Abstract
This systematic review and meta-analysis was performed to evaluate the effect of probiotics on serum high sensitivity-C reactive protein (hs-CRP) and oxidative stress biomarkers among patients with Diabetic Nephropathy (DN). Electronic databases were searched through May 10, 2020. Seven trials that included 340 patients were identified for analysis. Meta-analysis indicated that probiotics significantly reduced hs-CRP (WMD = -1.53 mg/L; 95% CI = -2.38, -0.69; P < 0.001) and Malondialdehyde (MDA) (WMD = -0.62 ɥmol/L; 95% CI = -1.18, -0.06; P = 0.030) levels in DN patients, whereas they increased Glutathione (GSH) (WMD = 73.84 ɥmol/L; 95% CI = 24.3, 123.29; P = 0.003) and Total Antioxidant Capacity (TAC) (WMD = 26.54 mmol/L; 95% CI = 6.23, 46.85; P = 0.010). Therefore, probiotics may improve hs-CRP and oxidative stress biomarkers in DN population.
Keywords: Probiotics, Oxidative stress, C-reactive protein, Diabetic nephropathies, Meta-analysis
Probiotics; Oxidative Stress; C-Reactive Protein; Diabetic Nephropathies; Meta-Analysis
1. Introduction
Diabetic nephropathy (DN) is characterized by albuminuria (>300 mg/day) and a reduced Glomerular Filtration Rate (GFR) [1]; it is the leading cause of the End-Stage Renal Disease (ESRD) worldwide. The incidence of DN in patients with Type 2 Diabetes Mellitus (T2DM) is 20–40% [2]. Thickening of the glomerular basement membrane, glomerulosclerosis, and expansion of the mesangial cells lead to kidney fibrosis in DN, however, the exact mechanisms implicated in the pathogenesis of DN are complex [3].
One possible explanation for DN pathogenesis is change in the intestinal biochemical environment, which promote an inflammatory gut dysbiosis based on gut-kidney axis interrelationships [4]. Also, several recent studies have suggested that gut-derived endotoxin (lipopolysaccharide, LPS) might be significantly involved in chronic inflammation, one of the classical markers of DN [5].
Given the dysregulation of this axis in DN progression, new therapies aim at restoring the balanced intestinal environment (eubiosis) using dietary prebiotics, probiotics, or synbiotics administration. Probiotics are defined as “living microorganisms that have beneficial effects on the host health”. Lactobacillus (L) spp., Bifidobacterium (B) spp., Streptococcus spp., Enterococcus spp., and Saccharomyces boulardii are the most conventional strains for supplementation [6, 7]. Previous investigations have confirmed that probiotics decrease Reactive Oxygen Species (ROS) and pro-inflammatory cytokines production in renal patients [8].
The effect of probiotics supplementation on the reduction of oxidative stress and the improvement of antioxidant biomarkers has been investigated in interventional studies [9, 10, 11, 12]. A meta-analysis also examined the effects of probiotics and synbiotics supplementation on oxidative stress indices in healthy subjects; the authors concluded that these supplements improve antioxidant resistance and increase the amount of antioxidant enzymes in the human body [13]. Another meta-analysis in Chronic Kidney Disease (CKD) patients indicated that microbial therapies have significant beneficial effect on serum levels of C-Reactive Protein (CRP), Total Glutathione (GSH), Malondialdehyde (MDA), and Total Antioxidant Capacity (TAC) [14].
Recently, a systematic review concluded that probiotic supplementation might improve systemic inflammation and oxidative stress status in subjects with DN without any considerable side effect [15]. Actually, the limited number of studies that investigated the issue have shown controversial results. Therefore, the objective of this systematic review and meta-analysis was to confirm the evidence that probiotics can alter inflammation and oxidative stress parameters compared to placebo in DN patients.
2. Materials and methods
2.1. Protocol registration
The review protocol has been registered in the International Prospective Register of Systematic Reviews (www.crd.york.ac.uk/PROSPERO) (Registration ID. CRD42020186189) and developed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [16].
2.2. Search strategy
Systematic searches of the literature were conducted in the Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials (CENTRAL), Ovid Cochrane Database of Systematic Reviews, Google Scholar, PubMed, ScienceDirect, ISI Web of Science, and Scopus up to May 10, 2020.
Two reviewers independently searched the aforementioned databases to identify RCTs, using the following MeSH and text keywords: ((“Diabetic Nephropathy” OR “Diabetic kidney disease” OR “DKD”) AND (“high sensitivity-C reactive protein” OR “hs-CRP” OR “oxidative stress” OR “total glutathione” OR “GSH” OR “malondialdehyde” OR “MDA” OR “total antioxidant capacity” OR “TAC” OR “nitric oxide” OR “NO”) AND (“Synbiotics” OR “Probiotics” OR “Prebiotics” OR “Probiotic”) AND ("Intervention Studies" OR "intervention" OR "controlled trial" OR "randomized" OR "randomised" OR "random" OR "randomly" OR "placebo" OR "assignment” OR "randomized controlled trial” OR "trial" OR "Clinical Trial” OR "RCT")). Furthermore, all references of previous relevant meta-analyses, systematic reviews, and selected Randomized Clinical Trials (RCTs) were manually reviewed to find any additional trials that had not been confined via online database searches.
2.3. Study selection criteria
Trial studies were included in our analyses if they met the following criteria: (1) being a RCT in either parallel or cross-over design, and at least two arms, (2) limited to DN patients aged ≥18 years and disease duration between 2-20 years, and (3) those which investigated the effect of probiotics (of any form, including capsule, milk, yogurt and honey) on plasma/serum biomarkers of oxidative stress (MDA, TAC, GSH, NO) and hs-CRP concentrations. The studies were excluded if: (1) outcomes had not been clearly stated, (2) they had a nonexperimental (case studies, case series, cross-sectional, case-control, cohort and other retrospective studies) design without clear inclusion and exclusion criteria, (3) they had uncontrolled body, and (4) they were preclinical studies with animal models.
The relevance of articles and abstracts for inclusion was reviewed by two independent reviewers. Then, one reviewer independently evaluated the full text of potentially relevant non-duplicated articles. Disagreements were resolved by discussion or third party opinion.
2.4. Data extraction
Two reviewers independently extracted data. Any discrepancies were resolved by a third author. The following details were abstracted using a standardized electronic abstraction form, including (1) study first author, (2) publication year and study location, (3) study design and duration, (4) baseline samples’ characteristics such as gender, disease duration, mean Body Mass Index (BMI) and age, (5) composition and dose of probiotics/placebo, and (6) outcome indicators. We contact the corresponding author of included studies if any related questions existed.
2.5. Risk of bias (quality) assessment
Two reviewers independently evaluated the risk of bias for included studies by using the Cochrane Risk-of-Bias (RoB) tool (version 5.0) [17]. The assessment included selection bias (method for random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting) and other sources of bias. The reviewers’ judgment was classified as “Low risk,” “High risk” or “Unclear risk” of bias. Any discrepancies were settled by a third assessor.
2.6. Statistical analysis
Meta-analysis was conducted using STATA software version 14 (Stata Corp LP, College Station, TX, USA). The effect of probiotics on selected parameters were analyzed using mean difference with standard deviation (SD); the random-effects model was used to compute Weighted Mean Differences (WMD) with 95% Confidence Intervals (CI). The conversion of median/range (or 95% CI) to the mean ± SD values was performed based on Hozo and colleagues [18] method. Forest plots showed the main results.
Subgroup analysis and I-square (I2) test were used to evaluate the between-study heterogeneity and to detect the source of heterogeneity by the following variables: study duration (≤10 vs. >10 weeks), disease duration (≤10 vs. >10 years), probiotics dose (≤5 billion CFU vs. >5 billion CFU), and mean BMI (≤30 vs. >30 kg/m2).
We also measured the potential for publication bias through the “Begg rank correlation” and the “Egger weighted regression” methods. A P-value of 0.05 was considered as level of statistical significance.
3. Results
3.1. Literature search
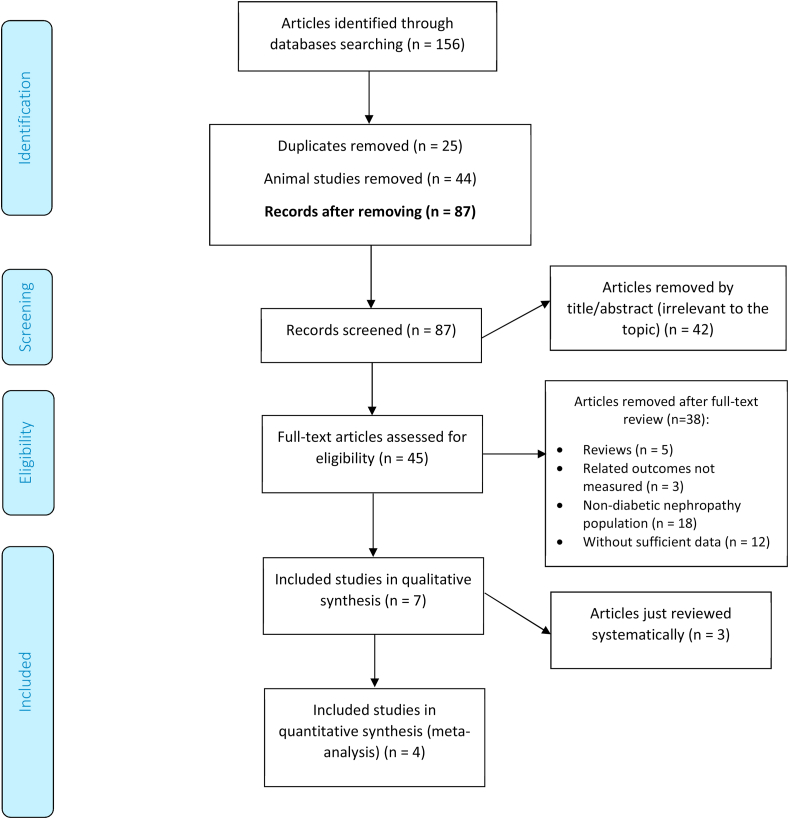
Figure 1 presents a diagram with the search strategy of the studies. We identified a total of 156 citations, of which 87 records remained after removing duplicates (n = 25) and animal studies (n = 44). After screening via titles and abstracts, 45 articles remained for further evaluation, of which 38 were excluded for the following reasons: reviews articles (n = 5); irrelevant outcomes (n = 3); Non-diabetic nephropathy population (n = 18) or insufficient data (n = 12). Finally, four eligible articles were entered in the data synthesis (220 participants) [9, 10, 11, 12] with publication range of 2017–2019; and three studies were just systematically reviewed (Table 1) [19-21].
Figure 1.
Flow diagram of the included and excluded studies.
Table 1.
Characteristic of randomized controlled trials that included for review; effects of probiotics on clinical manifestations of Diabetic Nephropathy.
| First author (publication year) | Country | Analyzed Sample size In/Co Male/Female |
Target population | Disease duration M (SD) |
BMI at base (M) | Age (M) | Study design Duration |
Intervention, Dose | Control, Dose | Probiotic content and numbers | Combined drug therapy, Type and % of subjects | Investigated markers |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbasi (2018)† | Iran | 20/20 19/21 |
Diabetic Nephropathy | 7.8 (3.5) | 26.6 | 55.2 | R, DB, PC Parallel 8 wks |
Probiotics soy milk 200 ml QD |
soy milk 200 ml QD |
lactobacillus plantarum A7 2 × 107 CFU/ml Totally 4 × 109 CFU |
NR | TG, TC, LDL-C, HDL-C, non HDL-C, Serum creatinine, Serum phosphorus, Serum genistein, eGFR |
| Abbasi (2017)† | Iran | 20/20 19/21 |
Diabetic Nephropathy | 7.8 (3.5) | 26.6 | 55.2 | R, DB, PC Parallel 8 wks |
Probiotics soy milk 200 ml QD |
soy milk 200 ml QD |
lactobacillus plantarum A7 2 × 107 CFU/ml Totally 4 × 109 CFU |
NR | Body Weight, BMI, WHR, IL-18, Serum sialic acid, Serum creatinine, Serum genistein, eGFR, Urinary albumin/creatinine ratio |
| Miraghajani (2019)† | Iran | 20/20 19/21 |
Diabetic Nephropathy | 7.8 (3.5) | 26.6 | 55.2 | R, DB, PC Parallel 8 wks |
Probiotics soy milk 200 ml QD |
soy milk 200 ml QD |
Lactobacillus plantarum A7 (KC355240, LA7) 2 × 107 CFU/ml Totally 4 × 109 CFU |
1) Anti-diabetic medications (In, 50 % of subjects; Co, 55 % of subjects) 2) Hypolipidemic agents (In, 95 % of subjects; Co, 100 % of subjects) 3) Hypertension drugs (In, 90 % of subjects; Co, 80 % of subjects) 4) Insulin (In, 50 % of subjects; Co, 45 % of subjects) |
Calorie, Protein, Fat, Carbohydrate, Fiber, Calcium, Magnesium, Potassium, NGAL, sTNFR1, Cys-C, PGRN, Weight, WHR, BMI |
| Mafi (2018) | Iran | 30/30 NR |
Diabetic Nephropathy | 18.1 (5.4) | 25.8 | 59.9 | R, DB, PC Parallel 12 wks |
Probiotic capsule QD |
Placebo capsule (contained only starch) QD |
Lactobacillus acidophilus strain ZT-L1, Bifidobacterium bifidum strain ZT-B1, Lactobacillus reuteri strain ZT-Lre, and Lactobacillus fermentum strain ZT-L3 (each 2 × 109 CFU/g) Totally 8 × 109 CFU per capsule |
NR | Body Weight, BMI, TAC, MDA, hs-CRP, FPG, Insulin, GSH, HOMA-IR, NO, QUICKI, TC, TG, LDL-C, HDL-C, VLDL-C, Hb A1C, TC/HDL ratio, AGEs, BUN, Serum creatinine, GFR, Urine protein, gene expression (IL-1, TNF-α, TGF-ß, PPAR-γ and LDLR) |
| Soleimani (2017) | Iran | 30/30 40/20 |
Diabetic Hemodialysis | 18.1 (5.4) | 26.2 | 56.7 | R, DB, PC Parallel 12 wks |
Probiotic capsule QD |
Placebo capsule (contained only starch) QD |
Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacterium bifidum (each 2 × 109 CFU/g) Totally 6 × 109 CFU per capsule |
1) ACEI or ARB drugs (In, 96.7 % of subjects; Co, 96.7 % of subjects) 2) Phosphate binder “Sevelamer” (In, 26.7 % of subjects; Co, 23.3 % of subjects) 3) Phosphate binder “Calcium carbonate” (In, 73.3 % of subjects; Co, 76.7 % of subjects) 4) Insulin (In, 25 % of subjects; Co, 25 % of subjects) |
Body Weight, BMI, MET, TAC, MDA, hs-CRP, FPG, Insulin, GSH, HOMA-IR, HOMA-B, NO, QUICKI, TC, TG, LDL-C, HDL-C, VLDL-C, Hb A1C, TC/HDL ratio, BUN, Serum creatinine, GFR, SGA score, Albumin, TIBC, Na, K |
| Arani (2019) | Iran | 30/30 NR |
Diabetic Nephropathy | 18.1 (5.4) | 26.2 | 56.7 | R, DB, PC Parallel 12 wks |
Probiotic honey 25 g QD |
Control honey 25 g QD |
Bacillus coagulans T4 (IBRC-N10791) (108 CFU/g) Totally 2.5 × 109 CFU |
NR | Body Weight, BMI, TAC, MDA, hs-CRP, FPG, Insulin, GSH, HOMA-IR, NO, QUICKI, TC, TG, LDL-C, HDL-C, VLDL-C, TC/HDL ratio, BUN, Serum creatinine |
| Miraghajani (2017) | Iran | 20/20 NR |
Diabetic kidney disease | 7.8 (3.5) | 26.6 | 55.2 | R, DB, PC Parallel 8 wks |
Probiotics soy milk 200 ml QD |
soy milk 200 ml QD |
Lactobacillus plantarum A7 (KC355240, LA7) 2 × 107 CFU/ml Totally 4 × 109 CFU |
1) Anti-diabetic medications (In, 50 % of subjects; Co, 55 % of subjects) 2) Hypolipidemic agents (In, 95 % of subjects; Co, 100 % of subjects) 3) Hypertension drugs (In, 90 % of subjects; Co, 80 % of subjects) 4) Insulin (In, 50 % of subjects; Co, 45 % of subjects) |
Calorie, Protein, Fat, Carbohydrate, Fiber, Cholesterol, MUFA, PUFA, Saturated fatty acid, Selenium, Vitamin E, and C, MDA, TAC, GSH, 8-iso-PGF2a, Oxidized glutathione, Glutathione peroxidase, Glutathione reductase |
Functional abbreviations: In, intervention group; Co, control group; M, mean; SD, standard deviation; QD, once a day; CFU, colony forming unit; RDBPC, randomized double blind placebo control trial; wks, weeks; NR, not reported.
Study outcome abbreviations: hs-CRP, high sensitive C-reactive protein; MDA, malondialdehyde; TAC, total antioxidant capacity; IL interleukin; FPG, fasting plasma glucose; GHQ, general health questionnaire; GSH, total glutathione; HOMA-IR, homeostasis model of assessment-estimated insulin resistance; HOMA-B, homeostasis model of assessment estimated B cell function; NO, nitric oxide; QUICKI, quantitative insulin sensitivity check index; TG, Triglycerides; TC, Total cholesterol; LDL, low density lipoprotein; VLDL, very low density lipoprotein; HDL, high density lipoprotein; TG, triacylglycerol; AGEs, advanced glycation end products; GFR, glomerular filtration rate; BUN, blood urea nitrogen; LDLR, low-density lipoprotein receptor; PPAR-γ, peroxisome proliferator-activated receptor gamma; TNF-α, tumor necrosis factor alpha; TGF-ß, transforming growth factor beta; HbA1c, hemoglobin A1c; NGAL, neutrophil gelatinase-associated lipocalin; sTNFR1, soluble tumor necrosis factor receptor 1; PGRN, Progranulin; Cys-C, cystatin C; METs, metabolic equivalents; SGA, subjective global assessment; TIBC, total iron binding capacity; Na, sodium; K, potassium; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; 8-iso-PGF2a, 8-iso-prostaglandin F2a.
Three selected studies were just systematically reviewed and not included in analysis due to irrelevant outcome markers.
3.2. Study characteristics
3.2.1. Meta-analyzed studies
Table 1 presents the summary data of the selected studies for meta-analyses. A total of 220 participants (110 as intervention group/110 as controls) were included. The mean age of participants ranged from 55 to 60 years old with a mean disease duration of 8–18 years for all trials. Mean BMI presented an overweight condition (25–30 kg/m2). Three studies did not report sexual distribution [10, 11, 12], however, Soleimani and colleagues [9] analyzed both sexes. All studies were conducted in Iran, had two-arm parallel design, and were related to DN patients [9, 10, 11, 12]. The study duration varied between 8 and 12 weeks. Three studies reported the number and percent of participants who consumed the related drugs for DN treatment [9, 10, 21]; 25–50 % of participants used exogenous insulin.
The administered probiotics were L. plantarum [10], Bacillus coagulans [12], and multistrain-based [9, 11]. Moreover, the daily dose of supplementation was ranged from 2.5 to 8 × 109 CFU.
3.2.2. Only systematically-reviewed studies
The three studies were published between “2017 and 2019” [19, 20, 21]. A total of 120 participants were enrolled (n = 57 for male and n = 63 for female). Soy milk enriched with probiotics was administered for 8 weeks (L. plantarum; total 4 × 109 CFU per day) in all three studies. One study reported the effects of supplementation on lipid profile and some renal markers [20], however, other authors discussed anthropometric measurements [19] and dietary factors [21]. The further characteristics of systematically-reviewed studies are summarized in Table 1.
3.3. Risk of bias (quality) assessment
The outcome assessors were blinded in all studies. Among the seven trials, adequate randomized sequence generation was reported for five trials [9, 10, 11, 12, 21] but was unclear in the remaining two studies [19, 20]. Four trials had a low risk of bias in allocation concealment [10, 12, 19, 22], whereas three trials had an unclear risk of bias [11, 20, 21]. While four trials had an unclear risk of bias in the blinding of participants and personnel [10, 11, 12, 21], three had a high risk of bias [9, 19, 20]. Furthermore, two studies had high risk of attrition bias [19, 20]. In total, two reports were assessed as high overall risk [19, 20], three as unclear [10, 11, 21], and two as low risk of bias [9, 12]. More details were presented in Figure 2.
Figure 2.
Risk of bias summary across the included studies. Each marker represents the level of risk: “+”, low risk; “-”, high risk, “?”, unclear risk.
3.4. Results of meta-analysis for serum oxidative stress markers
3.4.1. The effects of probiotics on GSH
In the pooled analysis of four studies with 220 participants (intervention and control, each 110) [9-12], effect of probiotics on serum GSH level (WMD = 73.84 ɥmol/L; 95% CI = 24.3, 123.29, P = 0.003) was statistically significant with a heterogeneity (I2) of 72.4 % (P = 0.012) (Figure 3a).
Figure 3.
Forest plot of randomized controlled trials investigating the effect of probiotics on serum oxidative stress markers “GSH (A), MDA (B), TAC (C), and NO (D) in Diabetic Nephropathy patients. The area of each square is proportional to the inverse of the variance of the WMD. Horizontal lines represent 95% CIs. Diamonds represent pooled estimates from fixed-effects analysis. GSH, total glutathione; MDA, malondialdehyde; TAC, total antioxidant capacity; NO, nitric oxide.
Due to heterogeneity, we conducted subgroup analysis to find possible sources (Table 2). Any subgroup could not explain the between-study heterogeneity. Unlike overall effect size, no subset was significant across the probiotics dose (≤5 billion CFU, P = 0.082; >5 billion CFU, P = 0.066).
Table 2.
Subgroup analysis to assess the effect of probiotics on hs-CRP and oxidative stress markers in diabetic nephropathy.
| Sub group by | No. of trials | WMD (95% CI) | P Value | P for heterogeneity | I2 (%) | P for between subgroup heterogeneity | |
|---|---|---|---|---|---|---|---|
| 1 | hs-CRP∗ | ||||||
| Total | 3 | -1.53 (-2.38, -0.69) | <0.001 | 0.878 | 0.0 | ||
| Probiotics Dose (billion CFU) | |||||||
| ≤5 | 1 | -1.70 (-3.43, 0.03) | 0.054 | - | - | 0.827 | |
| >5 | 2 | -1.48 (-2.45, -0.51) | 0.003 | 0.644 | 0.0 | ||
| Baseline BMI (kg/m2) | |||||||
| ≤30 | 2 | -1.48 (-2.45, -0.51) | 0.003 | 0.644 | 0.0 | 0.827 | |
| >30 | 1 | -1.70 (-3.43, 0.03) | 0.054 | - | - | ||
| 2 | GSH | ||||||
| Total | 4 | 73.84 (24.3, 123.29) | 0.003 | 0.012 | 72.4 | ||
| Study Duration (Weeks) | |||||||
| ≤10 weeks | 1 | 111.30 (76.04, 146.56) | <0.001 | - | - | 0.017 | |
| >10 weeks | 3 | 57.21 (0.32, 114.10) | 0.049 | 0.074 | 61.6 | ||
| Disease Duration (Years) | |||||||
| ≤10 years | 1 | 111.30 (76.04, 146.56) | <0.001 | - | - | 0.017 | |
| >10 years | 3 | 57.21 (0.32, 114.10) | 0.049 | 0.074 | 61.6 | ||
| Probiotics Dose (billion CFU) | |||||||
| ≤5 | 2 | 71.26 (-8.99, 151.50) | 0.082 | 0.004 | 88.1 | 0.799 | |
| >5 | 2 | 76.27 (-4.95, 157.49) | 0.066 | 0.122 | 58.1 | ||
| Baseline BMI (kg/m2) | |||||||
| ≤30 | 3 | 97.31 (57.08, 137.54) | <0.001 | 0.213 | 35.3 | 0.005 | |
| >30 | 1 | 29.40 (-13.18, 71.98) | 0.176 | - | - | ||
| 3 | MDA | ||||||
| Total | 4 | -0.62 (-1.18, -0.06) | 0.030 | <0.001 | 94.7 | ||
| Study Duration (Weeks) | |||||||
| ≤10 weeks | 1 | 0.01 (-0.06, 0.08) | 0.792 | - | - | <0.001 | |
| >10 weeks | 3 | -0.77 (-0.96, -0.58) | <0.001 | 0.405 | 0.0 | ||
| Disease Duration (Years) | |||||||
| ≤10 years | 1 | 0.01 (-0.06, 0.08) | 0.792 | - | - | <0.001 | |
| >10 years | 3 | -0.77 (-0.96, -0.58) | <0.001 | 0.405 | 0.0 | ||
| Probiotics Dose (billion CFU) | |||||||
| ≤5 | 2 | -0.89 (-1.32, -0.46) | 0.352 | <0.001 | 95.3 | <0.001 | |
| >5 | 2 | -0.33 (-1.03, 0.36) | <0.001 | 0.230 | 30.5 | ||
| Baseline BMI (kg/m2) | |||||||
| ≤30 | 3 | -0.61 (-1.31, 0.10) | 0.093 | <0.001 | 94.9 | <0.001 | |
| >30 | 1 | -0.70 (-0.99, -0.41) | <0.001 | - | - | ||
| 4 | TAC | ||||||
| Total | 4 | 26.54 (6.23, 46.85) | 0.010 | 0.544 | 0.0 | ||
| Study Duration (Weeks) | |||||||
| ≤10 weeks | 1 | 21.80 (0.15, 43.45) | 0.048 | - | - | 0.216 | |
| >10 weeks | 3 | 61.27 (2.66, 119.87) | 0.040 | 0.738 | 0.0 | ||
| Disease Duration (Years) | |||||||
| ≤10 years | 1 | 21.80 (0.15, 43.45) | 0.048 | - | - | 0.216 | |
| >10 years | 3 | 61.27 (2.66, 119.87) | 0.040 | 0.738 | 0.0 | ||
| Probiotics Dose (billion CFU) | |||||||
| ≤5 | 2 | 23.24 (2.23, 44.25) | 0.020 | 0.587 | 0.0 | 0.231 | |
| >5 | 2 | 73.21 (-5.85, 152.28) | 0.070 | 0.520 | 0.0 | ||
| Baseline BMI (kg/m2) | |||||||
| ≤30 | 3 | 25.39 (4.51, 46.27) | 0.017 | 0.563 | 0.0 | 0.642 | |
| >30 | 1 | 46.70 (-40.61, 134.01) | 0.294 | - | - | ||
| 5 | NO∗ | ||||||
| Total | 3 | 0.45 (-1.91, 2.80) | 0.711 | 0.270 | 23.6 | ||
| Probiotics Dose (billion CFU) | |||||||
| ≤5 | 1 | -0.50 (-2.05, 1.05) | 0.526 | - | - | 0.118 | |
| >5 | 2 | 2.87 (-1.06, 6.80) | 0.152 | 0.680 | 0.0 | ||
| Baseline BMI (kg/m2) | |||||||
| ≤30 | 2 | 2.87 (-1.06, 6.80) | 0.152 | 0.680 | 0.0 | 0.118 | |
| >30 | 1 | -0.50 (-2.05, 1.05) | 0.526 | - | - | ||
hs-CRP, high sensitive C-reactive protein; MDA, malondialdehyde; TAC, total antioxidant capacity; GSH, total glutathione; NO, nitric oxide.
All the studies which assessed the hs-CRP and NO had a duration of 12 weeks, and disease duration of >10 years; so subgroup analysis was not performed across the selected variables.
3.4.2. The effects of probiotics on MDA
The efficacy of probiotics on MDA was reported by four studies with 220 participants (intervention, 110; control, 110) [9-12]. The significant reduction was observed in patients who received treatment (WMD = -0.62 ɥmol/L; 95% CI = -1.18, -0.06, P = 0.030). Results showed a significant heterogeneity (I2 = 94.7 %, P < 0.001) (Figure 3b).
According to subgroup analysis, the impact of probiotics on MDA reduction towards the subsets of “study duration ≤10 weeks, disease duration ≤10 years, and baseline BMI ≤30 kg/m2” did not show any significant trend. When this variable subgrouped by probiotics dosage, a significant effect size was seen in those with >5 billion CFU (WMD = -0.33; 95% CI = -1.03, 0.36; P < 0.001) (Table 2).
3.4.3. The effects of probiotics on TAC
The pooled estimate demonstrated a significant improvement in serum TAC levels as a result of probiotics intervention in 220 DN patients (WMD = 26.54 mmol/L; 95% CI = 6.23, 46.85, P = 0.010) [9-12] (Figure 3c). No heterogeneity was recognized (I2 = 0.0 %, P = 0.544).
Subgroup analysis showed that the impact of probiotics on TAC towards the subsets of “study duration >10 weeks”, disease duration >10 years and “probiotics dose >5 billion CFU” (WMD = 61.27; 95% CI = 2.66, 119.87; P = 0.040 for study and disease duration, and WMD = 73.21; 95% CI = -5.85, 152.28; P = 0.070) was greater than overall results (Table 2).
3.4.4. The effects of probiotics on NO
There was no significant effect of probiotics on NO (WMD = 0.45 ɥmol/L; 95% CI = -1.91, 2.80, P = 0.711) after analyzing three studies with 120 participants (intervention, 60; control, 60) [9,11,12] with no heterogeneity (I2 = 23.6%, P = 0.270) (Figure 3d). A higher non-significant effect size was seen in those with >5 billion CFU probiotics dose and ≤30 kg/m2 baseline BMI (WMD = 2.87; 95% CI = -1.06, 6.80; P =0.152) (Table 2).
3.5. Results of meta-analysis for serum hs-CRP
Three RCTs [9, 11, 12] investigated the impact of probiotics administration on hs-CRP (subjects = 180; intervention, 90; control, 90). Overall, probiotics could make a 1.53 mg/L reduction in serum hs-CRP levels (95% CI = -2.38, -0.69, P < 0.001) (Figure 4). The results were significantly homogeneous (I2 = 0.0%, P = 0.878).
Figure 4.
Forest plot of randomized controlled trials investigating the effect of probiotics on serum inflammatory marker “hs-CRP” in Diabetic Nephropathy patients. The area of each square is proportional to the inverse of the variance of the WMD. Horizontal lines represent 95% CIs. Diamonds represent pooled estimates from fixed-effects analysis. hs-CRP, high sensitive C-reactive protein.
Subgroup analysis demonstrated that the impact of probiotics on hs-CRP reduction towards the subsets of “probiotics dose ≤5 billion CFU and baseline BMI >30 kg/m2” (WMD = -1.70; 95% CI = -3.43, 0.03; P = 0.054) is not statistically significant (Table 2).
3.6. Sensitivity analysis and publication bias
Sensitivity analysis showed that the effect of probiotics on the level of selected markers was not significant. Moreover, there was no evidence of publication bias for studies examining the effect of probiotics on GSH (P = 0.497 for Begg's test and P = 0.675 for Egger's test), MDA (P = 1.000 for Begg's test and P = 0.062 for Egger's test), TAC (P = 0.052 for Begg's test and P = 0.081 for Egger's test), NO (P = 0.602 for Begg's test and P = 0.536 for Egger's test), and hs-CRP (P = 0.117 for Begg's test and P = 0.052 for Egger's test).
4. Discussion
Previous studies have reported that gut microbiota modification - made by probiotics-may regulate systemic inflammation and oxidative stress in CKD patients [14]. Therefore, we systematically reviewed and quantitatively synthesized seven RCTs involving a total of 340 DN patients; the results showed that probiotics supplementation have a potentially beneficial effect on hs-CRP, GSH, MDA, and TAC. However, NO levels did not show any significant improvement in comparison with control group.
The reduction of pro-inflammatory cytokines via Nuclear Factor kappa B (NF-κB) pathway [23] and lowering oxidative stress [24] are the possible anti-diabetic effects of probiotics. Short-Chain Fatty Acids (SCFAs) protect against DN through multiple potential mechanisms of action [25]. SCFAs can decrease circulating endotoxins, and lowering inflammation and oxidative stress [26]. SCFAs may also improve insulin sensitivity via Glucose Transporter Type 4 (GLUT4) through the up-regulation of 5′-AMP-activated protein kinase signaling [27].
The mechanisms underlying the favorable effect of probiotics on DN are varied [28]. Alterations in the redox state in DN are triggered by the persistent state of hyperglycemia and the increase in Advanced Glycation End products (AGEs), making chronic inflammation, glomerular and tubular hypertrophy and favoring the appearance of oxidative stress [29]. DN patients typically experience an imbalance between prooxidant/antioxidant processes, and consequently higher level of ROS [30]. Recent evidence demonstrated that probiotics decrease ROS levels and regulate Nuclear factor erythroid 2–Related Factor 2 (Nrf 2) expression [31].
Based on our knowledge, this study is the first meta-analysis on the antioxidant and anti-inflammatory effects of probiotics supplementation in DN patients. Recently, a meta-analysis was conducted by AbdelQadir and colleagues [32] in DN patients. They included three trials to evaluate the effect of probiotics on hs-CRP and oxidative stress biomarkers. Similar to our results, the overall effect size for hs-CRP, MDA and TAC were significant. Unlike AbdelQadir and colleagues paper, we found significant results for GSH, perhaps due to the inclusion of four studies in the analysis.
Moreover, a systematic review was previously carried out and concluded that more investigations are needed for evaluating the probiotics on antioxidant and oxidative enzymes [33]. Our results showed that probiotics might decrease serum hs-CRP concentrations, however, Jia and colleagues [34] after evaluation of eight studies with 261 patients at CKD stage 3–5 with and without dialysis did not observe any significant changes for serum CRP levels (P = 0.55). Similarly, probiotic supplements did not show any significant effect on uric acid, CRP, Cr, and GFR of CKD patients [35]. The meta-analysis - conducted by Ardeshirlarijani and colleagues [36] on T2DM-indicated that probiotics intake results in significant improvement in serum levels of total antioxidant status (TAS) [SMD: 0.33, 95% CI: (0.11, 0.55)], GSH [SMD: 0.41, 95% CI: (0.26, 0.56)] and MDA [SMD: -0.54, 95% CI: (−0.83, −0.26)]. Similar to our results, no significant improvement was found in NO [SMD:-0.24, 95% CI: (−1.10, 0.62)] levels. Although we did not found any significant effect of probiotics for NO, a considerable change was seen for serum hs-CRP and other oxidative stress markers in DN patients.
It should be mentioned that in the subgroup analysis of trials based on the dose of probiotics, we found that higher doses (>5 billion CFU) were more effective in enhancing TAC/GSH levels than lower doses (≤5 billion CFU). This was similar to prior studies in which higher probiotic administrations were beneficial for improving antioxidant activity in the body [13].
The activation of inflammatory and oxidative stress mediators facilitates the progress of nephropathy to advanced stages [37, 38]. However, there are few acceptable markers of oxidative stress in the diagnosis and early prognosis of DN. Therefore, future researches should decipher the molecular aspects of oxidative stress in DN [39].
Three included studies reported the type and frequency of combined drug therapy among DN participants. There is a concern about pharmacological drug interactions with probiotics, which should be considered in relevant clinical trials. Some strains of probiotics have anti-diabetic and anti-hypertensive property [40, 41, 42]; therefore, these alive microbes may increase the effects of anti-diabetic and anti-hypertensive drugs such as exogenous insulin [43] and Angiotensin Converting Enzyme inhibitors (ACEis) [44]. It is important to know some medications may interact with certain probiotics such as antibiotics and antifungals (clotrimazole, ketoconazole, griseofulvin, and nystatin) [45].
4.1. Limitations
There are several important limitations in this meta-analysis. The number of included studies was small and qualified trials were performed in small sample sizes; three of seven trials were just systematically reviewed and it could overestimate the pooled effects. Studies included in this meta-analysis had follow-up periods ranging from 8 to 12 weeks, which were relatively short-term. As this was only an analysis of studies in age group between 50-60 years, it is unclear how probiotics affect oxidative stress status in youngsters and children. Usual dietary intakes were not assessed in terms of possible prebiotics and probiotics consumption through the normal dietary patterns of participants; it might introduce the high heterogeneity. The other causes of heterogeneity in the current study were the intra-individual strain differences, optimal dose of the probiotics, type of prebiotic used, and genotype of individuals. The methods and preparation of probiotic supplements in the included studies were different and it might have an influence on pooling the results. The response to probiotics intake might also have been influenced by a number of within-study factors, such as antibiotic use [46] and corticosteroid therapy [47]; moreover, the serum levels of hs-CRP is influenced by corticosteroid drugs [48]. Anyway, none of the seven included studies adjusted the mentioned confounders. Our evidence is not applicable to all species of probiotics because the majority of studies focused on one strain i.e. Lactobacillus. The subgroup analysis also had some limitations. The limited number of included studies resulted in the tiny subgroups, which weakened the generalizability of outcomes.
4.2. Strengths
The main strength of the current study is that we presented an exclusive investigation for DN; the high quality score of included studies also gave strength to the results. The prevalence of DN in now growing worldwide and the treatments are very limited, therefore the reported effects of probiotics can allow clinicians to use these compounds as adjunct therapy. Probiotics are considered as safe (GRAS status: generally recognized as safe) [49]. Moreover, it was found that the use of probiotic did not have any negative effect on the renal functions [50].
5. Conclusion
Our meta-analysis showed that probiotics consumption has a beneficial effect on inflammation and oxidative stress biomarkers by significantly reducing hs-CRP and MDA as well as increasing GSH and TAC in DN patients. However, there was no significant effect of probiotics on NO levels. Subgroup analyses indicated that the overall effects of probiotics on serum TAC levels may more be pronounced on probiotic dose >5 billion CFU/day. More trials with larger sample sizes are needed to characterize specific alterations of the intestinal microbiota in DN and to assess possible effects of probiotic, prebiotic, and synbiotic treatments in this disease.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This research was supported by Isfahan University of Medical Sciences, Isfahan, Iran. PROSPERO Registration ID. CRD42020186189.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
PRISMA Checklist
References
- 1.Rossing P., Frimodt-Møller M. Clinical features and natural course of diabetic nephropathy. Diab. Nephrop.: Springer. 2019:21–32. [Google Scholar]
- 2.Hu Y., Shi R., Mo R., Hu F. Nomogram for the prediction of diabetic nephropathy risk among patients with type 2 diabetes mellitus based on a questionnaire and biochemical indicators: a retrospective study. Aging. 2020;12 doi: 10.18632/aging.103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miranda-Díaz A.G., Pazarín-Villaseñor L., Yanowsky-Escatell F.G., Andrade-Sierra J. Oxidative stress in diabetic nephropathy with early chronic kidney disease. J. Diab. Res. 2016;2016 doi: 10.1155/2016/7047238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mikusic N.L.R., Kouyoumdzian N.M., Choi M.R. Gut microbiota and chronic kidney disease: evidences and mechanisms that mediate a new communication in the gastrointestinal-renal axis. Pflueg. Arch. Eur. J. Physiol. 2020:1–18. doi: 10.1007/s00424-020-02352-x. [DOI] [PubMed] [Google Scholar]
- 5.Trimarchi H., Coppo R. Podocytopathy in the mesangial proliferative immunoglobulin A nephropathy: new insights into the mechanisms of damage and progression. Nephrol. Dial. Transplant. 2019;34(8):1280–1285. doi: 10.1093/ndt/gfy413. [DOI] [PubMed] [Google Scholar]
- 6.Roobab U., Batool Z., Manzoor M.F., Shabbir M.A., Khan M.R., Aadil R.M. Sources, formulations, advanced delivery and health benefits of probiotics. Curr. Opin. Food Sci. 2020;32:17–28. [Google Scholar]
- 7.Moravejolahkami A., Chitsaz A. Mediterranean-style diet Co-supplemented with synbiotics improved quality of life, fatigue and disease activity in five secondary progressive multiple sclerosis patients. Ann. Med. Surg Case Rep.: AMSCR. 2019;2019(2) [Google Scholar]
- 8.Yener A.U., Sehitoglu M.H., Ozkan M.T., Bekler A., Ekin A., Cokkalender O. Effects of kefir on ischemia-reperfusion injury. Eur. Rev. Med. Pharmacol. Sci. 2015;19(5):887–896. [PubMed] [Google Scholar]
- 9.Soleimani A., Mojarrad M.Z., Bahmani F., Taghizadeh M., Ramezani M., Tajabadi-Ebrahimi M. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. 2017;91(2):435–442. doi: 10.1016/j.kint.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 10.Miraghajani M., Zaghian N., Mirlohi M., Feizi A., Ghiasvand R. The impact of probiotic soy milk consumption on oxidative stress among type 2 diabetic kidney disease patients: a randomized controlled clinical trial. J. Ren. Nutr. 2017;27(5):317–324. doi: 10.1053/j.jrn.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Mafi A., Namazi G., Soleimani A., Bahmani F., Aghadavod E., Asemi Z. Metabolic and genetic response to probiotics supplementation in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. Food Funct. 2018;9(9):4763–4770. doi: 10.1039/c8fo00888d. [DOI] [PubMed] [Google Scholar]
- 12.Arani N.M., Emam-Djomeh Z., Tavakolipour H., Sharafati-Chaleshtori R., Soleimani A., Asemi Z. The effects of probiotic honey consumption on metabolic status in patients with diabetic nephropathy: a randomized, double-blind, controlled trial. Prob. Antimicrob. Prot. 2019;11(4):1195–1201. doi: 10.1007/s12602-018-9468-x. [DOI] [PubMed] [Google Scholar]
- 13.Heshmati J., Farsi F., Shokri F., Rezaeinejad M., Almasi-Hashiani A., Vesali S. A systematic review and meta-analysis of the probiotics and synbiotics effects on oxidative stress. J. Funct. Foods. 2018;46:66–84. [Google Scholar]
- 14.Zheng H.J., Guo J., Wang Q., Wang L., Wang Y., Zhang F. Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020:1–22. doi: 10.1080/10408398.2020.1740645. [DOI] [PubMed] [Google Scholar]
- 15.Vlachou E., Ntikoudi A., Govina O., Lavdaniti M., Kotsalas N., Tsartsalis A. Effects of probiotics on diabetic nephropathy: a systematic review. Curr. Clin. Pharmacol. 2020 doi: 10.2174/1574884715666200303112753. [DOI] [PubMed] [Google Scholar]
- 16.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann. Intern. Med. 2009;151(4) doi: 10.7326/0003-4819-151-4-200908180-00136. W-65-W-94. [DOI] [PubMed] [Google Scholar]
- 17.Sterne J.A., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019:366. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 18.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5(1):13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbasi B., Ghiasvand R., Mirlohi M. Kidney function improvement by soy milk containing Lactobacillus plantarum A7 in type 2 diabetic patients with nephropathy: a double-blinded randomized controlled trial. Iran J. Kidney Dis. 2017;11(1):36–43. [PubMed] [Google Scholar]
- 20.Abbasi B., Mirlohi M., Daniali M., Ghiasvand R. Effects of probiotic soymilk on lipid panel in type 2 diabetic patients with nephropathy: a double-blind randomized clinical trial. Prog. Nutr. 2018;20:70–78. [Google Scholar]
- 21.Miraghajani M., Zaghian N., Mirlohi M., Ghiasvand R. Probiotic soy milk consumption and renal function among type 2 diabetic patients with nephropathy: a randomized controlled clinical trial. Prob. Antimicrob. Prot. 2019;11(1):124–132. doi: 10.1007/s12602-017-9325-3. [DOI] [PubMed] [Google Scholar]
- 22.Salehipour Z., Haghmorad D., Sankian M., Rastin M., Nosratabadi R., Dallal M.M.S. Bifidobacterium animalis in combination with human origin of Lactobacillus plantarum ameliorate neuroinflammation in experimental model of multiple sclerosis by altering CD4+ T cell subset balance. Biomed. Pharmacother. 2017;95:1535–1548. doi: 10.1016/j.biopha.2017.08.117. [DOI] [PubMed] [Google Scholar]
- 23.Bhardwaj R., Singh B.P., Sandhu N., Singh N., Kaur R., Rokana N. Probiotic mediated NF-κB regulation for prospective management of type 2 diabetes. Mol. Biol. Rep. 2020:1–13. doi: 10.1007/s11033-020-05254-4. [DOI] [PubMed] [Google Scholar]
- 24.Kooshki A., Tofighiyan T., Miri M. A synbiotic supplement for inflammation and oxidative stress and lipid abnormalities in hemodialysis patients. Hemodial. Int. 2019;23(2):254–260. doi: 10.1111/hdi.12748. [DOI] [PubMed] [Google Scholar]
- 25.Li Y.J., Chen X., Kwan T.K., Loh Y.W., Singer J., Liu Y. Dietary fiber protects against diabetic nephropathy through short-chain fatty acid–mediated activation of G protein–coupled receptors GPR43 and GPR109A. J. Am. Soc. Nephrol. 2020;31(6):1267–1281. doi: 10.1681/ASN.2019101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iddir M., Brito A., Dingeo G., Fernandez Del Campo S.S., Samouda H., La Frano M.R. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. 2020;12(6):1562. doi: 10.3390/nu12061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLeod K.H., Richards J.L., Yap Y.A., Mariño E. Elsevier; 2019. Dietary Short Chain Fatty Acids: How the Gut Microbiota Fight against Autoimmune and Inflammatory Diseases. Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; pp. 139–159. [Google Scholar]
- 28.Snelson M., Tan S.M., Higgins G.C., Lindblom R.S., Coughlan M.T. Exploring the role of the metabolite-sensing receptor GPR109a in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2020;318(3):F835–F842. doi: 10.1152/ajprenal.00505.2019. [DOI] [PubMed] [Google Scholar]
- 29.Pasupulati A.K., Chitra P.S., Reddy G.B. Advanced glycation end products mediated cellular and molecular events in the pathology of diabetic nephropathy. Biomol. Concepts. 2016;7(5-6):293–309. doi: 10.1515/bmc-2016-0021. [DOI] [PubMed] [Google Scholar]
- 30.Antunovic T., Stefanovic A., Gligorovic Barhanovic N., Miljkovic M., Radunovic D., Ivanisevic J. Prooxidant–antioxidant balance, hsTnI and hsCRP: mortality prediction in haemodialysis patients, two-year follow-up. Ren. Fail. 2017;39(1):491–499. doi: 10.1080/0886022X.2017.1323645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y., Wu Y., Wang Y., Xu H., Mei X., Yu D. Antioxidant properties of probiotic bacteria. Nutrients. 2017;9(5):521. doi: 10.3390/nu9050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.AbdelQadir Y.H., Hamdallah A., Sibaey E.A., Hussein A.S., Abdelaziz M., AbdelAzim A. Efficacy of probiotic supplementation in patients with diabetic nephropathy: a systematic review and meta-analysis. Clin. Nutr. ESPEN. 2020 doi: 10.1016/j.clnesp.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 33.Salehi-Abargouei A., Ghiasvand R., Hariri M. Prebiotics, prosynbiotics and synbiotics: can they reduce plasma oxidative stress parameters? a systematic review. Prob. Antimicrob. Prot. 2017;9(1):1–11. doi: 10.1007/s12602-016-9248-4. [DOI] [PubMed] [Google Scholar]
- 34.Jia L., Jia Q., Yang J., Jia R., Zhang H. Efficacy of probiotics supplementation on chronic kidney disease: a systematic review and meta-analysis. Kidney Blood Pres. Res. 2018;43(5):1623–1635. doi: 10.1159/000494677. [DOI] [PubMed] [Google Scholar]
- 35.Tao S., Tao S., Cheng Y., Liu J., Ma L., Fu P. Effects of probiotic supplements on the progression of chronic kidney disease: a meta-analysis. Nephrology. 2019;24(11):1122–1130. doi: 10.1111/nep.13549. [DOI] [PubMed] [Google Scholar]
- 36.Ardeshirlarijani E., Tabatabaei-Malazy O., Mohseni S., Qorbani M., Larijani B., Jalili R.B. Effect of probiotics supplementation on glucose and oxidative stress in type 2 diabetes mellitus: a meta-analysis of randomized trials. Daru. 2019:1–11. doi: 10.1007/s40199-019-00302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jabarpour M., Rashtchizadeh N., Argani H., Ghorbanihaghjo A., Ranjbarzadhag M., Sanajou D. The impact of dyslipidemia and oxidative stress on vasoactive mediators in patients with renal dysfunction. Int. Urol. Nephrol. 2019;51(12):2235–2242. doi: 10.1007/s11255-019-02319-7. [DOI] [PubMed] [Google Scholar]
- 38.Cachofeiro V., Goicochea M., De Vinuesa S.G., Oubiña P., Lahera V., Luño J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease: new strategies to prevent cardiovascular risk in chronic kidney disease. Kidney Int. 2008;74:S4–S9. doi: 10.1038/ki.2008.516. [DOI] [PubMed] [Google Scholar]
- 39.Sharma K., Gillum N., Boyd J.L., Schmid A. The RosR transcription factor is required for gene expression dynamics in response to extreme oxidative stress in a hypersaline-adapted archaeon. BMC Genom. 2012;13(1):351. doi: 10.1186/1471-2164-13-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wihansah RRaSb, Arief I., Batubara I. Anti-diabetic potency and characteristics of probiotic goat-milk yogurt supplemented with roselle extract during cold storage. Trop. Anim. Sci. J. 2018;41(3):191–199. [Google Scholar]
- 41.Wang Y., Wu Y., Sailike J., Sun X., Abuduwaili N., Tuoliuhan H. Fourteen composite probiotics alleviate type 2 diabetes through modulating gut microbiota and modifying M1/M2 phenotype macrophage in db/db mice. Pharmacol. Res. 2020;161:105150. doi: 10.1016/j.phrs.2020.105150. [DOI] [PubMed] [Google Scholar]
- 42.Ahtesh F.B., Stojanovska L., Apostolopoulos V. Anti-hypertensive peptides released from milk proteins by probiotics. Maturitas. 2018;115:103–109. doi: 10.1016/j.maturitas.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 43.Balakumar M., Prabhu D., Sathishkumar C., Prabu P., Rokana N., Kumar R. Improvement in glucose tolerance and insulin sensitivity by probiotic strains of Indian gut origin in high-fat diet-fed C57BL/6J mice. Eur. J. Nutr. 2018;57(1):279–295. doi: 10.1007/s00394-016-1317-7. [DOI] [PubMed] [Google Scholar]
- 44.Arief, Budiman C., Hanifah R., Soenarno M.S. Antihypertensive potency of goat milk yoghurt supplemented by probiotic and roselle extract. Int. J. Sci. Basic Appl. Res. 2016;30:207–214. [Google Scholar]
- 45.Mikawlrawng K., Kumar S., Bhatnagar K. Drug interactions, safety and efficacy of probiotics. Asian J. Med. Health. 2016:1–8. [Google Scholar]
- 46.Bäckhed F., Fraser C.M., Ringel Y., Sanders M.E., Sartor R.B., Sherman P.M. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe. 2012;12(5):611–622. doi: 10.1016/j.chom.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Kaur L., Gordon M., Baines P.A., Iheozor-Ejiofor Z., Sinopoulou V., Akobeng A.K. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2020;(3) doi: 10.1002/14651858.CD005573.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kilic H., Karalezli A., Hasanoglu H.C., Erel O., Ates C. The relationship between hs-CRP and asthma control test in asthmatic patients. Allergol. Immunopathol. 2012;40(6):362–367. doi: 10.1016/j.aller.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Žuntar I., Petric Z., Bursać Kovačević D., Putnik P. Safety of probiotics: functional fruit beverages and nutraceuticals. Foods. 2020;9(7):947. doi: 10.3390/foods9070947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sengul E., Gelen S.U., Yıldırım S., Çelebi F., Çınar A. Probiotic bacteria attenuates cisplatin-induced nephrotoxicity through modulation of oxidative stress, inflammation and apoptosis in rats. Asian Pac. J. Trop. Biomed. 2019;9(3):116. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist
Data Availability Statement
Data will be made available on request.