Abstract
Background
The medium-term effects of Coronavirus disease (COVID-19) on organ health, exercise capacity, cognition, quality of life and mental health are poorly understood.
Methods
Fifty-eight COVID-19 patients post-hospital discharge and 30 age, sex, body mass index comorbidity-matched controls were enrolled for multiorgan (brain, lungs, heart, liver and kidneys) magnetic resonance imaging (MRI), spirometry, six-minute walk test, cardiopulmonary exercise test (CPET), quality of life, cognitive and mental health assessments.
Findings
At 2–3 months from disease-onset, 64% of patients experienced breathlessness and 55% reported fatigue. On MRI, abnormalities were seen in lungs (60%), heart (26%), liver (10%) and kidneys (29%). Patients exhibited changes in the thalamus, posterior thalamic radiations and sagittal stratum on brain MRI and demonstrated impaired cognitive performance, specifically in the executive and visuospatial domains. Exercise tolerance (maximal oxygen consumption and ventilatory efficiency on CPET) and six-minute walk distance were significantly reduced. The extent of extra-pulmonary MRI abnormalities and exercise intolerance correlated with serum markers of inflammation and acute illness severity. Patients had a higher burden of self-reported symptoms of depression and experienced significant impairment in all domains of quality of life compared to controls (p<0.0001 to 0.044).
Interpretation
A significant proportion of patients discharged from hospital reported symptoms of breathlessness, fatigue, depression and had limited exercise capacity. Persistent lung and extra-pulmonary organ MRI findings are common in patients and linked to inflammation and severity of acute illness.
Funding
NIHR Oxford and Oxford Health Biomedical Research Centres, British Heart Foundation Centre for Research Excellence, UKRI, Wellcome Trust, British Heart Foundation.
Keywords: Coronavirus, SARS-CoV-2 infection, COVID-19, Survivors, Medium term, Follow up, Post-hospital discharge, Multiorgan effects, Magnetic Resonance Imaging, Mental health
Research in Context.
Evidence before this study
We searched PubMed from the inception of our study to September 4th, 2020, for articles published in English, with the search terms “COVID-19, multiorgan imaging, cardiac magnetic resonance, brain magnetic resonance, abdominal magnetic resonance, quality of life, cognitive assessment. cardiopulmonary exercise test, follow-up”. There was no single study which has comprehensively assessed multiple aspects of recovery in patients with SARS-CoV-2 infections including multiorgan magnetic resonance imaging, quality of life, exercise tolerance, mental health and cognition of patients after discharge from hospital. There was one brain MRI study of COVID-19 survivors; however, this did not formally assess cognition in patients. There were three studies of cardiac magnetic resonance imaging in the early and subacute phases of recovery in COVID-19. There were no MRI studies of abdominal organs (kidneys or liver) in COVID-19 survivors and no study on cardiopulmonary exercise testing in COVID-19 survivors at 2-3 months from disease onset. There were three studies of pulmonary function testing and three studies that have assessed mental health in COVID-19 survivors. There were no studies that systematically assessed cognition in post-hospital discharged COVID-19 patients.
Added value of this study
This is the first holistic study of post-hospital discharged COVID-19 patients to comprehensively assess the medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise tolerance, mental, cognitive and physical health. We have shown that a significant proportion of patients complained of symptoms of breathlessness and fatigue at 2-3 months after the onset of illness and that MRI changes could be seen in the brain, lungs, heart, liver and kidneys in a proportion of patients. An increased burden of self-reported symptoms of depression and dysexecutive cognitive profile were observed, as well as marked limitations in exercise tolerance. Serum markers of inflammation and severity of acute illness correlated with MRI evidence of multiorgan abnormalities and reduced exercise tolerance.
Implications of all the available evidence
Our results, showing multi-system changes, highlight the need to develop a multidisciplinary approach for the delivery of clinical care to patients who are discharged following COVID-19 hospital admissions. The high burden of self-reported mood symptoms, abnormalities in executive cognitive performance, perception of impaired quality of life and exercise tolerance limitations carry implications for individuals who are expected to return to work after hospital discharge. Further large-scale studies investigating the long-term effects of COVID-19 are warranted, to fully understand the burden of chronic illness among survivors of SARS-CoV-2 infections, and the long-term implications of multiorgan MRI abnormalities in patients.
Alt-text: Unlabelled box
1. Introduction
The global impact of Coronavirus disease or COVID-19 has been profound with hundreds of thousands of lives lost and millions affected. [1] Despite the high mortality seen among hospitalised patients, many have survived, with little known about the medium-to-long term effects of COVID-19 after discharge. Although predominantly a respiratory illness, emerging data suggests that multiorgan injury is common, particularly in those with moderate to severe infections. [2], [3], [4]
Studies have shown that the brain, heart, gastrointestinal system and the kidneys are particularly vulnerable to injury during the initial phase of illness. [2], [3], [4] The invasive potential and affinity of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) for multiple cell lines suggests that virus-mediated toxicity may play a central role in promoting multi-system damage. [5] An exaggerated and dysregulated immune response, endothelial damage, thromboinflammation and coagulopathy have also been implicated in causing injury to these organs. [6]
While it is clear that the extent of infection, inflammatory response and physiological reserve (influenced by obesity, age and comorbidities) are important determinants of clinical outcomes during the initial phase, reports of a chronic maladaptive inflammatory syndrome are also emerging. In particular, inflammatory changes in the lungs have been described in convalescing patients, months after discharge from hospital. [7] Whether extra-pulmonary organs are also susceptible to ongoing inflammation and injury is poorly understood, as are its effects on exercise tolerance, cognition, mental health and quality of life.
In a holistic study of survivors of moderate to severe COVID-19 infection discharged from hospital, at 2-3 months from disease onset, we aimed to investigate the prevalence of persistent multiorgan injury/inflammation and assess the effects of COVID-19 on physical, psychological, cognitive health and well-being.
2. Methods
2.1. Study population
Fifty-eight patients with moderate to severe laboratory-confirmed (SARS-CoV-2 polymerase chain reaction positive) COVID-19, admitted for treatment at the Oxford University Hospitals National Health Service Foundation Trust between 14th March – 25th May 2020, and 30 uninfected controls (SARS-CoV-2 immunoglobulin negative and asymptomatic) group-matched for age, sex, body mass index (BMI) and risk factors (smoking, diabetes and hypertension) from the community (during the same period) were prospectively enrolled in this observational cohort study (appendix, Figure 1, p27). Enrolment did not rely on the presence of multi-organ symptoms (neurological, cardiac, respiratory, gastrointestinal) and hence our patients were an unselected cohort of hospitalised individuals with moderate to severe COVID-19. Patients were approached by their medical team either at the time of discharge or after discharge and invited to participate in our study. Controls were identified from other ethically approved studies and by word of mouth as stated in our research ethics protocol. Flow chart of participant recruitment is listed in the appendix (appendix, Figure 1, p27). Enrolment of patients and controls occurred at a ratio of approximately 2:1. All controls were screened for symptoms of respiratory viral illness or history of contact with infected individuals prior to the study visit. Only those individuals who were asymptomatic for COVID-19 were invited and underwent a screening SARS-CoV-2 immunoglobulin test. Controls were prospectively enrolled in parallel to recruitment of COVID-19 patients and group-matched for baseline characteristics including age, gender, body mass index, risk factors such as smoking, hypertension, diabetes, coronary artery disease and stroke. Informed consent was obtained from all participants using an ethically approved consent form.
For further details on inclusion and exclusion criteria of patients and controls, refer to appendix, p2. COVID-19 severity on admission was defined as per the World Health Organisation (WHO) interim clinical guidance (appendix for definition, p2). Patients with severe/end-stage multi-system comorbidities and contraindications to magnetic resonance imaging were excluded.
This study was registered at ClinicalTrials.gov (NCT04510025) and approved in the United Kingdom by the North West Preston Research Ethics Committee (reference 20/NW/0235).
2.2. Study procedures
All subjects consented to have comprehensive multiorgan magnetic resonance imaging (MRI) of the brain, lungs, heart, liver, kidneys, six-minute walk (6MWT) test, spirometry, cardiopulmonary exercise test (CPET), series of questionnaires, and blood tests.
2.3. Multiorgan MRI protocol
A 70 minute multiorgan MRI scan was carried out at 3 Tesla (Prisma, Siemens Healthineers, Erlangen, Germany). Brain MRI included T1weighted imaging, T2-Fluid attenuated inversion recovery (FLAIR) to assess (e.g.) inflammation, diffusion-weighted imaging (DWI) to assess ischaemic injury, susceptibility-weighted imaging (SWI) to assess (e.g.) haemorrhage, and quantitative multi-inversion-delay arterial spin labelling (ASL) to assess cerebral blood flow. Lung imaging included a T2-weighted half‐Fourier‐acquisition single‐shot turbo spin‐echo (HASTE) to qualitatively assess the extent of lung parenchymal involvement. Cardiac assessment included cine imaging, T1 and T2 mapping, post-contrast T1 mapping and late gadolinium enhancement (LGE) imaging to assess biventricular volumes, function, myocardial oedema, diffuse and focal/patchy fibrosis. Liver imaging consisted of a single slice T1 map and multiecho gradient echo IDEAL, to quantify fibro-inflammation (T1), fat (proton density fat fraction, PDFF) and iron (T2*). Multiparametric renal imaging was also undertaken and consisted of a single coronal oblique slice T1 map to quantify fibro-inflammation and an R2* map for renal oxygenation assessment (details in appendix, p2-5).
2.4. MRI Image analysis
All images were analysed quantitatively and qualitatively by expert radiologist (XC), neuroradiologist (FS), cardiac MRI specialists (BR, MC), neuroimage analysts (NF, LG, FA, TO, SS, KM, SM, FKM, CW, CA, FL, JA, MJ) and physicists (EMT, FEM) in a blinded fashion. Lung MRIs were qualitatively assessed for parenchymal involvement by an expert radiologist (XC). Extent of lung parenchymal abnormalities was scored as 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%), or 4 (76–100%), respectively. Brain image processing was carried out using an adapted version of the processing pipeline created for the United Kingdom (UK) Biobank brain imaging analysis (https://www.fmrib.ox.ac.uk/ukbiobank/), based around tools from the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) software library [FSL (https://fsl.fmrib.ox.ac.uk/fsl)] (appendix, p3). Assessment of cardiac volumes, function, myocardial T1 maps, T2 maps, post-contrast T1 maps and LGE LGE images was undertaken using cvi42 software (Circle Cardiovascular Imaging Inc., Version 5.10.1, Calgary, Canada) (appendix, p4). Quantitative analyses of T1 and T2* for liver, spleen and renal images were carried out as described in the appendix (appendix, p5), including iron-correction for liver T1 with an algorithm related to LiverMultiScan cT1 (Perspectum, Oxford) but lacking cross-scanner standardisation and therefore not comparable to LiverMultiScan cT1 .
2.5. Spirometry
Spirometry, including forced vital capacity (FVC), and forced expiratory volume at the first second of exhalation (FEV1), was performed as per recommended guidance (appendix, p5). [8]
2.6. CPET
Symptom-limited CPET was undertaken using a cycle ergometer. Following two minutes of unloaded cycling, the work rate was increased to 20W, followed by a 10W/min ramp. [9] Participants were encouraged to reach their maximal work rate (appendix, p6).
2.7. Six-minute walk test
Participants were asked to walk for six-minutes in a pre-marked corridor. Borg scale, heart rate and oxygen saturation were measured immediately before and after the test (appendix, p5).
2.8. Questionnaires
Questionnaires were completed using an electronic data capture (CASTOR EDC, https://www.castoredc.com). Depression, anxiety and quality of life measures were assessed using the Patient Health Questionnaire (PHQ) depression module (PHQ-9) [10], General Anxiety Disorder Questionnaire (GAD-7) [11] and Short Form-36 (SF-36) survey [12]. Cognitive function was assessed using the Montreal Cognitive Asessment (MoCA) [13]. The Medical Research Council (MRC) dyspnoea [14] scale, Dyspnoea-12 score [15] and Fatigue Severity Scale (FSS) [16] were used to assess the extent of breathlessness and fatigue, respectively (appendix for details, p6).
2.9. Laboratory assessments
Blood-based testing consisted of a complete blood count, biochemical analysis, coagulation testing, assessment of liver and renal function, markers of cardiac injury and measures of electrolytes, C-reactive protein (CRP), procalcitonin, and lactate dehydrogenase.
2.10. Admission data collection and blood tests
Details on clinical symptoms or signs, vitals and laboratory findings during admission were extracted from electronic medical records. The severity of disease during hospital admission was graded as per the WHO ordinal scale for clinical improvement (appendix for definition, p2). [17]
2.11. Statistical analysis
Continuous variables were described using mean and standard deviation (SD) for parametric data and median with interquartile range (IQR) for non-parametric data in the tables. Normality was assessed by visual inspection of histograms. Differences between two groups were evaluated using Student's t-tests or Mann-Whitney U-tests as appropriate. Categorical variables were reported as frequency and percentages. Associations between two groups were compared using the Chi-square test or Fisher's exact test as appropriate. Pearson's or Spearman's correlation coefficients were used to describe the relationship between two variables where relevant. Analyses of brain imaging derived phenotypes (IDPs) were undertaken after Gaussianisation of all continuous variables which were then deconfounded i.e.,adjusting for age, sex, BMI, diastolic and systolic blood pressure, smoking and head size. Gaussianisation refers to the process of quantile normalisation or monotonic remapping of values resulting in a Gaussian distribution, to ameliorate both effects of outliers and highly skewed distributions. [18] The conventional level of statistical significance of 5% was used and not corrected for multiple comparisons. Statistical analyses were performed using SPSS Version 26.0 (IBM, Armonk, NY, USA).
2.12. Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, writing of the article, or the decision to submit for publication.
3. Results
Mean age of the patient group was 55±13 years and 34/58 (59%) were men (Table 1). Thirteen (22%) belonged to Black, Asian and Minority Ethnic groups. Twenty one patients (36%) had required intensive care unit (ICU) admission; 20/21 required mechanical ventilation (non-invasive ventilation or intubation). Four patients required renal replacement therapy or inotropic support. Median duration of hospitalisation was 8·5 days (IQR 5·0 – 17·0). Patients were assessed between 2-3 months from disease-onset at median interval of 2·3 months (IQR 2·06 – 2·53) and median 1·6 months from discharge (IQR 1·4 – 1·8). Baseline characteristics of patients and group-matched controls are listed in Table 1. At follow-up, patients had a mildly increased resting heart rate (p = 0·047), respiratory rate (p = 0·0004) and reduced oxygen saturation (0·008) relative to controls.
Table 1.
Demographics, baseline characteristics, vital signs at follow-up and admission details of patients and control participants.
| COVID-19 | CONTROL | p-value | |
|---|---|---|---|
| General demographics | |||
| Age, years | 55·4 (13·2) | 53·9 (12·3) | 0·62 |
| Sex | 1·00ᶞ | ||
| Female | 24/58 (41·4%) | 12/30 (40·0%) | |
| Male | 34/58 (58·6%) | 18/30 (60·0%) | |
| BMI, kg/m2 | 30·8 (26·2 - 36·4) | 27·3 (23·1 - 35·1) | 0·17⁺ |
| Black/Asian and minority ethnic groups | 13/58 (22·4%) | 1/30 (3·3 %) | 0·03 |
| Current/Ex-smoker | 20/58 (34·5%) | 7/30 (23·3%) | 0·34ᵋ |
| Type 1 Diabetes | 1/58 (1·7%) | 0/30 (0·0%) | 1·00ᵋ |
| Type 2 Diabetes | 8/58 (13·8%) | 3/30 (10·0%) | 0·74ᵋ |
| Hypertension | 22/58 (37·9%) | 9/30 (30·0%) | 0·49ᵋ |
| Coronary artery disease | 2/58 (3·4%) | 0/30 (0·0%) | 0·55ᵋ |
| Cerebrovascular Disease | 1/58 (1·7%) | 0/30 (0·0%) | 1·00ᵋ |
| Asthma | 20/58 (34·5%) | 6/30 (20·0%) | 0·22ᵋ |
| COPD | 3/58 (5·2%) | 0/30 (0·0%) | 0·55ᵋ |
| Previous cancer | 2/58 (3·4%) | 3/30 (10·0%) | 0·33ᵋ |
| Depression | 3/58 (5·2%) | 1/30 (3·3%) | 1·00 |
| Vital signs at follow-up | |||
| Heart rate, bpm | 76·3 (14·1) | 70·2 (12·1) | 0·047 |
| Systolic pressure, mmHg | 139·7 (16·5) | 137·2 (17·0) | 0·51 |
| Diastolic pressure, mmHg | 79·5 (71·8 - 86·8) | 71·5 (63·0 - 87·8) | 0·12⁺ |
| Temperature, oC | 36·6 (36·5 - 36·7) | 36·5 (36.4 - 36·6) | 0·047⁺ |
| Oxygen saturation, % | 96·0 (95·0 - 97·0) | 97·0 (96·0 - 98·0) | 0·008⁺ |
| Respiratory rate, respirations/minute | 18·0 (17·8 - 20·0) | 16·0 (13·8 - 18·0) | <0·001⁺ |
| Admission details | |||
| Median length of stay, days | 8·5 (5·0 - 17·0) | ||
| Readmitted | 10/58 (17·2%) | ||
| Required ITU admission | 21/58 (36·2%) | ||
| qSOFA52 | |||
| 0 | 17/58 (29·3%) | ||
| 1 | 38/58 (65·5%) | ||
| 2 | 3/58 (5·2%) | ||
| 3 | 0/58 (0·0%) | ||
| Ordinal scale for clinical improvement (WHO) | |||
| 1 | 0/58 (0·0%) | ||
| 2 | 4/58 (6·9%) | ||
| 3 | 22/58 (37·9%) | ||
| 4 | 5/58 (8·6%) | ||
| 5 | 15/58 (25·9%) | ||
| 6 | 7/58 (12·1%) | ||
| 7 | 5/58 (8·6%) | ||
| Signs and symptoms | |||
| Fever | 51/58 (87·9%) | ||
| Malaise | 51/58 (87·9%) | ||
| Shortness of breath | 51/58 (87·9%) | ||
| Cough | 35/58 (60·3%) | ||
| Dysgeusia | 29/58 (50·0%) | ||
| Anosmia | 26/58 (44·8%) | ||
| Diarrhoea | 17/58 (29·3%) | ||
| Chest pain | 16/58 (27·6%) | ||
| Headache | 13/58 (22·4%) | ||
| Vomiting | 9/58 (15·5%) | ||
| Fever on admission | 39/58 (67·2%) | ||
| <37·5ᵒC | 19/58 (32·8%) | ||
| 37·5°C - 38·0ᵒC | 12/58 (20·7%) | ||
| 38·1°C - 39ᵒC | 19/58 (32·8%) | ||
| >39°C | 8/58 (13·8%) | ||
| Treatment | |||
| Oxygen replacement | 54/58 (93·1%) | ||
| Nasal cannula | 14/58 (24·1%) | ||
| Simple face mask | 7/58 (12·1%) | ||
| Venturi face mask | 6/58 (10·3%) | ||
| High flow oxygen delivery | 7/58 (12·1%) | ||
| CPAP | 8/58 (13·8%) | ||
| Intubation | 12/58 (20·7%) | ||
| ECMO | 0/58 (0%) | ||
| Inotropic support | 4/58 (6·9%) | ||
| Renal replacement therapy | 2/58 (3·4%) | ||
| Antibiotics | 57/58 (98·3%) | ||
| Antivirals | 4/58 (6·9%) | ||
| Steroids | 16/58 (27·6%) | ||
| Acute organ injury | |||
| Acute liver injury* | 18/58 (31·0%) | ||
| Acute kidney injuryΘ | 6/58 (10·3%) | ||
| Acute cardiac injuryж | 3/38 (7·8%) | ||
| Pulmonary embolism | 7/58 (12·1%) | ||
| Central | 1/58 (1·7%) | ||
| Peripheral | 6/58 (10·3%) | ||
Data are mean (SD), median (IQR) and n/N (%), where N is the total number of participants with available data. p-values from independent Student's t-test, Mann-Whitney U test (⁺), Chi square (ᶞ) or Fisher's exact test (ᵋ). COPD = chronic obstructive pulmonary disease. ITU = intensive treatment unit. qSOFA52 = quick sequential organ failure assessment52. CPAP = Continuous positive airway pressure. ECMO = extracorporeal membrane oxygenation. WHO = world health organisation. * defined as blood levels of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) above 3x the upper reference limit (>135 IU/L or >126 IU/L, respectively), alkaline phosphatase or gamma-glutamyltransferase above 2x the upper reference limit (>260 IU/L or >80 IU/L, respectively). Θ defined as an increase in serum creatinine of at least 26 umol/L within 48 hours, or 1·5 to 2-fold increase from baseline.
ж defined as an acute rise in hypersensitive troponin I above the 99th percentile upper reference limit (>34 ng/L). Control subjects were matched for co-morbidities as closely as possible.
MRI data (Table 2) were available for up to 54/58 patients [brain MRI (n = 54), lung MRI (n = 53) and cardiac and abdominal MRI (n = 52)] and 28/30 controls (study flowchart and summary of missing data in appendix Figure 1, p27, and appendix Table 7, p25).
Table 2.
Relevant MRI parameters in patients and controls.
| COVID-19 | CONTROL | p-value | |
|---|---|---|---|
| Lung MRI | |||
| Lung parenchymal abnormalities, % | 32/53 (60·4%) | 3 (10·7%) | <0·0001ᵋ |
| 0 | 21/53 (39·6%) | 25/28 (89·3%) | 0·0003ᵋ |
| 1-25% | 3/53 (5·7%) | 0/28 (0·0%) | |
| 26 - 50% | 8/53 (15·1%) | 2/28(7·1%) | |
| 51 - 75% | 9/53 (17·0%) | 0/28 (0·0%) | |
| > 75% | 12/53 (22·6%) | 1/28 (3·6%) | |
| Cardiac MRI | |||
| T1, T2, post contrast T1 mapping analysis | |||
| Native T1 (basal myocardium), ms | 1179·7 (34·4) | 1149·3 (24·0) | 0·0001 |
| > 1197 ms (>2SD from control mean) | 13/50 (26·0%) | 1/28 (3·7%) | 0·015ᵋ |
| Native T1 (mid myocardium), ms | 1173·1 (33·6) | 1150·2 (32·4) | 0·004 |
| > 1215 ms (>2SD from control mean) | 4/51 (7·8%) | 0/28 (0·0%) | 0·29ᵋ |
| Native T1 (apical myocardium), ms | 1177·4 (44·7) | 1168·3 (53·2) | 0·42 |
| > 1275 ms (>2SD from control mean) | 1/50 (2·0%) | 1/28 (3·6%) | 1·00ᵋ |
| Extracellular volume (basal myocardium), % | 30·4 (28·3 - 31·3) | 28·3 (26·8 - 31·5) | 0·12 |
| Extracellular volume (mid myocardium), % | 30·1 (27·2 - 31·4) | 29·4 (27·1 - 30·7) | 0·41⁺ |
| Extracellular volume (apical myocardium), % | 28·7 (27·0 - 31·6) | 29·7 (27·2 - 31·5) | 0·51⁺ |
| T2 (basal myocardium), ms | 41·7 (2·2) | 41·6 (2·2) | 0·80 |
| T2 (mid myocardium), ms | 41·8 (2·2) | 41·1 (2·3) | 0·21 |
| T2 (apical myocardium), ms | 43·5 (3·0) | 43·7 (3·5) | 0·81⁺ |
| Late gadolinium enhancement analysis | |||
| % LGE enhancement, % of left ventricular mass | 0·8 (0·5 - 1·9) | 0·6 (0·3 - 1·0) | 0·023+ |
| Myocarditis pattern | 6/52 (11·5%) | 2/28 (7·4%) | 0·47ᵋ |
| Myocardial infarction | 1/52 (1·9%) | 0 (0·0%) | |
| LV/RV insertion point | 7/52 (13·5%) | 1/28 (3·7%) | |
| Mixed | 0 (0%) | 0 (0%) | |
| Other | 0 (0%) | 0 (0%) | |
| Liver analysis | |||
| T1, ms | 832·4 (127·4) | 778·1 (98·2) | 0·059 |
| T2*, ms | 17·7 (4·4) | 17·2 (3·49) | 0·60 |
| Iron-corrected liver T1, ms¶ | 861·0 (99·2) | 803·9 (106·6) | 0·019 |
| > 1016ms (>2SD from control mean) | 5/52 (9·6%) | 1/28 (3·6%) | 0·66ᵋ |
| Average proton density fat fraction, % | 4·9 (3·1 - 9·5) | 3·7 (2·1 - 6·5) | 0·18⁺ |
| Extracellular volume, % | 27·3 (23·0 - 31·2) | 27·3 (17·6 - 33·6) | 0·60+ |
| Renal analysis | |||
| T1 map anaylsis | |||
| Average cortex, ms | 1597·5 (91·2) | 1523·1 (65·5) | 0·0003 |
| > 1652 ms (>2SD from control mean)) | 15/51 (29·4%) | 0/28 (0·0%) | 0·001ᵋ |
| Average corticomedullary differentiation, ms | 385·4 (335·8 - 456·4) | 470·8 (431·5 - 496·3) | 0·0002⁺ |
| Brain analysis& | |||
| T2-FLAIR volumes | |||
| White matter hyperintensities, mm³ | 2305·0 (1402·0 - 4021·0) | 1457·0 (654·2 - 2700·5) | 0·085& |
| Periventricular white matter hyperintensities, mm³ | 1884·0 (1172·0-3303·0) | 1305·0 (525·0 - 2284·8) | 0·066& |
| Deep white matter hyperintensities, mm³ | 330·5 (141·0 - 863·0) | 213·0 (83·5 - 416·8) | 0·20& |
| Susceptibility-weighted imaging, T2* | |||
| Left thalamus, ms | 44·2 (42·0 - 46·1) | 42·8 (39·9 - 45·3) | 0·047& |
| Right thalamus, ms | 43·9 (41·7 - 45·8) | 42·4 (40·2 - 45·0) | 0·034& |
| Left and right thalamus, ms | 43.9 (42.0 - 45.8) | 42.6 (40.3 - 45.2) | 0·022& |
| Diffusion weighted imaging, Mean diffusivity | |||
| Right posterior thalamic radiation, x10⁻⁶ mm²/s | 842·0 (804·5 - 871·2) | 813·0 (787·0 - 832·8) | 0·20& |
| Left posterior thalamic radiation, x10⁻⁶ mm²/s | 831·0 (814·5 - 851·5) | 811·0 (792·2 - 828·8) | 0·042& |
| Right sagittal stratum, x10⁻⁶ mm²/s | 840·0 (799·5 - 863·5) | 813·0 (789·2 - 828·5) | 0·022& |
| Left sagittal stratum, x10⁻⁶ mm²/s | 789·0 (776·5 - 814·0) | 787·0 (767·2 - 791·5) | 0·078& |
| Left and right (averaged) sagittal stratum, x10⁻⁶ mm²/s | 810·0 (791·0 - 834·0) | 791·5 (779·5 - 808·8) | 0·020& |
Data are median (IQR) for non-parametric data and mean (SD) for parametric data, and n/N (%), where N is the total number of participants with available data. p-values from independent Student's t-test, Mann-Whitney U test (⁺), or Fisher's exact test (ᵋ). Brain image derived phenotypes (IDPs) were Gaussianised and deconfounded for typical brain confounders. p-values for brain measurements were derived from a Gaussianised deconfounded model (&) and relate to independent Student's t-test comparison of this data. Raw data are presented in the table for ease of interpretation. All other parameters are listed in the appendix Table 2. An in-house algorithm was used to calculate iron-corrected T1, so these values cannot be compared to the LiverMultiScan cT1. FLAIR = Fluid attenuated inversion recovery. LV = left ventricle. LGE = Late gadolinium enhancement. RV = right ventricle. Control subjects were matched for co-morbidities as closely as possible.
3.1. Lung health and exercise tolerance
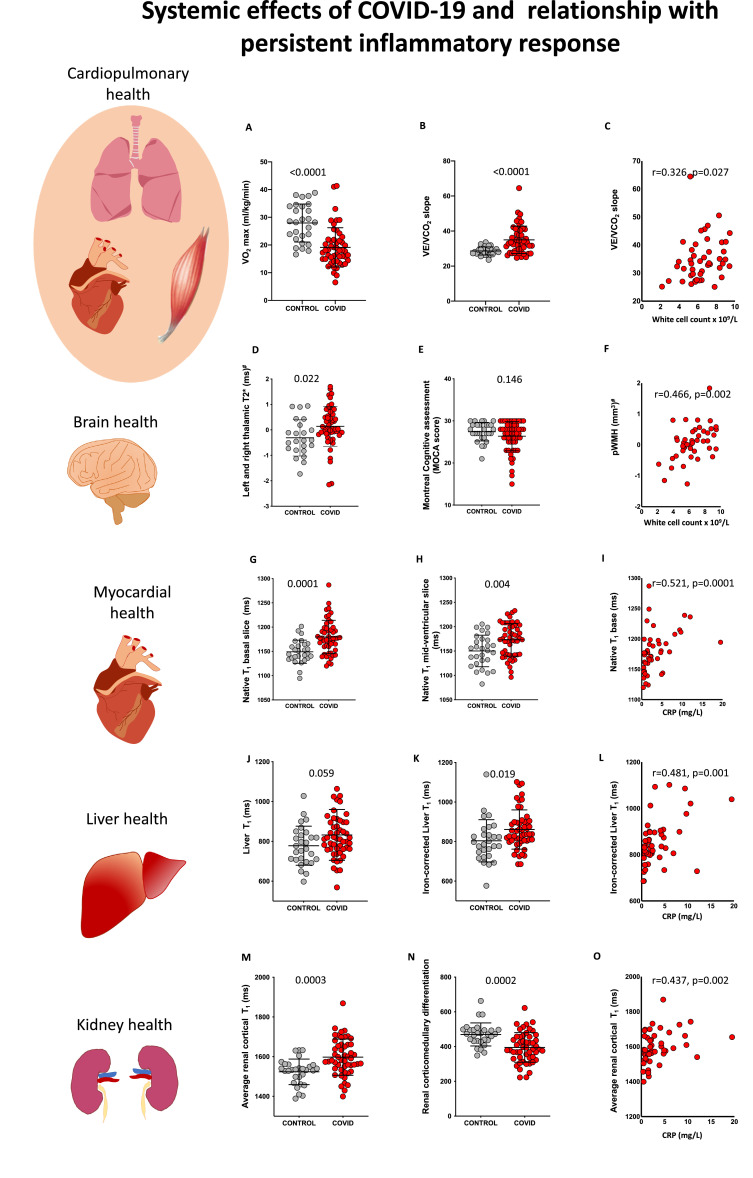
During hospital admission, 54/58 (93%) patients had abnormal chest X-ray or computed tomography (CT). At ~2–3 months, persistent parenchymal abnormalities on lung MRI were present in 32/53 (60%) patients (Table 2, appendix Figure 2, p28). Thirty-six (64%) experienced symptoms of significant breathlessness (MRC dyspnoea score ≥ 2) and 30/55 (55%) complained of fatigue (FSS ≥ 4). On average, COVID-19 survivors had a significantly lower FEV1 (p = 0·0004), FVC (p < 0·0001) and higher FEV1/FVC ratio (p = 0·027) at follow-up (Table 3). Abnormalities were noted in FEV1 % predicted in 6/56 (11%) and FVC % predicted in 7/56 (13%). Patients covered a shorter distance on 6MWT than controls (405±118m vs 517± 106m, p < 0.0001). Four (7%) patients desaturated at the end of the test. During CPET, patients achieved lower peak oxygen uptake (VO2, represented as % of predicted VO2 max, p < 0·0001)) (Fig. 1), oxygen uptake efficiency slope (p = 0.001) and higher ventilatory equivalent for carbon dioxide (VE/VCO2 slope, p < 0.0001) (Table 3, Fig. 1) compared to controls. VE/VCO2 slope, a marker of ventilatory efficiency, was worse in those with MRI lung parenchymal abnormalities versus those without (Median 35, IQR (32–43) versus 32, IQR (29–34), p = 0·007). CPET was stopped early in 15/51 (29%) patients due to fatigue and myalgia and 5/51 (10%) of patients due to breathlessness. VE/VCO2 and six-minute walk distance correlated with markers of inflammation (white cell count, C-reactive protein (CRP) and pro-calcitonin) in patients (Fig. 1, appendix Table 4, p21).
Table 3.
Spirometry and cardiopulmonary exercise test results from patients and controls.
| Spirometry | COVID-19 | CONTROL | p-value |
|---|---|---|---|
| FVC, % predicted | 108·3 (22·8) | 131·4 (21·8) | <0·0001 |
| < 80% | 7/56 (12·5%) | 0/28 | 0·090ᵋ |
| FEV1, % predicted | 101·4 (19·7) | 118·7 (22·1) | 0·0004 |
| < 80% | 6/56 (10·7%) | 1/28 (3·6%) | 0·42ᵋ |
| FEV1/FVC | 0·77 (0·73 - 0·80) | 0·75 (0·70 - 0·78) | 0·027⁺ |
| FEF25, % predicted | 97·0 (27·6) | 110·1 (30·4) | 0·020 |
| FEF50, % predicted | 81·0 (23·2) | 86·9 (24·5) | 0·13 |
| FEF75, % predicted | 54·5 (42·8 - 70·0) | 54·0 (48·5 - 69·5) | 0·60⁺ |
| Peak expiratory flow, % predicted | 105·7 (27·7) | 114·5 (24·7) | 0·16 |
| Cardiopulmonary exercise test | |||
| VO2 peak, % of predicted VO2 max | 80·5 (23·1) | 112·7 (27·0) | <0·0001 |
| < 80% | 28/51 (54·9%) | 2/27(7·4%) | <0·0001ᵋ |
| Anaerobic threshold (% of predicted VO2 max) | 40·7 (36·2 - 47·5) | 46·8 (43·3 - 51·3) | 0·0005⁺ |
| VE/VCO2 Slope | 33·4 (29·2 - 40·3) | 28·2 (26·7 - 30·0) | <0·0001⁺ |
| Oxygen Uptake Efficiency Slope | 1·9 (1·6 - 2·4) | 2·7 (2·0 - 3·2) | 0·001⁺ |
Data are median (IQR) for non-parametric data and mean (SD) for parametric data, and n/N (%), where N is the total number of participants with available data. p-values from independent Student's t-test, Mann-Whitney U test (⁺), or Fisher's exact test (ᵋ). FVC = Forced vital capacity. FEV1 = Forced expiratory volume in 1 second. FEF25, FEF50, FEF75 = Forced expiratory flow at 25%, 50% and 75% of forced expiration, respectively.VO2 = oxygen consumption. VE/VCO2 = ventilatory equivalent for carbon dioxide. Control subjects were matched for co-morbidities as closely as possible.
Fig. 1.
Systemic effects of COVID-19 and relationship with inflammatory response. A, B: Comparison of cardiopulmonary exercise test (CPET) parameters (VO2 max and VE/VCO2) between comorbidity-matched control and COVID-19 survivors. C: Relationship between VE/VCO2 and white cell count in COVID-19. D, E: Comparison of susceptibility weighted T2* signal (left and right thalamus) and MoCA scores between control and COVID-19 survivors. F: Relationship between periventricular white matter hyperintensity volume (pWMH) volume and white cell count in COVID-19. G, H: Comparison of myocardial native T1 (base and mid ventricle) between control and COVID-19 survivors. I: Relationship between basal native T1 and C-reactive protein (CRP). J, K: Comparison of liver T1 and iron-corrected liver T1 between control and COVID-19 survivors (these values cannot be compared to the LiverMultiScan cT1). L: Relationship between iron-corrected liver T1 and CRP in COVID-19. M, N: Comparison of average cortical kidney T1 and corticomedullary differentiation in control and COVID-19 survivors. O: Relationship between average cortical kidney T1 and CRP in COVID-19 (p-values for comparisons are from Student's t-tests for all variables; Spearman's correlation coefficient and p-values are reported for correlations, # signifies p-values were derived from comparison of variables that were Gaussianised and deconfounded).
3.2. Brain health and cognition
During hospital admission, one patient developed a right occipital stroke; follow-up brain MRI revealed signs of a mature infarct. Blinded qualitative assessment (by expert neuroradiologist - FS) of brain MRI at 2-3 months did not reveal group differences in burden of small vessel disease, white matter hyperintensities, haemorrhage or ischaemic changes between patients and controls (appendix Table 3, p20). No statistically significant differences were seen in quantitative measurements of grey matter volumes (globally and regionally), white matter volumes and cerebral perfusion (appendix Table 2, p12). Compared to controls, patients had a higher T2* signal on susceptibility-weighted imaging in the left and right thalamus (p = 0·022) (Fig. 1, Appendix Fig. 3) and increased mean diffusivity in the left posterior thalamic radiation (p = 0·042) and left and right averaged sagittal stratum (0·020) (Table 2). Patients tended to have higher periventricular white matter hyperintensity volume (p = 0.066) on T2-FLAIR imaging compared to controls.
Cognitive performance in the executive/visuospatial domain was impaired among patients compared to controls (MoCA visuospatial score ≤4 in 40% patients versus 16% in controls, p = 0·01). Among patients, 28% (16/58) had a total MoCA score that was abnormal according to the established cut-off of <26 compared to 17% (5/30) of controls. Median MoCA scores in patients (27, IQR 25-29), however, were not statistically significantly different from controls (28, IQR 27-29, p = 0·146) (Fig. 1). Periventricular white matter hyperintensities (pWMH) and right thalamic T2* correlated with white cell count (pWMH: r = 0·47, p = 0·002; right thalamic T2*: r = 0·29 p = 0.05) in patients (appendix Table 4, p21), but not with cognitive performance.
3.3. Cardiac health
During admission, 38/58 (66%) were screened for cardiac involvement with troponin (high sensitivity Troponin I). Three (8%) were found to have an elevated troponin during admission (>34 ng/L). At follow-up, troponin was normal in all patients (appendix Table 1, p9). Left ventricular function was normal and comparable between groups. Right ventricular ejection fraction in patients ranged from 43 to 79%, and on average was normal and not different from controls (p = 0·85, appendix Table 2, p12). Slice-averaged basal and mid-ventricular native T1, a marker of fibrosis or inflammation on cardiac MRI, were significantly elevated in patients (p = 0·0001, p = 0·004, respectively) (Table 2, Fig. 1). Basal myocardial T1 was elevated (> 2 SD from average control T1) in 26% (13/50) of patients. Mid myocardial T1 was elevated in 8% (4/51) and average of base and mid myocardial T1 in 24% (12/50) of patients. Native T2, a marker of oedema, was not different between patients and controls (p = 0·80, 0·21 and 0·81 for basal, mid and apical values, respectively). Extracellular volume fraction (a measure of diffuse fibrosis) tended to be higher in the base but the numerical differences failed to reach statistical significance (Table 2). Focal fibrosis burden was mildly increased in patients. Both basal and mid-ventricular myocardial T1 correlated moderately (appendix, p2 for definition) with CRP and pro-calcitonin in patients (appendix Table 4, p21, Fig. 1), but not in controls (p > 0·1).
3.4. Liver health
Acute liver injury (appendix, p2) was seen in 31% (18/58) of patients. At 2-3 months, 11% had persistent liver injury (non-specific pattern) on blood tests (appendix Table 1, p9). On MRI, another 10% (5/52) of patients had signs of liver injury evidenced by increased iron-corrected liver T1 (Table 2, Fig. 1). Iron-corrected liver T1 is a histologically validated imaging biomarker of hepatic fibro-inflammation, [19] which has subsequently been developed in LiverMultiScan where it is increasingly being used to monitor the response of hepatitis to novel therapies. [20] By contrast, no statistically significant differences were seen in liver fat (p=0·18), iron (p=0·60) and extracellular volume fraction (a marker of diffuse fibrosis, p=0·60) between both groups. Iron-corrected liver T1 correlated moderately with systemic markers of inflammation (white cell count, neutrophil, monocyte count and CRP) in patients (appendix Table 4, p21), but also in controls.
3.5. Haematological system and spleen
Haematological abnormalities including lymphocytopenia and thrombocytopenia were seen in 47% (27/58) and 2% (1/58) of patients at admission respectively and an elevated CRP during admission (>10mg/L) was seen in 98% (57/58). At follow-up, all abnormalities in lymphocyte and platelet count returned to normal. However, the higher CRP in patients versus controls approached statistical significance (p = 0·058) (appendix, Table 1, p9). There were no statistically significant differences in splenic volume and tissue characteristics on MRI between patients and controls (p = 0.47, 0.37 and 0.93 for spenic volume, T1 and T2*, respectively) (appendix Table 2, p12).
3.6. Kidney health
Six (10%) patients developed acute kidney injury (Table 1 for breakdown and definition, appendix p2), of whom two required renal replacement therapy during admission. At 2-3 months, 3% (2/58) of patients had residual renal impairment which was not present prior to COVID-19. On average, creatinine and estimated glomerular filtration rate were not significantly different between patients and controls (p = 0·16 and 0·70, respectively, appendix Table 1, p9). Despite this, both average (left and right) renal cortical T1 and corticomedullary differentiation, markers of renal injury/fibro-inflammation, were abnormal in patients (Table 2, Fig. 1). A significantly higher renal cortical T1 (>2 SD than control mean) was seen in 29% (15/51) of patients. Patients with acute kidney injury during admission had a higher average renal cortical T1 (1711±90ms versus 1582±81ms, p=0·001) and lower corticomedullary differentiation (318±59 versus 405±83, p=0·016; appendix Figure 2, p28) compared to those without. Kidney oxygenation did not differ between patients and controls (p=0·51, appendix Table 2, p12). Average renal cortical T1 had a moderate correlation with markers of inflammation (CRP, pro-calcitonin) in patients at follow-up (appendix Table 4, p21).
3.7. Mental health and quality of life
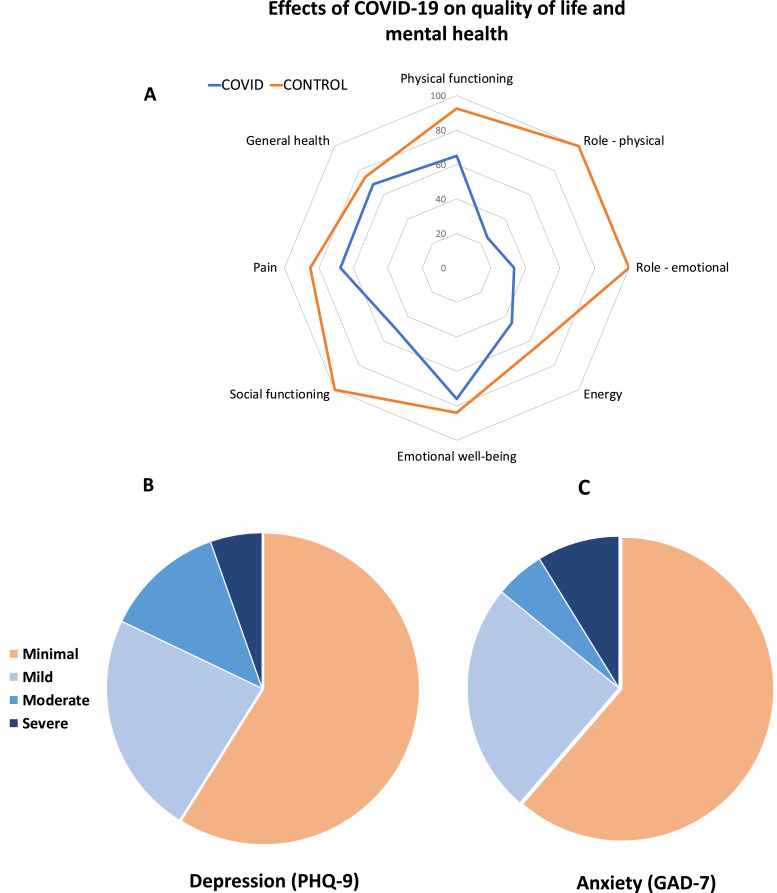
At 2-3 months, patients had higher cumulative self-reported symptom scores for depression (PHQ-9 median score 3 versus 1·5, p = 0·009) compared to controls (Table 4). Symptom score for anxiety also tended to be higher (GAD-7 median score 2·0 vs 0·5, p = 0·066) in patients compared to controls. 19% of patients reported symptoms of moderate to severe depression and 14% of patients had symptoms of moderate to severe anxiety (Fig. 2). Patients also reported a significantly reduced quality of life in all domains (Table 4, Fig. 2). Importantly, impairment in both physical and emotional health imposed significant role limitations among COVID-19 survivors. The severity of depression and anxiety did not consistently associate with markers of inflammation (except for monocyte count) or multiorgan injury among patients (appendix Table 4, p21). However, a moderate correlation was seen between extent of mood symptoms (PHQ-9 score) and anxiety (GAD-7 score) and ongoing breathlessness (MRC dyspnoea score) (PHQ-9 and MRC dyspnoea score: r=0·58, p<0·0001, GAD-7 and MRC dyspnoea score: r=0·41, p = 0·002).
Table 4.
Anxiety (GAD-7), depression (PHQ-9), quality of life (SF-36) and symptom (dyspnoea, fatigue) burden in patients and controls.
| COVID-19 | CONTROL | p-value | |
|---|---|---|---|
| GAD-7 | |||
| Score | 2·0 (0·0 - 7·5) | 0·5 (0·0 - 4·3) | 0·066+ |
| 0 – 4 (Minimal) | 35/57 (61·4%) | 23/30 (76·7%) | |
| 5 – 9 (Mild) | 14/57 (24·6%) | 6/30 (20·0%) | |
| 10 – 14 (Moderate) | 3/57 (5·3%) | 1/30 (3·3%) | |
| ≥15 (Severe) | 5/57 (8·8%) | 0/30 (0·0%) | |
| Moderate or worse anxiety | |||
| ≥10 (Moderate or more) | 8/57 (14%) | 1/30 (3.3%) | 0.16ᵋ |
| PHQ-9 | |||
| Score | 3·0 (1·0 - 7·5) | 1·5 (0·0 - 5·0) | 0·009+ |
| 0 - 4 (Minimal) | 33/57 (57·9%) | 21/30 (70·0%) | |
| 5 - 9 (Mild)– | 13/57 (22·8%) | 8/30 (26·7%) | |
| 10 - 14 (Moderate)– | 7/57 (12·3%) | 1/30 (3·3%) | |
| ≥15 (Moderately severe or severe) | 4/57 (7·0%) | 0/30 (0·0%) | |
| Moderate or worse mood symptoms | |||
| ≥10 (Moderate or more) | 11/57 (19·3%) | 1/30 (3·3%) | 0.051ᵋ |
| SF-36 Domains | |||
| Physical Functioning | 65·0 (45·0 - 90·0) | 92·5 (83·8 - 100·0) | <0·0001⁺ |
| Role Limitations Due to Physical Health | 25·0 (0·0 - 75·0) | 100·0 (100·0 - 100·0) | <0·0001⁺ |
| Role Limitations Due to Emotional Health | 33·3 (0·0 - 100·0) | 100·0 (100·0 - 100·0) | <0·0001⁺ |
| Energy | 45·0 (25·0 - 70·0) | 65·0 (55·0 - 80·0) | <0·0001⁺ |
| Emotional Well-Being | 76·0 (62·0 - 88·0) | 84·0 (72·0 - 92·0) | 0·044⁺ |
| Social Functioning | 50·0 (37·5 - 87·5) | 100·0 (62·5 - 100·0) | 0·0002⁺ |
| Pain | 67·5 (35·0 - 90·0) | 85·0 (67·5 - 100·0) | 0·003⁺ |
| General Health | 68·8 (43·8 - 81·3) | 75·0 (60·9 - 87·5) | 0·022⁺ |
| Dyspnoea - 12 symptom score | |||
| Median (IQR) | 4·0 (1·0 - 11·0) | 0·0 (0 -1·5) | <0·0001+ |
| Fatigue Severity Scale | |||
| Median (IQR) | 34 (18 - 49) | 17 (11 - 24) | 0·001+ |
| /≥4 | 30/55 (54·5%) | 5/29 (17·2%) | 0·010ᵋ |
| Medical Research Council Dyspnoea Scale | |||
| MRC grade 2 - 5 | 36/56 (64·3%) | 3/29 (10·3%) | <0·0001ᵋ |
Data are median (IQR) and n/N (%), where N is the total number of participants with available data. p-values from Mann-Whitney U test (+) or Fisher's exact test (ᵋ). GAD-7 = Generalised anxiety disorder-7 assessment. PHQ-9 = Patient health questionnaire-9 assessment. SF-36 = Short form 36. MRC = Medical Research Council Scale. Control subjects were matched for co-morbidities as closely as possible.
Fig. 2.
A: Quality of life (Short Form-36) radar plot for patients and controls. B,C: Burden of depression and anxiety among patients. A: The radar plot demonstrates that patients with COVID-19 (blue line) were more likely to experience impairment in energy, general health, physical health, social and emotional well-being and increased pain when compared to controls (orange line). Both physical and emotional factors caused significant role limitations among patients. B, C: 19% of hospitalised COVID-19 patients had moderate to severe self-reported symptoms of depression and 14% of hospitalised COVID-19 patients had moderate to severe self-reported symptoms of anxiety.
3.8. Severity of disease and persistent inflammation
Severity of illness during admission (WHO ordinal scale) (appendix Table 4, p21, Table 5, p23) correlated moderately with inflammatory markers (Procalcitonin, CRP, white cell count, neutrophil count monocyte count) at follow-up, signs of persistent inflammation/injury in the lungs, liver, kidneys, and exercise tolerance. Notably, even in patients who were not critically ill (i.e, those who were not intubated or ventilated or receiving vasopressor/ionotropic support or renal replacement therapy), MRI evidence of lung, cardiac, kidney and brain abnormalities could be seen (appendix Table 6, p24). Hospitalised-patients with more severe disease were more likely to also experience persistent breathlessness (r = 0·268, p = 0·046), but severity of illness did not predict risk of depression or anxiety.
4. Discussion
The present holistic study uniquely characterised the medium-term effects of COVID-19 infection on multiple vital organs, functional capacity, mental health and cognition in post-hospital survivors of moderate to severe infection. The key findings of our study are: First, at 2-3 months from disease-onset, a proportion of patients displayed abnormalities in the lungs, brain, heart, liver and kidneys on MRI. Second, the severity of acute illness during admission correlated with some markers of multiorgan injury at follow-up. Third, limitations in exercise tolerance (CPET and six-minute walk distance) and imaging biomarkers associated with blood inflammatory markers. Deconditioning as assessed by CPET, symptoms of persistent breathlessness, and fatigue were prominent among patients and interfered with activities of daily living and quality of life. Finally, patients had a higher burden of self-reported mood symptoms which related to symptoms of persistent breathlessness at follow-up.
Studies examining the temporal evolution of lung abnormalities on serial high-resolution CT scans have revealed that persistent inflammatory changes may be seen in up to 71% of COVID-19 survivors at two to three months post discharge. [7,21] Consistent with this, we observed a high proportion of parenchymal abnormalities on lung MRI, albeit at a lower frequency than that seen on CT. Previous investigations have shown that survivors of SARS pneumonia can be left with more permanent lung damage [22] and abnormalities in lung function for months to even years after infection. In our study, 13% of patients exhibited abnormalities on spirometry (FVC) at 2–3 months. Although we were unable to assess diffusion capacity (DLCO) in our patients, our findings are in line with a recent report by Mo and colleagues, who demonstrated similar anomalies on spirometry and additionally described an impairment in DLCO in up to 47% of cases. [23]
Occult neurological injury has been suspected in COVID-19 due to a high burden of non-specific neurological symptoms. [24] Although neurological symptoms were frequent (~50%) in our unselected cohort, imaging evidence of severe neurologic injury on MRI was rare. Nevertheless, patients demonstrated increased bilateral thalamic T2* signal on susceptibility-weighted imaging and increased mean diffusivity in posterior thalamic radiations and sagittal stratum. Susceptibility-weighted imaging is often used to detect blood breakdown products and calcification. [25] That these abnormalities could reflect a higher burden of microvascular events among COVID-19 survivors is tentatively supported by a slightly increased volume of white matter hyperintensities among patients. This would be consistent with the higher frequency of cerebrovascular events reported by others. [24] While the exact pathophysiology underlying the cerebrovascular disease is yet to be clarified, it is possible that a combination of hypercoagulable state acutely and chronic neuroinflammatory processes, supported by the association of white matter hyperintensity volumes, [26] T2* abnormalities and inflammatory markers, play an important role. The cognitive profile observed (primarily dysexecutive) among patients is also consistent with a vascular pattern, replicating previous reports of dysexecutive syndrome in COVID-19 survivors [27]. Although this cross-sectional data limit the extent to which causal associations can be made, our findings suggest a potential link between COVID-19 and future risk of cognitive decline, [28] given that both white matter hyperintensities [29], [30], [31] and vascular injury [32] on brain MRI are emerging as potent predictors of dementia risk in individuals.
Evidence of acute myocardial injury can be seen in up to a third of hospitalised patients with moderate to severe SARS-CoV-2 infection and associates with fatal outcomes. [2,33] Cardiac MRI can to be particularly useful in providing a diagnosis in patients with suspected cardiac involvement. [34] In a recent study by Puntmann and colleagues, [35] cardiac MRI showed evidence of a high burden of inflammation (60% of patients), as seen by elevated native T1, T2 and some biventricular impairment in convalescing patients, a third of whom required hospitalisation. In our study of previously hospitalised patients, only 26% had a significantly elevated native T1. Native T2 and cardiac function did not differ from our risk-factor matched cohort, consistent with an earlier study [34]. A point worth noting is that differences in prevalence estimates on MRI studies may arise from variations in ‘reference ranges’, methodological differences, and patient characteristics. Our approach to use a risk-factor matched control group (prospectively enrolled under identical scan conditions) as reference suggests that abnormal MRI tissue characteristics in 26% of patients could not be explained by the comorbidities alone. Furthermore, myocardial native T1 moderately correlated with serum markers of inflammatory response (CRP, white cell count, neutrophil count and procalcitonin), indicating myocardial tissue abnormalities were linked to inflammation.
Several independent investigations [3,4] have confirmed a high prevalence of acute liver injury in hospitalised patients. Potential mechanisms include hyperinflammatory syndrome, hypoxia-mediated metabolic derangements, venous thrombosis and drug-induced hepatitis. [36] Direct infection of cholangiocytes has also been suggested, as SARS-CoV-2 may injure the bile ducts by binding to ACE2 receptors. [37] We observed that 11% of patients had persistent blood biomarker evidence of liver injury at 2–3 months, and another 10% demonstrated increased iron-corrected liver T1, a marker of liver fibro-inflammation [19]. Iron-corrected liver T1 also correlated with serum biomarkers of inflammation, providing further evidence that multiorgan health is worse in those with a higher burden of inflammation.
The kidneys are amongst the most common targets of SARS-CoV2, with acute kidney injury reported in 0.5–37% of hospitalised patients. [[2], [3], [4],38,39] Direct infection of renal cells may occur via ACE2 receptors which are enriched in podocytes and endothelial cells. [40] Associated histopathological abnormalities include prominent lymphocytic endothelitis, acute tubular necrosis, diffuse erythrocyte aggregation, peritubular obstruction and podocyte injury. [40] We showed that 29% of patients had abnormal renal tissue characteristics on MRI. In particular, renal cortical T1, a marker of renal inflammation/injury was prolonged and accompanied by a loss of corticomedullary differentiation, reminiscent of other post-inflammatory glomerulonephritides. [41] Patients with more severe disease (i.e, acute kidney injury, need for higher oxygen support) and higher inflammatory burden were more likely to have abnormal cortical T1 and corticomedullary differentiation at follow-up.
Chronic inflammation represents a sustained reaction of the immune system to an inflammatory stimulus (i.e, viral nucleic acid) accompanied by tissue damage. [42] The association of multiple imaging markers of organ abnormalities and inflammatory response in patients, but not in controls, raises the possibility that persistent inflammation could play a role in mediating multiorgan abnormalities. [43] Another explanation is that COVID-19 recovery is slow, resulting in persistence of tissue abnormalities and increased CRP even at 3 months from disease onset. Although critical illness has also been shown in prior studies to associate with systemic inflammation [44], we found that MRI evidence of multiorgan abnormalities was not limited to patients with critical disease alone. Further efforts to understand the mechanisms underlying multiorgan damage, and strategies to arrest them could limit the long-term detrimental effects of COVID-19 on vital organs.
Insights from earlier studies of SARS survivors [45] have raised concerns that limitations in exercise tolerance may persist for months after infection. In our study, patients achieved a shorter six-minute walk distance, lower peak VO2 and lower % of predicted VO2 max at the anaerobic threshold (VT1). VE/VCO2 slope, a measure of ventilatory efficiency, was worse in patients with parenchymal abnormalities and both VE/VCO2 and six-minute walk distance correlated with markers of systemic inflammation. Of note, many patients stopped CPET early because of generalised muscle ache and fatigue rather than breathlessness. These findings suggest that muscle wasting, secondary to a catabolic state induced by severe illness [46] and potentially inflammation [47], may also contribute to exercise limitations in the patients with moderate to severe infection.
In addition to coping with the debilitating acute effects of COVID-19, survivors experience a range of mental stressors whilst in-hospital and after discharge. We and others [48,49] have observed a high-level of self-reported symptoms of depression among survivors. Infection-triggered cytokine dysregulation and the neurotropic potential of SARS-CoV-2 have widely been speculated to induce psychopathological sequelae among patients, consistent with neuroinflammatory mechanisms implicated in other psychiatric disorders. [50] Here, although the burden of ongoing symptoms of breathlessness associated with mood and anxiety symptoms, we did not see a consistent association between disease severity and depression. Given the limited sample size of our study, a more focussed approach in a larger cohort could yield further insights into such relationships and offers the potential to identify novel targets for neuropsychiatric therapeutic modulation.
The relatively small sample size of this single-centre study, cross-sectional nature of some assessments and lack of correction for multiple comparisons are important limitations which curtail the generalisability of our findings and accuracy of prevalence estimates. The lack of pre-COVID-19 imaging limits our ability to make causal inferences about the mechanism of MRI tissue abnormalities. However, this is the first exploratory study to comprehensively assess multiple vital organs, mental, cognitive and physical health in patients with COVID-19 post-hospital discharge. These findings underscore the need for further large scale investigations as is currently planned by Public Health England through the Post-HOSPitalisation COVID-19 (PHOSP-COVID) [51] national consortium and its two MRI sub-studies C-MORE and COVERSCAN. The use of lung MRI could have underestimated the prevalence of lung injury. Controls in our study were not hospitalised, thus group differences may not be COVID-19 specific. There were ethnoracial differences between the control and patients enrolled in this study which may have contributed to prevalence estimates of multiorgan injury. Finally, whether the findings on MRI have any long term clinical implications remains to be determined by further longitudinal follow-up studies.
Our findings underscore the need to further understand the pathophysiological mechanisms underpinning multiorgan MRI tissue abnormalities and to provide a holistic-integrated multidisciplinary model of clinical care for patients recovering from COVID-19 post hospital discharge.
Disclaimer
The views expressed are those of the authors and not necessarily those of the National Health Service, NIHR, or the United Kingdom Department of Health.
Data sharing statement
Individual de-identified participant data will be made available when the trial is complete, or upon requests directed to the corresponding author; after approval of a proposal, data can be shared through a secure online platform.
Author contribution
BR and SN had the idea for and designed the study with the help of all co-authors and had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. BR and HL were involved in application of ethics. MM, BR, CK, RM and MC collected the data. PJ, EMT, RM, KM, SS, SM, CM were involved in developing the MRI protocol. EOO, MC, LG, SM, FA, TO, FKM, CW, CA, FL, JA, MJ, SS, EMT, XC, BR, MW, AL, FEM performed the analysis. BR, MC and SN drafted the paper. All authors critically revised the manuscript for important intellectual content and gave final approval for the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of Competing Interest
Dr. Raman reports grants from NIHR Oxford Biomedical Research Centre, grants from United Kingdom Research Innovation Award, during the conduct of the study. Dr. Cassar reports grants from NIHR Oxford Biomedical Research Centre, during the conduct of the study. Dr. Tunnicliffe reports grants from NIHR Oxford Biomedical Research Centre, during the conduct of the study; shareholding in Perspectum, outside the submitted work; In addition, Dr. Tunnicliffe has a patent Systems and methods for gated mapping of T1 values in abdominal visceral organs GB2497668B licensed to Perspectum, a patent Multi-parametric magnetic resonance diagnosis and staging of liver disease GB2498254B licensed to Perspectum, and a patent Processing MR relaxometry data of visceral tissue to obtain a corrected value of relaxometry data based on a normal iron content for the visceral tissue GB2513474B licensed to Perspectum. Dr. Okell reports grants from Wellcome Trust/Royal Society, during the conduct of the study; personal fees from SBGNeuro, personal fees from Oxford University Press, personal fees from Siemens Healthineers, outside the submitted work; In addition, Dr. Okell has a patent Combined angiography and perfusion using radial imaging and arterial spin labeling pending, a patent Off-resonance Correction for Pseudo-continuous Arterial Spin Labeling pending, a patent Estimation of blood flow rates issued, a patent Fast analysis method for non-invasive imaging of blood flow using vessel-encoded arterial spin labelling with royalties paid to Siemens Healthineers, and a patent Quantification of blood volume flow rates from dynamic angiography data with royalties paid to Siemens Healthineers. Dr. Lewandowski reports non-financial support from Perspectum as a minority share-holder. Dr. Jenkinson reports personal fees from Oxford University Innovations, outside the submitted work. Dr. Channon reports grants funding from the British Heart Foundation and the National Institute for Health Research. Dr. Ferreira reports grants from British Heart Foundation, grants from National Institute Health Research Oxford Biomedical Research Centre, during the conduct of the study. Dr. Piechnik has a patent US patent 61/387,591 licensed to Siemens, a patent US patent 61/630,508 licensed to Perspectum, and a patent US patent 61-630,510 licensed to Perspectum. Dr. Pavlides reports other from Perspectum, outside the submitted work. Dr. Neubauer reports grants from Oxford NIHR Biomedical Research Centre, grants from UKRI, during the conduct of the study; personal fees and other from Perspectum Diagnostics, outside the submitted work; In addition, Dr. Neubauer has a patent Multi-parametric magnetic resonance diagnosis & staging of liver disease licensed to Perspectum.
Acknowledgments
Acknowledgements
We thank our patients and their families who have sought to help others understand about the effects of COVID-19. We are grateful to the University of Oxford and Oxford University Hospital Trust for their support of this study. We would like to acknowledge OCMR staff, Ms Polly Whitworth, Ms Joanna Leal, Ms Claudia Nunes, Ms Harriet Nixon, Ms Injung Jang, Ms Maryam Khan, Ms Gillian Roberts, Mr Mike Mcdonald, Ms Jasmine Taylor, Dr Ahmed Moola, Ms Sarah White, Dr Innes Abdel Assam, for their help with this work. We acknowledge the support of Siemens in providing WIP 1048 for cardiac T1 mapping and a licence for the use of ASL.
Funding
NIHR Oxford and Oxford Health Biomedical Research Centres, British Heart Foundation Centre for Research Excellence, UKRI, Wellcome Trust, British Heart Foundation. The authors’ work was supported by NIHR Oxford and Oxford Health Biomedical Research Centre, Oxford British Heart Foundation (BHF) Centre of Research Excellence (RE/18/3/34214), United Kingdom Research Innovation and Wellcome Trust. This project is part of a tier 3 study (C-MORE) within the collaborative research programme entitled PHOSP-COVID Post-hospitalisation COVID-19 study: a national consortium to understand and improve long-term health outcomes. Funded by the Medical Research Council and Department of Health and Social Care/National Institute for Health Research Grant (MR/V027859/1) ISRCTN number 10980107. This work also arises from one of the national "COVID-19 Cardiovascular Disease Flagship Projects" designated by the NIHR-BHF Cardiovascular Partnership. P.J. thanks the Dunhill Medical Trust for support.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100683.
Appendix. Supplementary materials
References
- 1.World Health Organisation. WHO coronavirus disease (COVID-19) dashboard. Accessed on August 29, 2020. https://covid19.who.int/
- 2.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet North Am Ed. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet North Am Ed. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W-j, Ni Z-y, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puelles VG, Lütgehetmann M, Lindenmeyer MT. Multiorgan and Renal Tropism of SARS-CoV-2. N Engl J Med. 2020 doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A, Madhavan MV, Sehgal K. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y-m, Shang Y-m, Song W-b. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020 doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham BL, Steenbruggen I, Miller MR. Standardization of spirometry 2019 update. An official American thoracic society and European respiratory society technical statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mezzani A. Cardiopulmonary exercise testing: basics of methodology and measurements. Ann Am Thoracic Soc. 2017;14(Supplement 1):S3–S11. doi: 10.1513/AnnalsATS.201612-997FR. [DOI] [PubMed] [Google Scholar]
- 10.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 12.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992:473–483. [PubMed] [Google Scholar]
- 13.Nasreddine ZS, Phillips NA, Bédirian V. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 14.Bestall J, Paul E, Garrod R, Garnham R, Jones P, Wedzicha J. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yorke J, Moosavi SH, Shuldham C, Jones PW. Quantification of dyspnoea using descriptors: development and initial testing of the Dyspnoea-12. Thorax. 2010;65(1):21–26. doi: 10.1136/thx.2009.118521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organisation. COVID-19 Therapeutic Trial Synopsis Accessed Aug 2, 2020. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf (accessed August 2 2020).
- 18.Miller KL, Alfaro-Almagro F, Bangerter NK. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci. 2016;19(11):1523–1536. doi: 10.1038/nn.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavlides M, Banerjee R, Tunnicliffe EM. Multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease severity. Liver Int. 2017;37(7):1065–1073. doi: 10.1111/liv.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomaides-Brears HB, Lepe R, Banerjee R, Duncker C. Multiparametric MR mapping in clinical decision-making for diffuse liver disease. Abdominal Radiol. 2020:1–16. doi: 10.1007/s00261-020-02684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zha L, Shen Y, Pan L. Follow-up study on pulmonary function and radiological changes in critically ill patients with COVID-19. J Infect. 2020;(20) doi: 10.1016/j.jinf.2020.05.040. S0163-445330317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hui D, Joynt G, Wong K. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mo X, Jian W, Su Z. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55(6) doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varatharaj A, Thomas N, Ellul MA, CoroNerve Study Group Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875–882. doi: 10.1016/S2215-0366(20)30287-X. Epub 2020 Jun 25. Erratum in: Lancet Psychiatry. 2020 Jul 14; PMID: 32593341; PMCID: PMC7316461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mittal S, Wu Z, Neelavalli J, Haacke EM. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. Am J Neuroradiol. 2009;30(2):232–252. doi: 10.3174/ajnr.A1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verhaaren BF, Debette S, Bis JC. Multiethnic genome-wide association study of cerebral white matter hyperintensities on MRI. Circ Cardiovasc Genet. 2015;8(2):398–409. doi: 10.1161/CIRCGENETICS.114.000858. Epub 2015 Feb 7. PMID: 25663218; PMCID: PMC4427240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helms J, Kremer S, Merdji H. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: a study of 62,354 COVID-19 cases. Lancet Psychiatry. 2020 doi: 10.1016/S2215-0366(20)30462-4. S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brickman AM, Provenzano FA, Muraskin J. Regional white matter hyperintensity volume, not hippocampal atrophy, predicts incident Alzheimer disease in the community. Arch Neurol. 2012;69(12):1621–1627. doi: 10.1001/archneurol.2012.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolfson L, Wakefield DB, Moscufo N. Rapid buildup of brain white matter hyperintensities over 4 years linked to ambulatory blood pressure, mobility, cognition, and depression in old persons. J Gerontol Series A. 2013;68(11):1387–1394. doi: 10.1093/gerona/glt072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tabei K-i, Kida H, Hosoya T, Satoh M, Tomimoto H. Prediction of cognitive decline from white matter hyperintensity and single-photon emission computed tomography in Alzheimer's disease. Front Neurol. 2017;8:408. doi: 10.3389/fneur.2017.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miwa K, Tanaka M, Okazaki S. Multiple or mixed cerebral microbleeds and dementia in patients with vascular risk factors. Neurology. 2014;83(7):646–653. doi: 10.1212/WNL.0000000000000692. [DOI] [PubMed] [Google Scholar]
- 33.Guo T, Fan Y, Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knight DS, Kotecha T, Razvi Y. COVID-19: Myocardial injury in survivors. Circulation. 2020;142(11):1120–1122. doi: 10.1161/CIRCULATIONAHA.120.049252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puntmann VO, Carerj ML, Wieters I. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H, Liu L, Zhang D. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395(10235):1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chai X, Hu L, Zhang Y. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020 2020.02.03.931766. [Google Scholar]
- 38.Hirsch JS, Ng JH, Ross DW. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arentz M, Yim E, Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su H, Yang M, Wan C, Yi L, Tang F, Zhu H. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham-Brown M, Singh A, Wormleighton J. Association between native T1 mapping of the kidney and renal fibrosis in patients with IgA nephropathy. BMC Nephrol. 2019;20(1):256. doi: 10.1186/s12882-019-1447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furman D, Campisi J, Verdin E. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang L, Liu S, Liu J. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Trans Targeted Ther. 2020;5(1):1–8. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffith DM, Lewis S, Rossi AG. Systemic inflammation after critical illness: relationship with physical recovery and exploration of potential mechanisms. Thorax. 2016;71(9):820–829. doi: 10.1136/thoraxjnl-2015-208114. [DOI] [PubMed] [Google Scholar]
- 45.Ong KC, Ng A-K, Lee L-U. Pulmonary function and exercise capacity in survivors of severe acute respiratory syndrome. Eur Respir J. 2004;24(3):436–442. doi: 10.1183/09031936.04.00007104. [DOI] [PubMed] [Google Scholar]
- 46.Herridge MS, Tansey CM, Matté A. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 47.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98(3):911–917. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 48.Mazza MG, De Lorenzo R, Conte C. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu C, Hu X, Song J. Mental health status and related influencing factors of COVID-19 survivors in Wuhan, China. Clin Transl Med. 2020;10(2):e52. doi: 10.1002/ctm2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.NIHR Leicester Biomedical Research Centre. Post-HOSPitalisation COVID-19 study – a national consortium to understand and improve long-term health outcomes. Accessed on 9th September 2020. https://www.leicesterbrc.nihr.ac.uk/themes/respiratory/research/phosp-covid/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.