Abstract
Background
Telomere length (TL) is considered a biological marker of aging and may indicate age-related disease susceptibility. Adults and children show a fixed ranking and tracking of TL over time. However, the contribution of an individual's initial birth TL to their later life TL is unknown. We evaluated change and tracking of TL from birth to child- and adulthood.
Methods
Telomere length at birth was measured using qPCR in two independent prospective birth cohorts. After a median follow-up period of 4 years in ENVIRONAGE (n = 273) we assessed leukocyte telomere length (LTL) and after 23 years in EFPTS (n = 164) buccal TL was assessed. Correlations and multivariable regression models were applied to study telomere tracking and determinants of TL change from birth onwards.
Findings
In children, LTL at the age of 4 correlates with TL at the start of life both in cord blood (r = 0.71, P < 0.0001;) and placenta (r = 0.60, P < 0.0001) and was –11.2% and –33.1% shorter, respectively. In adulthood, buccal TL at the age of 23 correlates with placental TL (r = 0.46, P < 0.0001) and was –35.9% shorter. TL attrition was higher in individuals with longer birth TL. However, based on TL ranking, individuals do not tend to change dramatically from TL rank after 4 or 23 years of follow-up. Finally, longer maternal TL associates with lower telomere attrition in the next generation.
Interpretation
The high prediction of newborn TL for later life TL, and stable TL ranking from birth onwards underscores the importance of understanding the initial setting of newborn TL and its significance for later life.
Funding
European Research Council (ERC-StG310898) and Flemish Scientific Fund (12X9620N).
Keywords: Newborn telomere length, Telomere tracking, Telomere dynamics, Early life aging
Research in context.
Evidence before this study
So far, the main focus of telomere research has been on adults, whereas telomere research on newborns remains rather limited. Animal based studies suggest that telomere length in early life predicts lifespan, and it is hypothesised that early life telomere length may predict later life telomere length and may indicate susceptibility for later life diseases. We searched PubMed for studies published before September 20, 2020 with search terms “telomere tracking, newborn”, “cord blood, placenta telomere length, tracking”. In adults and children telomere tracking over time suggests that an individual's telomere length at a given time point is predictive for their telomere length at a later time point in life.
Added value of this study
We studied newborn telomere length tracking and changes from birth to child- and adulthood in two birth cohorts. In this study we were able to show the strong predictive potential of newborn telomere length for telomere length later in life. Irrespective of the studied biological matrix (cord leukocytes or placenta), newborn telomere length predicts telomere length in leukocytes at the age of 4 years. Furthermore, we explored in an independent cohort placenta telomere length and showed that it was predictive for buccal telomere length at the age of 23 years. Finally, we showed that a higher telomere attrition occurs in boys compared with girls and that newborns from mothers with longer telomeres show a slower telomere attrition during the first 4 years of life.
Implications of all available evidence
Our results shed new insights on the consequences of newborn telomere length. Telomere length at birth is the most important predictor for later life telomere length. These results may have an important impact on the interpretation of current adult population-based telomere length studies. As observations in adults show that telomere length is associated with age-related health and disease conditions, our results show that these observations may find their origin to some extent at birth. Telomere length related health and disease later in life may be programmed at birth. Therefore, understanding the setting of initial telomere length may further gain insights in the developmental origins of health and disease.
Alt-text: Unlabelled box
1. Introduction
Telomeres, the nucleoprotein ends of human chromosomes, are essential genomic protectors, preventing chromosome ends being recognized as broken [1]. They consist of conserved tandem TTAGGG repeats connected with shelterin proteins [2]. DNA replication cannot fully be completed after each cell division, leading telomeres to shorten over time. Telomere length (TL) has been considered a biomarker of aging, and short telomeres are associated independently of chronological age with cardiovascular disease risks and mortality [3,4]. It has been stated that TL may be a biological indicator for an individual's lifespan and disease-susceptibility [5].
Telomere length shows a high variability in same-aged individuals, and a comparable variability in TL is present at birth [6]. Based on child- and adult longitudinal follow-up studies, it is shown that during these different phases of life, humans have a fixed telomere ranking and telomere tracking (i.e. individuals with a long/short TL at a given time point in life will preserve his or her long/short TL at a later phase in life) [7,8].
Therefore, is it assumed and most likely that an individual's later life TL is highly determined by its TL at birth and by TL attrition in early life. In the latter case, TL at birth can be considered to be an essential programmed biological marker that may shape a part of the later life human aging-phenotype. This makes evaluating and defining the genetic and non-genetic landscape affecting newborn TL more relevant in understanding the concept of fetal programming of health and disease later in life [6,9,10]. We therefore evaluated the predictive role of TL at birth for later life TL in transition to child- and adulthood in two independent longitudinal prospective cohort studies.
2. Methods
2.1. Study populations
The Environmental Influence on Ageing in early life study (ENVIRONAGE) is an ongoing prospective birth cohort initiated in 2010 [11]. Mother-newborn pairs are recruited at birth and invited for a follow-up visit around the age of 4 years. At birth, cord blood and placental tissue are collected. Furthermore, a blood sample is collected from consenting mothers at delivery. Between October 2014 and October 2019, 439 mother-child pairs had a follow-up examination. 293 (69.3%) children and parents gave consent for a venous blood sample. Based on available biological samples and exclusion of improper TL measurements (n = 6 cord blood, n = 1 placenta, n = 2 child at 4-year follow-up, n = 5 mothers at baseline), the final ENVIRONAGE population size to study telomere tracking consists of 273 children with matching baseline (cord blood) and follow-up (leukocyte) TL (see Fig. 1a for inclusion/exclusion of participants). Furthermore, for these children data were available for 242 placenta TLs, and 211 maternal TLs at delivery. For 186 of these participants a match between placenta TL and maternal TL was available. The ENVIRONAGE study protocol is approved by the ethical committees of Hasselt University (Diepenbeek, Belgium, reference no. B371201216090 and B371201524537) and East-Limburg Hospital (Genk, Belgium) and has been carried out according to the Helsinki declaration. Mothers provided written informed consent at birth and follow-up. The selected population is representative for the baseline ENVIRONAGE population and the reproductive segment of the population at large [12] as for parental age, sex, gestational age and birth weight but slightly differed in maternal education and newborn ethnicity (Supplementary Table 1).
Fig. 1.
Flowchart for selecting participants in ENVIRONAGE (a) and EFPTS (b). For ENVIRONAGE 912 mother-child pairs were eligible for a follow-up study. In total 439 mother-child pairs participated due to a loss of contact from 145 (15.6%), 321 refused participation (35.2%) and 7 (0.8%) could not participate due to language difficulties. A total of 293 (66.7%) mothers and children provided consent for blood drawn, resulting in 273 children with baseline TL (cord blood) and follow-up LTL. The EFPTS study comprised 242 twins with both a placental and buccal swab sample from the Loos et al., 2001 study [14], of which 164 twins had a proper TL determined in both placenta and buccal swab.
The East Flanders Prospective Twin Survey (EFPTS) is a population based register of multiple births started in 1964 in the East Flanders province of Belgium [13]. Due to its prospective character with long follow-up period from birth to later life (23-years), and the availability of biological samples at both occasions, the EFPTS was used as a validation cohort upon the findings from birth till childhood based on ENVIRONAGE. We obtained historically stored placental tissue from 242 participants born between 1969 and 1982, who participated at a 23-year follow-up visit from 1997 to 2000 with an available buccal swab (see Fig. 1b for inclusion/exclusion of participants) [14]. We excluded 78 participants, of whom 38 no qualitative buccal DNA could be extracted, 29 we could not extract a sufficient amount of buccal DNA to perform the qPCR assay. Finally, 11 participants were excluded of which the buccal or placental TL showed a high variation between the technical qPCR triplicates. Using data recorded by the obstetrician at birth and questionnaires during later stages and at follow-up, basic perinatal and demographic characteristics were obtained. The selected participants did not differ from the baseline characteristics according to parental age, sex, gestational age and birth weight, but slightly differed in maternal education, parity and zygosity-chorionicity (Supplementary Table 2). The EFPTS study was approved by the Ethics Committee of the Faculty of Medicine of Leuven University (Leuven, Belgium, reference no. B32220096237), and written informed consent was obtained from all participants.
2.2. Perinatal, postnatal and demographic factors
For ENVIRONAGE detailed population characteristics are collected using medical records, filled-out questionnaires by the mothers at delivery and follow-up. Data on parental ages, newborn sex, gestational age, birth weight and maternal education are collected. Maternal education at delivery is coded “low” when mothers did not obtain a diploma, “middle” when obtained a high school diploma and “high” when obtained a college or university degree. Gestational complications are defined as present when mothers experienced one or more of the following conditions during pregnancy: gestational diabetes, hypertension, preeclampsia or infectious diseases. Newborn prenatal smoke exposure was coded as present when mothers actively smoked during pregnancy. Postnatal smoke exposure was coded as present when one or both parents indicated to smoke indoors during the follow-up period. At the follow-up visit at the age of 4, height was measured to the nearest centimeter and weight to the nearest 0.1 kg. Body-mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Adverse health conditions of the child were identified as the experience of one or more of the following conditions: diagnosed asthma, hay fever, topical corticosteroid treated eczema or immunodeficiency.
For EFPTS maternal education, gestational complications and prenatal smoke exposure are coded using similar definitions in ENVIRONAGE. Postnatal smoke exposure was defined by the current smoking status of the followed-up adult and was indicated as present when actively smoking. Adverse health conditions were defined as the presence of one or more of the following conditions during follow-up: hypertension, diabetes, heart-related condition (congenital malformation of cardiac septum), and kidney-related inflammation (tubulointerstitial nephritis).
2.3. Relative average telomere length
Detailed sample collection procedures and TL-assay specifications are provided in Supplementary Text 1. TL was measured using an adapted qPCR method described by Cawthon, 2009 [15]. TL at birth for ENVIRONAGE [16] and EFPTS [17] have been evaluated previously, however for this study all TLs were re-analysed in triplicate with inclusion of follow-up TLs and maternal TL (ENVIRONAGE) to ensure that matching samples for individuals were assayed at the same occasion and using the same method. ENVIRONAGE telomeres were measured in a single batch, in which samples from the same individuals are matched on the same qPCR plate (cord blood, placenta, child leukocytes at follow-up, and maternal leukocytes at delivery). EFPTS telomeres were measured in a separate batch in which baseline (placental) and follow-up (buccal) samples were matched per individual on the same qPCR plate. Telomere lengths were expressed as the ratio of telomere copy number to single-copy gene number (T/S) relative to the mean T/S ratio of the entire sample for ENVIRONAGE and EFPTS separately. Assay reliability parameters are provided in Supplementary Text 1.
2.4. Statistical analysis
Data was analysed using the SAS 9.4 enterprise software. Differences between groups were evaluated using paired and unpaired t-tests. We expressed telomere characteristics as a % difference with 95%CI. Pearson correlations were used to estimate the correlation between newborn TL and LTL at childhood in ENVIRONAGE. As TL was not normally distributed in EFPTS, Spearman correlation was used to study TL tracking from birth to adulthood.
Change in TL over time was calculated as the difference between follow-up TL and birth TL (TLfollow-up–TLbirth). Using multivariable adjusted general linear models, determinants of TL change rates from birth to childhood (ENVIRONAGE) and adulthood (EFPTS) were studied. The TL change rate was calculated as the % change in TL between follow-up and baseline divided by follow-up time, and this was corrected for the regression to the mean effect as formulated by Verhulst and colleagues [18]. Newborn and parental factors included in the models were: birth TL (cord blood or placenta), gestational age, birth weight, newborn sex, maternal age, paternal age. In addition, for ENVIRONAGE, maternal LTL at delivery was included as a potential determinant. Twins from the EFPTS were analysed as individuals, and given the relatedness between twin members, we used a mixed-effects model, with family as random intercept and adjustment for zygosity and chorionicity as a fixed effect. Effect estimates of the multivariable adjusted models are presented as %change in TL/year with corresponding 95% CI. Finally, telomere ranking [7] was studied by dividing newborns into quintiles of the TL distribution at birth and follow-up. The change in ranking was calculated as follows: follow-up quintile – birth quintile.
2.5. Sensitivity and secondary analyses
As a sensitivity analysis we evaluated the impact of gestational complications and the experience of adverse health conditions during the follow-up on the correlations between newborn TL and later life TL in ENVIRONAGE and EFPTS. Furthermore, as a secondary analysis we additionally evaluated whether gestational complications, maternal education at delivery, prenatal smoke exposure, postnatal smoke exposure, BMI at follow-up and adverse health conditions during follow-up were associated with TL change rates.
2.6. Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and accepts responsibility to submit for publication.
3. Results
3.1. ENVIRONAGE and EFPTS study populations
Demographic characteristics for the ENVIRONAGE and EFPTS study participants are provided in Table 1. ENVIRONAGE included 136 newborn girls (49.8%), and newborns had a mean (SD) gestational age of 39.2 (1.4) weeks and a birth weight of 3380 (461) grams. At delivery the mean age of mothers and fathers were 30.2 (4.4) and 32.7 (5.6) years, respectively. In the ENVIRONAGE cohort, median follow of the newborns was 4.5 years (5th–95th percentile: 4.1–5.4).
Table 1.
Descriptive characteristics of study participants.
| Characteristic | ENVIRONAGE (n = 273) | EFPTS (n = 164)1 |
|---|---|---|
| Newborn | ||
| Gestational age | 39.2 ± 1.4 | 37.5 ± 2.2 |
| Birth weight, g | 3380 ± 461 | 2619 ± 496 |
| Girls, n | 136 (49.8%) | 82 (50.0%) |
| Zygosity - Chorionicity | ||
| Dizygotic - Dichorionic | NA | 48 (29.3%) |
| Monozygotic - Dichorionic | NA | 54 (32.9%) |
| Monozygotic - Monochorionic | 62 (37.8%) | |
| Adverse health conditions during follow-up, n | 29 (10.6%) | 28 (17.1%) |
| Prenatal smoke exposure, n | 35 (12.8%) | 23 (14.5%) |
| Postnatal smoke exposure, n | 95 (34.8%) | 54 (32.9%) |
| Follow-up age, years | 4.6 ± 0.4 | 23.2 ± 3.3 |
| Follow-up BMI, kg/m2 | 16.0 ± 1.2 | 21.5 ± 2.7 |
| Telomere length | ||
| Baseline cord blood, T/S ratio | 1.13 ± 0.24 | NA |
| Baseline placenta, T/S ratio2 | 1.50 ± 0.34 | 1.27 ± 0.58 |
| Follow-up leukocyte, T/S ratio | 1.00 ± 0.17 | NA |
| Follow-up buccal, T/S ratio | NA | 0.81 ± 0.24 |
| Maternal | ||
| Age | ||
| Baseline age, years | 30.2 ± 4.4 | 27.0 ± 4.3 |
| Follow-up age, years | 35.3 ± 4.4 | 50.1 ± 5.4 |
| Education at delivery | ||
| Low, n | 26 (9.5%) | 68 (42.8%) |
| Middle, n | 77 (28.2%) | 34 (21.4%) |
| High, n | 170 (62.3%) | 57 (35.8%) |
| Gestational complications, n | 24 (8.8%) | 30 (18.9%) |
| Telomere length | ||
| Baseline leukocyte, T/S ratio3 | 0.82 ± 0.17 | NA |
| Paternal | ||
| Age | ||
| Baseline age, years | 32.7 ± 5.6 | 29.3 ± 5.2 |
| Follow-up age, years | 37.2 ± 5.6 | 52.5 ± 6.2 |
Data presented as mean ± SD or number of participants (%). Telomere lengths are normalized and measured for ENVIRONAGE and EFPTS participants separately.
Data available for n = 159 on prenatal smoke, maternal education and gestational complications.
Data available for n = 242 in ENVIRONAGE.
Data available for n = 211.
Our subpopulation of the EFPTS cohort comprised 82 newborn (50.0%) girls, and the newborn twins had a mean gestational age of 37.5 (2.2) weeks with a birthweight of 2619 (496) grams. The study included 47 twin pairs (68.1% monozygotic and 31.9% dizygotic) and 70 participants without their matching twin pair (74.3% monozygotic and 25.7% dizygotic). Mothers and fathers were on the average 27.0 (4.3) and 29.3 (5.2) years old, respectively. The median follow of the EFTPS newborns was 22.9 years (5th–95th percentile: 18.3–28.4).
3.2. Telomere length characteristics
In ENVIRONAGE, cord blood and placental TLs were correlated (r = 0.56, P<0.0001) and placental TL was 24.9% (95% CI: 22.5 to 27.3%; P<0.0001) longer. Cord blood and placental TLs were not correlated with gestational age (r=–0.03, P = 0.64 and r = 0.03, P = 0.66, respectively), but were shorter in boys compared with girls (–5.0%; 95% CI: –9.2 to –0.7%; P = 0.022 and –4.5; 95% CI: –9.8 to 0.9%; P = 0.10, respectively). Telomere length at the age of 4-years was shorter in boys compared with girls (–6.2%; 95% CI: –9.8 to –2.5%; P = 0.0011). Maternal LTL at delivery was –26.0% (95% CI: –28.9 to –23.1%; P<0.0001) and –44.8% (95% CI: –48.2 to –41.4%; P<0.0001) shorter compared with cord blood and placental TL of their child, respectively. Maternal LTL was correlated with cord blood TL at birth (r = 0.16; P = 0.023) and child LTL at the age of 4 (r = 0.38; P<0.0001), but not with placental TL at birth (r = 0.11; P = 0.14). Furthermore, maternal LTL was correlated with chronological age at delivery (r=–0.16, P = 0.018).
In the EFPTS study, placental TL was not correlated with gestational age (r = 0.10, P = 0.19) and was not different in boys compared with girls (–3.1%; 95% CI: –17.0 to 10.8%; P = 0.66). At the age of 23-years, buccal TL correlated with chronological age (r=–0.35, P<0.0001) and no difference was observed between boys compared with girls (–2.4%; 95% CI: –11.5 to 6.8%; P = 0.61).
3.3. Telomere length tracking from birth to child- and adulthood
Cord blood TL is strongly correlated with child LTL at the age of 4 years, and explained up to 50.5% (R2=0.505) (95%CI: 41.8 to 58.5%; P<0.0001) of the LTL variation at childhood (Fig. 2a). A similar correlation between cord blood TL and child LTL was observed for boys (Pearson r = 0.70, P<0.0001) and girls (Pearson r = 0.72, P<0.0001). Placental TL, as a surrogate for newborn TL, explained up to 35.4% (R2=0.354) (95%CI: 26.0 to 44.7%; P<0.0001) of the observed child LTL variation at the age of 4-years (Fig. 2b). The correlation between placental TL and child blood LTL was similar in boys (Pearson r = 0.62, P<0.0001) and girls (Pearson r = 0.57, P<0.0001). Excluding mothers that experienced gestational complications and children with adverse health related conditions did not alter the observed correlations (Supplementary Table 3).
Fig. 2.
Telomere tracking from birth to child- and adulthood. a) Pearson correlation between cord blood TL at birth and LTL at the age of 4. b) Pearson correlation between placental TL at birth and LTL at the age of 4. c) Spearman correlation between placental TL at birth and buccal TL at the age of 23. Telomere lengths are normalized and measured for ENVIRONAGE (panel a and b) and EFPTS (panel c) participants separately.
Our observations from birth to childhood were further studied in the EFPTS study to track telomere length from birth to adulthood, where placental TL was correlated with buccal TL (Spearman r = 0.46, P<0.0001) 23 years later in life (Fig. 2c). In boys (Spearman r = 0.40, P = 0.0002) and girls (Spearman r = 0.50, P<0.0001) placental TL correlated with buccal TL. Exclusion of gestational complications and adverse health conditions during follow-up slightly increased the observed correlations (Supplementary Table 3).
3.4. Telomere length change rates from birth to child- and adulthood
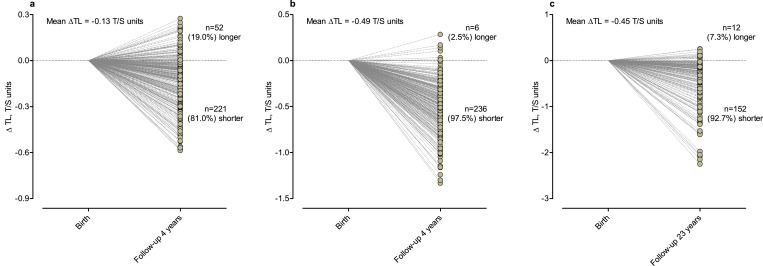
The mean difference between cord blood TL and LTL at the age of 4-years was –0.13 T/S units (–11.2%; 95% CI: –13.0 to –9.5%; P<0.0001; with mean TL decrease of –2.1% /year), with 81% of the children showing a decrease in TL over the first 4-years of life (Fig. 3a). Using placental TL as baseline measure (Fig. 3b) a mean decrease of –0.49 T/S units (–33.1%; 95% CI: –35.4 to –30.8%; P<0.0001; with mean TL decrease of –6.9% /year) was observed and 97.5% showed a shorter TL at the age of 4-years compared with baseline placenta TL. For EFPTS participants, the difference between placental TL and buccal TL in adulthood was –0.45 T/S units (–35.9%; 95% CI: –42.1 to –29.7%; P<0.0001; with mean TL decrease of –1.3% /year), with 92.8% of the participants displaying a shorter buccal TL 23 years later (Fig. 3c).
Fig. 3.
Change of newborn TL over time. a) Change of cord blood TL at birth compared with LTL at the age of 4-years. b) Change of placental TL at birth compared with LTL at the age of 4 years. c) Change of placental TL at birth compared with buccal TL at the age of 23 years.
Using multivariable adjusted linear models (Table 2), a stronger TL attrition rate in %TL/year was observed in newborns form the ENVIRONAGE birth cohort with longer TL at birth. For each SD longer cord blood TL, a –0.88% higher telomere loss per year (95%CI: –1.19 to –0.57%/year; P<0.0001) was observed as shown in Table 2. Each SD increase in placental TL at birth was associated with a –1.19% (95%CI: –1.47 to –0.91%/year; P<0.0001) higher telomere loss per year (Table 2). Furthermore, in full adjusted models, boys showed a higher telomere loss per year compared with girls (–0.93%/year; P = 0.0028 for cord blood and –0.72%/year; P = 0.012 for placenta) during childhood. Furthermore, children born from mothers with a longer LTL at delivery showed a lower telomere loss per year for each SD increment in maternal LTL (0.95%/year; P<0.0001 for cord blood and 0.98%/year; P<0.0001 for placenta) during childhood after adjustment for newborn TL. Models not adjusting for baseline TL (Supplementary Table 4), showed a weaker effect of newborn sex on TL change, especially when using placenta as baseline TL. Maternal LTL remained associated with TL change in baseline TL unadjusted models, with a slight decrease in effect size (Supplementary Table 4).
Table 2.
Determinants of telomere change from birth to childhood (ENVIRONAGE) and young adulthood (EFPTS).
| MODEL 1 |
MODEL 2 |
MODEL 3 |
MODEL 4† |
|||||
|---|---|---|---|---|---|---|---|---|
| Study | Estimate (95%CI) | P-value | Estimate (95%CI) | P-value | Estimate (95%CI) | P-value | Estimate (95%CI) | P-value |
| ENVIRONAGE | ||||||||
| Cord blood TL change(n = 273) | ||||||||
| Cord blood TL, T/S ratio | –0.72 (–0.98; –0.34) | <0.0001 | –0.73 (–1.01; –0.45) | <0.0001 | –0.71 (–0.99; –0.43) | <0.0001 | –0.88 (–1.19; –0.57) | <0.0001 |
| Gestational age, weeks | NA | NA | 0.15 (–0.17; 0.47) | 0.36 | 0.13 (–0.18; 0.45) | 0.41 | 0.21 (–0.13; 0.55) | 0.22 |
| Birth weight, g | NA | NA | –0.06 (–0.38; 0.27) | 0.73 | –0.06 (–0.38; 0.27) | 0.74 | 0.04 (–0.30; 0.39) | 0.81 |
| Newborn sex, boys vs girls* | NA | NA | –0.55 (–1.12; 0.01) | 0.056 | –0.51 (–1.08; 0.05) | 0.075 | –0.93 (–1.53; –0.32) | 0.0028 |
| Maternal age at delivery, years | NA | NA | NA | NA | 0.27 (–0.12; 0.65) | 0.18 | 0.28 (–0.13; 0.70) | 0.18 |
| Paternal age at delivery, years | NA | NA | NA | NA | 0.01 (–0.37; 0.40) | 0.95 | –0.05 (–0.47; 0.36) | 0.81 |
| Maternal LTL at delivery, T/S | NA | NA | NA | NA | NA | NA | 0.95 (0.64; 1.26) | <0.0001 |
| Placenta TL change(n = 242) | ||||||||
| Placental TL, T/S ratio | –0.88 (–1.14; –0.62) | <0.0001 | –0.88 (–1.15; –0.61) | <0.0001 | –0.89 (–1.16; –0.62) | <0.0001 | –1.19 (–1.47; –0.91) | <0.0001 |
| Gestational age, weeks | NA | NA | 0.11 (–0.19; 0.41) | 0.47 | 0.11 (–0.19; 0.41) | 0.48 | 0.09 (–0.22; 0.41) | 0.56 |
| Birth weight, g | NA | NA | –0.16 (–0.46; 0.14) | 0.30 | –0.17 (–0.48; 0.13) | 0.27 | –0.13 (–0.46; 0.19) | 0.27 |
| Newborn sex, boys vs girls* | NA | NA | –0.40 (–0.93; 0.14) | 0.15 | –0.38 (–0.92; 0.16) | 0.16 | –0.72 (–1.27; –0.16) | 0.012 |
| Maternal age at delivery, years | NA | NA | NA | NA | –0.04 (–0.41; 0.33) | 0.83 | 0.22 (–0.18; 0.62) | 0.27 |
| Paternal age at delivery, years | NA | NA | NA | NA | 0015 (–0021; 0.52) | 0.41 | –0.13 (–0.52; 0.27) | 0.52 |
| Maternal LTL at delivery, T/S | NA | NA | NA | NA | NA | NA | 0.98 (0.70; 1.27) | <0.0001 |
| EFPTS(n = 164) | ||||||||
| Placental TL, T/S ratio | –0.32 (–0.43; –0.21) | <0.0001 | –0.32 (–0.43; –0.20) | <0.0001 | –0.32 (–0.43; –0.20) | <0.0001 | NA | NA |
| Gestational age, weeks | NA | NA | 0.00 (–0.16; 0.16) | 0.97 | 0.00 (–0.16; 0.16) | 0.96 | NA | NA |
| Birth weight, g | NA | NA | –0.01 (–0.17; 0.14) | 0.86 | 0.00 (–0.16; 0.16) | 0.99 | NA | NA |
| Newborn sex, boys vs girls* | NA | NA | –0.03 (–0.30; 0.23) | 0.80 | –0.02 (–0.29; 0.24) | 0.86 | NA | NA |
| Maternal age at delivery, years | NA | NA | NA | NA | 0.05 (–0.20; 0.29) | 0.70 | NA | NA |
| Paternal age at delivery, years | NA | NA | NA | NA | –0.10 (–0.35; 0.15) | 0.42 | NA | NA |
Estimates presented as a %TL change/year with 95%CI for each SD increment in explanatory variable from the general linear models. All EFPTS models were analysed in a mixed model with a random intercept for family and zygosity and chorionicity as fixed effect.
Estimate presented for boys compared with girls.
Model 4 comprises n = 211 for cord blood TL change and n = 186 for placental TL change.
In the EFPTS study, a longer TL at birth was associated with a higher telomere loss per year from birth to adulthood. Each SD increment in placental TL at birth resulted in a –0.32% higher telomere loss per year (95%CI: –0.43 to –0.20%/year; P<0.0001). From birth to adulthood, boys did not show a higher telomere loss per year compared with girls. Models not adjusting for baseline TL did not showed different results (Supplementary Table 4).
Secondary analyses, evaluating both prenatal and postnatal risk factors (Supplementary Table 5) did not show an association between TL change rates and maternal education, prenatal smoke exposure, gestational complications, postnatal adverse health conditions, postnatal smoke exposure and later life BMI in both ENVIRONAGE and EFPTS.
3.5. Change in telomere length ranking from birth to child- and adulthood
Based on cord blood TL at birth, 37.4% of the newborns did not change from quintile and 48.4% showed only a change of 1 quintile (23.4% shifted downwards and 24.9% shifted upwards) during childhood (Fig. 4a). A similar change in TL rank during childhood was observed when using placenta TL at birth (Fig. 4b), in which 36.4% did not change rank, 19.0% shifted 1 quintile downwards and 21.5% shifted 1 quintile upwards.
Fig. 4.
Change of TL ranking over child-and adulthood, using quintiles of the TL distribution at birth and follow-up. ∆Quintiles=difference in quintile at birth and follow-up (Qfollow-up – Qbirth). a) Change in cord blood TL rank of the first 4 years of life. b) Change of placental TL rank of the first 4 years of life. c) Change of placental TL rank of the first 23 years of life.
In the EFPTS study in which TL rank change was evaluated over a 23-year period (Fig. 4c), 36.0% of the participants did not change rank and 14.0% shifted 1 quintile downwards and 22.6% shifted 1 quintile upwards. Results were comparable for boys and girls both in ENVIRONAGE and EFPTS (Supplementary Fig. 1).
The fraction of newborns that showed a strong downward shift in ranking were more likely to belong to the highest quintiles of the TL distribution at birth, whereas newborns showing the strongest upward shift in rank were more likely from the lowest quintiles of the TL distribution at birth (Supplementary Tables 6–8).
4. Discussion
In this study we evaluated the importance of newborn TL for later life TL and TL dynamics in the transition from birth to child- and adulthood. The main findings of our study based on two independent longitudinal birth cohorts can be summarized as follows: First, newborn TL strongly predicts child- and adulthood TL. Second, newborn TL is the strongest predictor of TL change over time. Third, differences in TL change are observed in boys and girls, and are depending on maternal LTL. Fourth, although having a long TL at birth results in a higher change in TL over time, individuals tend not to change dramatically in TL ranking from birth to child- and adulthood.
Telomere length at birth explained up to 50% (R2=0.505) of the variation in LTL observed in childhood, when using blood as a biological matrix. Comparing TL from different biological matrices (placenta, cord blood, blood and buccal cells), at the same time (birth), or after a shorter (childhood) and longer (adulthood) period of time, showed a decreasing, but significant intercorrelation, respectively. This indicates that although TL dynamics and regulatory processes are tissue-specific (including proliferative differences), TL in different tissues contain predictive information of the overall telomeric state of an individual. Our results suggest that tissue specific average TLs may act as a surrogate for the overall TL state in the human body at any given time point (even as from birth), over and beyond the progress of life.
What may be the consequences of these findings? First, it can be stated that the genetic and non-genetic factors explaining initial TL may be more important than exposures during the life course for later life TL than initially thought. Our results confirm the importance of initial TL for later TL as suggested in previous hypotheses [6,8,19]. Recent progresses have been made to elucidate important newborn TL associated environmental, and socio-demographic factors [10,16,17,[20], [21], [22]]. Second, as later life TL may be programmed at birth, the observed health and disease conditions related to shorter telomeres, may find to some extent their onset at birth. Therefore, our results may give some first indications in understanding the role of newborn telomere biology in shaping a part of the later life aging-phenotype.
Between tissue TL correlations are in line with strong correlations of TL in different tissues observed in cross-sectional analyses of adults [23]. The observed correlation between initial TL and later life TL (when comparing blood TLs, R2 up to 0.50) was weaker compared with telomere tracking data later in life from adults [7] (R2 up to 0.93) and children [8] (R2 up to 0.82). This may be the result of the higher telomere attrition rates observed early in life [8,24,25], the potentially more important role of environmental exposures that accelerate TL shortening in this phase of development compared with the later life phase [7,17], and by the fact that cord blood constitutes different blood cells than peripheral blood used at follow up. In this regard low correlations (ranging from 0.12 to 0.14) between saliva telomeres from 8.6 months to 3-years are observed [26]. Furthermore, the strength of correlation of TL between time points may be influenced by the TL measurement method, as lower TL correlations between time points are observed when measuring telomeres using the qPCR method (correlations in adults ranging from 0.38 up to 0.79) [27], [28], [29], compared with the terminal restriction fragment method (TRF). So even a higher contribution of the initial TL to later life TL may be expected. A stronger mean decrease in TL% /year was observed averaged over the 4-year period in ENVIRONAGE compared with the averaged 23-year period in EFPTS, which may be in line with studies showing a stronger telomere decrease early in life, compared with later life [8,24,25].
A higher TL attrition is observed with increasing TL at birth. This is also evidenced by multiple longitudinal analyses of TL in adults with durations of follow-up ranging from 6 to 10 years [30], [31], [32]. Having longer telomeres may indicate more targets for oxidative telomere damage resulting in a higher TL loss, whereas shorter telomeres may be preferential for telomerase action [33]. It is postulated that this observation is partly explained by the regression-to-the-mean phenomenon. In our analyses we adjusted the TL attrition rate for the regression-to-the-mean effect as postulated by Verhulst and colleagues [18], and we observe that after regression-to-the-mean correction the explained variation in TL attrition by baseline TL was 8%, 15% and 14% for cord and placenta TL in ENVIRONAGE and placenta TL in EFPTS, respectively. However, this study shows that most individuals do not tend to change strongly from TL ranking (more than 1 quintile) in the earlier stages of life. This ranking is less strong compared with a higher persistent ranking previously observed in adults [7]. This may reflect that TL change is a bit more dynamic earlier in life compared with later life. After adjusting for baseline TL, a higher attrition is observed in boys compared with girls in the ENVIRONAGE birth cohort, which may explain the bigger TL difference observed between boys and girls at the follow-up compared with the birth difference. In a middle-aged population a higher telomere attrition after baseline TL adjustment was observed in men compared with women [31]. Several hypotheses have been proposed to explain the telomere sex difference in adults, and we and others [6,34] observed that this difference is already present from birth. In addition, we show now that based on data from the ENVIRONAGE birth cohort the sex difference is more pronounced during childhood. Biological explanations for the telomere length and dynamics related differences between boys and girls might be explained by sex-related hormonal conditions operative during the intrauterine life and throughout the life course [35]. For instance, the steroid hormone estrogen is known to activate telomerase and to have antioxidant properties [36,37]. Besides sex related differences are the TL and its attrition heritable traits, with heritability estimates around 70% for TL [38] and 28% for attrition [39]. In this study we confirm previous findings that newborn TL is related to maternal TL [6,40], and furthermore we observed that telomere attrition during childhood relates to maternal telomeres, indicative of an heritable factor in the initial TL setting and dynamic features early in life.
We observed that in childhood (4 years) and early childhood (23 years) TL for some individuals are longer than TL at birth. Different possibilities can explain these observations. First, using different baseline tissues that show a difference in baseline TL explains that we observed 19% of the individuals with increased TL when comparing cord blood with LTL and only 2.5% when comparing placental TL with LTL, as we show that placental TL is on the average 24.9% longer. Second, this observation can be an artefact related to measurement error. This is supported by studies in which lengthening of telomeres within individuals are less frequently observed when measured with the Southern blot method, which show overall less measurement error compared with qPCR [41]. Computational models confirm that lengthening of telomeres is largely an artefact of measurement error [41,42], but it indicates that some biological mechanisms are plausible [42]. Potential lengthening of LTL may be related to dynamics in hematopoietic cells in which telomerase and clonal expansion of lymphocytes with longer telomeres may arise under different conditions [43].
Our study has several strengths. Birth TL for ENVIRONAGE and TLs for EFPTS have been measured previously, however for this specific study we re-measured all telomeres at birth and follow-up to reduce technical measurement errors. Our results on TL tracking were based on two independent birth cohorts, one with tracking from birth to childhood and one on tracking from birth to adulthood. We were able to study telomere tracking using different biological matrices at birth and in later life, showing the predictive role of newborn TL for later life TL, irrespectively from the biological matrix. Nevertheless, in this regard we acknowledge the fact that the absolute estimates of telomere attrition rates may to some extent be influenced by the difference in TL in the used sample type at birth and follow-up. A more precise attrition model can be provided when e.g. peripheral blood at both birth and follow-up is available, however this lack of a matched biological sample between birth and follow-up is due to ethical restrictions that no peripheral blood can be drawn from newborns in ENVIRONAGE. Furthermore, no buccal swabs were collected in EFPTS at birth. We acknowledge the following limitations. As the placenta contains a fetal and maternal side, using placental TL at baseline may be biased by maternal tissue contamination. In this regard ENVIRONAGE placental biopsies are taking directly underneath the fetal side and histological examination confirmed that the majority of cells are trophoblast derived (Supplementary Fig. 2) [44]. For EFPTS the same biopsies used for zygosity determination were used, confirming the presence of fetal tissue. As fetal tissue may be predominantly present, placental TL may reflect to a large extent fetal derived cells and tissue, which is in line with the observation that TL in placenta did not differ between the maternal and fetal side [45]. Measuring TL in long-term stored biological samples is more challenging due to a higher loss of biological quality over time, resulting in a higher measurement variation and a higher sample loss for the EFPTS study. No maternal and paternal DNA for EFPTS is available, and due to ethical restrictions no paternal DNA and consequently no paternal telomeres were available for ENVIRONAGE, which could gain more insights into the parent-offspring TL and TL dynamics relationship. We acknowledge that although our follow-up sample was representative for the entire ENVIRONAGE population and population at large that the participating mothers are slightly more likely to be from European origin and are more likely to have a higher education. Furthermore, as the EFPTS is a twin study, the generalizability is more limited, however we argue that telomere biology and attrition do not substantially differ between twins and singletons. Finally, we measure an average telomere length which provides a broad general view of the telomeric state of an individual, and does not provide information on telomere integrity, dysfunctionalities or the amount of short or critically short telomeres, that may relate to specific telomere pathologies and diseases [46]. Our results on TL should therefore be viewed in the context of a population-based perspective rather than an individual-based perspective. In future perspective, our results may impact current interpretations on adult population-based telomere studies, as the contribution of the initial TL appears to play an important role. Further prospective evaluations based on long follow-up studies should elucidate the role of initial TL in regard to health and disease outcomes later in life. This to further proof the concept of prenatal programming of diseases in relation to TL and whether telomere induced biological aging may find their origins at birth. Furthermore, the role of experiencing similar environmental contexts and exposures throughout the life course in the observation of telomere tracking may warrant future investigations.
In conclusion we show that TL at birth is a major predictor of later life TL and predicts TL change in the transition from birth to child- and adulthood. Irrespectively from their TL change, individuals tend not to change dramatically from TL rank over time as from birth onwards.
Contributors
T.S.N. coordinates the ENVIRONAGE birth cohort and designed the current study together with D.S.M. C.D., E.T. and E.M.B. coordinated the EFPTS study and samples. C.V.D.S. and D.S.M. performed the telomere assay. D.S.M. processed and statistically analysed all data and performed the quality control of the database. D.S.M. and T.S.N. wrote the first draft of the manuscript. All authors were involved in data interpretation and critical revision of the manuscript. All authors read and approved the final version of the manuscript.
Declaration of Interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This research was funded by European Research Council (ERC-StG310898) and the Flemish Scientific Fund (FWO). Dries Martens holds a postdoctoral grant by the Flemish Scientific Fund (FWO grant 12X9620N). The researchers would like to thank all the ENVIRONAGE follow-up study researchers.
Data sharing
The data that support the findings of this study are available upon request from the corresponding author.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.103164.
Appendix. Supplementary materials
References
- 1.Shay J.W., Wright W.E. Telomeres and telomerase: three decades of progress. Nat Rev Genet. 2019;20:299–309. doi: 10.1038/s41576-019-0099-1. [DOI] [PubMed] [Google Scholar]
- 2.O'Sullivan R.J., Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. 2010;11:171–181. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Meyer T., Nawrot T., Bekaert S., De Buyzere M.L., Rietzschel E.R., Andres V. Telomere length as cardiovascular aging biomarker: JACC review topic of the week. J Am Coll Cardiol. 2018;72:805–813. doi: 10.1016/j.jacc.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q., Zhan Y., Pedersen N.L., Fang F., Hagg S. Telomere length and all-cause mortality: a meta-analysis. Ageing Res Rev. 2018;48:11–20. doi: 10.1016/j.arr.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Steenstrup T., Kark J.D., Verhulst S. Telomeres and the natural lifespan limit in humans. Aging. 2017;9:1130–1142. doi: 10.18632/aging.101216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Factor-Litvak P., Susser E., Kezios K. Leukocyte telomere length in newborns: implications for the role of telomeres in human disease. Pediatrics. 2016;137 doi: 10.1542/peds.2015-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benetos A., Kark J.D., Susser E. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell. 2013;12:615–621. doi: 10.1111/acel.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benetos A., Verhulst S., Labat C. Telomere length tracking in children and their parents: implications for adult onset diseases. FASEB J Off Publ Fed Am Soc Exp Biol. 2019;33:14248–14253. doi: 10.1096/fj.201901275R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Entringer S., de Punder K., Buss C., Wadhwa P.D. The fetal programming of telomere biology hypothesis: an update. Philos Trans R Soc Lond B Biol Sci. 2018;373 doi: 10.1098/rstb.2017.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martens D.S., Cox B., Janssen B.G. Prenatal air pollution and newborns' predisposition to accelerated biological aging. JAMA Pediatr. 2017;171:1160–1167. doi: 10.1001/jamapediatrics.2017.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janssen B.G., Madhloum N., Gyselaers W. Cohort profile: the environmental influence on early ageing (ENVIRONAGE): a birth cohort study. Int J Epidemiol. 2017;46 doi: 10.1093/ije/dyw269. 1386-137m. [DOI] [PubMed] [Google Scholar]
- 12.Cox B., Martens E., Nemery B., Vangronsveld J., Nawrot T.S. Impact of a stepwise introduction of smoke-free legislation on the rate of preterm births: analysis of routinely collected birth data. Bmj. 2013;346:f441. doi: 10.1136/bmj.f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derom C., Thiery E., Rutten B.P.F. The east Flanders prospective twin survey (EFPTS): 55 years later. Twin Res Hum Genet Off J Int Soc Twin Stud. 2019;22:454–459. doi: 10.1017/thg.2019.64. [DOI] [PubMed] [Google Scholar]
- 14.Loos R.J., Beunen G., Fagard R., Derom C., Vlietinck R. The influence of zygosity and chorion type on fat distribution in young adult twins consequences for twin studies. Twin Res. 2001;4:356–364. doi: 10.1375/1369052012524. [DOI] [PubMed] [Google Scholar]
- 15.Cawthon R.M. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucl Acids Res. 2009;37:e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martens D.S., Janssen B.G., Bijnens E.M. Association of parental socioeconomic status and newborn telomere length. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bijnens E.M., Zeegers M.P., Derom C. Telomere tracking from birth to adulthood and residential traffic exposure. BMC Med. 2017;15:205. doi: 10.1186/s12916-017-0964-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verhulst S., Aviv A., Benetos A., Berenson G.S., Kark J.D. Do leukocyte telomere length dynamics depend on baseline telomere length? An analysis that corrects for 'regression to the mean'. Eur J Epidemiol. 2013;28:859–866. doi: 10.1007/s10654-013-9845-4. [DOI] [PubMed] [Google Scholar]
- 19.Aviv A., Shay J.W. Reflections on telomere dynamics and ageing-related diseases in humans. Philos Trans R Soc Lond B Biol Sci. 2018;373 doi: 10.1098/rstb.2016.0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosquet Enlow M., Bollati V., Sideridis G. Sex differences in effects of maternal risk and protective factors in childhood and pregnancy on newborn telomere length. Psychoneuroendocrinology. 2018;95:74–85. doi: 10.1016/j.psyneuen.2018.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drury S.S., Esteves K., Hatch V. Setting the trajectory: racial disparities in newborn telomere length. J Pediatr. 2015;166:1181–1186. doi: 10.1016/j.jpeds.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Entringer S., Epel E.S., Lin J. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am J Obstet Gynecol. 2013;208:134 e1-7. doi: 10.1016/j.ajog.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniali L., Benetos A., Susser E. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013;4:1597. doi: 10.1038/ncomms2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aubert G., Baerlocher G.M., Vulto I., Poon S.S., Lansdorp P.M. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frenck R.W., Jr., Blackburn E.H., Shannon K.M. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci USA. 1998;95:5607–5610. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosquet Enlow M., Kane-Grade F., De Vivo I., Petty C.R., Nelson C.A. Patterns of change in telomere length over the first three years of life in healthy children. Psychoneuroendocrinology. 2020;115 doi: 10.1016/j.psyneuen.2020.104602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martens D.S., Wei F.F., Cox B. Retinal microcirculation and leukocyte telomere length in the general population. Sci Rep. 2018;8:7095. doi: 10.1038/s41598-018-25165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bendix L., Thinggaard M., Fenger M. Longitudinal changes in leukocyte telomere length and mortality in humans. J Gerontol Ser A Biol Sci Med Sci. 2014;69:231–239. doi: 10.1093/gerona/glt153. [DOI] [PubMed] [Google Scholar]
- 29.Rosero-Bixby L., Rehkopf D.H., Dow W.H. Correlates of longitudinal leukocyte telomere length in the Costa Rican longevity study of healthy aging (CRELES): on the importance of DNA collection and storage procedures. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0223766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aviv A., Chen W., Gardner J.P. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol. 2009;169:323–329. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Needham B.L., Wang X., Carroll J.E. Sociodemographic correlates of change in leukocyte telomere length during mid- to late-life: the multi-ethnic study of atherosclerosis. Psychoneuroendocrinology. 2019;102:182–188. doi: 10.1016/j.psyneuen.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nordfjall K., Svenson U., Norrback K.F., Adolfsson R., Lenner P., Roos G. The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hockemeyer D., Collins K. Control of telomerase action at human telomeres. Nat Struct Mol Biol. 2015;22:848–852. doi: 10.1038/nsmb.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wojcicki J.M., Olveda R., Heyman M.B. Cord blood telomere length in Latino infants: relation with maternal education and infant sex. J Perinatol Off J Calif Perinat Assoc. 2016;36:235–241. doi: 10.1038/jp.2015.178. [DOI] [PubMed] [Google Scholar]
- 35.Benetos A., Dalgard C., Labat C. Sex difference in leukocyte telomere length is ablated in opposite-sex co-twins. Int J Epidemiol. 2014;43:1799–1805. doi: 10.1093/ije/dyu146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aviv A. Telomeres, sex, reactive oxygen species, and human cardiovascular aging. J Mol Med. 2002;80:689–695. doi: 10.1007/s00109-002-0377-8. [DOI] [PubMed] [Google Scholar]
- 37.Kyo S., Takakura M., Kanaya T. Estrogen activates telomerase. Cancer Res. 1999;59:5917–5921. [PubMed] [Google Scholar]
- 38.Broer L., Codd V., Nyholt D.R. Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur J Hum Genet. 2013;21:1163–1168. doi: 10.1038/ejhg.2012.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hjelmborg J.B., Dalgard C., Moller S. The heritability of leucocyte telomere length dynamics. J Med Genet. 2015;52:297–302. doi: 10.1136/jmedgenet-2014-102736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herlin M., Broberg K., Igra A.M., Li H., Harari F., Vahter M. Exploring telomere length in mother-newborn pairs in relation to exposure to multiple toxic metals and potential modifying effects by nutritional factors. BMC Med. 2019;17:77. doi: 10.1186/s12916-019-1309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steenstrup T., Hjelmborg J.V., Kark J.D., Christensen K., Aviv A. The telomere lengthening conundrum–artifact or biology? Nucl Acids Res. 2013;41:e131. doi: 10.1093/nar/gkt370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bateson M., Nettle D. The telomere lengthening conundrum - it could be biology. Aging Cell. 2017;16:312–319. doi: 10.1111/acel.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hodes R.J., Hathcock K.S., Weng N.P. Telomeres in T and B cells. Nat Rev Immunol. 2002;2:699–706. doi: 10.1038/nri890. [DOI] [PubMed] [Google Scholar]
- 44.Janssen B.G., Byun H.M., Cox B. Variation of DNA methylation in candidate age-related targets on the mitochondrial-telomere axis in cord blood and placenta. Placenta. 2014;35:665–672. doi: 10.1016/j.placenta.2014.06.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Martin I., Janssen A.B., Jones R.E. Telomere length heterogeneity in placenta revealed with high-resolution telomere length analysis. Placenta. 2017;59:61–68. doi: 10.1016/j.placenta.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calado R.T., Young N.S. Telomere diseases. N Engl J Med. 2009;361:2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.