknosis of surrounding myocardial cells. These findings formally correspond to pAMR2 (pathology Antibody Mediated Reaction grade 2) according to the nomenclature of 2013 ISHLT (International Society for Heart and Lung transplantation) [76]. This inflammation pattern is different from acute mediated cardiac rejection, which is characterized by perivascular and/or interstitial infiltrates, consisting predominantly of CD3, CD4 and CD8-positive lymphocytes or neutrophils in severe cases but not of macrophages [76]. The prevalence of macrophages and CD4-positive T-lymphocytes without involvement of CD8-positive T-lymphocytes suggests that COVID-19 disease and antibody-mediated transplant rejection could share some pathogenic mechanisms, possibly due to inflammatory mediated endothelial damage through a cytokine-storm rather than a direct viral damage. In particular, these morphological observations lend further support to the hypothesis that complement activation could mediate endotheliitis by recruitment of macrophages.
In conclusion, our data provide evidence that endotheliitis of small epicardial and intramyocardial vessels poses an important pathogenetic mechanism of myocardial injury in COVID-19 disease. Cardiac inflammatory neuropathy with identical immunophenotype as detected in cardiac vessels may contribute to arrhythmias in COVID-19-patients. The crescentic expansion of inflammatory reaction towards the small cardiac vessels could well explain the increased troponin levels with normal epicardial coronary arteries in the absence of acute coronary syndrome in patients with COVID-19.
Data sharing statement
Digital links to all study relevant immunohistochemical stains (such as ACE2, CD4, TMPRSS) are available upon request through the IT section of the Department of Pathology and Molecular Pathology. Individual participant data that underlie the results reported in this article will only be available after de-identification upon justified request addressed to the corresponding author.
Contributors
All authors read and approved the final version of the manuscript.
U.M. Designed the study, collected samples, analyzed immunohistochemical and morphological data, drafted the paper.
ASZ. Designed the study, interpreted clinical information and correlation, drafted the paper.
SMS. Interpreted clinical information and correlation, drafted the paper
XZ: provided antibodies for fluorescence labelled immunohistochemistry, interpreted results on immunohistochemistry and drafted the paper.
GC: performed RNA analyses with qRT-PCR and in-situ hybridization
FR: Interpreted clinical information and correlation, drafted the paper
RAS: Designed the study, interpreted clinical information and correlation, drafted the paper.
HM. Designed the study, interpreted clinical and pathological data, drafted the paper.
ZV: Designed the study, analyzed immunhistochemical and morphological data, interpreted clinical and pathological data, drafted the paper.
Declaration of Competing Interests
The authors have no financial or personal interests to declare. All authors had access to the study date and take responsibility for the accuracy of the analysis, contributed to the manuscript preparation and to the decision to submit the manuscript for publication. All authors approved the manuscript and agree to adhere to all terms outlined in EBioMedicine.
Acknowledgments
Experiments were covered by the Internal University Research Funds of the Department of Pathology and Molecular Pathology, University Hospital Zurich Switzerland.
The authors thank to following contributions on the study:
The Laboratory of Special Technics of the Department of Pathology and Molecular Pathology, University Hospital Zurich Switzerland, especially to Mr André Fitsche and to Mrs Christine Mittmann for testing the additional antibodies and performing the complete immunohistochemichal experiments for the study, and also toMrs Daniela Meir, Mrs Annabelle Marks and to Mr Fabian Baron for excellent technical assistance in the postmortal analyses.
The Swiss Reference centre for Infectious diseases in the Pathology, Cantonal Hospital Liestal Switzerland for performing the qRT-PCR SARS-COV2 analysis on selected paraffin embedded tissues.
To all patients’ families who approved performing the autopsies.
Footnotes
Homepage. http://www.klinische-pathologie.usz.ch
Conflict of interest statement: There is no conflict of interest to disclose (neither financial nor non-financial).
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.103182.
Appendix. Supplementary materials
Supplementary Figure 1: Summary of immunohistological findings (MPO, CD147, IL-6, TMPRSS2, Caspase 3) in the coronary tree and epicardial nerves in COVID-19 positive and COVID-19 negative control patients. The COVID-19 negative control groups were selected as influenza A positive and a second cohort with cardiovascular diseases.
Supplementary Figure 2: Representative immunostains (MPO, Caspase 3,TMPRSS2, CD147, IL-6) from COVID-19 positive patients. There are increased IL-6 and CD147 positive inflammatory cells and a pronounced endothelial TMPRSS2 expression.
Supplementary Figure 3: Representative immunostains (MPO, Caspase 3,TMPRSS2, CD147, IL-6) from COVID-19-negative control patients with cardiovascular disease. Note the lack or only scattered presence of inflammatory cells and the pronounced TMPRSS2 expression in the control cohort.
Supplementary Figure 4: Representative immunostains (MPO, Caspase 3,TMPRSS2, CD147, IL-6) from COVID-19-negative control patients with influenza A / viral pneumonia infection. Note the lack or only scattered presence of inflammatory cells and the pronounced TMPRSS2 expression in the control cohort.
Supplementary Figure 5: Immunfluorescence labelled immunistochemistry for SARS-Cov2 viral components in small intramyocardial vessels. Upper row images: anti FITC labelled antibody against the S component. a: negative control without primary antibody, b/c: positive cases without (b) and with (c) amplification kit. Middle row images: anti FITC labelled antibody against the NP component. d: negative control without primary antibody, e/f: positive cases without (e) and with (f) amplification kit. Lower row images: Goat anti mouse Alexa Fluor 488 labelled antibody against the NP component. g: negative control without primary antibody, h/i: positive cases without (h) and with (i) amplification kit.
Supplementary Table 1. Additional markers. Distribution and intensity of TMPRSS2, CD147 and IL-6 in different anatomic locations in COVID-19 patients, patients from control cohort with cardiovascular diseases and control patients with Influenza A. Abbreviations: Cor. Coronary, A/V arterioles/venules, Cap. Capillaries, N nerves, NA not available.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A Novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Long B., Brady W.J., Koyfman A., Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38(7):1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedro David, Wendel Garcia T.F., Philippe Guerci, Dorothea Monika Heuberger, Jonathan Montomoli, Ferran Roche-Campo, Reto Andreas Schuepbach, Matthias Peter Hilty, RISC-19-ICU-Investigators Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: initial report of the international RISC-19-ICU prospective observational cohort. EClin Med. 2020 doi: 10.1016/j.eclinm.2020.100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y., Roever L., Tse G., Liu T. 2019-novel coronavirus-related acute cardiac injury cannot be ignored. Curr Atheroscler Rep. 2020;22(3):14. doi: 10.1007/s11883-020-00842-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirano T., Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52(5):731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20(7):389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian W., Jiang W., Yao J., Nicholson C.J., Li R.H., Sigurslid H.H. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirschenbaum D., Imbach L.L., Ulrich S., Rushing E.J., Keller E., Reimann R.R. Inflammatory olfactory neuropathy in two patients with COVID-19. Lancet. 2020;396(10245):166. doi: 10.1016/S0140-6736(20)31525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Passarelli P.C., Lopez M.A., Mastandrea Bonaviri G.N., Garcia-Godoy F., D'Addona A. Taste and smell as chemosensory dysfunctions in COVID-19 infection. Am J Dent. 2020;33(3):135–137. [PubMed] [Google Scholar]
- 13.Matusiak M., Schurch C.M. Expression of SARS-CoV-2 entry receptors in the respiratory tract of healthy individuals, smokers and asthmatics. Respir Res. 2020;21(1):252. doi: 10.1186/s12931-020-01521-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116(6):1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pohlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88(2):1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 19.Dong M., Zhang J., Ma X., Tan J., Chen L., Liu S. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchandot B., Sattler L., Jesel L., Matsushita K., Schini-Kerth V., Grunebaum L. COVID-19 related coagulopathy: a distinct entity? J Clin Med. 2020;9(6) doi: 10.3390/jcm9061651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noris M., Benigni A., Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020;98(2):314–322. doi: 10.1016/j.kint.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Yongwen Z.F., Diao Bo, Wang Rongshuai, Wang Gang, Wang Chenhui, Tan Yingjun, Liu Liang, Wang Changsong, Liu Ying, Liu Yueping, Yuan Zilin, Ren Liang, Wu Yuzhang. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. medRxiv. 2020 [Google Scholar]
- 25.Diao Bo C.W., Wang Rongshuai, Feng Zeqing, Tan Yingjun, Wang Huiming, Wang Changsong, Liu Liang, Liu Ying, Liu Yueping, Wang Gang, Yuan Zilin, Ren Liang, Wu Yuzhang, Chen Yongwen. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv. 2020 doi: 10.1038/s41467-021-22781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020 doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang D., Comish P., Kang R. The hallmarks of COVID-19 disease. PLoS Pathog. 2020;16(5) doi: 10.1371/journal.ppat.1008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 29.Meduri G.U., Headley S., Kohler G., Stentz F., Tolley E., Umberger R. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107(4):1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 30.Liu J., Babka A.M., Kearney B.J., Radoshitzky S.R., Kuhn J.H., Zeng X. Molecular detection of SARS-CoV-2 in formalin-fixed, paraffin-embedded specimens. JCI Insight. 2020;5(12) doi: 10.1172/jci.insight.139042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nina Maria Burkhard-Koren M.H., Maccio Umberto, Ruschitzka Frank, Schuepbach Reto A, Zinkernagel Annelies S, Hardmeier Thomas, Varga Zsuzsanna, Moch. Holger. Higher prevalence of pulmonary macrothrombi in SARS-CoV-2 than in influenza A: autopsy results from ‘Spanish flu’ 1918/1919 in Switzerland to Coronavirus disease 2019. J Pathol: Clin Res. 2020 doi: 10.1002/cjp2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fox S.E., Lameira F.S., Rinker E.B., Vander Heide R.S. Cardiac endotheliitis and multisystem inflammatory syndrome after COVID-19. Ann Intern Med. 2020 doi: 10.7326/L20-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonow R.O., Fonarow G.C., O'Gara P.T., Yancy C.W. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 34.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J Pathol. 2020 doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y., Zhou W., Yang L., You R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol Res. 2020;157 doi: 10.1016/j.phrs.2020.104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu M., Wang T., Zhou Y., Zhao Y., Zhang Y., Li J. Potential role of ACE2 in coronavirus disease 2019 (COVID-19) prevention and management. J Transl Int Med. 2020;8(1):9–19. doi: 10.2478/jtim-2020-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pons S., Fodil S., Azoulay E., Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. 2020;24(1):353. doi: 10.1186/s13054-020-03062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tersalvi G., Vicenzi M., Calabretta D., Biasco L., Pedrazzini G., Winterton D. Elevated troponin in patients with coronavirus disease 2019: possible mechanisms. J Card Fail. 2020;26(6):470–475. doi: 10.1016/j.cardfail.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kochi A.N., Tagliari A.P., Forleo G.B., Fassini G.M., Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31(5):1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vonck K., Garrez I., De Herdt V., Hemelsoet D., Laureys G., Raedt R. Neurological manifestations and neuro-invasive mechanisms of the severe acute respiratory syndrome coronavirus type 2. Eur J Neurol. 2020;27(8):1578–1587. doi: 10.1111/ene.14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desforges M., Le Coupanec A., Dubeau P., Bourgouin A., Lajoie L., Dube M. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1) doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakakura K., Nakano M., Otsuka F., Ladich E., Kolodgie F.D., Virmani R. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ. 2013;22(6):399–411. doi: 10.1016/j.hlc.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 46.De Filippo O., D'Ascenzo F., Angelini F., Bocchino P.P., Conrotto F., Saglietto A. Reduced rate of hospital admissions for ACS during Covid-19 outbreak in Northern Italy. N Engl J Med. 2020;383(1):88–89. doi: 10.1056/NEJMc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mafham M.M., Spata E., Goldacre R., Gair D., Curnow P., Bray M. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396(10248):381–389. doi: 10.1016/S0140-6736(20)31356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piccolo R., Bruzzese D., Mauro C., Aloia A., Baldi C., Boccalatte M. Population trends in rates of percutaneous coronary revascularization for acute coronary syndromes associated with the COVID-19 outbreak. Circulation. 2020;141(24):2035–2037. doi: 10.1161/CIRCULATIONAHA.120.047457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roffi M., Guagliumi G., Ibanez B. The obstacle course of reperfusion for ST-segment-elevation myocardial infarction in the COVID-19 pandemic. Circulation. 2020;141(24):1951–1953. doi: 10.1161/CIRCULATIONAHA.120.047523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng Q., Hu B., Zhang Y., Wang H., Zhou X., Hu W. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. 2020;311:116–121. doi: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ni Wentao Xiuwen Y., Liu Jie, Bao Jing, Li Ran, Xu Yu, Guo Wei, Hu Yi, Gao Zhancheng. Acute myocardial injury at hospital admission is associated with all-cause mortality in COVID-19. J Am Coll Cardiol. 2020;76(1):124–125. doi: 10.1016/j.jacc.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Babapoor-Farrokhran S., Gill D., Walker J., Rasekhi R.T., Bozorgnia B., Amanullah A. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253 doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manka R., Karolyi M., Polacin M., Holy E.W., Nemeth J., Steiger P. Myocardial edema in COVID-19 on cardiac MRI. J Heart Lung Transplant. 2020;39(7):730–732. doi: 10.1016/j.healun.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rali A.S., Ranka S., Shah Z., Sauer A.J. Mechanisms of myocardial injury in coronavirus disease 2019. Card Fail Rev. 2020;6:e15. doi: 10.15420/cfr.2020.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang K., Chen W., Zhou Y.-.S., Lian J.-.Q., Zhang Z., Du P. SARS-CoV-2 invades host cells via a novel route: cD147-spike protein. BioRxiv. 2020;2020(03) 14.988345. [Google Scholar]
- 57.Radzikowska U., Ding M., Tan G., Zhakparov D., Peng Y., Wawrzyniak P. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy. 2020 doi: 10.1111/all.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoel H., Heggelund L., Reikvam D.H., Stiksrud B., Ueland T., Michelsen A.E. Elevated markers of gut leakage and inflammasome activation in COVID-19 patients with cardiac involvement. J Intern Med. 2020 doi: 10.1111/joim.13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monteil V., Kwon H., Prado P., Hagelkruys A., Wimmer R.A., Stahl M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905–913. doi: 10.1016/j.cell.2020.04.004. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meinhardt Jenny, Dittmayer Carsten, Franz Jonas, Thomas Carolina, Mothes Ronja, Laue Michael, Schneider Julia, Brünink Sebastian, Greuel Selina, Lehmann Malte, Hassan Olga, Aschman Tom, Schumann Elisa, Chua Robert Lorenz, Conrad Christian, Eils Roland, Stenzel Werner, Windgassen Marc, Rößler Larissa, Goebel Hans-Hilmar, Gelderblom Hans R., Martin Hubert, Nitsche Andreas, Schulz-Schaeffer Walter J., Hakroush Samy, Winkler Martin S., Tampe Björn, Scheibe Franziska, Körtvélyessy Péter, Reinhold Dirk, Siegmund Britta, Kühl Anja A., Elezkurtaj Sefer, Horst David, Oesterhelweg Lars, Tsokos Michael, Ingold-Heppner Barbara, Stadelmann Christine, Drosten Christian, Corman Victor Max, Radbruch Helena, Heppner. Frank L. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2020 doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 61.Nienhold R., Ciani Y., Koelzer V.H., Tzankov A., Haslbauer J.D., Menter T. Two distinct immunopathological profiles in autopsy lungs of COVID-19. Nat Commun. 2020;11(1):5086. doi: 10.1038/s41467-020-18854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nathalie Schwab R.N., Jasmin Haslbauer, Sachs Melanie, Willi Niels, Matter Matthias S., Tzankov Alexandar, Mertz Kirsten, Cathomas Gieri. Immunohistochemistry for SARS-CoV-2 in autoptic pulmonary tissue. Abstr 18, Der Pathol. 2020;6:689. [Google Scholar]
- 63.Massoth L.R., Desai N., Szabolcs A., Harris C.K., Neyaz A., Crotty R. Comparison of RNA in situ hybridization and immunohistochemistry techniques for the detection and localization of SARS-CoV-2 in human tissues. Am J Surg Pathol. 2020 doi: 10.1097/PAS.0000000000001563. [DOI] [PubMed] [Google Scholar]
- 64.Sekulic M., Harper H., Nezami B.G., Shen D.L., Sekulic S.P., Koeth A.T. Molecular detection of SARS-CoV-2 infection in FFPE samples and histopathologic findings in fatal SARS-CoV-2 cases. Am J Clin Pathol. 2020;154(2):190–200. doi: 10.1093/ajcp/aqaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ko C.J., Harigopal M., Gehlhausen J.R., Bosenberg M., McNiff J.M., Damsky W. Discordant anti-SARS-CoV-2 spike protein and RNA staining in cutaneous perniotic lesions suggests endothelial deposition of cleaved spike protein. J Cutan Pathol. 2020 doi: 10.1111/cup.13866. [DOI] [PubMed] [Google Scholar]
- 66.Drago F., Javor S., Ciccarese G., Cozzani E., Parodi A. A case of complete adult-onset kawasaki disease: a review of pathogenesis and classification. Dermatology. 2015;231(1):5–8. doi: 10.1159/000381911. [DOI] [PubMed] [Google Scholar]
- 67.Naoe S., Takahashi K., Masuda H., Tanaka N. Kawasaki disease. With particular emphasis on arterial lesions. Acta Pathol Jpn. 1991;41(11):785–797. doi: 10.1111/j.1440-1827.1991.tb01620.x. [DOI] [PubMed] [Google Scholar]
- 68.Loomba R.S., Villarreal E., Flores S. COVID-19 and Kawasaki syndrome: should we really be surprised? Cardiol Young. 2020;30(7):1059–1060. doi: 10.1017/S1047951120001432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sandhaus H., Crosby D., Sharma A., Gregory S.R. Association between COVID-19 and Kawasaki disease: vigilance required from otolaryngologists. Otolaryngol Head Neck Surg. 2020;163(2):316–317. doi: 10.1177/0194599820930238. [DOI] [PubMed] [Google Scholar]
- 70.Xu S., Chen M., Weng J. COVID-19 and Kawasaki disease in children. Pharmacol Res. 2020;159 doi: 10.1016/j.phrs.2020.104951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shaigany S., Gnirke M., Guttmann A., Chong H., Meehan S., Raabe V. An adult with Kawasaki-like multisystem inflammatory syndrome associated with COVID-19. Lancet. 2020;396(10246):e8–e10. doi: 10.1016/S0140-6736(20)31526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sokolovsky S., Soni P., Hoffman T., Kahn P., Scheers-Masters J. COVID-19 associated Kawasaki-like multisystem inflammatory disease in an adult. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Loupy A., Lefaucheur C. Antibody-mediated rejection of solid-organ allografts. N Engl J Med. 2018;379(12):1150–1160. doi: 10.1056/NEJMra1802677. [DOI] [PubMed] [Google Scholar]
- 75.Berry G.J., Burke M.M., Andersen C., Bruneval P., Fedrigo M., Fishbein M.C. The 2013 international society for heart and lung transplantation working formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J Heart Lung Transpl. 2013;32(12):1147–1162. doi: 10.1016/j.healun.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 76.Stewart S., Winters G.L., Fishbein M.C., Tazelaar H.D., Kobashigawa J., Abrams J. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transpl. 2005;24(11):1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Summary of immunohistological findings (MPO, CD147, IL-6, TMPRSS2, Caspase 3) in the coronary tree and epicardial nerves in COVID-19 positive and COVID-19 negative control patients. The COVID-19 negative control groups were selected as influenza A positive and a second cohort with cardiovascular diseases.
Supplementary Figure 2: Representative immunostains (MPO, Caspase 3,TMPRSS2, CD147, IL-6) from COVID-19 positive patients. There are increased IL-6 and CD147 positive inflammatory cells and a pronounced endothelial TMPRSS2 expression.
Supplementary Figure 3: Representative immunostains (MPO, Caspase 3,TMPRSS2, CD147, IL-6) from COVID-19-negative control patients with cardiovascular disease. Note the lack or only scattered presence of inflammatory cells and the pronounced TMPRSS2 expression in the control cohort.
Supplementary Figure 4: Representative immunostains (MPO, Caspase 3,TMPRSS2, CD147, IL-6) from COVID-19-negative control patients with influenza A / viral pneumonia infection. Note the lack or only scattered presence of inflammatory cells and the pronounced TMPRSS2 expression in the control cohort.
Supplementary Figure 5: Immunfluorescence labelled immunistochemistry for SARS-Cov2 viral components in small intramyocardial vessels. Upper row images: anti FITC labelled antibody against the S component. a: negative control without primary antibody, b/c: positive cases without (b) and with (c) amplification kit. Middle row images: anti FITC labelled antibody against the NP component. d: negative control without primary antibody, e/f: positive cases without (e) and with (f) amplification kit. Lower row images: Goat anti mouse Alexa Fluor 488 labelled antibody against the NP component. g: negative control without primary antibody, h/i: positive cases without (h) and with (i) amplification kit.
Supplementary Table 1. Additional markers. Distribution and intensity of TMPRSS2, CD147 and IL-6 in different anatomic locations in COVID-19 patients, patients from control cohort with cardiovascular diseases and control patients with Influenza A. Abbreviations: Cor. Coronary, A/V arterioles/venules, Cap. Capillaries, N nerves, NA not available.