Abstract
Maternal nutritional programming by caloric exposure during pregnancy and lactation results in long-term behavioral modification in the offspring. Here, we characterized the effect of maternal caloric exposure on synaptic and brain morphological organization and its effects on depression-like behavior susceptibility in rats’ offspring. Female Wistar rats were exposed to chow or cafeteria (CAF) diet for 9 weeks (pre-pregnancy, pregnancy, and lactation) and then switched to chow diet after weaning. By postnatal day 60, the male Wistar rat offspring were tested for depressive-like behavior using operational conditioning, novelty suppressed feeding, sucrose preference, and open-field test. Brain macro and microstructural morphology were analyzed using magnetic resonance imaging deformation-based morphometry (DBM) and western blot, immunohistochemistry for NMDA and AMPA receptor, synaptophysin and myelin, respectively. We found that the offspring of mothers exposed to CAF diet displayed deficient motivation showing decrease in the operant conditioning, sucrose preference, and suppressed feeding test. Macrostructural DBM analysis showed reduction in the frontomesocorticolimbic circuit volume including the nucleus accumbens (NAc), hippocampus, and prefrontal cortex. Microstructural analysis revealed reduced synaptic terminals in hippocampus and NAc, whereas increased glial fibrillary acidic protein in hippocampus and lateral hypothalamus, as well as a decrease in the hippocampal cell number and myelin reduction in the dentate gyrus and hilus, respectively. Also, offspring exhibited increase of the GluR1 and GLUR2 subunits of AMPA receptor, whereas a decrease in the mGluR2 expression in hippocampus. Our findings reveal that maternal programming might prime depression-like behavior in the offspring by modulating macro and micro brain organization of the frontomesocorticolimbic circuit.
Subject terms: Molecular neuroscience, Depression
Introduction
Depression is one of the leading causes of disability worldwide affecting >300 million people of all ages1,2. Depressive subjects show anhedonia or low motivation for natural or social stimuli, which become resistant to brain therapy and classical pharmacology approaches3.
Major depressive disorder (MDD) is characterized by an age-dependent brain dysfunction and structural alterations in selective regions of the reward circuit4–9. The reward circuit integrates dopaminergic neurons located in the ventral tegmental area (VTA) that innervate the nucleus accumbens (NAc), the prefrontal cortex (PFC), central, and basolateral amygdala (BLA) and the hippocampus and dorsal striatum10. Glutamate neurons also originating in the VTA and substantia nigra (SN) of the midbrain innervate limbic sites, including the NAc and dorsal striatum11–13. Major volume brain changes in adult and adolescence in MDD subjects have been documented, including hippocampus atrophy7,14 thinner cortical gray matter in the orbitofrontal and medial cortex (OFC), anterior and posterior cingulate, insula, and temporal lobes9,15–17. Of note, genome-wide association studies (GWAS) of MDD and schizophrenia cohorts have identified genetic variants linked to brain volume alterations during development18, supporting the notion that brain macrostructural changes might potentially lead to MDD.
Aberrant brain morphological organization has also been observed in murine models of depression-like behavior. For instance, depression-like behavior models in rats show microstructural alterations linked to a decrease in hippocampal synaptophysin and NR2A subunit19, and dendritic atrophy in the CA1 and CA3 regions of the hippocampus6,10,20–23. Also, GWAS analysis of MDD and schizophrenia cohorts identified single-nucleotide polymorphisms in genes that encode synaptic plasticity and myelin repair proteins18. In addition to macrostructural and microstructural alterations of the reward system identified in MDD subjects or murine models, neurobiological causes underlying aberrant brain plasticity are unknown.
Epidemiological data and basic research studies in humans and animal models, respectively, have identified that maternal obesity24,25 and/or hypercaloric diet exposure during embryonic development26–29, a physiological process known as maternal programming, modulates establishment of functional and structural neuronal connectome of the reward system27,28,30, potentially leading to depression susceptibility in the offspring26–29. For example, maternal nutritional programming by exposure to a hypercaloric diet during pregnancy primed an altered glutamatergic neurotransmission in the reward system28,30,31, disruption in structural and functional integrity of the hippocampus32,33 and reduction in dendritic complexity in BLA of the offspring33. In humans, prolonged consumption of caloric diets during adolescence favors defective emotional behaviors11,34, which correlates with failure in hippocampal neurogenesis in murine models32,35. In this study, we hypothesized that maternal programming by hypercaloric diet exposure would produce macro- and microstructural alterations linked to brain volume changes and aberrant glutamatergic synaptic plasticity of the reward circuit in the offspring, leading to depression-like behavior early in life. For this, we studied behavior and global structural changes using magnetic resonance imaging (MRI), coupled with selective molecular and histological characterization of glutamatergic synaptic markers.
Materials and methods
A full description of all experimental procedures is provided in the Supplemental Materials.
Animals and housing
Programing and mating experiments were performed using males and virgin females from 10 to 12 weeks old Wistar rats, respectively. Animals were handled according to the NOM-062-ZOO-1999 guide for the care and use of laboratory animals, with approval of the Universidad Autónoma de Nuevo León Animal Care Committee (BI0002) (Supplemental information).
Maternal nutritional programming model in offspring by cafeteria (CAF) diet exposure
Female Wistar rats were fed with chow (Control) or CAF diets for 9 weeks (pre-pregnancy, pregnancy, and lactation) as reported24. CTRL-CTRL and CAF-CTRL offspring were fed with control diet and CAF-CAF offspring were fed with CAF diet after weaning at postnatal day 21 (Fig. S1). Control chow and CAF diet formulas and caloric density are found in Table S1. At 2 months of age, we performed behavioral tests to characterize motivation. We registered body weight of all offspring at birth (~15 rats/litter) and at the age of 3 weeks, we killed female offspring. Body weight were quantified in offspring from 3rd to 7th week.
Depression-like behavioral phenotyping
Individual animal behavior analysis was conducted on two cohorts, the first one for MRI, immunohistochemistry, and histology, and the second one for western blot analysis of synaptic markers (Fig. S2). For behavioral phenotyping we used the Skinner box for operational conditioning, as previously reported28,36, the preference to sucrose test, novelty suppressed feeding and open-field test as described in Supplemental information
Ex-vivo fixation and MRI analysis by deformation-based morphometry (DBM)
Rats were anesthetized with 1 mL pentobarbital (PiSA Agropecuaria) i.p. overdose and transcardially perfused following standardized methods as described in Supplemental information. For MRI acquisition, the skulls were submerged and fixed inside plastic tubes filled with Fomblin (a chemically inert perfluoropolyether fluorocarbon; Solvay Solexis, Inc.) and imaging was performed in a 16 cm bore 7 T Bruker scanner, T1w sequence name: UANL_Camacho_FatRat, resolution 1.25 mm (Pharmascan 70/16), Bruker FLASH, slice thickness = 0.0853 mm, TR/TE = 30.76/8.64 ms, flip angle = 20 degrees, averages = 1, matrix = 376 × 376, spacing = 0.0853 mm, Pixel bandwidth = 74 Hz, FOV = 300 × 300 mm, no. of slices = 376. Morphological analysis was performed by converting DICOM to MINC format, and then preprocessed using an in-house pipeline based on MINC-Tools and the pydpiper pipeline (https://github.com/Mouse-Imaging-Centre/pydpiper) (see Supplemental information). All analyses were performed using pydpiper37, R statistics38, R studio39, and the RMINC40 and tidyverse41 packages.
Histological analysis
Following MRI analysis, coronal sections from the brains were cut in a cryostat and stained with Hematoxilyn & Eosin, Kluver–Barrera stain, and synaptic markers were also evaluated through immunohistochemistry (Supplemental information).
Membrane and cytoplasmic fractions isolation from the brain samples
Brains from the second cohort of subjects were dissected from the skull and the NAc, the PFC and the hippocampus were isolated using the Paxinos and Watson atlas (AP 1, 3, and 3.8 mm from bregma, respectively) as we reported previously32 (Supplemental information).
Western blot analysis
Samples were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis to identify changes in synaptic markers including NMDA, AMPA, synaptophysin, mGlur2, and Glur5 (see Supplemental information for details).
Quantification and statistical analysis
Data are presented as mean ± SEM for all data. All statistical analyses including testing the normality of data distribution were performed using GraphPad Prism 7.01 and IBM SPSS statistics version 22 software and a corrected p value <0.05 was considered as significant. All results were tested for normality using Shapiro–Wilk test. For differences between three groups in the behavioral tests and protein concentration one-way Analysis of variance (ANOVA) followed by Tukey’s multiple comparison test was used and effect size was calculated in R language with the pwr package. For significant differences in high vs low responders during operant conditioning we used Chi-square per sample test. The data are shown as the mean ± SEM and significant differences p < 0.05. The statistical analysis on MBD was performed using the log-transformed Jacobian determinants as the dependent variable, “group” as the independent variable (between subjects) and as covariate we included “batch order” (rats were trained in batches). We compared the three groups using a GLM and analyses were corrected for multiple comparisons using the false-discovery rate (FDR) at 5%42. Furthermore, we extracted the t alues from significant peaks at NAc and hippocampus to create scatterplots and correlation with the immunohistochemistry data. Details about the statistical analysis are available in the open-access R script (see above).
Results
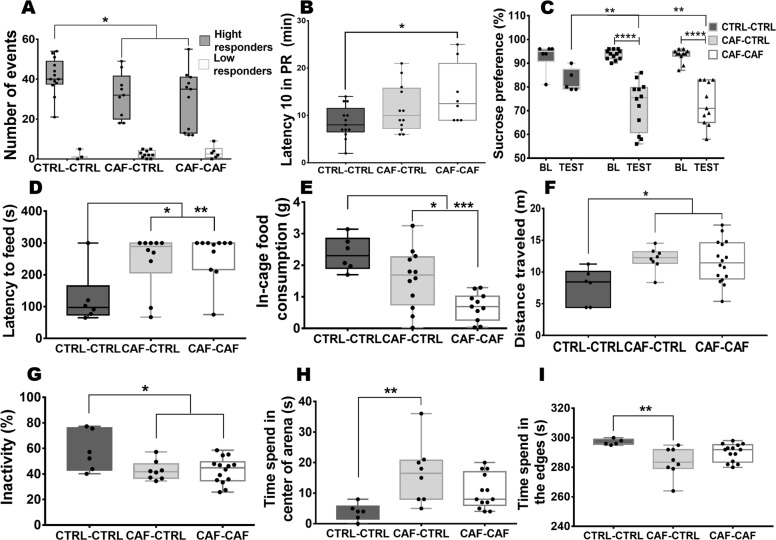
Individual behavioral phenotyping was performed in two cohorts of subjects to determine the effect of nutritional programming on motivation for rewards in offspring. Initially, we found that nutritional programming by CAF diet decreased offspring weight (Fig. S3; F2,49 = 20.88, p = 0.0005). only if the offspring continues the CAF diet after weaning (Fig. S3, p ≤ 0.0001). Motivation for natural rewards in rats displayed low and high responder subjects (Fig. 1A; F2,46 = 112.2, p = 0.0001), similarly as we reported recently23. In contrast to control subjects, fetal programming by CAF diet exposure decreases the motivation of high responder subjects (Fig. 1A; CTRL-CTRL vs. CAF-CTRL*p = 0.0190, effect size result was 0.96). In fact, fetal programming and CAF exposure after weaning reproduces low motivation for rewards in the offspring (Fig. 1A; CTRL-CTRL vs. CAF-CAF *p = 0.0170, effect size result was 0.94). In addition, offspring exposed to CAF experiences a delay in the lever presses performance (Fig. 1B; F2,30 = 3.652, p = 0.0381) compared with the CTRL-CTRL (Fig. 1B; *p = 0.0316, effect size result was 0.87), showed only significant in offspring exposed to CAF after weaning (Fig. 1A; CTRL-CTRL vs CAF-CAF *p = 0.0316). Analysis of the total high and low responder subjects show no significant difference 53% high responder and 47% low responder CAF-CTRL (X2;; p = 0.8185), and 65% high responder and 35% low responder CAF-CAF (X2; p = 0.2253), on the other hand, control group show more high responder (81%) than low responder (19%)(X2;; *p = 0.01242) (Table S2). Differences in motivation behavior in the offspring were also identified during the sucrose preference test (Fig. 1C; F2,46 = 4.784 p = 0.0130), showing a significant decrease in the percentage of sucrose intake in the CAF-CTRL and CAF-CAF groups compared with their baseline (Fig. 1C, ANOVA post hoc Tukey ****p ≤ 0.0001) and a significant decrease in the CAF-CTRL and CAF-CAF compared with the CTRL-CTRL in the test day (Fig. 1C, p = 0.0015 and p = 0.0074, respectively). Next, we examined hyponeophagia by NSFT (Fig. 1D; F2,24 = 5.833, p = 0.0086) programmed offspring displays longer time to reach the pellet in the center of the arena (Fig. 1D; CAF-CTRL and CAF-CAF vs. CTRL-CTRL, *p = 0.0234 and **p = 0.0088), and also show a decrease in individual food consumption following 18 h fasting (Fig. 1E; F2,26 = 11.55, p = 0.0003) CAF-CTRL and CAF-CAF vs. CTRL-CTRL (*p = 0169, ***p = 0.0002, respectively). Finally, offspring exposed to CAF increased their locomotor activity (Fig. 1F; F2,27 = 4.551, p = 0.0198) in contrast with the CTRL-CTRL group (Fig. 1F CTRL-CTRL vs CAF-CAF *p = 0.0280 and CTRL-CTRL vs CAF-CTRL *p = 0.0288), less inactive (Fig. 1G; F2,27 = 4.551, p = 0.0198; CAF-CAF *p = 0.0235, CAF-CTRL *p = 0.0431 vs CTRL-CTRL) and only the programed offspring fed with chow diet after weaning, exhibited longer time in the center of the arena when compared with edges and control subjects (Fig. 1H, I; F2,24 = 5.815, p = 0.0087, F2,24 = 5.797, p = 0.0088, CAF-CTRL and CAF-CAF vs CTRL-CTRL **0.0063 and **p = 0.0069 respectively). These data propose that fetal programming decreases motivation for natural rewards and increases latency to feed in the offspring.
Fig. 1. Behavioral testing of depression-like behavior in the offspring.
A Offspring was nutritionally programmed as described, and subjects were trained using the operational conditioning protocol. The number of events (action of the lever) were obtained during the PR protocol (1 h × 5 days). Graph shows response to reinforcers of two subject groups, high responders (>10 lever presses) and low responders (<10 lever presses) per training session. Results are expressed as mean ± SEM, following by ANOVA two ways, Post hoc Tukey. *p < 0.05 vs the control. n = 16–19/group). B Latency to PR. Graph shows the time the subject invests to reach the threshold of 10 events during the PR schedule. Results are expressed as mean ± SEM, following by ANOVA two ways, Post hoc Tukey. *p < 0.05 vs the control. n = 16–19/group). C Basal sucrose intake was measure for 72 h under ad libitum food and water exposure. Sucrose preference was performed by quantifying water and 2% w/v sucrose intake for 20 min after food and water deprivation for 16 h. The percentage of preference for sucrose in the offspring was quantified according to % PS = [IS ÷ (SI + WI)] × 100. Results are expressed as mean ± SEM, following by ANOVA post hoc Tukey. ****p < 0.0001 vs the control. n = 6–12/group. D Latency to feed was determine comparing time required to reach the food of the center of the arena in the Control, CAF-CTRL and CAF-CAF groups. Results are expressed as mean ± SEM, following by ANOVA post hoc Tukey. *p < 0.05 **p ≤ 0.01 vs the control. n = 6–12/group. E Food intake in cage after fasting in the CAF-CTRL, CAF-CAF, and control groups. Results are expressed as mean ± SEM, following by ANOVA post hoc Tukey ***p < 0.05 vs the control. n = 6–12/group. F–I Comparison of total distance traveled, inactivity and time spend in on the edges and in the center of the open-field test. Results are expressed as mean ± SE following by ANOVA post hoc Tukey. *p < 0.05, **p < 0.005, ***p < 0.001, ****p < 0.0001 vs the control group, n = 6–16/group.
Programmed offspring show brain macrostructural alterations
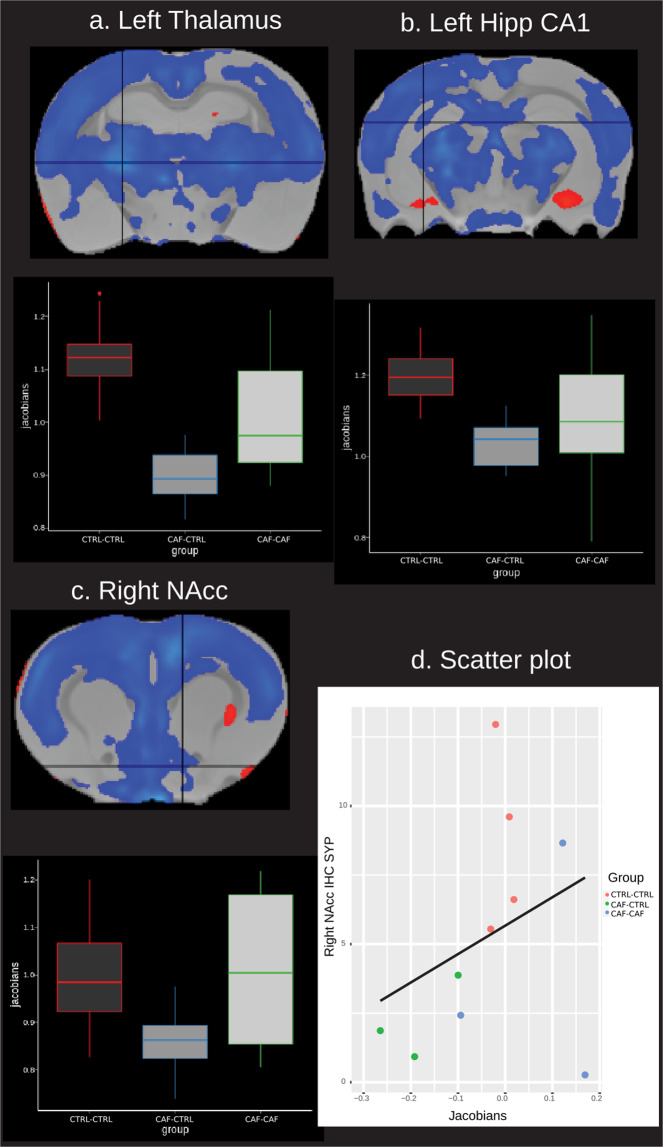
We found significant differences in local volume across all groups. The pairwise analysis showed significant whole-brain local volume differences in the CAF-CTRL group compared with the CTRL-CTRL group (Fig. S4). The most consistent difference was lower local volume, however, there were localized clusters with higher volume (Table S3 and Table S4). The CAF-CAF group showed lower local volume compared with the CTRL-CTRL group in more localized clusters but only at FDR 10% and not 5%. There were no significant differences between CAF-CAF and CAF- CTRL groups. By using Fischer-344 rat anatomical atlas43, we identified that the left thalamus, left hippocampal CA1 and the right NAc core displayed the lowest volumes in the CAF-CTRL group. Notably, the peak volume of right NAc was proportionally correlated to synaptophysin expression (Fig. 2) (see data from immunohistochemistry below).
Fig. 2. DBM of brain volume in the offspring.
Boxplots show the relative volume (y axis = Jacobians) in each group (x axis) in several significant peaks. The peaks are shown in the crosshairs. a ROI = left thalamus, b left hippocampus CA1, c right NAc, d scatter plot between immunohistochemistry results in the right nucleus accumbens (y axis) and right nucleus accumbens peak volume (x axis). No significant correlation was found in d. Blue-light blue = lower volume; red-yellow = higher volume. Results are significant at FDR 5%.
Brain microstructural defects in offspring linked to fetal programming
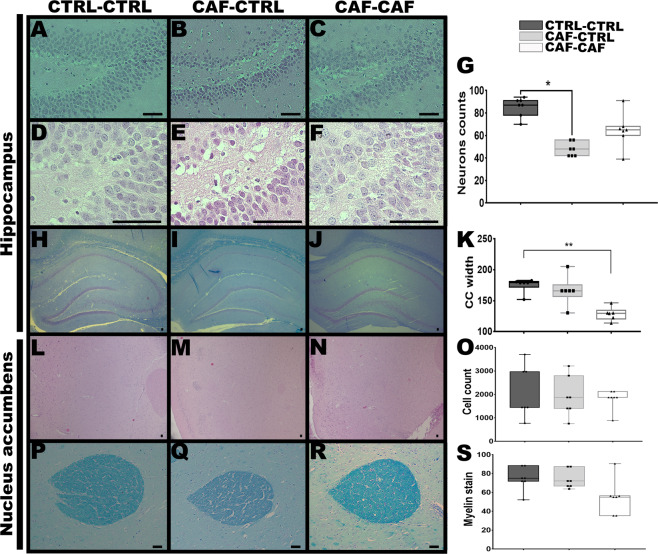
We tested if maternal nutritional programming sets histological and synaptic protein expression defects in offspring. We specifically selected brain regions showing macrostructural alterations in the MRI analysis (Fig. S3, Table S3, and Table S4). First, hippocampal H-E staining identified a decrease in the total number of cell nucleus in the dentate gyrus (DG) of the offspring programmed by CAF diet (Fig. 3A–G; F2,9 = 5.978, p = 0.0223), showing only significant difference in CAF-CTRL compared to the CTRL-CTRL Fig. 3G *p = 0.0184), it also indicated pyknotic cells, chromatin condensation and cellular disorganization (Fig. 3A–G). Likewise, the Klüver–Barrera staining for myelin displayed that the offspring programmed by CAF diet and exposed to CAF diet after weaning showed a decrease in the area of corpus callosum at level of CA1 of hippocampus (Fig. 3H–K; F2,9 = 5.993, p = 0.0221, *p = 0.0267). Histological and myelin characterization of NAc does not show significant changes following maternal programming by CAF diet (Fig. 3L–S).
Fig. 3. Histological analysis of hippocampus in the offspring.
A–G Representative H&E stain of brain coronal slices comparing DG cellularity of the Control, CAF-CTRL, and CAF-CAF groups (400 and 1000 magnification). Results are expressed as mean ± SEM. following by ANOVA post hoc Tukey *p < 0.05 vs the control group, n = 4 per group). H–K Representative Luxol fast blue stain of brain coronal slices comparing corpus callosum of the CTRL-CTRL, CAF-CAF, and control groups (500 magnification). L–O Representative H&E stain of brain coronal slices of the NAc cellularity of the Control, CAF-CTRL, and CAF-CAF groups (400 magnification). P–S Representative histological image (coronal plane) of anterior commissure at NAc of the CTRL-CTRL, CAF-CAF, and control groups treated with luxol fast blue and cresyl-violet for myelin stain. Results are expressed as mean ± SEM. following by ANOVA post hoc Tukey. *P < 0.05, **P < 0.005, ***P < 0.001, ****P < 0.0001 vs the control group, (n = 4 per group). Scale bar = 50 μm.
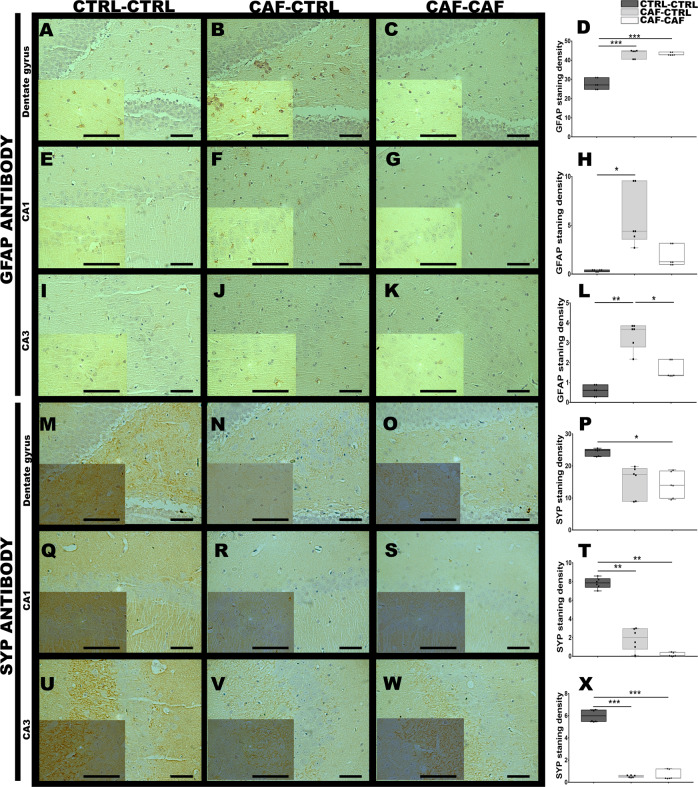
Defects in synaptic markers in the offspring of mothers exposed to CAF diet were also determined using the subjects identified in the MRI analysis. Immunohistochemical imaging of brain regions showing macrostructural alterations revealed significant increase in the glial fibrillary acidic protein (GFAP) in the DG (Fig. 4A–D; F2,6 = 44.82, p = 0.0002), CA1 (Fig. 4E–H; H(2) = 7.318, p = 0.0043, *p = 0.0206) and CA3 (Fig. 4I–L; H(2) = 7.2, p = 0.0036, *p = 0.0219) regions of the hippocampus in subjects exposed to fetal programming by CAF diet (CAF-CTRL group, ***p = 0.0004, *p = 0.0206, and **p = 0.0036, respectively) and in the offspring exposed to CAF after weaning (CAF-CAF group, ***p = 0.0004, p = 0.5326 and **p = 0.0448, respectively)). Notably, a significant decrease in the synaptic marker synaptophysin in the DG (Fig. 4M–P; F2,6 = 5.606, p = 0.0424, CTRL-CTRL vs CAF-CAF p = 0.0497), and a substantial decrease in the CA1 (Fig. 4Q–T; F2,4 = 35.81, p = 0.0028, CTRL-CTRL vs CAF-CAF and CAF-CTRL p = 0.0027, p = 0.0074, respectively) and CA3 (Fig. 4U–X; F2,4 = 44.98, p = 0.0018, CTRL-CTRL vs CAF-CAF, and CAF-CTRL p = 0.0021, p = 0.0032, respectively) regions of hippocampus was identified in offspring of mothers exposed to CAF diet and in subjects exposed to CAF after weaning compared with the control. Also, there were no significant changes in the immunohistochemical stain of synaptophysin and no changes in the expression of GFAP marker in NAc when compared with the control group (Fig. S5A–H). Finally, the lateral hypothalamus showed a profound decrease in synaptophysin and GFAP immune signal in subjects exposed to fetal programming (Fig. S5I-P; F2,7 = 57.35, p = 0.0001, CTRL-CTRL vs CAF-CAF and CAF-CTRL, ****p = 0.0001 and ***p = 0.0001, respectively).
Fig. 4. Maternal programming activates gliosis and decreases synaptophysin expression in depression-like behavior subjects.
Coordinates for hippocampus was obtained from brain previously scanned by MRI. Brain slices were obtained as described in Methods. Immunostaining of sections using anti-GFAP antibody from dentate gyrus A–D, CA1 E–H, and CA3 I–L of hippocampal regions were tested for GFAP immunostaining. Immunostaining for synaptophysin was performed in hippocampus using an anti-SYP antibody of the Control, CAF-CTRL and CAF-CAF. M–P Dentate gyrus, Q–T CA1, U–X CA3 region. Results are expressed as mean ± SEM. following by ANOVA post hoc Tukey. *P < 0.05, **P < 0.005, ***P < 0.001, ****P < 0.0001 vs the control group (n = 4 per group). Scale bar = 50 μm.
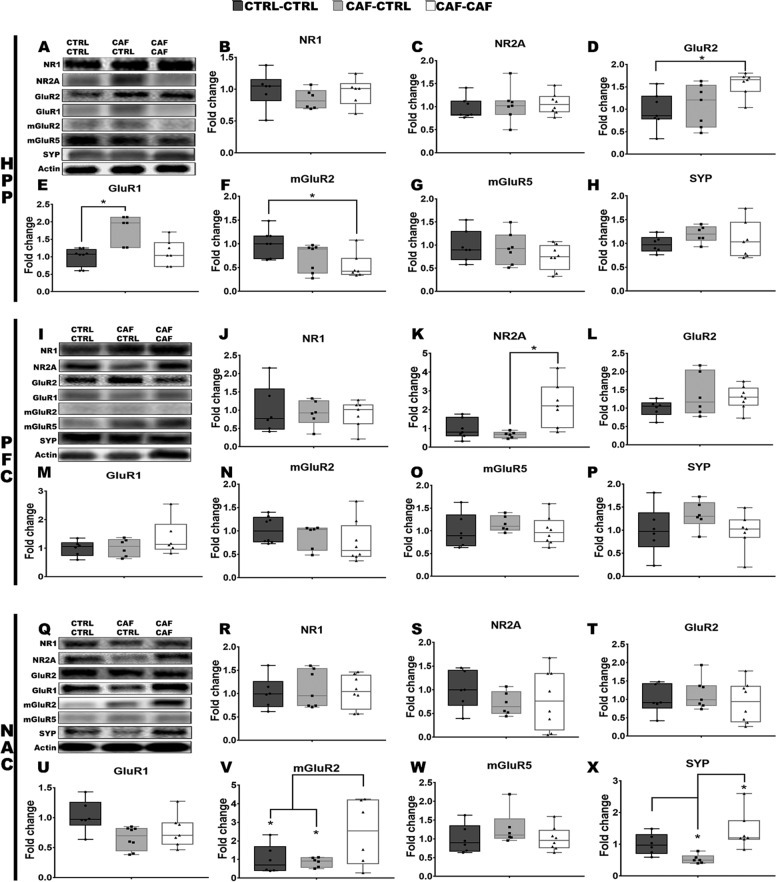
Synaptic defects of selective brain regions showing major structural changes evidenced by MRI analysis including hippocampus, PFC, and NAc in the offspring were detected by western blot analysis in the second batch of offspring subjects exposed to maternal programming. We focused our analysis on the glutamatergic neurotransmission markers including the NR1 and NR2A subunits (NMDA receptor), the GluR1 and GluR2 subunits (AMPA receptor), the mGluR2 and mGluR5 (metabotropic receptors) and synaptophysin for synaptic terminals (Fig. 5). Hippocampal analysis in the offspring of programmed mothers showed a significant increase in the GluR1 (Fig. 5E; F2,9 = 4.098, p = 0.0543, CAF-CTRL vs CTRL-CTRL *p = 0.0484) subunit protein expression, and an increase in the GluR2 (Fig. 5D; F2,18 = 4.626, p = 0.0239, CAF-CAF vs CTRL-CTRL *p = 0.0268) subunit as well as a decrease in the mGluR2 (Fig. 5D; F2,16 = 3.566, p = 0.0524, CAF-CAF vs CTRL-CTRL *p = 0.0428) expression in the offspring exposed to CAF after weaning. No changes were found in NR1, NR2A subunits, and mGluR5 and synaptophysin protein expression (Fig. 5B, C, G, H). PFC shows NR2A subunit upregulation in the offspring exposed to CAF after weaning (Fig. 5K; F2,13 = 4.477, p = 0.0332, CAF-CAF vs CAF-CTRL *p = 0.0310). Also, we did not find protein expression changes in NR1, GluR1, GluR2, mGluR2, mGluR5, or synaptophysin protein expression in PFC (Fig. 5J, L–P). Finally, NAc of programmed offspring subjects integrated a downregulation of synaptophysin in the offspring of mothers exposed to CAF diet (Fig. 5X; F2,12 = 7.408, p = 0.0080; CTRL-CTRL vs CAF-CTRL *p = 0.0407)), and an upregulation of mGluR2 and synaptophysin protein levels in the offspring exposed to CAF after weaning (Fig. 5V, X; *0.04901 and *p = 0.0144, respectively) (Fig. 5V, X). No changes were identified in the protein expression of NR1, NR2A, GluR1, GluR2, and mGluR5 (Fig. 5R, S, T, U, W). These results propose that caloric exposure during fetal development negatively regulates synaptic terminals expression in selective brain regions of the offspring.
Fig. 5. Characterization of the glutamatergic neurotransmission markers in depression-like behavior subjects.
The second cohort of subjects was diagnosed as depression-like behavior and PFC, NAc, and hippocampus were isolated to perform western blot analysis for glutamatergic receptors markers including the NR1 and NR2A subunits (NMDA receptor), GluR2 and GluR1 subunits (AMPA receptor), mGluR2 and mGluR5 (metabotropic receptors) and synaptophysin (SYP). A–H hippocampus (HPP), I–P prefrontal cortex (PFC), and Q–X Nucleus accumbens (NAC). The graphs show normalized data of the mean ± SEM following by Tukey multiple comparation test. *p < 0.05 vs the control group. n = 5–8/group.
Discussion
Maternal overnutrition during pregnancy leads to several alterations in the offspring’s behavior such anxiety44, and addiction28,30, however, neurobiological causes of programmed offspring behavior induced by caloric exposure are not completely understood. Initially, we identified that maternal programming CAF-CAF diet exposure decreased body weight from week 4 to week 7 of age and the rats did not recover to control values (Fig. 1A), corresponding with our previous report29. We identified that maternal programming by CAF diet exposure led to depression-like behavior in the offspring, showing lower volume in many regions, among which the thalamus, hippocampus, NAc core, and hypothalamus, important regions in the frontostriatomesolimbic system, were correlated with their own alterations in SYP and GFAP expressions. Also, maternal programming led to a cell number and myelin reduction in the DG and hilus of hippocampus, respectively, and an increase in the GLUR1 receptor expression. This evidence proposes that maternal exposure to high-fat diet formula primes lower brain volume and changes in synaptic markers of glutamatergic neurotransmission which associates to depression-like phenotype in the offspring.
In accordance with a previous study45, our results show that fetal programming by CAF diet exposure during pregnancy contributes to hyperactivity in male offspring measured by distance traveled46. Following behavioral phenotyping of depression-like behavior for rats47, our results show for the first time that, despite no anxiolytic effect by fetal programming, the offspring experienced defects in motivation for natural rewards during the operant conditioning test, and a reduced preference for hedonic sucrose intake and latency to eat following fasting. In fact, defective motivation for natural rewards and anhedonia-like behavior have been reported in rats expose to chronic mild stress20,48–53, after chronic consumption of a high-fat food11 or even followed by exposure to sweet beverages26,54. In order to explain the effect of maternal programing of CAF diet exposure on depression-like behavior phenotype, we initially focused our analysis on aberrant brain microstructural alterations by MRI technology. Structural defects of selective brain regions are a hallmark of neuropsychiatric disorders including depression55,56, showing lower brain volume within the corticolimbic circuit diagnosed in the MDD subjects57. We found that maternal programming (CAF-CTRL group) led to dramatic brain volume loss in the left thalamus, left hippocampal CA1 and in the right NAc core. Also, the occipital cortex and the frontal association cortex displayed the major volume decrease of CAF diet programed offspring (CAF-CTRL group). Despite caloric exposure after weaning (CAF-CAF group), there were fewer brain volume changes when compared with the maternal programming group (CAF-CTRL), at least at this age. Depression-like behavior has been identified to show a time-dependent atrophy of CA1, CA3, and DG brain regions50, such as, reduced cortical thickness in prefrontal cortex and orbitofrontal, and smaller hippocampal volume and larger pallidal volume in children and adolescence diagnosed with MDD9. Conversely, increased cortical thickness in the bilateral posterior dorsolateral prefrontal cortex and right superior parietal cortex were found in MDD adult patients15. This evidence suggests a detrimental effect of caloric fetal programming on lower brain volume leading to depression-like behavior priming in the offspring.
To identify potential molecular or cellular causes of the lower brain volume measured with MRI in programmed subjects showing depression-like behavior, we initially focused our analysis in the lateral hypothalamus, based on its major brain volume loss. We found a direct decrease in GFAP levels and atrophy in the lateral hypothalamus of subjects exposed to CAF diet during pregnancy and lactation (CAF-CTRL group) and after weaning (CAF-CAF group). However, we did not find a significant correlation with MRI brain volume, potentially owing to low sample analyzed by immunohistochemistry. Our results agree with recent evidence showing a decrease in GFAP levels in hypothalamus following maternal high-fat diet exposure58,59, and also, deficient GFAP expression after immune activation by LPS inoculation59–61. We identified a correlation between the lower volume in the right NAc core and a decrease in synaptophysin immunosignal. However, in contrast to the lateral hypothalamus, we observed a prominent increase in the GFAP expression in the left hippocampal CA1 and the right NAc core. Selective increase in GFAP immunoreactivity was reported in layer I of the dorsolateral prefrontal cortex of brain post mortem biopsies of depressed subjects62. Conversely, studies in depressed post mortem patients63 and animal models of chronic stress reported a time-dependent reduction in glial cell number in the CA1 of hippocampus, potentially owing to a selective neuroadaptive response to stress20. Also, glial alterations in MDD might presumably be related to active release of S100B by astrocytes64 or microglia activation during programming65. Hippocampal shrinkage may be explained by a decrease in glial cells number and/or loss in the number of neurons due to a neurotoxic effect of glucocorticoids66, which are increased in depressed patients4,67, and have been found in murine models exposed to perinatal high-fat diet68. Similarly, we show that the hippocampus of offspring programmed by CAF diet displayed a decrease in cellular number in the DG coupled to pyknotic cells, chromatin condensation, cellular disorganization and a postweaning CAF diet intake decrease in the width of the body of the corpus callosum, which have also been implicated in MDD in humans69–71. Depression induced by chronic mild stress in murine models reported decrease cellular number and size in the CA1, thinner layers of cells in hippocampus50 and a decreased number of apical dendrites in CA1 and CA320, which is also reported for mice or rats programming by a high-fat diet33,35. We conceive that an increase in the glial cells number found in our study potentially may compensate the synaptic loss and myelin decreases in the offspring showing anhedonia included in depression-like behavior.
Finally, we characterized microstructural changes of selected brain regions identified by MRI in subjects diagnosed with depression-like behavior by analyzing glutamatergic transmission markers which have been reported in both murine models and postmortem brains of depressive patients12,28,48,72–74. We found a substantial increase of the GluR1 and GLUR2 subunits of AMPA receptor in the hippocampus, which correlated with a low cell number at DG only in subjects programmed by CAF diet (CAF-CTRL group). Also, we found a decrease in the mGluR2 expression in hippocampus of subjects programmed by CAF diet and upregulation in NAc of subjects exposed to CAF diet after weaning (CAF-CAF group). Clinical observations in post mortem hippocampal samples from depressed subjects showed decreased expression of genes that encode AMPARs subunits75. A low expression of the subunit GluR1 and a high expression of the subunit GluR2, seem to be related to psychiatric disorders76. We speculate that a decrease of synaptic terminals evidenced by synaptophysin immunosignal following caloric exposure in offspring was compensated by upregulation of AMPA subunits expression. We have reported that glutamatergic receptor expression such as NMDA subunits become regulated by modifying in vivo glycolytic metabolism in hippocampus77, which also regulates neuronal death78. As reported, the offspring of mothers exposed to CAF diet integrates changes in the metabolic and hormonal plasma profile29, which correlates with metabolic defects found in a fetal programming model by high-fat diet exposure79–81, suggesting metabolic and hormonal molecular priming during embryonic development. In fact, we have recently reported that the offspring programmed by CAF diet show increased DNA methylation into the NAc36. This evidence supports “the fetal origins hypothesis” of chronic psychiatric diseases during development and its effects on major behavioral and brain abnormalities82. Our results also suggest that when the offspring was exposed to a “second-hit” stressor (postweaning CAF diet), behavior and brain volume might contrast from the fetal programming subjects, confirming a postnatal regulation of metabolic and behavioral traits by external stimuli. In this context, plasma lipidomic analysis of the offspring of mothers exposed to CAF diet showed a plasma decrease in the 22:6 lipid specie (n-3 PUFA), whereas an increase in the 20:4 specie (n-6 PUFA) (data unpublished). Some reports have identified lower levels of the n-3 polyunsaturated fatty acids (PUFA), eicosapentaenoic acid and docosahexaenoic acid whereas higher levels of the n-6 PUFA and araquidonic acid in the blood of subjects showing depressive or anxiety symptoms83. Notably, low levels of 22:6 lipid specie has been reported in the post mortem orbitofrontal cortex of patients with major depressive subjects84. Again, these evidence confirm the effect of fetal programming by high-fat diet on metabolic and behavioral traits in the offspring.
Our findings suggest that caloric diet programming reduces motivation for natural rewards, which relates with lower brain volume in the lateral hypothalamus and in the right NAc core showing defects in synaptophysin expression. This supports the role of CAF diet during gestation and lactation on setting a depression-like phenotype in young offspring.
Supplementary information
Acknowledgements
We thank Jurgen Germann for his feedback and processing support of the MRI. This research was enabled in part by support provided by CobraLab (http://cobralab.ca) and Compute Canada (www.computecanada.ca). Also, we thank to M.C. Juan José Ortíz Retana and the Laboratorio Nacional de Imagenología por Resonancia Magnética, UNAM. México. This work was supported by the National Council of Science and Technology in Mexico (CONACYT) (Grant number: 255317) and 781759 CONACYT for L.A. Trujillo-Villarreal.
Author contributions
L.A.T.-V., L.F.-M., M.M.C., E.E.G.-V., and A.C.-M. conceived and designed the study. L.A.T.-V., M.A.P.-C., G.A.D., M.M.C., A.C.-M., and E.E.G.-V. carried out the MRI analysis. L.A.T.-V., V.J.R.-D., I.A.M.-M., L.F.-M., and A.C.-M. carried out the immunohistochemical, histological, and western blot analysis. L.A.T.-V., V.J.R.-D., I.A.M.-M., L.F.-M., M.A.P.-C., G.A.D., M.M.C., A.C.-M., E.E.G.-V. discussed and wrote the paper. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alberto Camacho-Morales, Email: alberto.camachomr@uanl.edu.mx.
Eduardo E. Garza-Villarreal, Email: egarza@comunidad.unam.mx
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41398-020-01157-x).
References
- 1.World Health Organization. Depression and other common mental disorders: global health estimates. [Internet]. 1–24. Available from: http://apps.who.int/iris/bitstream/10665/254610/1/WHO-MSD-MER-2017.2-eng.pdf (2017).
- 2.World Health Organization. “Depression: let’s talk” says WHO, as depression tops list of causes of ill health [Internet]. Available from: http://www.who.int/en/news-room/detail/30-03-2017--depression-let-s-talk-says-who-as-depression-tops-list-of-causes-of-ill-health (2017).
- 3.Spijker J, Bijl RV, De Graaf R, Nolen WA. Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Acta Psychiatr. Scand. 2001;103:122–130. doi: 10.1034/j.1600-0447.2001.103002122.x. [DOI] [PubMed] [Google Scholar]
- 4.Mcintosh AL, Gormley S, Tozzi L, Frodl T, Harkin A. Recent advances in translational magnetic resonance imaging in animal models of stress and depression. Front. Cell Neurosci. 2017;11:1–15. doi: 10.3389/fncel.2017.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert C, et al. Anhedonia in depression and schizophrenia: a transdiagnostic challenge. CNS Neurosci. Ther. 2018;24:615–623. doi: 10.1111/cns.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, et al. Effects of electroconvulsive stimulation on long-term potentiation and synaptophysin in the hippocampus of rats with depressive behavior. J. Ect. 2012;28:111–117. doi: 10.1097/YCT.0b013e31824a47ca. [DOI] [PubMed] [Google Scholar]
- 7.Campbell S, Marriott M, Macqueen GM. Lower hippocampal volume in patients suffering From Depression: A Meta-Analysis. Am. J. Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 8.Sharma A, Wolf DH, Satterthwaite TD. Connectome-wide analysis reveals common dimensional reward deficits across mood and psychotic disorders. Am. J. Psychiatry. 2017;174:657–666. doi: 10.1176/appi.ajp.2016.16070774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merz EC, He X, Noble KG. Anxiety, depression, impulsivity, and brain structure in children and adolescents. NeuroImage Clin. 2018;20:243–251. doi: 10.1016/j.nicl.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat. Neurosci. Rev. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma S, Fulton S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int J. Obes. 2013;37:382–389. doi: 10.1038/ijo.2012.48. [DOI] [PubMed] [Google Scholar]
- 12.Miladinovic T, Nashed MG, Singh G. Overview of glutamatergic dysregulation in central pathologies. Biomolecules. 2015;5:112–141. doi: 10.3390/biom5043112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uys JD, Reissner KJ. Glutamatergic neuroplasticity in cocaine addiction. Prog. Mol. Biol. Trans. Sci. 2011;98:367–400. doi: 10.1016/B978-0-12-385506-0.00009-0. [DOI] [PubMed] [Google Scholar]
- 14.Santos MAO, Bezerra LS, Carvalho ARMR, Brainer-Lima AM. Global hippocampal atrophy in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Trends psychiatry Psychother. 2018;40:369–378. doi: 10.1590/2237-6089-2017-0130. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, et al. Gray matter abnormalities in non-comorbid medication-naive patients with major depressive disorder or social anxiety disorder. EBioMedicine. 2017;21:228–235. doi: 10.1016/j.ebiom.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suh JS, et al. Cortical thickness in major depressive disorder: a systematic review and meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2019;88:287–302. doi: 10.1016/j.pnpbp.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Schmaal L, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry. 2017;22:900–909. doi: 10.1038/mp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott L, et al. Genome-wide association studies of brain structure and function in the UK Biobank. bioRxiv. 2018;562:210–216. doi: 10.1038/s41586-018-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arcego DM, et al. Impact of high-fat diet and early stress on depressive-like behavior and hippocampal plasticity in adult male rats. Mol. Neurobiol. 2018;55:2740–2752. doi: 10.1007/s12035-017-0538-y. [DOI] [PubMed] [Google Scholar]
- 20.Qiao H, An SC, Ren W, Ma XM. Progressive alterations of hippocampal CA3-CA1 synapses in an animal model of depression. Behav. Brain Res. 2014;275:191–200. doi: 10.1016/j.bbr.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, et al. Increased hippocampal fissure width is a sensitive indicator of rat hippocampal atrophy. Brain Res. Bull. 2018;137:91–97. doi: 10.1016/j.brainresbull.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Schoenfeld TJ, McCausland HC, Morris HD, Padmanaban V, Cameron HA. Stress and loss of adult neurogenesis differentially reduce hippocampal volume. Biol. Psychiatry. 2017;82:914–923. doi: 10.1016/j.biopsych.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci. 2007;30:111–118. doi: 10.1016/j.tins.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Van Lieshout RJ, Robinson M, Boyle MH. Materal pre-pregnancy body mass index and internalizing and externalizing problems in offsprning. Can. J. Psychiatry. 2013;58:151–159. doi: 10.1177/070674371305800305. [DOI] [PubMed] [Google Scholar]
- 25.Marmorstein NR, Iacono WG. Associations between depression and obesity in parents and their late-adolescent offspring: a community-based study. Psychosom. Med. 2017;78:861–866. doi: 10.1097/PSY.0000000000000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vendruscolo LF, Gueye AB, Darnaude M, Ahmed SH, Cador M. Sugar overconsumption during adolescence selectively alters motivation and reward function in adult rats. PLoS ONE. 2010;5:1–10. doi: 10.1371/journal.pone.0009296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montalvo-Martínez L, Maldonado-Ruiz R, Cárdenas-Tueme M, Reséndez-Pérez D, Camacho A. Maternal overnutrition programs central inflammation and addiction-like behavior in offspring. Biomed. Res. Int. 2018;2018:8061389. doi: 10.1155/2018/8061389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camacho A, Montalvo-Martinez L, Cardenas-Perez RE, Fuentes-Mera L, Garza-Ocañas L. Obesogenic diet intake during pregnancy programs aberrant synaptic plasticity and addiction-like behavior to a palatable food in offspring. Behav. Brain Res. 2017;330:46–55. doi: 10.1016/j.bbr.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Cardenas-Perez RE, et al. Maternal overnutrition by hypercaloric diets programs hypothalamic mitochondrial fusion and metabolic dysfunction in rat male offspring. Nutr. Metab. 2018;15:1–16. doi: 10.1186/s12986-018-0279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peleg-Raibstein D, et al. Enhanced sensitivity to drugs of abuse and palatable foods following maternal overnutrition. Transl. Psychiatry. 2016;6:1–11. doi: 10.1038/tp.2016.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teegarden SL, Scott AN, Bale TL. Early life exposure to a high fat diet promotes long-term changes in dietary preferences and central reward signaling. Neuroscience. 2010;162:924–932. doi: 10.1016/j.neuroscience.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lépinay AL, et al. Perinatal high-fat diet increases hippocampal vulnerability to the adverse effects of subsequent high-fat feeding. Psychoneuroendocrinology. 2015;53:82–93. doi: 10.1016/j.psyneuen.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Janthakhin AY, Rincel M, Darnaud M, Ferreira G. Maternal high-fat diet leads to hippocampal and amygdala dendritic remodeling in adult male offspring. Psychoneuroendocrinology. 2017;83:49–57. doi: 10.1016/j.psyneuen.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Zemdegs J, et al. High-fat diet-induced metabolic disorders impairs 5-HT function and anxiety-like behavior in mice. Br. J. Pharmacol. 2016;173:2095–2110. doi: 10.1111/bph.13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boitard C, et al. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus. 2012;22:2095–2100. doi: 10.1002/hipo.22032. [DOI] [PubMed] [Google Scholar]
- 36.Cruz-Carrillo G, et al. Fetal programming by methyl donors modulates central inflammation and prevents food addiction-like behavior in rats. Front. Neurosci. 2020;14:1–15. doi: 10.3389/fnins.2020.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedel M, van Eede MC, Pipitone J, Mallar Chakravarty M, Lerch JP. Pydpiper: a flexible toolkit for constructing novel registration pipelines. Front. Neuroinform. 2014;8:1–21. doi: 10.3389/fninf.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Core Team. (2013) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing;. p. ISBN 3-900051-07-0. Available from: http://www.r-project.org/.
- 39.RStudio Team. (2015) RStudio: Integrated Development for R. RStudio. Inc. Boston, MA;. Available from: http://www.rstudio.com/.
- 40.Lerch J., Hammill C., van Eede M. C., Cassel D. RMINC: Statistical Tools for Medical Imaging NetCDF (MINC) Files [Internet]. 2017. Available from: http://mouse-imaging-centre.github.io/RMINC.
- 41.Wickham H. tidyverse: Easily Install and Load the “Tidyverse” [Internet]. 2017. Available from: https://cran.r-project.org/package=tidyverse.
- 42.Delint-Ramirez I, et al. Genetic obesity alters recruitment of TANK-binding kinase 1 and AKT into hypothalamic lipid rafts domains. Neurochem Int. 2015;80:23–32. doi: 10.1016/j.neuint.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Near J. Fischer-344 rat brain template and anatomical atlas [Internet]. Douglas Institute: CIC Pavilion. Available from: https://www.nearlab.xyz/fischer344atlas.
- 44.Peleg-Raibstein D, Luca E, Wolfrum C. Maternal high-fat diet in mice programs emotional behavior in adulthood. Behav. Brain Res. 2012;233:398–404. doi: 10.1016/j.bbr.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 45.Kang SS, Kurti A, Fair DA, Fryer JD. Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring. J. Neuroinflamm. 2014;11:1–12. doi: 10.1186/1742-2094-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schulz D. Acute food deprivation separates motor-activating from anxiolytic effects of caffeine in a rat open field test model. Behav. Pharmacol. 2018;29:543–546. doi: 10.1097/FBP.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 47.Belovicova K, Bogi E, Csatlosova K, Dubovicky M. Animal tests for anxiety-like and depression-like behavior in rats. Interdiscip. Toxicol. 2017;10:40–43. doi: 10.1515/intox-2017-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin M., Hou G., Zhao Y., Yuan T. Recovery of chronic stress-triggered changes of hippocampal glutamatergic transmission. 2018, 1–11 (2018). [DOI] [PMC free article] [PubMed]
- 49.Shen J, Xu L, Qu C, Sun H, Zhang J. Resveratrol prevents cognitive deficits induced by chronic unpredictable mild stress: Sirt1/miR-134 signalling pathway regulates CREB/BDNF expression in hippocampus in vivo and in vitro. Behav. Brain Res. 2018;349:1–7. doi: 10.1016/j.bbr.2018.04.050. [DOI] [PubMed] [Google Scholar]
- 50.Li Y, et al. Detection of volume alterations in hippocampal subfields of rats under chronic unpredictable mild stress using 7T MRI: a follow-up study. J. Magn. Reson. Imaging. 2017;46:1456–1463. doi: 10.1002/jmri.25667. [DOI] [PubMed] [Google Scholar]
- 51.Bambico FR, et al. Rostrocaudal subregions of the ventral tegmental area are differentially impacted by chronic stress. Psychopharmacol. (Berl.). 2019;236:1917–1929. doi: 10.1007/s00213-019-5177-8. [DOI] [PubMed] [Google Scholar]
- 52.Mateus-Pinheiro A, et al. The Sweet Drive Test: refining phenotypic characterization of anhedonic behavior in rodents. Front. Behav. Neurosci. 2014;8:1–10. doi: 10.3389/fnbeh.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiborg O. Chronic mild stress for modeling anhedonia. Cell Tissue Res. 2013;354:155–169. doi: 10.1007/s00441-013-1664-0. [DOI] [PubMed] [Google Scholar]
- 54.Gueye AB, et al. Unlimited sucrose consumption during adolescence generates a depressive-like phenotype in adulthood. Neuropsychopharmacology. 2018;43:2627–2635. doi: 10.1038/s41386-018-0025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanner AM. Structural MRI changes of the brain in depression. Clin. EEG Neurosci. 2004;35:46–52. doi: 10.1177/155005940403500111. [DOI] [PubMed] [Google Scholar]
- 56.Kalyan-masih P., et al. Western High-Fat Diet Consumption during Adolescence Increases Susceptibility to Traumatic Stress while Selectively Disrupting Hippocampal and Ventricular Volumes. eNeuro3, ENEURO.0125-16.2016 (2016). [DOI] [PMC free article] [PubMed]
- 57.Sacher J, et al. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J. Affect Disord. 2012;140:142–148. doi: 10.1016/j.jad.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 58.Ogassawara TB, et al. Food deprivation in F0 generation and hypercaloric diet in F1 generation reduce F2 generation astrogliosis in several brain areas after immune challenge. Int J. Dev. Neurosci. 2018;64:29–37. doi: 10.1016/j.ijdevneu.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 59.Molina J, et al. Reduced astrocytic expression of GFAP in the offspring of female rats that received hypercaloric Reduced astrocytic expression of GFAP in the offspring of female rats that received hypercaloric diet. Nutr. Neurosci. 2018;23:411–421. doi: 10.1080/1028415X.2018.1512783. [DOI] [PubMed] [Google Scholar]
- 60.Becskei C, et al. Inhibitory effects of lipopolysaccharide on hypothalamic nuclei implicated in the control of food intake. Brain Behav. Immun. 2008;22:56–64. doi: 10.1016/j.bbi.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 61.Id, A. J., Gao, F., Coppola G., Vogel Z. & Kozela, E. miRNA expression profiles and molecular networ ks in resting and LPS-activated BV-2 microglia-effect of cannabinoids. PLoS ONE14, 1–25 (2019).. [DOI] [PMC free article] [PubMed]
- 62.Davis S, et al. Glial fibrillary acidic protein in late life major depressive disorder: an immunocytochemical study. J. Neurol. Neurosurg. Psychiatry. 2002;73:556–560. doi: 10.1136/jnnp.73.5.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cobb JA, et al. Density of GFAP-immunoreactive astrocytes is decreased in left hippocampi in major depressive disorder. Neuroscience. 2017;316:209–220. doi: 10.1016/j.neuroscience.2015.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schroeter ML, Abdul-Khaliq H, Krebs M, Diefenbacher A, Blasig IE. Serum markers support disease-specific glial pathology in major depression. J. Affect. Disord. 2008;111:271–280. doi: 10.1016/j.jad.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 65.Maldonado-Ruiz R, et al. Priming of hypothalamic ghrelin signaling and microglia activation exacerbate feeding in rats’ offspring following maternal overnutrition. Nutrients. 2019;11:1–17. doi: 10.3390/nu11061241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol. Psychiatry. 2000;48:766–777. doi: 10.1016/S0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- 67.Frodl T, et al. Reduced expression of glucocorticoid-inducible genes GILZ and SGK-1: high IL-6 levels are associated with reduced hippocampal volumes in major depressive disorder. Transl. Psychiatry. 2012;2:1–8. doi: 10.1038/tp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sasaki A, de Vega W, Sivanathan S, St-Cyr S, McGowan P. Maternal high-fat diet alters anxiety behavior and glucocorticoid signaling in adolescent offspring. Neuroscience. 2014;272:92–101. doi: 10.1016/j.neuroscience.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 69.Walterfang M, et al. Corpus callosum size and shape in individuals with current and past depression. J. Affect. Disord. 2009;115:411–420. doi: 10.1016/j.jad.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 70.Macmaster F, Macmaster FP, Carrey N, Marie L. Corpus callosal morphology in early onset adolescent depression Corpus callosal morphology in early onset adolescent depression. J. Affect. Disord. 2012;145:256–259. doi: 10.1016/j.jad.2012.04.047. [DOI] [PubMed] [Google Scholar]
- 71.Van Velzen LS. White matter disturbances in major depressive disorder: a coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol. Psychiatry. 2019;25:1511–1525. doi: 10.1038/s41380-019-0477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chaki S. mGlu2/3 receptor antagonists as novel antidepressants. Trends Pharm. Sci. 2017;38:569–580. doi: 10.1016/j.tips.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 73.Musazzi L, Treccani G, Popoli M. The action of antidepressants on the glutamate system: regulation of glutamate release and glutamate receptors. Biol. Psychiatry. 2013;73:80–88. doi: 10.1016/j.biopsych.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 74.Erreger K, Dravid SM, Banke TG, Wyllie DJA, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J. Physiol. 2005;563:345–358. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duric V, et al. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int J. Neuropsychopharmacol. 2013;16:69–82. doi: 10.1017/S1461145712000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmidt MV, et al. Individual stress vulnerability is predicted by short-term memory and ampa receptor subunit ratio in the hippocampus. J. Neurosci. 2010;30:16949–16958. doi: 10.1523/JNEUROSCI.4668-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Camacho A, Montiel T, Massieu L. Sustained metabolic inhibition induces an increase in the content and phosphorylation of the NR2B subunit of N-methyl-d-aspartate receptors and a decrease in glutamate transport in the rat hippocampus in vivo. Neuroscience. 2007;145:873–886. doi: 10.1016/j.neuroscience.2006.12.069. [DOI] [PubMed] [Google Scholar]
- 78.Camacho A, Montiel T, Massieu L. The anion channel blocker, 4,4′-dinitrostilbene-2,2′-disulfonic acid prevents neuronal death and excitatory amino acid release during glycolysis inhibition in the hippocampus in vivo. Neuroscience. 2006;142:1005–1017. doi: 10.1016/j.neuroscience.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 79.Mathias JR, et al. Rat maternal obesity and high fat diet program offspring metabolic syndrome. Am. J. Obstet. Gynecol. 2014;211:1–13. doi: 10.1016/j.ajog.2014.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Masuyama H, Hiramatsu Y. Effects of a high-fat diet exposure in utero on the metabolic syndrome-like phenomenon in mouse offspring through epigenetic changes in adipocytokine gene expression. Endocrinology. 2012;153:2823–2830. doi: 10.1210/en.2011-2161. [DOI] [PubMed] [Google Scholar]
- 81.Srinivasan M, Katewa SD, Palaniyappan A, Pandya JD, Patel MS. Maternal high-fat diet consumption results in fetal malprogramming predisposing to the onset of metabolic syndrome-like phenotype in adulthood. Am. J. Physiol. Endocrinol. Metab. 2006;291:E792–E799. doi: 10.1152/ajpendo.00078.2006. [DOI] [PubMed] [Google Scholar]
- 82.Barker DJP. The origins of the developmental origins theory. J. Intern. Med. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 83.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol. Psychiatry. 2010;68:140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 84.McNamara RK, et al. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol. Psychiatry. 2007;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.