Abstract
Background
Pineapple peel is a waste component of pineapple with valuable source of metabolites as phytoactive compounds in ameliorating metabolic-related disorders. This study investigated the atheroprotective and neuroprotective effects of peel extract of Ananas comosus fruit (PEAC) in normal diet (ND) and high-fat diet (HFD) fed rats.
Methods
Male Wistar rats were fed ND or HFD for 9 weeks, and beginning from the 6th week animals were also orally treated with PEAC (200 mg/kg). Memory performance was assessed using Y-maze test (YMT) and novel object recognition test (NORT) while anxiolytic-like effect was assessed on the elevated plus maze (EPM). Serum cholesterol, triglycerides and HDL-C were determined, while LDL-C and atherogenic risk calculated. Serum and brain tissue malondialdehyde, reduced glutathione, catalase were determined. Brain acetylcholinesterase activity and interleukin-6 level were also determined.
Results
PEAC significantly attenuated HFD-induced reduction in correct alternation in YMT, and discrimination index in NORT. Also, PEAC demonstrated anxiolytic-like activity in EPM test. PEAC significantly improved lipid profile and decreased risk of atherogenicity in ND and HFD-fed rats. In addition, PEAC improves serum and brain antioxidant status by decreasing malondialdehyde and increasing GSH and catalase. PEAC significantly impaired HFD-induced brain acetylcholinesterase activity and IL-6 levels.
Conclusion
These findings suggest that peel extract of Ananas comosus fruit may protect against diet-induced behavioral disturbances via atheroprotective, antioxidants and anti-inflammatory activities.
Keywords: Pineapple, Peel, Atheroprotective, Memory, Anti-inflammatory
Highlight
-
•
This study investigated health promoting potentials of Pineapple peels.
-
•
High-fat diet induced neurobehavioral deficits in mice.
-
•
Pineapple peel extract prevented HFD-induced memory impairment and anxiety-like behavior.
-
•
Pineapple peel extract demonstrated atheroprotective, anti-inflammatory and antioxidants properties in rats.
1. Introduction
Obesity is now well recognized as a complicated public health disease and one of the most important reasons for reduced life expectancy within the “modern” world [1]. According to Obesity Medicine Association (OMA), obesity is defined as a “chronic, relapsing, multi-factorial, neurobehavioral disease, wherein an increase in body fat promotes adipose tissue dysfunction and abnormal fat mass physical forces, resulting in adverse metabolic, biomechanical, and psychosocial health consequences” [2]. Abnormal or excess fat accumulation in adipose tissue which is ultimately expressed as obese phenotype is determined by the interaction of genetic, environmental and psycho-social factors acting through the physiological mediators of energy intake and expenditure [3,4]. Obesity-related co-morbid conditions may include dyslipidemia, coronary artery disease, liver diseases, reproductive disorder, hypertension, type 2 diabetes mellitus, respiratory disorders, cancer, and mood disorders [3,5].
Dyslipidemia is seen in approximately 60–70% of patients with obesity. The lipid abnormalities in obesity is characterized by elevated serum triglyceride (TG), cholesterol, apolipoprotein B and Low-density lipoprotein cholesterol (LDL-C) and a reduced high density lipoprotein (HDL) [6]. Elevated serum triglycerides is caused by increased hepatic production of very low density lipoprotein (VLDL) particles or it may be due to a reduction in the clearance of triglyceride rich lipoproteins [7,8]. The pathophysiological changes in lipid metabolism as seen in obesity are the elevated fasting and postprandial triglycerides [7]. Hypertriglyceridemia is the underlying cause of lipid abnormalities including the small-dense LDL which enhances atherogenicity [9]. TG-rich lipoproteins and remnant cholesterol have been shown to be present in coronary, cerebral and peripheral atherosclerosis [7]. Remnant-mediated atherogenesis mechanisms in obesity include postprandial activation of leucocytes, generation of oxidative stress and production of cytokines [7].
The health consequences of obesity extend to cognitive function, with evidence for reduced memory [10] and executive function [11] as well as increased impulsivity [12]. Proposed mechanisms underpinning reduced cognitive function in obesity include oxidative stress, metabolic dysfunction, cardiovascular disease, and systemic inflammation, which have been reported to alter brain structure and volume [13]. Finally, substantial evidence exists in the literature for an association between blood fatty acids and cognitive impairment, particularly in Alzheimer’s disease [14,15].
Obesity is a strong predictor of chronic medical conditions and obesity-related chronic conditions could increase the risk of anxiety [[16], [17], [18]]. Plausible biopsychosocial mechanisms linking anxiety and obesity have also been proposed [19,20]. Several shared biological pathways in dysregulation by obesity such as immuno-inflammatory processes, oxidative stress, neurotransmitter balance, and neuroprogression have all been associated with anxiety [21]. Other reported mediators of the relationship between psychiatric disorders and obesity are behavioral and include current and past unhealthy dietary patterns, lower rates of physical activity, and increased sedentary behaviors documented among obese individuals [22,23].
Feeding rodents with High-fat diet for a prolonged period is shown to be associated with neurobehavioral and neuroimmunological changes associated with obesity [24]. Peripheral inflammation which is capable of signaling brain-based disorders has been reported in HFD [24]. Also, HFD enhances formation of reactive oxygen species in the peripheral which causes oxidative stress and brain dysfunction resulting in impaired learning and memory [25]. Chronic feeding with HFD for 3–6 weeks duration was shown to induce alterations in spatial memory and hippocampal expression of JNK, P38, ERK and Akt [26]. Herbs, spices and fruits with rich sources of polyphenols that have shown antioxidative and anti-inflammatory properties are being investigated to ameliorating the oxido-inflammatory consequences of HFD models of obesity [25,27].
Ananas comosus, popularly known as pineapple is one of the herbaceous perennial plant of bromeliaceae family native to tropic and sub-tropic countries [28]. A. comosus is rich in nutrients including calcium, potassium as well as vitamin C and vitamin A [29]. It is reported that the solvent extracts of the various parts of A. comosus exhibit antibacterial, antiviral, antifungal, antiparasitic, antiobesity, uterotonic, and anti-inflammatory properties [[30], [31], [32]]. Fresh pineapple is rich in bromelain, a mixture of different thiol endopeptidases and other components like phosphatase, glucosidase, peroxidase, cellulase, and several protease inhibitors [33]. The fruit is becoming a favorable fruit in dyslipidemia and obesity with potential in diminishing the severity of cardiovascular syndromes as a result of potent anti-inflammatory bromelain content [32,34]. Pineapple peel is a waste component of pineapple with source of metabolites for therapeutics, including antioxidant, anti-inflammatory and anti-microbial properties [34] and also cosmeceutical applications [35]. In this study, Ananas comosus fruit peel extract was evaluated for memory impairment, anxiety-like behavior, and oxidative stress-induced by High-Fat diet feeding in rats.
2. Materials and methods
2.1. Reagents
5′, 5′-Dithiobis 2-nitrobenzoate (DTNB), Trichloroacetic acid (TCA), Thiobarbituric acid (TBA), Griess reagent ((sulfanilamide and N-(1-naphthyl) ethylenediamine dihydrochloride), were all products of Sigma-Aldrich St. Louis, USA. Interleukin −6 Biolegend ELISA kits (San Diego, USA). All other solvents and reagents were analytical grade reagents.
2.2. Preparation of Ananas comosus fruit peel extract
Ananas comosus (pineapple) was bought from Oje Market, Ibadan, the Oyo State capital, Nigeria. A. comosus was peeled in the lab using a sterile knife, the peel was chopped into smaller pieces, and then oven dried at 40 °C for 72 h. Dried peel were ground using a mechanical grinder. 200 g of the powdered peel was soaked in 80% methanol for 48 h. The extract was filtered using a muslin cloth and doubly filtered with whatmann filter paper. The filtrate was concentrated under reduced pressure in rotary evaporator and dried further in vacuum over to a constant weight. The extract was greenish brown semi solid, tagged Peel Extract of A. comosus (PEAC).
2.3. Experimental animals
Animals used were 28 male Wistar rats of about 70–90 g after one week acclimatization in the experimental animal house, Department of Pharmacology and Therapeutics, University of Ibadan. The animals were kept in good hygienic conditions in polypropylene plastic cages with wood shavings as bedding. All animals were allowed free access to water and fed with standard commercial rat chow pellets (Vital feeds Ltd, Ibadan, Nigeria) for animals on basal diet or formulated high-fat diet for the other experimental groups. All experiments were carried out with strict compliance to The “Principle of Laboratory Animal Care” (NIH Publication No. 85–23) and approved institutional protocols on animal handling.
2.4. Basal diet and experimental high fat diet composition
Basal diet was composed of normal rat chow diet obtained from Vital feeds Ltd (Ibadan, Nigeria) with the following composition: Crude protein (13%), Fat (8%), Crude fibre (15%), Calcium (0.9%), phosphorus (0.35%), metabolisable energy (2600 kcal/kg). The high fat diet was made up of the commercial animal feed supplemented with 44% animal fat (lard) and methionine (0.3%).
2.5. Animal design
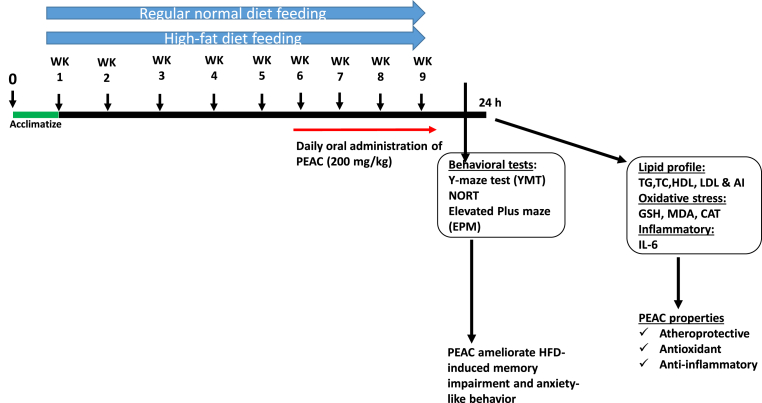
The rats were initially fed for five weeks with either normal diet (ND) for groups 1 and 2 or HFD for groups 3 and 4. The body weight were taken weekly. At the sixth week, there was weight differences in ND and HFD groups, and also behavioral symptoms were beginning to appear as described by Wu et al. (2018). Treatment commenced at the beginning of the 6th week with vehicle (10 mL/kg) for groups 1 and 3 or PEAC (200 mg/kg, p.o) for groups 2 and 4, respectively. The dose of PEAC was selected based on the acute toxicity test (data not shown). As shown in Fig. 1, the rats were fed for nine weeks and treated with PEAC between 09 and 11 h daily for four weeks altogether. Four hours after the last administration of PEAC, the animals were subjected to behavioral procedure to test for memory performance and anxiety-like behaviors.
Fig. 1.
Experimental design and treatment.
2.6. Behavioral procedure
2.6.1. Test for memory performance using Y-maze paradigm
The effect of peel extract of Ananas comosus (PEAC) on spatial working memory of normal diet and high fat diet-fed rats were assessed using the Y-maze paradigm as previously described [36]. They were placed individually at arm A of the Y-maze and allowed to explore all the three arms freely for 5 min. The number and sequence of arm entries were recorded and the apparatus was cleaned after each test with 70% ethanol to remove animal clue. An entry was scored when the four paws of the animals were completely in the arm of the Y-maze. The percentage alternation, which is a measure of memory function, and was calculated by dividing the total number of alternations by the total number of arm entries, subtracted by two, and then multiplied by 100 [36].
2.6.2. Test for spatial memory using novel object recognition test (NORT)
This test was carried out as earlier described [37]. The NORT consists of two phases: the trial phase and the test phase. NORT trial phase started 4 h after the last administration of PEAC and test phase was conducted after an interval of 4 h. The trial phase was carried out by placing each rat in the middle of two identical objects (A and B) on opposite sides (at a distance of 8 cm from the walls and 34 cm from each other) of the open-field chamber for 5 min. Thereafter, the animals were returned to their cages for an interval of 4 h. In the test phase, object B was replaced with object C, which was novel to the animals and different from either object A or B. The rat was then left to explore objects A and C for a period of 5 min. The apparatus was cleaned after each test and the time spent (in seconds) in exploring each of the objects was recorded in both phases. The discrimination index (DI) was calculated as the difference in time of exploration of the novel and familiar object divided by the total amount of time spent with both objects [37].
2.6.3. Elevated plus-maze (EPM) test for anxiety
The EPM test was used to assess the anxiety-like behavior in rats, 4 h after the last administration of PEAC according to the procedure described by Lister [38]. The animals were placed individually at the center of the maze with its head facing an open arm and allowed to explore the maze for 5 min. The parameters measured were frequency and duration of arm entries. An entry was scored when the four paws of the animals was completely be into one arm of the EPM. Ethanol solution (10%) was used to clean the plus maze after each test [38].
2.7. Biochemical assays
The rats were sacrificed 24 h after the last administration of PEAC after an overnight fast. The animals were sacrificed through cervical dislocation after the collection of blood samples from the retro-orbital plexus and the brain was excised and rinsed in cold Tris-KCl buffer. The brain, liver, heart, kidneys and spleen were harvested and weighed. The blood was centrifuged at 4000 rpm for 15 min at room temperature, serum was obtained and aliquots for determination of lipid profile and oxidative stress parameters. The whole brain was homogenized in 0.1 M cold sodium phosphate buffer (pH 7.4) using the Teflon homogenizer. The homogenate was centrifuged at 4 °C for 10 min at 10,000 rpm. The supernatant were kept in aliquots for later determination of oxidative stress parameters, acetylcholinesterase and interleukin-6.
2.7.1. Estimation of serum lipid profile
Serum total cholesterol, triglyceride and HDL were estimated using Randox diagnostic kits (Randox Laboratories Limited, Antrim, United Kingdom). Low density lipoprotein (LDL) was calculated as LDL = (TC-HDL)-(TG/5) [39].
2.7.2. Evaluation of the risk of atherogenicity
The risk of atherogenicity was determined by calculating the atherogenic index [40], HDL/LDL ratio [42] and coronary risk index [41].
2.7.3. Estimation of oxidative stress parameters in serum and brain supernatant
The LPO end product malondialdehyde (MDA) concentration was measured in serum and brain supernatant using the thiobarbituric reacting substance (TBARS) assay [43]. Reduced glutathione (GSH) concentration in the serum and brain supernatant was determined using the Ellman’s reagent [44]. Assay for catalase enzyme activity in serum and brain supernatants of was determined using the colorimetric assay based on the yellow complex formation with H2O2 and molybdate [45].
2.7.4. Estimation of acetyl-cholinesterase activity
The procedure described by Ellman et al. [46] was used to estimate AChE activity in the supernatant of the brain tissues. Briefly, 50 μL aliquots of brain supernatant was diluted 50 μL of phosphate buffer (0.1 M, pH 7.4) followed by addition of 50 μL of DTNB (0.0001 M) in a 96-well plate. The initial absorbance was first measured after 5 min of incubation with DTNB. Thereafter, 50 μL of acetylthiocholine iodide (0.028 M) was added to the mixture for 3 min and the absorbance again measured at 405 nm in a microplate reader (Micro READ 1000, Belgium). The rate of acetyl-cholinesterase activity (μmol/min/mg tissue) was calculated as described below [46]:
| R = 5.74 × 10−4 x A/Co |
Where.
R Rate in moles of substrate hydrolyzed/min/g tissue.
A Change in absorbance/min.
Co = Original concentration of the tissue.
2.7.5. Estimation of Interleukin-6 (IL-6)
The concentration of IL-6 (BioLegend, USA) in the brain supernatant was determined according to the manufacturer’s instruction. IL-6 ELISA (BioLegend ELISA MAX™ Deluxe kit, USA) with sensitivity limit of 4 pg/mL. All the measurements were done at room temperature in accordance with BioLegend instructions using microplate reader with 450 nm filter(Micro READ 1000, Belgium). The concentration of IL-6 from the tissue was extrapolated from the standard curve of IL-6 standards included in the assay kits. The level of IL-6 in the brain was expressed as pg/mg protein.
2.8. Data analysis
All data were presented as mean ± standard error of mean (SEM) and statistical significance was taken for p < 0.05. Data were analyzed using one-way analysis of variance (ANOVA), significant main effects were further analyzed by Newman-Keuls post hoc test for multiple comparison of treatment groups with GraphPad Prism® software version 5.01 (GraphPad Software, Inc. La Jolla, CA 92037 USA).
3. Results
3.1. The effect of PEAC on body weight and organ weights
Following administration of HFD for 9 weeks, HFD group had the highest body weight gain, although not significantly different from ND group only (Table 1). Treatment with PEAC did not significantly reduced body weight gained in HFD-fed rats. As shown in Table 1, no statistical significant differences could be found in the brain, liver, heart or spleen among treatment groups.
Table 1.
Effect of PEAC on body weight and organ weights.
| Weight (g) | ND | PEAC | HFD | HFD + PEAC |
|---|---|---|---|---|
| Initial body weight | 88.2 ± 8.6 | 72.8 ± 7.2 | 72.4 ± 10.6 | 92.6 ± 3.9 |
| Final body weight | 191.8 ± 8.8 | 181.6 ± 6.4 | 184.8 ± 4.2 | 203.2 ± 11.5 |
| Weight gain | 103.6 ± 19.2 | 108.8 ± 15.0 | 112.4 ± 9.9 | 110.6 ± 9.4 |
| Brain | 1.69 ± 0.03 | 1.58 ± 0.05 | 1.66 ± 0.03 | 1.63 ± 0.06 |
| Liver | 5.82 ± 0.27 | 5.76 ± 0.32 | 6.39 ± 0.18 | 6.01 ± 0.32 |
| Heart | 0.58 ± 0.02 | 0.59 ± 0.02 | 0.58 ± 0.03 | 0.66 ± 0.09 |
| Kidneys | 1.0 ± 0.03 | 0.93 ± 0.03 | 1.08 ± 0.03 | 1.10 ± 0.05 |
| Spleen | 0.94 ± 0.05 | 0.74 ± 0.07 | 0.76 ± 0.04 | 0.72 ± 0.03 |
Values are expressed as mean ± SEM (n = 6).
3.2. Effect of PEAC on memory performance in normal diet and high fat diet-fed rats
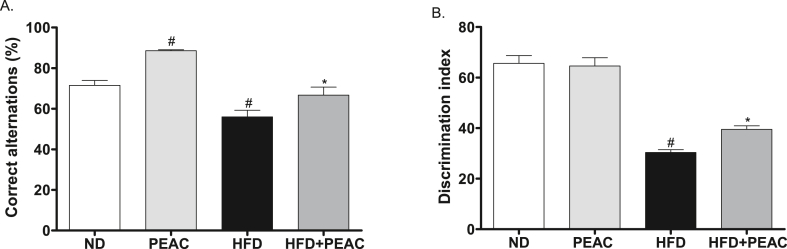
The effect of PEAC on spatial working memory of normal diet and high fat diet-fed rats is presented in Fig. 2A. The high fat diet-fed group significantly impaired spatial working memory performance as evidenced by decreased % correct alternation in the Y-maze test when compared with the vehicle [F (3, 20) = 22.84, p < 0.0001]. However, treatment with PEAC (200 mg/kg, p.o) daily for 4 weeks significantly attenuated spatial memory impairment caused by HFD in rats. PEAC (200 mg/kg) also significantly (p < 0.05) increased the percentage of correct alternation in basal diet-fed rats when compared with animals that received basal diet alone.
Fig. 2.
Effect of Peel extract of Ananas comosus (PEAC) on (A) spatial (B) non-spatial working memory of normal diet and high fat diet-fed rats measured in YMT and NORT, respectively. Values are expressed as mean ± SEM (n = 6). #p < 0.05 compared to ND group, ∗p < 0.05 compared with HFD group (One-way ANOVA, NewMann Keuls).
The effect of PEAC on non-spatial working memory performance of normal diet and high fat diet-fed rats is presented in Fig. 2B. The high fat diet-fed group significantly impaired non-spatial working memory performance as shown by decreased discrimination in the novel object recognition test when compared with vehicle [F (3, 20) = 54.60, p < 0.0001]. However, treatment with PEAC (200 mg/kg, p.o) daily for 4 weeks significantly attenuated non-spatial memory impairment caused by HFD in rats.
3.3. Anxiolytic-like effect of PEAC in normal diet and high fat diet-fed rats
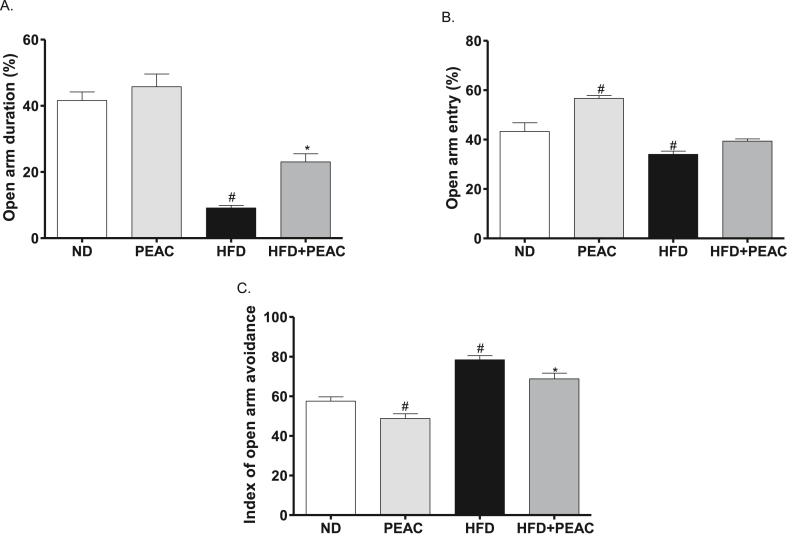
The open arms duration percentage [F(3,20) = 41.43, p < 0.001; Fig. 3A] was significantly decreased by HFD group when compared with the vehicle (ND) while the HFD treated with PEAC group increased this parameter compared with the HFD group. The open arms entries percentage [F(3,20) = 23.10, p < 0.0001; Fig. 3B] was significantly increased and decreased by PEAC and HFD groups respectively compared to the normal diet group but no significant change by HFD treated with PEAC (p > 0.05 vs HFD). With regard to the index open arms avoidance [F(3,20) = 29.33, p < 0.0001; Fig. 3C] there was significant decrease and increase in PEAC and HFD groups respectively compared to the normal diet group while HFD treated with PEAC group significantly decreased the parameter as compared with the HFD group.
Fig. 3.
Elevated plus maze test on anxiolytic effect of peel extract of Ananas comosus (PEAC) on normal diet and high fat diet-fed rats. (A) OADP, open arms duration percentage; (B) OAEP, open arms entries percentage; (C) IOAA, index open arms avoidance; Values are expressed as mean ± SEM (n = 6). #p < 0.05 compared to ND group, ∗p < 0.05 compared with HFD group (One-way ANOVA, NewMann Keuls).
3.4. Effect of PEAC on lipid profile in normal diet and high fat diet-fed rats
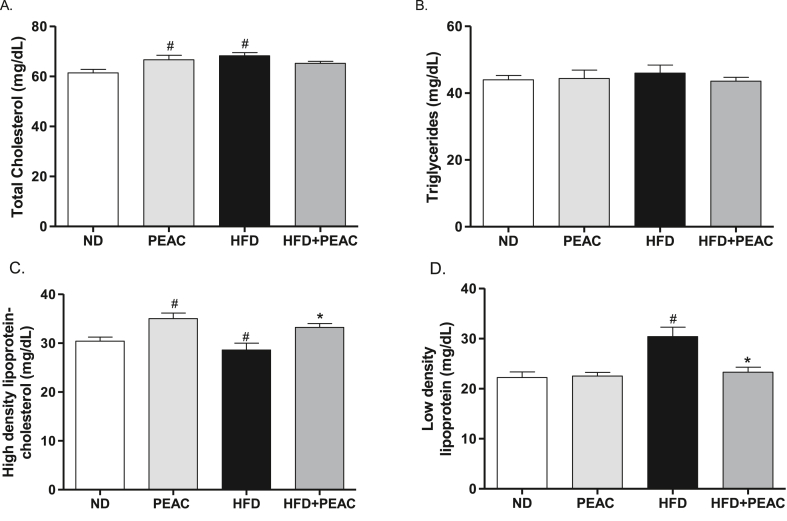
The effect of PEAC on serum lipid profiles is shown in Fig. 4 (A-D). Total cholesterol (TC) [F(3,19) = 4.623, p = 0.0164; Fig. 4A] and Low density lipoprotein (LDL) [F(3,19) = 9.727, p = 0.0007; Fig. 4D] were significantly elevated in HFD-fed rats compared to normal diet fed rats. No significant effect was observed in the triglycerides level [F(3,19) = 0.2967, p = 0.8272; Fig. 4B]. HDL-C was significantly [F(3,19) = 7.135, p = 0.0029; Fig. 4C] reduced in HFD-fed rats compared with normal diet fed rats (Fig. 4C). However, treatment with PEAC significantly improves HDL levels in normal diet and HFD-fed animals. Also PEAC administration to HFD-fed rats significantly reversed the elevated LDL in HFD-fed rats (Fig. 4D).
Fig. 4.
Antihyperlipidemic effect of peel extract of Ananas comosus (PEAC) on normal diet and high fat diet-fed rats. (A) Total cholesterol; (B) Triglycerides; (C) High density lipoprotein; (D) Low density lipoprotein. Values are expressed as mean ± SEM (n = 5). #p < 0.05 compared to ND group, ∗p < 0.05 compared with HFD group (One-way ANOVA, NewMann Keuls).
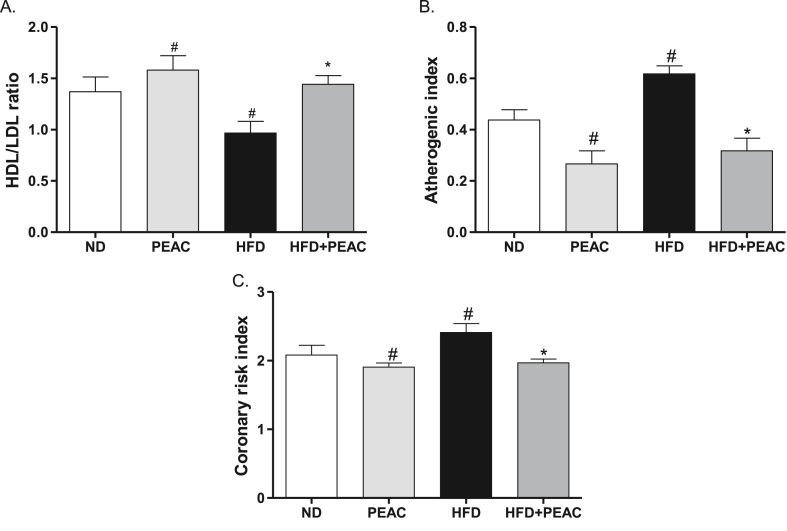
3.5. Effect of PEAC on risk of atherogenicity in normal diet and high fat diet-fed rats
The HFD group showed a significantly (p < 0.05) decreased HDL/LDL ratio when compared to the normal diet group (Fig. 5A). PEAC significantly [F(3,19) = 4.579, p = 0.0169; Fig. 5A] increased the HDL/LDL ratio both in the normal diet and high fat diet-fed rats. HFD feeding of rats significantly increased the atherogenic index of the animals [F(3,19) = 12.75, p = 0.0002; Fig. 5B]. Treatment of both normal diet fed and HFD-fed rats with PEAC significantly reduced the atherogenic indices (Fig. 5B). As shown in Fig. 5C, the HFD group showed a significantly [F(3,19) = 4.502, p = 0.0179; Fig. 5C] increased coronary risk index when compared to the normal diet group. However, PEAC significantly decreased the coronary risk index in both the normal diet and high-fat diet-fed rats (Fig. 5C).
Fig. 5.
Antiatherogenic effect of peel extract of Ananas comosus (PEAC) on normal diet and high fat diet-fed rats. (A) HDL/LDL ratio; (B) Atherogenic index; (C) Coronary risk index. Values are expressed as mean ± SEM (n = 5). #p < 0.05 compared to ND group, ∗p < 0.05 compared with HFD group (One-way ANOVA, NewMann Keuls).
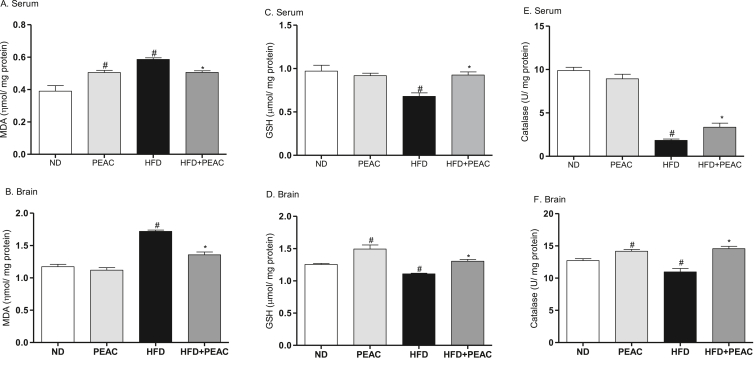
3.6. Effect of PEAC on serum and brain oxidative stress parameters in normal diet and high fat diet-fed rats
As shown in Fig. 6A&B, there was significant increase in the index of lipid peroxidation (malondialdehyde) in the serum [F(3,19) = 16.85, p < 0.0001; Fig. 6A] and brain [F(3,19) = 58.15, p < 0.0001; Fig. 6B] of HFD-fed rats. Administration of PEAC significantly increased the serum malondialdehyde levels (Fig. 6A) in normal diet-fed rats but significantly attenuated the HFD-induced lipid peroxidation in serum and brain. GSH concentration was significantly reduced in the serum [F(3,19) = 8.366, p = 0.0014; Fig. 6C] and brain [F(3,19) = 19.25, p < 0.0001; Fig. 6D] of HFD-fed rats. PEAC significantly increased GSH level in brain of ND rats (Fig. 6D) and attenuated GSH depletion in serum and brain tissues of HFD-fed rats (p < 0.05; Fig. 6C&D). Catalase enzymes activities were significantly reduced in serum [F(3,18) = 94.20, p < 0.0001; Fig. 6E] and brain [F(3,19) = 17.81, p < 0.0001; Fig. 6F] of HFD-fed rats. In ND-fed rats that received PEAC, catalase activity was increased in the brain tissue (Fig. 6F). Also PEAC reversed the effect of HFD-induced reduction of catalase activities in serum and brain tissues of HFD-fed rats (Fig. 6 E&F).
Fig. 6.
Effect of peel extract of Ananas comosus (PEAC) on oxidative stress parameters in normal diet and high fat diet-fed rats. (A) Serum malondialdehyde; (B) Brain malondialdehyde; (C) Serum reduced glutathione; (D) Brain reduced glutathione; (E) Serum catalase; (F) Brain catalase. Values are expressed as mean ± SEM (n = 5). #p < 0.05 compared to ND group, ∗p < 0.05 compared with HFD group (One-way ANOVA, NewMann Keuls).
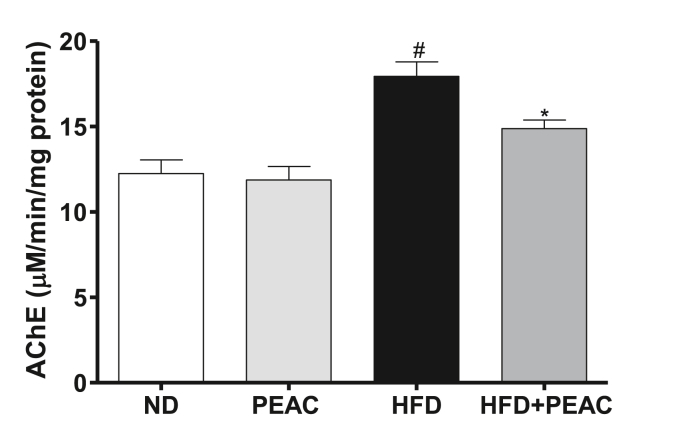
3.7. Effect of PEAC on brain acetylcholinesterase level in normal diet and high fat diet-fed rats
The effect of PEAC on the activity of acetyl-cholinesterase in the brain of normal diet and high fat diet fed rats is presented in Fig. 7. There was significant increase [F(3,19) = 13.97, p < 0.0001; Fig. 7] in brain acetylcholinesterase enzyme activities in HFD group compared to the normal diet. However, treatment with PEAC significantly decreased the AChE in HFD-fed rats as compared with the HFD group. PEAC did not alter AChE levels in ND-fed rats.
Fig. 7.
Effect of peel extract of Ananas comosus (PEAC) on acetylcholinesterase (AChE) in normal diet and high fat diet-fed rats. Values are expressed as mean ± SEM (n = 5). #p < 0.05 compared to ND group, ∗p < 0.05 compared with HFD group (One-way ANOVA, NewMann Keuls).
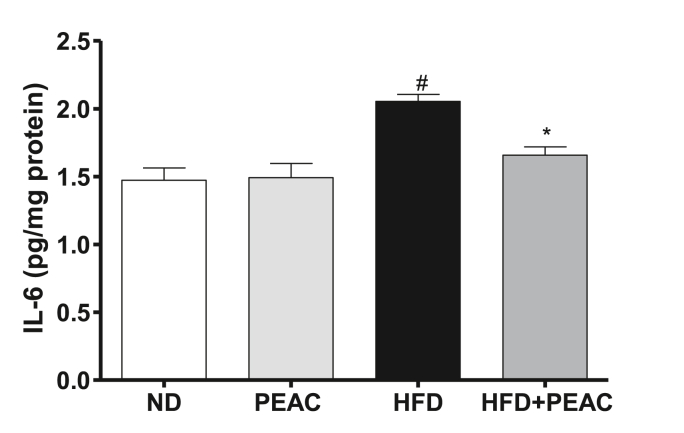
3.8. Effect of PEAC on brain interleukin-6 (IL-6) level in normal diet and high fat diet-fed rats
IL-6 level was significantly [F(3,23) = 11.22, p = 0.0002; Fig. 8] elevated in the HFD-fed rats. No significant elevation in ND-fed rats. Treatment with PEAC significantly (p < 0.05) reduced elevated IL-6 in brains of HFD-fed rats.
Fig. 8.
Effect of peel extract of Ananas comosus (PEAC) on brain interleukin-6 (IL-6) in normal diet and high fat diet-fed rats. Values are expressed as mean ± SEM (n = 5). #p < 0.05 compared to ND group, ∗p < 0.05 compared with HFD group (One-way ANOVA, NewMann Keuls).
4. Discussion
Recent evidence shows an association between obesity and cognitive decline [10]. Consumption of diets with increased sugar and fats have demonstrated some impairment in spatial and working memory in rodents [47]. The long-term consumption of a HFD impairs brain function through inflammation, oxidative stress, and induction of insulin resistance [48]. Oxidative stress and systemic inflammation contributes to the underlying complications of obesity [49]. This study reported the antiatherogenic, antiamnesic, anxiolytic-like, and antioxidant effects of peel extract of Ananas comosus fruit in normal diet and high-fat diet-fed rats. High-fat diet represents an important model to demonstrate the effect of obesity on cognition and anxiety-like effect in rodents [50,51]. Diet-induced obesity in rodents is characterized by hyperlipidemia, oxidative stress and systemic inflammation [52].
It has long been shown that a high fat diet increase LDL and total cholesterol in plasma, while HDL is reduced [53]. The observation is similar in this study as HFD feeding to rats induced hyperlipidemia that is evidenced by elevated total cholesterol, triglycerides, and low density lipoprotein and low high density lipoprotein. However, PEAC administration significantly reduced the cholesterol and LDL and increased HDL in the HFD-fed rats. Hypercholesteronemia has been shown to be associated with increased risk of developing later life dementia [54], it then appears that PEAC may be beneficial for treating patients with hypercholesterolemia. Some earlier studies have demonstrated the anti-hyperlipidemic effects of the pineapple juice [32,55,56] or the peel extract [56]. Bromelain and phenolic compounds found in pineapple peel has been reportedly shown to decrease cholesterol in obesity by breakdown of cholesterol plaques [57]. Also presence of high amount of soluble fibres in pineapple peels can also lower cholesterol by binding to cholesterol in the stomach and small intestine, ultimately reducing its absorption [58,59].
High Density Lipoprotein reverses cholesterol transport by scavenging excess cholesterol from peripheral tissues to the liver for its metabolism and excretion [60]. In addition, HDL can reduce or neutralize the atherogenic effects of oxidized LDL in artery walls [61]. A major component of the negative effects of a high fat diet is the HDL/LDL profile [62]. Treatment with PEAC significantly improved the HDL/LDL ratio in both normal diet and HFD-fed rats. Oxidative modification of LDL plays an immense role in the initial development of atherosclerosis and promotes further accumulation of free radicals in the arterial wall. Low HDL/LDL ratio indicates high LDL concentration in the plasma and can be proatherogenic [63].
Furthermore, PEAC improved the lipid profile of animals fed with the HFD diet and lowered their atherogenic index closer to the levels of the control animals. A decrease in atherogenic index is a positive physiological effect. Atherogenic index normally provides evidence for possible deposition of foam cells or plaque or fatty infiltration into important organs such as the heart, coronary vessels, aorta, liver and kidneys [64]. The higher the atherogenic index, the higher is the risk of the above organs for oxidative damage [65]. PEAC reduced the atherogenic index and coronary risk index which are often considered more important than individual lipoprotein as indicators of coronary heart disease risk [41,42]. Hence, it is plausible to suggest that PEAC has atheroprotective property, possibly partly due to the presence of bromelain and other phenolic compounds [32,34].
In this study, rats fed HFD for nine weeks displayed behavioral impairment on Y-maze test, NORT and EPM platforms. HFD-fed rats showed reduced correct alternation and discrimination index on the YMT and NORT, respectively. High-fat diets have been previously shown to impair spatial learning on the Y-maze paradigm [66,67] and non-spatial memory measured on the NORT paradigm [68]. Four weeks administration of PEAC reversed the HFD-induced memory impairment in rats. Previous study had reported the cognitive enhancing activity of pineapple juice in scopolamine-induced amnesia model [69].
The EPM, which is one of the most widely used models of animal anxiety, is based on the observation that rats avoid open elevated alleys and the assumption that this avoidance is generated by fear. Our results showed that a HFD increased the levels of anxiety in the tested animals, and oral administration of the PEAC significantly decreased the index of open arm avoidance and increased the % amount of open-arm exploration exhibited by the HFD-treated rats, which suggested anxiolytic effects. It has been reported that HFD exacerbates behavioral anomalies in various animal models of anxiety-like behaviors [51]. Neurobehavioral changes, including anxiety, may be mediated by oxido-inflammatory stress damage to the brain [70]. The mechanism(s) of how a HFD produces memory impairments is still under much scrutiny. Spatial memory is of course reliant on a functioning hippocampus and we suggest that the diets are having degenerative effects on this region. Our findings of changes in brain AChE levels suggests that high fat in the diet for a period of 9 weeks disrupted the cholinergic system. The administration of PEAC to HFD-fed rats decreased acetylcholinesterase activity in the brain. Increased acetylcholinesterase activity is associated with rapid breakdown of acetylcholine and subsequent memory deficits and oxidative stress [71,72]. Prolonged HFD feeding can induce upregulation of AChE expression and consequent assembly and stimulation of Ca2+ influx-mediated release of prooxidant amyloid beta peptides [73,74].
A balance between free radical production and antioxidant capacity is critical, as oxidative stress results from the upregulation and accumulation of oxidative products and downregulation of antioxidant enzymes in obese subjects [75], [76]. Oxidative stress markers and antioxidant concentrations have been evaluated in HFD studies to consider the role of oxidative stress in anxiety-like behavior [21]. The brain is very susceptible to damage from oxidative stress because of its oxygen consumption, relatively low antioxidant defenses, and high fat contents [77]. The present study demonstrated a significant increase in lipid peroxidation and decreased levels of antioxidants in rats fed HFD for nine weeks. Treatment with PEAC for four weeks ameliorated the oxidative stress in blood, suggesting that polyphenolic compounds may scavenge and inhibit ROS overproduction to overcome the adverse consequences of a HFD. In our study, the HFD significantly decreased serum GSH, while oral administration of the PEAC extract significantly increased GSH. GSH is important in the elimination of free radicals, and it acts as substrate for glutathione peroxidase (GPx) to neutralize hydrogen peroxide and organic hydroperoxides in lipid cellular membranes against oxidative damage. The PEAC may have upregulated GSH content via improving glutathione reductase (GR) activity. The current study showed that measurements of MDA levels in serum and brain were increased in the HFD group, whereas oral administration with the PEAC decreased the MDA levels. Concurrent treatment with PEAC produced higher activity of CAT in the serum and brain of HFD-fed rats, which indicates the ability of PEAC to scavenge ROS or protect the SOD/CAT system that eliminates superoxide radicals. Extract of pineapple peel have shown presence of phenolic compounds like ferulic acid, gallic acid, epicatechin, catechin, syringic acid [33]. The protection observed after PEAC treatment of HFD-fed rats suggested a therapeutic impact on HFD-induced metabolic disruption via the normalization of ROS.
Moreover, HFD-induced obesity is associated with chronic low-grade inflammation, which is characterized by increased inflammatory cytokines, such as interleukin-6, interleukin-1β, and tumor necrosis factor-α [77]. A high fat diet can act as an inflammatory insult to the body triggering synthesis and secretion of pro-inflammatory cytokines which will lead to even more cytokine production as well as increased reactive oxygen species (ROS) and nitrogen species. One of the pro-inflammatory cytokines, interleukin-6 (IL-6), controls hepatic C-reactive protein (CRP) production, a commonly used marker for inflammation, especially for obesity issues and cardiovascular disease [78]. IL-1β, IL-6, and TNF- α are pro-inflammatory cytokines which orchestrate the inflammatory response to many stimuli, both systemically and in the brain. IL-1β and IL-6 receptors are located all over the brain, especially in the hippocampus, a brain region involved in the anxiogenic/depressive phenotype [79]. The HFD significantly increased brain levels of IL-6 while orally administered PEAC significantly decreased the levels of IL-6 in HFD-fed rats. These anti-inflammatory properties of PEAC might probably be as a result of the presence of bromelain and flavonoids in the peels of A. comosus fruits [32,34]. The limitation of this study is our inability to investigate the phenolic and flavonoid composition of the ethanol extract of peels of A. comosus, however, some earlier reports have shown the presence of important phenolic compounds in the peel. Further investigation is needed to elucidate the anti-inflammatory effects of the peel and its bioactive constituents in adipocytes.
Taken together, the present study demonstrated memory impairment and anxiogenic effects of a high-fat diet in rat and beneficial atheroprotective, memory enhancing, anxiolytic-like, antioxidant and anti-inflammatory effects of peel extract of Ananas comosus fruit (pineapple). Hence, we identified pineapple peel as a potential natural source of functional food ingredients that may be useful in preventing obesity-related complications.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Abayomi M. Ajayi: Conceptualization, Methodology, Supervision, Investigation, Formal analysis, Writing - original draft. Kayode A. John: Investigation, Writing - original draft, report writing. Ilerioluwa B. Emmanuel: Formal analysis, Writing - original draft. Emmanuel O. Chidebe: Investigation, Writing - original draft. Aduragbenro D.A. Adedapo: Conceptualization, Methodology, Supervision, Writing - review & editing.
Acknowledgement
Authors acknowledge the assistance of Oyetola Oyebanji for the behavioral experiments.
References
- 1.Çakmur H. Obesity as a growing public health problem. In: Gordeladze Jan Oxholm., editor. Chapter 2. IntechOpen; 2017. (Adiposity - epidemiology and treatment modalities). [Google Scholar]
- 2.Bays H., Scinta W. Adiposopathy and epigenetics: an introduction to obesity as a transgenerational disease. Curr Med Res Opin. 2015;31(11):2059–2069. doi: 10.1185/03007995.2015.1087983. [DOI] [PubMed] [Google Scholar]
- 3.Bray G.A., Kim K.K., Wilding J.P.H. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18(7):715–723. doi: 10.1111/obr.12551. [DOI] [PubMed] [Google Scholar]
- 4.Susmita B., Anbarasu A. Obesity : a critical review. Int J Pharmacol Biol Sci. 2011;2(4):B582–B592. [Google Scholar]
- 5.Park H.J., Kim J.H., Shim I. Anti-obesity effects of ginsenosides in high-fat diet-fed rats. Chin J Integr Med. 2019;25(12):895–901. doi: 10.1007/s11655-019-3200-x. [DOI] [PubMed] [Google Scholar]
- 6.Feingold K.R., Grunfeld C. Obesity and dyslipidemia. In: Feingold K.R., Anawalt B., Boyce A., editors. Endotext [internet]. South dartmouth (MA) MDText.com, Inc.; 2000. https://www.ncbi.nlm.nih.gov/books/NBK305895 [Updated 2018 Apr 10] Available from. [Google Scholar]
- 7.Klop B., Elte J.W.F., Cabezas M.C. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5(4):1218–1240. doi: 10.3390/nu5041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huet F., Roubille C., Roubille F. Is hypertriglyceridemia atherogenic? Curr Opin Lipidol. 2019;30(4):291–299. doi: 10.1097/MOL.0000000000000622. [DOI] [PubMed] [Google Scholar]
- 9.Leguisamo N.M., Lehnen A.M., Machado U.F., Okamoto M.M., Markoski M.M., Pinto G.H., Schaan B.D. GLUT4 content decreases along with insulin resistance and high levels of inflammatory markers in rats with metabolic syndrome. Cardiovasc Diabetol. 2012;11(1):1. doi: 10.1186/1475-2840-11-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vekic J., Zeljkovic A., Stefanovic A., Jelic-Ivanovic Z., Spasojevic-Kalimanovska V. Obesity and dyslipidemia. Metabolism: Clinical and Experimental. 2019;92:71–81. doi: 10.1016/j.metabol.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Cheke L.G., Simons J.S., Clayton N.S. Higher body mass index is associated with episodic memory deficits in young adults. Q J Exp Psychol. 2016;69(11):2305–2316. doi: 10.1080/17470218.2015.1099163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prickett C., Brennan L., Stolwyk R. Examining the relationship between obesity and cognitive function: a systematic literature review. Obes Res Clin Pract. 2015;9(2):93–113. doi: 10.1016/j.orcp.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Cunnane S.C., Schneider J.A., Tangney C., Tremblay-Mercier J., Fortier M., Bennett D.A., Morris M.C. Plasma and brain fatty acid profiles in mild cognitive impairment and alzheimer’s disease. J Alzheim Dis. 2012;29(3):691–697. doi: 10.3233/JAD-2012-110629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conquer J.A., Tierney M.C., Zecevic J., Bettger W.J., Fisher R.H. Fatty acid analysis of blood plasma of patients with Alzheimer’s disease, other types of dementia, and cognitive impairment. Lipids. 2000;35(12):1305–1312. doi: 10.1007/s11745-000-0646-3. [DOI] [PubMed] [Google Scholar]
- 15.Cook R.L., O’Dwyer N.J., Donges C.E., Parker H.M., Cheng H.L., Steinbeck K.S., Cox E.P., Franklin J.L., Garg M.L., Rooney K.B., O’Connor H.T. Relationship between obesity and cognitive function in young women: the food, mood and mind study. Journal of Obesity. 2017;2017 doi: 10.1155/2017/5923862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’sullivan J.B. Body weight and subsequent diabetes mellitus. J Am Med Assoc: JAMA, J Am Med Assoc. 1982;248(8):949–952. [PubMed] [Google Scholar]
- 17.Beuther D.A., Sutherland E.R. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175(7):661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroenke Kurt, Spitzer Robert L., Williams Janet B.W., Monahan Patrick O., Lö we Bernd. Anxiety disorders in primary Care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;151(10):678–685. doi: 10.7326/0003-4819-146-5-200703060-00004. [DOI] [PubMed] [Google Scholar]
- 19.Lopresti A.L., Drummond P.D. Obesity and psychiatric disorders: commonalities in dysregulated biological pathways and their implications for treatment. Prog Neuro Psychopharmacol Biol Psychiatr. 2013;45:92–99. doi: 10.1016/j.pnpbp.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Sinha R., Jastreboff A.M. Stress as a common risk factor for obesity and addiction. Biol Psychiatr. 2013;73(9):827–835. doi: 10.1016/j.biopsych.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeJesus R.S., Breitkopf C.R., Ebbert J.O., Rutten L.J.F., Jacobson R.M., Jacobson D.J., Fan C., St Sauver J. Associations between anxiety disorder diagnoses and body mass index differ by age, sex and race: a population based study. Clin Pract Epidemiol Ment Health. 2016;12(1):67–74. doi: 10.2174/1745017901612010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Da Silva M.A., Singh-Manoux A., Brunner E.J., Kaffashian S., Shipley M.J., Kivimäki M., Nabi H. Bidirectional association between physical activity and symptoms of anxiety and depression: the whitehall II study. Eur J Epidemiol. 2012;27(7):537–546. doi: 10.1007/s10654-012-9692-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsiao P.Y., Jensen G.L., Hartman T.J., Mitchell D.C., Nickols-Richardson S.M., Coffman D.L. Food intake patterns and body mass index in older adults: a review of the epidemiological evidence. Journal of Nutrition in Gerontology and Geriatrics. 2011;30(3):204–224. doi: 10.1080/21551197.2011.591266. [DOI] [PubMed] [Google Scholar]
- 24.Wu H., Liu Q., Kalavagunta P.K., Huang Q., Lv W., An X., Chen H., Wang T., Heriniaina R.M., Qiao T., Shang J. Normal diet vs High fat diet-A comparative study: behavioral and neuroimmunological changes in adolescent male mice. Metab Brain Dis. 2018;33(1):177–190. doi: 10.1007/s11011-017-0140-z. [DOI] [PubMed] [Google Scholar]
- 25.Ganji A., Salehi I., Nazari M., Taheri M., Komaki A. Effects of Hypericum scabrum extract on learning and memory and oxidant/antioxidant status in rats fed a long-term high-fat diet. Metab Brain Dis. 2017;32(4):1255–1265. doi: 10.1007/s11011-017-0022-4. [DOI] [PubMed] [Google Scholar]
- 26.Abbasnejad Z., Nasseri B., Zardooz H., Ghasemi R. Time-course study of high fat diet induced alterations in spatial memory, hippocampal JNK, P38, ERK and Akt activity. Metab Brain Dis. 2019;34(2):659–673. doi: 10.1007/s11011-018-0369-1. [DOI] [PubMed] [Google Scholar]
- 27.Lambert K., Hokayem M., Thomas C., Fabre O., Cassan C., Bourret A., Bernex G., Feuillet-Coudray C., Notarnicola C., Mercier J., Avignon A., Bisbal C. Combination of nutritional polyphenols supplementation with exercise training counteracts insulin resistance and improves endurance in high-fat diet-induced obese rats. Sci Rep. 2018;8(1):1–10. doi: 10.1038/s41598-018-21287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaudhary V., Kumar V., Sunil Vaishali, Singh K., Kumar R., Kumar V. Pineapple (Ananas cosmosus) product processing: a review. J Pharmacogn Phytochem. 2019;8(3):4642–4652. [Google Scholar]
- 29.Lawal D. Medicinal, pharmacological and phytochemical potentials of Annona comosus Linn. peel-a review. Bayero Journal of Pure and Applied Sciences. 2013;6(1):101–104. [Google Scholar]
- 30.Monji F., Adaikan P.G., Lau L.C., Bin Said B., Gong Y., Tan H.M., Choolani M. Investigation of uterotonic properties of Ananas comosus extracts. J Ethnopharmacol. 2016;193:21–29. doi: 10.1016/j.jep.2016.07.041. [DOI] [PubMed] [Google Scholar]
- 31.Dabesor A.P., Asowata-Ayodele A.M., Umoiette P. Phytochemical compositions and antimicrobial activities of Ananas comosus peel (M.) and Cocos nucifera kernel (L.) on selected food borne pathogens. American Journal of Plant Biology. 2017;2(2):73–76. [Google Scholar]
- 32.El-Shazly S.A., Ahmed M.M., AL-Harbi M.S., Alkafafy M.E., El-Sawy H.B., Amer S.A.M. Physiological and molecular study on the anti-obesity effects of pineapple (Ananas comosus) juice in male Wistar rat. Food Science and Biotechnology. 2018;27(5):1429–1438. doi: 10.1007/s10068-018-0378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azizan A., Lee A.X., Hamid N.A.A., Maulidiani M., Mediani A., Ghafar S.Z.A., Zolkeflee N.K.Z., Abas F. Potentially bioactive metabolites from pineapple waste extracts and their antioxidant and α-glucosidase inhibitory activities by 1H NMR. Foods. 2020;9(2) doi: 10.3390/foods9020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maulana M., Ikram M., Ridwani S., Putri S.P., Fukusaki E. GC-MS based metabolite profiling to monitor ripening-specific metabolites in pineapple ( Ananas comosus ) Metabolites. 2020;10(134):1–15. doi: 10.3390/metabo10040134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei J., Bai W., Liu T., Tian X. Functional connectivity changes during a working memory task in rat via NMF analysis. Front Behav Neurosci. 2015;9:1–13. doi: 10.3389/fnbeh.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W., Li S., Dong H. ping, Lv S., Tang Y. yuan. Differential impairment of spatial and nonspatial cognition in a mouse model of brain aging. Life Sci. 2009;85(3–4) doi: 10.1016/j.lfs.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Lister R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 38.Friedwald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 39.Harnafi H., Caid H.S., el Houda Bouanani N., Aziz M., Amrani S. Hypolipemic activity of polyphenol-rich extracts from Ocimum basilicum in Triton WR-1339-induced hyperlipidemic mice. Food Chem. 2008;108(1):205–212. [Google Scholar]
- 40.Joerin L., Kauschka M., Bonnländer B., Pischel I., Benedek B., Butterweck V. Ficus carica leaf extract modulates the lipid profile of rats fed with a high-fat diet through an increase of HDL-C. Phytother Res. 2014;28(2):261–267. doi: 10.1002/ptr.4994. [DOI] [PubMed] [Google Scholar]
- 41.Singh S.V., Shrivastava A., Chaturvedi U., Singh S.C., Shanker K., Saxena J.K. A mechanism-based pharmacological evaluation of efficacy of Flacourtia indica in management of dyslipidemia and oxidative stress in hyperlipidemic rats. J Basic Clin Physiol Pharmacol. 2016;27(2):121–129. doi: 10.1515/jbcpp-2015-0017. [DOI] [PubMed] [Google Scholar]
- 42.Ádám-Vizi V., Seregi A. Receptor independent stimulatory effect of noradrenaline on Na,K-ATPase in rat brain homogenate. Role of lipid peroxidation. Biochem Pharmacol. 1982;31(13):2231–2236. doi: 10.1016/0006-2952(82)90106-x. [DOI] [PubMed] [Google Scholar]
- 43.Jollow D.J. Glutathione thresholds in reactive metabolite toxicity. Arch Toxicol Suppl. 1980;3:95–110. doi: 10.1007/978-3-642-67389-4_8. [DOI] [PubMed] [Google Scholar]
- 44.Goth L. A simple method for determination of serum catalase activity and revision of reference range. Clinica chimica acta. 1991;196(2–3):143–151. doi: 10.1016/0009-8981(91)90067-m. [DOI] [PubMed] [Google Scholar]
- 45.Ellman G.L., Courtney K.D., Andre J.V., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 46.Abbott K.N., Arnott C.K., Westbrook R.F., Tran D.M. The effect of high fat, high sugar, and combined high fat-high sugar diets on spatial learning and memory in rodents: a meta-analysis. Neurosci Biobehav Rev. 2019;107:399–421. doi: 10.1016/j.neubiorev.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Spinelli M., Fusco S., Mainardi M., Scala F., Natale F., Lapenta R., Mattera A., Rinaudo M., Puma D.D.L., Ripoli C., Grassi A. Brain insulin resistance impairs hippocampal synaptic plasticity and memory by increasing GluA1 palmitoylation through FoxO3a. Nat Commun. 2017;8(1):1–14. doi: 10.1038/s41467-017-02221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng T., Lyon C.J., Bergin S., Caligiuri M.A., Hsueh W.A. Obesity, inflammation, and cancer. Annu Rev Pathol. 2016;11:421–449. doi: 10.1146/annurev-pathol-012615-044359. [DOI] [PubMed] [Google Scholar]
- 49.Zemdegs J., Quesseveur G., Jarriault D., Pénicaud L., Fioramonti X., Guiard B.P. High-fat diet-induced metabolic disorders impairs 5-HT function and anxiety-like behavior in mice. Br J Pharmacol. 2016;173(13):2095–2110. doi: 10.1111/bph.13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hicks J.A., Hatzidis A., Arruda N.L., Gelineau R.R., De Pina I.M., Adams K.W., Seggio J.A. Voluntary wheel-running attenuates insulin and weight gain and affects anxiety-like behaviors in C57BL6/J mice exposed to a high-fat diet. Behav Brain Res. 2016;310:1–10. doi: 10.1016/j.bbr.2016.04.051. [DOI] [PubMed] [Google Scholar]
- 51.Othman Z.A., Ghazali W., Syaheedah W., Noordin L., Yusof M., Aiman N., Mohamed M. Phenolic compounds and the anti-atherogenic effect of bee bread in high-fat diet-induced obese rats. Antioxidants. 2020;9(1):33. doi: 10.3390/antiox9010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shanmugasundaram K.R., Visvanathan A., Dhandapani K., Srinivasan N., Rasappan P., Gilbert R., Alladi S., Kancharla S., Vasanthi N. Effect of high-fat diet on cholesterol distribution in plasma lipoproteins, cholesterol esterifying activity in leucocytes, and erythrocyte membrane components studied: importance of body weight. Am J Clin Nutr. 1986;44(6):805–815. doi: 10.1093/ajcn/44.6.805. [DOI] [PubMed] [Google Scholar]
- 53.Carlsson C.M., Nondahl D.M., Klein B.E., McBride P.E., Sager M.A., Schubert C.R. Increased atherogenic lipoproteins are associated with cognitive impairment: effects of statins and subclinical atherosclerosis. Alzheimer disease and associated disorders. 2009;23(1):11. doi: 10.1097/wad.0b013e3181850188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daher C.F., Abou-Khalil J., Baroody G. Effect of acute and chronic grapefruit, orange, and pineapple juice intake on blood lipid profile in normolipidemic rat. Med Sci Mon Int Med J Exp Clin Res. 2005;11(12):BR465–BR472. 2005. [PubMed] [Google Scholar]
- 55.Ezz M.K., Hassan R.E., Esmat A.Y. Pineapple juice supplementation activates thyroid gland and attenuates hyperlipidemia in rats. Int J Biosci. 2017;10:173–184. [Google Scholar]
- 56.Emmanuel E.U., Onagbonfeoana E.S., Adanma O.C., Precious O.C., Faith A.I., Ndukaku O.Y. In vivo and in vitro antioxidant and hypolipidemic activity of methanol extract of pineapple peels in wistar rats. Int J Biosci. 2016;8(6):64–72. [Google Scholar]
- 57.Manzoor Z., Nawaz A., Mukhtar H., Haq I. Bromelain: methods of extraction, purification and therapeutic applications. Braz Arch Biol Technol. 2016;59 [Google Scholar]
- 58.Ackom N.B., Tano-Debrah K. Processing pineapple pulp into dietary fibre supplement. Afr J Food Nutr Sci. 2012;12(6) [Google Scholar]
- 59.Hu H., Zhao Q. Optimization extraction and functional properties of soluble dietary fiber from pineapple pomace obtained by shear homogenization-assisted extraction. RSC Adv. 2018;8(72):41117–41130. doi: 10.1039/c8ra06928j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trajkovska K.T., Topuzovska S. High-density lipoprotein metabolism and reverse cholesterol transport: strategies for raising HDL cholesterol. Anatol J Cardiol. 2017;18(2):149. doi: 10.14744/AnatolJCardiol.2017.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brites F., Martin M., Guillas I., Kontush A. Antioxidative activity of high-density lipoprotein (HDL): mechanistic insights into potential clinical benefit. BBA clinical. 2017;8:66–77. doi: 10.1016/j.bbacli.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vijaimohan K., Jainu M., Sabitha K.E., Subramaniyam S., Anandhan C., Devi C.S. Beneficial effects of alpha linolenic acid rich flaxseed oil on growth performance and hepatic cholesterol metabolism in high fat diet fed rats. Life Sci. 2006;79(5):448–454. doi: 10.1016/j.lfs.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 63.Alique M., Luna C., Carracedo J., Ramírez R. LDL biochemical modifications: a link between atherosclerosis and aging. Food Nutr Res. 2015;59(1):29240. doi: 10.3402/fnr.v59.29240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Subramaniam S., Subramaniam R., Rajapandian S., Uthrapathi S., Gnanamanickam V.R., Dubey G.P. Anti-atherogenic activity of ethanolic fraction of Terminalia arjuna bark on hypercholesterolemic rabbits. Evid base Compl Alternative Med. 2011;2011 doi: 10.1093/ecam/neq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abid R., Mahmood R., Santosh Kumar H.S. Hypolipidemic and antioxidant effects of ethanol extract of Cassia fistula fruit in hyperlipidemic mice. Pharmaceut Biol. 2016;54(12):2822–2829. doi: 10.1080/13880209.2016.1185445. [DOI] [PubMed] [Google Scholar]
- 66.Cui Y., Shu Y., Zhu Y., Shi Y., Le G. High-fat diets impair spatial learning of mice in the Y-maze paradigm: ameliorative potential of α-lipoic acid. J Med Food. 2012;15(8):713–717. doi: 10.1089/jmf.2011.1970. [DOI] [PubMed] [Google Scholar]
- 67.Kosari S., Badoer E., Nguyen J.C., Killcross A.S., Jenkins T.A. Effect of western and high fat diets on memory and cholinergic measures in the rat. Behav Brain Res. 2012;235(1):98–103. doi: 10.1016/j.bbr.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 68.Francis H.M., Mirzaei M., Pardey M.C., Haynes P.A., Cornish J.L. Proteomic analysis of the dorsal and ventral hippocampus of rats maintained on a high fat and refined sugar diet. Proteomics. 2013;13(20):3076–3091. doi: 10.1002/pmic.201300124. [DOI] [PubMed] [Google Scholar]
- 69.Momtazi-borojeni A.A., Sadeghi-Aliabadi H., Rabbani M., Ghannadi A., Abdollahi E. Cognitive enhancing of pineapple extract and juice in scopolamine-induced amnesia in mice. Research in pharmaceutical sciences. 2017;12(3):257. doi: 10.4103/1735-5362.207198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yan B., Guo J., Liu X., Li J., Yang X., Ma P., Wu Y. Oxidative stress mediates dibutyl phthalate induced anxiety-like behavior in Kunming mice. Environ Toxicol Pharmacol. 2016;45:45–51. doi: 10.1016/j.etap.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 71.Melo J.B., Agostinho P., Oliveira C.R. Involvement of oxidative stress in the enhancement of acetylcholinesterase activity induced by amyloid beta-peptide. Neurosci Res. 2003;45(1):117–127. doi: 10.1016/s0168-0102(02)00201-8. [DOI] [PubMed] [Google Scholar]
- 72.Amri Z., Ghorbel A., Turki M., Akrout F.M., Ayadi F., Elfeki A., Hammami M. Effect of pomegranate extracts on brain antioxidant markers and cholinesterase activity in high fat-high fructose diet induced obesity in rat model. BMC Compl Alternative Med. 2017;17(1):339. doi: 10.1186/s12906-017-1842-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Inestrosa N.C., Alvarez A., Perez C.A., Moreno R.D., Vicente M., Linker C., Garrido J. Acetylcholinesterase accelerates assembly of amyloid-β-peptides into Alzheimer’s fibrils: possible role of the peripheral site of the enzyme. Neuron. 1996;16(4):881–891. doi: 10.1016/s0896-6273(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 74.Kar S., Slowikowski S.P., Westaway D., Mount H.T. Interactions between β-amyloid and central cholinergic neurons: implications for Alzheimer’s disease. J Psychiatry Neurosci. 2004;29:427–441. [PMC free article] [PubMed] [Google Scholar]
- 75.Lozano I., Van der Werf R., Bietiger W., Seyfritz E., Peronet C., Pinget M., Jeandidier N., Maillard E., Marchioni E., Sigrist S. High fructose and high-fat diet-induced disorders in rats: impact on diabetes risk, hepatic and vascular complications. Nutr Metab. 2016;13:1. doi: 10.1186/s12986-016-0074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.AbdulKadir N.A.A., Rahmat A., Jaafar H.Z. Protective effects of Tamarillo (Cyphomandra betacea) extract against high fat diet induced obesity in Sprague–Dawley rats. J Obes. 2015 doi: 10.1155/2015/846041. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dutheil S., Ota K.T., Wohleb E.S., Rasmussen K., Duman R.S. High-fat diet induced anxiety and anhedonia: impact on brain homeostasis and inflammation. Neuropsychopharmacology. 2016;41:1874–1887. doi: 10.1038/npp.2015.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sakr H.F., Abbas A.M., Khalil K., Shata A.M. Modulatory effect of concomitant administration of sitagliptin and vitamin E on inflammatory biomarkers in rats fed with high fat diet: role of adiponectin. J Physiol Pharmacol. 2019;70(6) doi: 10.26402/jpp.2019.6.13. [DOI] [PubMed] [Google Scholar]
- 79.Lotrich F.E. Inflammatory cytokine-associated depression. Brain Res. 2015;1617:113–125. doi: 10.1016/j.brainres.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]