Highlights
-
•
A persistent ultrasound-assisted hydrothermal synthesis has been developed.
-
•
Ultrasonication can promote the production of Co3O4/N-GO hybrids.
-
•
The ultrasonicated Co3O4/N-GO hybrids are effective catalyst for Al-air battery.
-
•
Ultrasound allows Co3O4 particles uniformly grow on the dispersed graphene oxides.
Keywords: Aluminum air battery, Ultrasound-assisted hydrothermal synthesis, Oxygen reduction reaction catalyst, Co3O4/N-GO hybrid
Abstract
A persistent ultrasound-assisted hydrothermal method has been developed to prepare cobalt oxide incorporated nitrogen-doped graphene (Co3O4/N-GO) hybrids. The electrochemical behaviors and catalytic activity of the prepared hybrids have been systematically investigated as cathode materials for Al-air battery. The results show that ultrasonication can promote the yield ratio of Co3O4 from 63.1% to 70.6%. The prepared Co3O4/N-GO hybrid with ultrasonication exhibits better ORR activity over that without ultrasonication. The assembled Al-air battery using the ultrasonicated Co3O4/N-GO hybrid exhibited an average working voltage of 1.02 V in 4 M KOH electrolyte at 60 mA∙cm−2, approximately 60 mV higher than that using hybrid without ultrasonication. This should be attributed to large number density of fine Co3O4 particles growing on the dispersed GO sheets under the persistent ultrasonication. The related ultrasonic mechanism has been discussed in details.
1. Introduction
Storage and conversion of renewable resources are promising to meet the most urgent goals for clean energy production and environmental remediation [1], [2]. Aluminum-air battery is one of the promising and efficient energy storage and supply systems, delivering renewable energy with metals and the inexhaustible O2 in air [3], [4], [5]. However, there are many technical and scientific issues that hinder the large-scale development and application of Al-air batteries.
The oxygen reduction reaction (ORR) activity is one of the most important concerns for the Al-air battery with high energy efficiency [5]. Precious metal catalysts (e.g. Pt, Au, Ag) have superior ORR catalytic performance [6], [7]. However, their scarcity, high cost and poor tolerance limit the large-scale application. Thus, it is crucial to develop low-cost and efficient ORR catalysts for Al-air battery. Transition metal oxides have been reported to be cheap and effective ORR catalysts [8], [9], and among them, Co3O4 has attracted great interests [10], [11], [12]. However, careful adjustment should be done to promote the electrical conductivity and stability of the Co3O4-containing ORR catalysts.
One of the effective strategies is using highly stable and conductive catalyst carriers or supporters, for instance, the widely employed graphene oxide (GO) [13], [14], [15], [16]. Furthermore, additional nitrogen doping could greatly increase the electron mobility and introduce chemically active sites for catalyst deposition [17], [18]. Thus, hybrid air-breathing cathodes consisting of cobalt oxide and nitrogen doped graphene oxide, exhibit high ORR activity in alkaline solutions [19], [20], [21], [22], [23]. However, cobalt oxide nanoparticles are easy to aggregate into random bundles among the graphene layers [24]. Such agglomeration gives rise to the reduction of ORR activity for the air-breathing cathodes, due to the decreased number density of active sites.
Many efforts have been made to avoid the agglomeration of catalyst particles. Ultrasound is the prevailing approach to disperse the particles [25], [26], [27]. In general, an ultrasonication for 0.5 ~ 1.5 h can yield dispersive and fine nano particles [28], [29], [30], [31]. Similar ultrasonication treatment for precursors prior to hydrothermal synthesis has been applied to prepare hybrid catalysts nanoparticles [32], [33]. However, the agglomeration of reacting precursors or nanoparticles and catalyst carriers possibly reappears during the long-time hydrothermal synthesis process [34]. Thus, an ultrasonication upon hydrothermal synthesis is proposed to achieve a sufficient dispersion of hybrid catalyst particles and carriers, which has been scantly investigated.
In this work, a persistently ultrasound-assisted hydrothermal process (HU) is developed to prepare a hybrid catalyst incorporating cobalt oxide with nitrogen-doped graphene oxide (HU-Co3O4/N-GO). This hybrid is characterized using XPS, FESEM, TEM and electrochemical measurement. In addition, a full Al-air battery is constructed to evaluate the ORR activity of the as-prepared hybrid catalyst.
2. Experimental section
2.1. Material synthesis
Graphene oxide used was commercial product (Tu ling Co., Ltd). Analytical grade C4H6CoO4·4H2O, NH4OH, Na2SO4, KOH, and activated carbon and acetylene black were supplied from Sinopharm Chemical Reagent Co., Ltd. De-ionized (DI) water was used as a solvent in the synthesis process.
The schematic diagram for the synthesis route of HU-Co3O4/N-GO hybrid is shown in Fig. 1. Firstly, graphene oxide (450 mg) was dispersed in Co(CH3COO)2 aqueous solution (0.2 mol L−1, 225 ml) at room temperature, then added NH4OH (4.2 ml, 30% solution) for nitration under ultrasonication. Then, the dispersed precursors were transferred to a Teflon-lined and sealed autoclave with a capacity of 300 ml. A persistently ultrasound-assisted hydrothermal synthesis was carried out at 150 °C for 10 h in a self-designed apparatus, where a 40 kHz ultrasound with a fixed power of 600 W was transmitted into a stainless reactor with periodically repeated switch on for 30 min and off for 5 min via silicone fluid media. The underlying synthesis process for Co3O4/N-GO hybrid is considered as following: Co2+ cations can homogeneously coat onto GO sheets due to the electrostatic interaction. During the ultrasound-assisted hydrothermal process, N atoms from NH4OH are doped into the graphene oxide. The precursor covered on the surface of nitrogen can be doped on the graphene oxide surface and finally transformed into Co3O4/N-GO hybrid via the following reaction:
| (1) |
Fig. 1.
Schematic synthetic procedure of HU-Co3O4/N-GO hybrid.
Finally, the HU-Co3O4/N-GO hybrid was collected after centrifugation, washed with ethanol and DI water and lyophilization on the reaction products. Similar synthesis was also conducted without ultrasonic assistance to prepare H-Co3O4/N-GO hybrid. HU-Co3O4 and HU-N-GO were also prepared without the addition of GO and Co (CH3COO)2, respectively. A blank H-N-GO was also prepared for a direct comparison.
2.2. Material characterizations
Powder XRD (X-ray diffraction) patterns for the synthesized samples were performed using an Ultima Ⅳ X-ray diffractometer with Cu Kα (λ = 1.54 Å) radiation. The chemical states of C, N, O, and Co elements in the samples were detected by XPS (X-ray photoelectron spectroscopy measurements, AXISULTRA-DLD, Al Kα X-ray radiation source at 150 W). The yield of Co3O4 synthesized by hydrothermal reaction was determined by ICP (inductively coupled plasma emission spectrometer, Optima-7000DV). The morphology observation was conducted using a Zeiss Auriga scanning electron microscope (FESEM) coupled with energy dispersive spectra (EDS) and a JEM-2100 transmission electron microscope (TEM).
2.3. Electrochemical measurements
The electrochemical measurements were performed in a three-electrode system using a CHI660E electrochemical workstation. Catalyst powders coated on the glassy carbon electrode (geometric diameter: 3 mm) were used as the working electrode in the aqueous solution of 0.1 M KOH saturated with O2. Hg/HgO electrode and platinum foil served as the reference electrode and the counter electrode, respectively. Cyclic voltammetry (CV) and linear sweep voltammetry (LSV) were carried out at a scan rate of 50 mV s−1 and 5 mV s−1, respectively. Electrochemical impedance spectroscopy (EIS) was conducted in the scanning range from 0.1 Hz and 100 kHz at 1.023 V.
2.4. Al-air battery tests
Al-air batteries were assembled with the commercial pure Al anode and self-made air-breathing cathodes loaded with the as-prepared hybrid catalysts. The surface area of electrodes was 1 cm2, and 4 M KOH aqueous solution was used as electrolyte. The discharge tests were conducted using a LANHE battery testing system at the current densities of 20, 40 and 60 mA cm−2, respectively.
3. Results and discussion
3.1. Production of synthesized Co3O4
Table 1 lists the calculated production of the Co3O4 after hydrothermal synthesis with or without ultrasonication based on ICP measurement. The total amount of Co was 2.652 g in each trial for hydrothermal synthesis. The residual Co in the collected filtrates was 0.857 g, 1.065 g, 0.780 g and 0.979 g in HU-Co3O4, H-Co3O4, HU-Co3O4/N-GO hybrid and H-Co3O4/N-GO hybrid, respectively. Consequently, the Co3O4 production in HU-Co3O4, H-Co3O4, HU-Co3O4/N-GO and H-Co3O4/N-GO hybrid were 2.445 g (67.7%), 2.161 g (59.8%), 2.5502 g (70.6%) and 2.2785 g (63.1%), respectively. It suggests that the ultrasonication can promote the hydrothermal reaction for Co3O4 production. Furthermore, the catalyst carrier N-GO also has a slight promotion on the Co3O4 production.
Table 1.
The calculated production of the Co3O4 after hydrothermal synthesis with or without ultrasonication via ICP measurement.
| Item (g) | HU-Co3O4 | H-Co3O4 | HU-Co3O4/N-GO | H-Co3O4/N-GO |
|---|---|---|---|---|
| Total amount of Co | 2.625 (±0.034) | |||
| Residual Co in filtrate | 0.857 (±0.012) | 1.065 (±0.018) | 0.780 (±0.022) | 0.979 (±0.017) |
| Co production | 1.768 (±0.012) | 1.560 (±0.018) | 1.845 (±0.022) | 1.646 (±0.017) |
| Theoretical Co3O4 production | 3.576 (±0.034) | |||
| Actual Co3O4 production | 2.408 (±0.016) | 2.125 (±0.024) | 2.514 (±0.030) | 2.242 (±0.023) |
| Yield ratio | 67.3% (±0.4) | 59.4% (±0.7) | 70.3% (±0.8) | 62.7% (±0.6) |
3.2. XRD and XPS analysis
Fig. 2(a) shows the XRD pattern of the prepared HU-Co3O4/N-GO hybrid. The diffraction peaks at 31.2°, 36.7°, 59.2° and 65.1° can be readily indexed to the (220), (311), (511) and (440) planes of face centered cubic Co3O4, respectively [13]. It indicates a successful synthesis of cubic Co3O4 in the ultrasound-assisted hydrothermal process.
Fig. 2.
a) XRD patterns of HU-Co3O4/N-GO hybrid. b) Survey scan spectrum of the HU-Co3O4/N-GO hybrid, c) C 1s, d) N 1s, e) O 1s, and f) Co 2p.
The atomic valence states of the HU-Co3O4/N-GO hybrid were studied by XPS characterization. As shown in Fig. 2(b), the principal peaks of C, N, O, and Co appear on the full survey spectrum of the HU-Co3O4/N-GO hybrid. Fig. 2(c)–(f) show the high-resolution C 1s, N 1s, O 1s and Co 2p fitted spectra, respectively. There are two peaks in the C 1s fitted spectra, as shown in Fig. 2(c). The peak at 284.7 eV is conformed to the C C/C–C network and the other at 288.2 eV corresponds to C–O in graphene oxide. Fig. 2(d) shows the N 1s fitted spectra. The peak at 399.1 eV and 400.9 eV are assigned to pyrrolic nitrogen and graphitic nitrogen on the graphene oxide surface. The high resolution of O 1s fitted spectra consists of metal–oxygen bonds for the Co3O4 phase and carbon–oxygen bonds for graphene oxide with peaks at 530.9 eV and 531.8 eV, respectively. The peaks at 778.6 and 795.9 eV correspond to the Co 2p3/2 and Co 2p1/2 spin-orbit peaks of Co3O4, as shown in Fig. 2(f). The Co 2p3/2 peak can be further divided into Co3+ and Co2+ peaks. There is a peak around the main peak of Co 2p1/2, representing the Co3O4 phase. The XPS result is consistent with that in previous literature [35].
3.3. The morphology and structure of the hybrids
Fig. 3(a) shows the SEM images of as-prepared HU-Co3O4/N-GO hybrids and the corresponding EDS mapping analysis. The EDS mapping shows a uniform distribution of C, N, O and Co throughout the observed particle. The EDS point shooting analysis (spectrum 1 and 2) further confirms that the Co content of the particle reaches 31.2 ~ 34.7 at. %. It indicates that the synthesized Co3O4 grows on the N-GO sheets. Fig. 3(b) and (c) show the representative TEM images of H-Co3O4/N-GO and HU-Co3O4/N-GO, respectively. There are just a few cubic Co3O4 particles growing on the N-GO sheet without ultrasonication, as shown in Fig. 3(b). A large number density of fine cubic Co3O4 particles grow on the flat N-GO sheets in HU-Co3O4/N-GO sample (Fig. 3(c)). It clearly reveals that ultrasonication greatly promotes the production of Co3O4 during hydrothermal synthesis, which is quite consistent with the calculated result based on the ICP measurement in Table 1.
Fig. 3.
a) SEM image of HU-Co3O4/N-GO hybrid with corresponding elemental mapping. b) TEM image of H-Co3O4/N-GO hybrid. c) TEM image of HU-Co3O4/N-GO hybrid.
Fig. 4 shows the TEM images of initial GO, H-N-GO, HU-N-GO, H-Co3O4 and HU-Co3O4, in order to further clarify the ultrasonic mechanism for the growth of Co3O4 on the N-GO sheets. The initial graphene oxide is crumpled sheet with curled edges, as shown in Fig. 4(a). The H-N-GO presents as thick and large sheets (Fig. 4(b)), while the N-GO transforms into the dispersed and thin sheets with ultrasonication during hydrothermal process, as shown in Fig. 4(c). It can be found that a short period ultrasonication prior to hydrothermal reaction fails to achieve high dispersion of the N-GO sheet. A persistent assistance of ultrasonication can sufficiently ensure a dispersion of fine N-GO sheets during the long-term hydrothermal synthesis. Furthermore, the cubic Co3O4 particle clusters (~700 nm) can be observed in the H-Co3O4 sample, as shown in Fig. 4(d). It suggests a severe agglomeration of Co3O4 particle (~220 nm) during the normal hydrothermal synthesis without the addition of N-GO and ultrasonication. The agglomeration of cubic Co3O4 particles is greatly retarded after the persistent assistance of ultrasonication in hydrothermal reaction, as shown in Fig. 4(e). Furthermore, fine Co3O4 nanoparticles (~30 nm) are clearly identified in HU-Co3O4 sample, as shown in Fig. 4(f).
Fig. 4.
TEM images of the prepared catalysts. (a) Initial GO; (b) N-GO; (c) HU-N-GO; (d) H-Co3O4; (e) and (f) HU-Co3O4.
It can be clearly concluded that the agglomeration of N-GO sheets and cubic Co3O4 particles is easily to appear during the long-term hydrothermal synthesis, even though a dispersion of N-GO sheets and precursors has been achieved prior to hydrothermal process. Ultrasonication-assisted hydrothermal synthesis can be effective to disperse the N-GO sheets and refine the cubic Co3O4 particles, as shown in Fig. 4. The N-GO sheets are effective sites for the nucleation and growth of Co3O4 particles. Thus, a persistent dispersion of N-GO sheets assisted by ultrasonication is in favor of the dispersion of Co3O4. As a result, the number density of Co3O4 growing on N-GO sheets greatly increases, as shown in Fig. 3c.
3.4. Electrocatalytic performance for ORR
Fig. 5 shows the electrochemical behaviors including CV, LSV and EIS of the as-prepared hybrids in alkaline solution. Both HU-Co3O4/N-GO and H-Co3O4/N-GO show oxygen reduction peaks, as shown in Fig. 5(a). The HU-Co3O4/N-GO has a 45 mV higher ORR peak than the H-Co3O4/N-GO, which manifests a high electrocatalytic activity toward ORR. Furthermore, the HU-Co3O4/N-GO hybrid exhibits a half-wave potential (0.76 V vs. RHE), 50 mV higher than the H-Co3O4/N-GO (0.71 V vs. RHE), according to the LSV curves in Fig. 5(b). The calculated ORR Tafel slope for HU-Co3O4/N-GO is 135 mV dec−1, a bit lower than that of H-Co3O4/N-GO (160 mV dec−1), as shown in Fig. 5(c). It fully demonstrates that HU-Co3O4/N-GO shows better ORR activity than H-Co3O4/N-GO. Fig. 5(d) shows the Nyquist plots of the HU-Co3O4/N-GO and H-Co3O4/N-GO at 1.023 V. The Nyquist plots consist of two capacitive circles and straight line, which can be fitted by the equivalent circuit model in Fig. 5(d). Re is resistance of electrolyte, Rint∥CPEint represents the interphase contact resistance and related capacitance in the electrode. Rct∥CPEdl is the charge-transfer resistance and related capacitance, and W1 is ionic diffusion impedance. According to the fitted results in Table 2, HU-Co3O4/N-GO has lower Re, Rint and Rct values than the H-Co3O4/N-GO, suggesting the fast electron transportation and high ORR activity. Thus, HU-Co3O4/N-GO hybrid exhibits a better electrocatalytic activity than H-Co3O4/N-GO. This means that ultrasonication can effectively alleviate the agglomeration degree of graphene oxide and make the Co3O4 uniformly growing on graphene oxide. Therefore, it is in favor of increasing the number density of active catalytic sites accessible to oxygen, hence greatly promoting ORR activities [36].
Fig. 5.
(a) CV curves of HU-Co3O4/N-GO and H-Co3O4/N-GO in an oxygen-saturated 0.1 M KOH solutions; (b) LSV curves of HU-Co3O4/N-GO and H-Co3O4/N-GO in an oxygen-saturated 0.1 M KOH solutions at a scan rate of 5 mV s−1; (c) Tafel plots derived from LSV curves; (d) Nyquist plot of HU-Co3O4/N-GO and H-Co3O4/N-GO air electrodes in 1 M KOH solutions between 0.1 Hz and 100 kHz at 1.023 V and the equivalent circuit.
Table 2.
Impedance parameters of HU-Co3O4/N-GO and H-Co3O4/N-GO electrodes obtained by fitting the electrochemical impedance spectra (EIS) at 1.023 V.
| Hybrid | Re Ω cm2 | Rint Ω cm2 | Rct Ω cm2 |
|---|---|---|---|
| HU-Co3O4/N-GO | 0.61 | 2.22 | 0.80 |
| H-Co3O4/N-GO | 0.63 | 2.40 | 3.10 |
3.5. Al-air battery tests
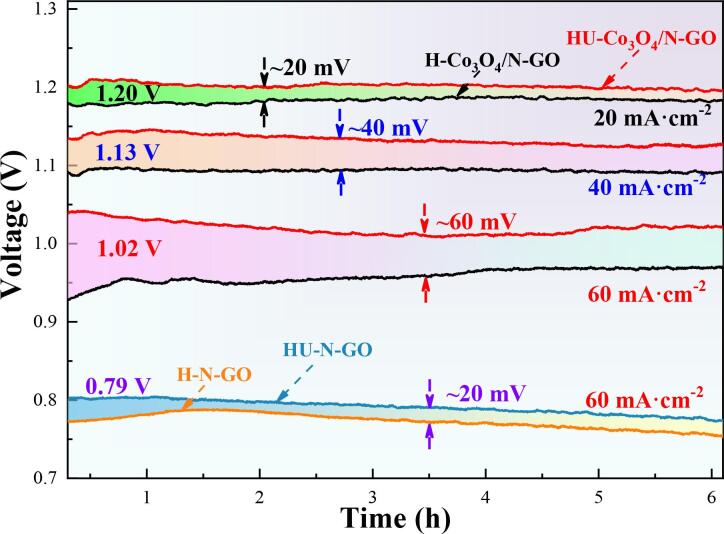
Fig. 6 shows the discharge curves of the assembled Al-air batteries with the prepared hybrids as ORR electrocatalysts in 4 M KOH discharged at current densities ranging from 20 to 60 mA∙cm−2 for 6 h. The assembled Al-air batteries exhibit stable working voltage and low degree of polarization during discharge. The Al-air battery with HU-Co3O4/N-GO as ORR electrocatalyst has a voltage plateau of approximately 1.20 V, 1.13 V and 1.02 V, which are about 20, 40 and 60 mV higher than that with H-Co3O4/N-GO at 20, 40 and 60 mA∙cm−2, respectively. It suggests that HU-Co3O4/N-GO hybrid has superior catalytic activity over the H-Co3O4/N-GO hybrid as cathode materials for alkaline Al-air battery. Thus, the persistent ultrasonication is beneficial for the catalytic performance of the Co3O4/N-GO hybrid. This is well consistent with the measured electrochemical behaviors in Fig. 5. Furthermore, Al-air battery with HU-N-GO catalyst has an average working voltage of ~0.79 V (at 60 mA∙cm−2), 20 mV higher than that with H-N-GO. This clearly implies that the catalytic activity of N-GO is slightly enhanced with the persistent assistance of ultrasonication, as shown in Fig. 6. However, such enhancement is much smaller than that (~60 mV) on the Co3O4/N-GO hybrid induced by ultrasonication. This implies that Co3O4 particles play dominating role in the catalytic activity of as-prepared hybrid, and the improved catalytic activity induced by ultrasonication is primarily attributed to the modification of Co3O4. Such improvement can be of relative great significance for a better discharge performance with a lower material cost for Al-air battery. Firstly, the preparation of the hybrid N-GO/Co3O4 ORR catalyst, even though with ultrasonication, is still quite cost-effective in battery operation compared to use of the precious metal-based catalysts. Secondly, more effective utilization of Co3O4 recourse with ultrasonication preparation also means a trade-off reduction in materials cost (using less Co3O4) for Al battery construction, compared to the convention hydrothermal preparation method that usually consumes more expensive Co-containing raw materials.
Fig. 6.
Discharge curves of the assembled Al-air batteries with the prepared hybrids as the ORR electrocatalysts in 4 M KOH using at current densities ranging from 20 to 60 mA∙cm−2.
Fig. 7 schematically illustrates the mechanism for persistently ultrasound-assisted hydrothermal synthesis of Co3O4/N-GO hybrids. Ultrasonication improves both the yield and number density of Co3O4 particles. To the authors’ best knowledge, disperse and fine particles with high number density are more important than the yield, regarding the ORR activity, since a high yield of Co3O4 may contribute to a rapid growth of coarse Co3O4 particles with low number density, which is detrimental to electrocatalytic activity (low number density of catalytic sites). Ultrasonication prior to hydrothermal synthesis can perform sufficient dispersion of both graphene oxides and precursors for Co3O4 via the outstanding ultrasonic cavitation and streaming effects [25], [37], [38]. However, the dispersed graphene oxides gradually agglomerate to large and thick sheets (Fig. 4(b)) during a 10 h hydrothermal process at 150 °C. Since the agglomerated GO serve as effective substrates for the nucleation of Co3O4 particles, it would further result in the agglomeration of the latterly precipitated Co3O4 particles, as shown in Fig. 4(d). A persistent assistance of ultrasonication in hydrothermal synthesis can effectively maintain a sufficient dispersion of the precursors and GO, as shown in Fig. 4c. Thus, the latterly precipitated Co3O4 particles effectively and uniformly nucleate and grow on the dispersed GO under the persistent assistance of ultrasonication. Furthermore, the significant ultrasonic cavitation can enhance the nucleation events of Co3O4 particles by activating the surface of solid particles [39], [40] (possibly graphene oxides in this work). In addition, the enhanced mass transfer would also be beneficial for uniform distribution of precursors [41], [42], [43], refining Co3O4 particles via the eliminating the solute segregation upon the solid-liquid interface. This explains that the persistently ultrasound-assisted hydrothermal synthesis process creates large number density of fine cubic Co3O4 growing on the dispersed GO sheet, as shown in Fig. 3c. In a word, the persistently ultrasound-assisted hydrothermal synthesis can efficiently prepare well dispersed and fine Co3O4/N-GO hybrids, which exhibit better electrocatalytic (ORR) activity as cathode material for Al-air battery.
Fig. 7.
Schematic illustration of the persistent ultrasound-assisted hydrothermal synthesis of Co3O4/N-GO hybrid.
4. Conclusions
In this work, a persistently ultrasound-assisted hydrothermal method has been developed to prepare cobalt oxide incorporated nitrogen-doped graphene hybrids. The electrochemical behaviors and catalytic activity of the prepared hybrids have been systematically investigated for cathode materials of Al-air battery. The results show that ultrasonication can promote the yield ratio of Co3O4 from 63.1% to 70.6%. It can effectively disperse and refine the cubic Co3O4 growing nitrogen-doped graphene oxides, as well as increase the number density of Co3O4 particles. The half-wave potential of the Co3O4/N-GO hybrid with ultrasonication is 50 mV higher than that without ultrasonication. The assembled Al-air battery using the ultrasonicated Co3O4/N-GO hybrid exhibits an average working voltage of 1.02 V in 4 M KOH electrolyte, approximately 60 mV higher than that using the hybrid without ultrasonication at 60 mA∙cm−2. This should be ascribed that the persistent ultrasonication in hydrothermal synthesis can maintain a favorable dispersion of graphene oxides, allowing the effective and uniform nucleation and growth of Co3O4 particles.
CRediT authorship contribution statement
Zengjie Wang: Writing - original draft, Methodology. Hongpeng Zhou: Investigation. Jilai Xue: Conceptualization, Supervision. Xuan Liu: Writing - review & editing. Shizhe Liu: Validation. Xiang Li: Visualization. Dingyong He: Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is supported by National Natural Science Foundation of China (51674025 and 51874035, No. 51704020) and Fundamental Research Funds for Central Universities of China (No. FRF-TP-19-034A2).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2020.105457.
Contributor Information
Jilai Xue, Email: jx@ustb.edu.cn.
Xuan Liu, Email: xuanliu@ustb.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Chu S., Cui Y., Liu N. The path towards sustainable energy. Nat. Mater. 2017;16(1):16–22. doi: 10.1038/nmat4834. [DOI] [PubMed] [Google Scholar]
- 2.Cano Z.P., Banham D., Ye S., Hintennach A., Lu J., Fowler M., Chen Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy. 2018;3(4):279–289. [Google Scholar]
- 3.Liu Y., Chen F., Ye W., Zeng M., Han N.a., Zhao F., Wang X., Li Y. High-performance oxygen reduction electrocatalyst derived from polydopamine and cobalt supported on carbon nanotubes for metal-air batteries. Adv. Funct. Mater. 2017;27(12):1606034. doi: 10.1002/adfm.v27.1210.1002/adfm.201606034. [DOI] [Google Scholar]
- 4.Stamenkovic V.R., Strmcnik D., Lopes P.P., Markovic N.M. Energy and fuels from electrochemical interfaces. Nat. Mater. 2017;16(1):57–69. doi: 10.1038/nmat4738. [DOI] [PubMed] [Google Scholar]
- 5.Ryu J., Park M., Cho J. Advanced technologies for high-energy aluminum-air batteries. Adv. Mater. 2019;31(20):1804784. doi: 10.1002/adma.201804784. [DOI] [PubMed] [Google Scholar]
- 6.Holewinski A., Idrobo J.-C., Linic S. High-performance Ag–Co alloy catalysts for electrochemical oxygen reduction. Nat. Chem. 2014;6(9):828–834. doi: 10.1038/nchem.2032. [DOI] [PubMed] [Google Scholar]
- 7.Liu J., Jiao M., Lu L., Barkholtz H.M., Li Y., Wang Y., Jiang L., Wu Z., Liu D.-J., Zhuang L., Ma C., Zeng J., Zhang B., Su D., Song P., Xing W., Xu W., Wang Y., Jiang Z., Sun G. High performance platinum single atom electrocatalyst for oxygen reduction reaction. Nat. Commun. 2017;8:15938. doi: 10.1038/ncomms15938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai Y., Wang Q., Wang M., Li J., Fang J., Zhang Z. Facile synthesis of mesoporous Fe-N-C electrocatalyst for high performance alkaline aluminum-air battery. J. Electroanal. Chem. 2017;801:72–76. [Google Scholar]
- 9.Xue Y., Miao H.e., Sun S., Wang Q., Li S., Liu Z. (La1−xSrx)0.98MnO3 perovskite with A-site deficiencies toward oxygen reduction reaction in aluminum-air batteries. J. Power Sources. 2017;342:192–201. [Google Scholar]
- 10.Wang Y., Zhou T., Jiang K., Da P., Peng Z., Tang J., Kong B., Cai W.-B., Yang Z., Zheng G. Reduced mesoporous Co3O4 nanowires as efficient water oxidation electrocatalysts and supercapacitor electrodes. Adv. Energy Mater. 2014;4(16):1400696. doi: 10.1002/aenm.201400696. [DOI] [Google Scholar]
- 11.Liu K., Huang X., Wang H., Li F., Tang Y., Li J., Shao M. Co3O4–CeO2/C as a highly active electrocatalyst for oxygen reduction reaction in Al–air batteries. Acs Appl. Mater. Inter. 2016;8(50):34422–34430. doi: 10.1021/acsami.6b12294. [DOI] [PubMed] [Google Scholar]
- 12.Han X., He G., He Y., Zhang J., Zheng X., Li L., Zhong C., Hu W., Deng Y., Ma T.-Y. Engineering catalytic active sites on cobalt oxide surface for enhanced oxygen electrocatalysis. Adv. Energy Mater. 2018;8:1870043. [Google Scholar]
- 13.Eom W., Kim A., Park H., Kim H., Han T.H. Graphene-mimicking 2D porous Co3O4 nanofoils for lithium battery applications. Adv. Funct. Mater. 2016;26(42):7605–7613. [Google Scholar]
- 14.Dong Y., Deng Y., Zeng J., Song H., Liao S. A high-performance composite ORR catalyst based on the synergy between binary transition metal nitride and nitrogen-doped reduced graphene oxide. J. Mater. Chem. A. 2017;5(12):5829–5837. [Google Scholar]
- 15.Deng J., Deng D., Bao X. Robust catalysis on 2D materials encapsulating metals: concept, application, and perspective. Adv. Mater. 2017;29(43):1606967. doi: 10.1002/adma.201606967. [DOI] [PubMed] [Google Scholar]
- 16.Zhou D., Cai Z., Lei X., Tian W., Bi Y., Jia Y., Han N., Gao T., Zhang Q., Kuang Y., Pan J., Sun X., Duan X. NiCoFe-layered double hydroxides/N-doped graphene oxide array colloid composite as an efficient bifunctional catalyst for oxygen electrocatalytic reactions. Adv. Energy Mater. 2018;8(9):1701905. [Google Scholar]
- 17.Imran Jafri R., Rajalakshmi N., Ramaprabhu S. Nitrogen doped graphene nanoplatelets as catalyst support for oxygen reduction reaction in proton exchange membrane fuel cell. J. Mater. Chem. 2010;20(34):7114. doi: 10.1039/c0jm00467g. [DOI] [Google Scholar]
- 18.Sheng Z.-H., Shao L., Chen J.-J., Bao W.-J., Wang F.-B., Xia X.-H. Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis. ACS Nano. 2011;5(6):4350–4358. doi: 10.1021/nn103584t. [DOI] [PubMed] [Google Scholar]
- 19.Liu X., Liu W., Ko M., Park M., Kim M.G., Oh P., Chae S., Park S., Casimir A., Wu G., Cho J. Metal (Ni, Co)-metal oxides/graphene nanocomposites as multifunctional electrocatalysts. Adv. Funct. Mater. 2015;25(36):5799–5808. [Google Scholar]
- 20.Higgins D., Zamani P., Yu A., Chen Z. The application of graphene and its composites in oxygen reduction electrocatalysis: a perspective and review of recent progress. Energ. Environ. Sci. 2016;9(2):357–390. [Google Scholar]
- 21.Zitolo A., Ranjbar-Sahraie N., Mineva T., Li J., Jia Q., Stamatin S., Harrington G.F., Lyth S.M., Krtil P., Mukerjee S., Fonda E., Jaouen F. Identification of catalytic sites in cobalt-nitrogen-carbon materials for the oxygen reduction reaction. Nat. Commun. 2017;8:957. doi: 10.1038/s41467-017-01100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang Y., Li Y., Wang H., Zhou J., Wang J., Regier T., Dai H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011;10:780–786. doi: 10.1038/nmat3087. [DOI] [PubMed] [Google Scholar]
- 23.Peng S.-H., Chen T.-H., Lee C.-H., Lu H.-C., Lue S.J. Optimal cobalt oxide (Co3O4): Graphene (GR) ratio in Co3O4/GR as air cathode catalyst for air-breathing hybrid electrolyte lithium-air battery. J. Power Sources. 2020;471 [Google Scholar]
- 24.Mei J., Liao T., Ayoko G.A., Bell J., Sun Z. Cobalt oxide-based nanoarchitectures for electrochemical energy applications. Prog. Mater. Sci. 2019;103:596–677. [Google Scholar]
- 25.Meskin P.E., Ivanov V.K., Barantchikov A.E., Churagulov B.R., Tretyakov Y.D. Ultrasonically assisted hydrothermal synthesis of nanocrystalline ZrO2, TiO2, NiFe2O4 and Ni0.5Zn0.5Fe2O4 powders. Ultrason. Sonochem. 2006;13:47–53. doi: 10.1016/j.ultsonch.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Mohandes F., Salavati-Niasari M. Sonochemical synthesis of silver vanadium oxide micro/nanorods: Solvent and surfactant effects. Ultrason. Sonochem. 2013;20(1):354–365. doi: 10.1016/j.ultsonch.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Askarinejad A., Morsali A. Direct ultrasonic-assisted synthesis of sphere-like nanocrystals of spinel Co3O4 and Mn3O4. Ultrason. Sonochem. 2009;16:124–131. doi: 10.1016/j.ultsonch.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Fouad D.E., Zhang C., Mekuria T.D., Bi C., Zaidi A.A., Shah A.H. Effects of sono-assisted modified precipitation on the crystallinity, size, morphology, and catalytic applications of hematite (α-Fe2O3) nanoparticles: a comparative study. Ultrason. Sonochem. 2019;59:104713. doi: 10.1016/j.ultsonch.2019.104713. [DOI] [PubMed] [Google Scholar]
- 29.Sanati S., Rezvani Z. Ultrasound-assisted synthesis of NiFe- layered double hydroxides as efficient electrode materials in supercapacitors. Ultrason. Sonochem. 2018;48:199–206. doi: 10.1016/j.ultsonch.2018.05.035. [DOI] [PubMed] [Google Scholar]
- 30.Montazerozohori M., Masoudiasl A., Farokhiyani S., Joohari S., McArdle P. Sonochemical synthesis of a new cobalt(II) complex: Crystal structure, thermal behavior, Hirshfeld surface analysis and its usage as precursor for preparation of CoO/Co3O4 nanoparticles. Ultrason. Sonochem. 2017;38:134–144. doi: 10.1016/j.ultsonch.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Chen T.-W., Chinnapaiyan S., Chen S.-M., Ali M.A., Elshikh M.S., Mahmoud A.H. A feasible sonochemical approach to synthesize CuO@CeO2 nanomaterial and their enhanced non-enzymatic sensor performance towards neurotransmitter. Ultrason. Sonochem. 2020;63 doi: 10.1016/j.ultsonch.2019.104903. [DOI] [PubMed] [Google Scholar]
- 32.Ida S., Wilson P., Neppolian B., Sathish M., Mahammed Shaheer A.R., Ravi P. Tuning the type of nitrogen on N-RGO supported on N-TiO2 under ultrasonication/hydrothermal treatment for efficient hydrogen evolution – a mechanistic overview. Ultrason. Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2019.104866. [DOI] [PubMed] [Google Scholar]
- 33.Liu X., Zhuo M., Zhang W., Gao M., Liu X.-H., Sun B., Wu J. One-step ultrasonic synthesis of Co/Ni-catecholates for improved performance in oxygen reduction reaction. Ultrason. Sonochem. 2020;67 doi: 10.1016/j.ultsonch.2020.105179. [DOI] [PubMed] [Google Scholar]
- 34.Nicolosi V., Chhowalla M., Kanatzidis M.G., Strano M.S., Coleman J.N. Liquid exfoliation of layered materials. Science. 2013;340:1226419. [Google Scholar]
- 35.Bao L., Li T., Chen S., Peng C., Li L., Xu Q., Chen Y., Ou E., Xu W. 3D graphene frameworks/Co3O4 composites electrode for high-performance supercapacitor and enzymeless glucose detection. Small. 2017;13:1602077. doi: 10.1002/smll.201602077. [DOI] [PubMed] [Google Scholar]
- 36.Mistry H., Varela A.S., Kühl S., Strasser P., Cuenya B.R. Nanostructured electrocatalysts with tunable activity and selectivity. Nat. Rev. Mater. 2016;1:16009. [Google Scholar]
- 37.Liu X., Zhang C., Zhang Z., Xue J., Le Q. The role of ultrasound in hydrogen removal and microstructure refinement by ultrasonic argon degassing process. Ultrason. Sonochem. 2017;38:455–462. doi: 10.1016/j.ultsonch.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 38.Liu X., Xue J., Zhao Q., Le Q., Zhang Z. Effects of radiator shapes on the bubble diving and dispersion of ultrasonic argon process. Ultrason. Sonochem. 2018;41:600–607. doi: 10.1016/j.ultsonch.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 39.Liu X., Yin S.Q., Zhang Z.Q., Le Q.C., Xue J.L. Effect of limestone ores on grain refinement of as-cast commercial AZ31 magnesium alloys. T Nonferr. Metal. Soc. 2018;28:1103–1113. [Google Scholar]
- 40.Qian M., Ramirez A., Das A. Ultrasonic refinement of magnesium by cavitation: clarifying the role of wall crystals. J. Cryst. Growth. 2009;311:3708–3715. [Google Scholar]
- 41.Liu X., Zhang Z., Hu W., Le Q., Bao L., Cui J., Jiang J. Study on hydrogen removal of AZ91 alloys using ultrasonic argon degassing process. Ultrason. Sonochem. 2015;26:73–80. doi: 10.1016/j.ultsonch.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 42.Sajjadi Baharak, Asgharzadehahmadi Seyedali, Asaithambi Perumal, Raman Abdul Aziz Abdul, Parthasarathy Rajarathinam. Investigation of mass transfer intensification under power ultrasound irradiation using 3D computational simulation: a comparative analysis. Ultrason. Sonochem. 2017;34:504–518. doi: 10.1016/j.ultsonch.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 43.Gnana Sundara Raj B., Kim H.-Y., Kim B.-S. Ultrasound assisted formation of Mn2SnO4 nanocube as electrodes for high performance symmetrical hybrid supercapacitors. Electrochim. Acta. 2018;278:93–105. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.