Highlights
-
•
Air volume in the ipsilateral maxillary sinus varies substantially during a RT treatment course.
-
•
Aeration changes cannot be predicted from evaluation of changes in the first five fractions.
-
•
No conclusive dosimetric consequences of aeration changes are present for photon or proton therapy.
Keywords: Sinonasal cancer, Maxillary sinus, Aeration maxillary sinus, IMRT, Proton therapy
Abstract
Introduction
The aim was to characterise patterns and predictability of aeration changes in the ipsilateral maxillary sinus during intensity-modulated radiotherapy (IMRT) for sinonasal cancer (SNC), and in a sample evaluate the dosimetric effects of aeration changes for both photon and proton therapy.
Materials and methods
The study included patients treated with IMRT for SNC in a single institution in 2009–2017. The volume of air in the ipsilateral maxillary sinus was recorded in 1578 daily cone beam computer tomography (CBCT) from 53 patients. Patterns of changing air volumes were categorised as ‘stable’, increasing’, ‘decreasing’, or ‘erratic’. For the prediction analysis, categorisation was performed based both on the entire treatment course and the first five fractions (F1–5). Photon and proton therapy plans were generated for four patients, the one from each category with the largest aeration variation. Synthetic CT images were generated for each CBCT and all plans were recalculated on the daily synthetic CTs.
Results
The absolute volume of air varied considerably during the treatment course, ranging from 0 to 25.9 cm3. Changes within a single participant varied in the range of 0–18.7 cm3. In the categorisation of patterns, most patients had increasing aeration of the sinus. Generally, patterns of aeration could not be predicted from F1–5. Patients categorised as increasing in F1–5 had the best prediction, with 78% predicted correctly as increasing for the entire treatment course. The numeric correlation coefficients for target coverage and air volume were low for 3/4 scenarios (photons 0.03–0.23, protons 0.26–0.48). No straightforward correlation between the dosimetric effect and the volume changes could be detected in the sample test of four patients for neither photon nor proton therapy.
Conclusion
The variation of aeration was large and unpredictable. No clear dosimetric consequences of the aeration variation were evident for neither IMRT nor proton therapy for the patients investigated.
1. Introduction
Sinonasal cancer (SNC) comprises tumours originated in the epithelium of the nasal cavity or the paranasal sinuses, and radiotherapy (RT) is a key element in the curative treatment. The sinonasal region is characterised by multiple cavities, all delimited by bone and covered by epithelial mucosa. All cavities are interconnected, and the maxillary sinus communicates with the lateral wall of the nasal cavity through the ostiomeatal complex. Numerous studies have evaluated maxillary sinus pathology in computed tomography (CT) imaging [1], [2], [3], [4]. Pathological findings encompassed mucosal thickening, partly or complete opacification, fluid-air level, polyps/cysts, and calcifications. Fluid may be accumulated in the maxillary sinus secondary to acute or chronic rhinosinutitis or inflammatory conditions [3]. Inflammation or infections are typically caused by common viral infections or obstruction of the ostiomeatal complex [5]. Other causes for changing opacification may be tumour growth [6] and surgery, introducing conditions such as oedema, inflammation and secondary infections. The majority of patients treated for SNC undergo surgery immediately prior to RT [7], [8], [9], [10], [11].
The anatomical changes in the maxillary sinus are important for the RT dose delivery during a five to six week long treatment course. Important organs at risk (OARs) are located close to sinonasal tumours, including the brain, the brainstem, the pituitary gland and the optic pathway. Radiation of OARs might cause permanent and potentially severe late toxicity. As described in a cohort of SNC patients [12], [13], late toxicity might include cognitive impairment, deteriorated visual acuity, hypopituitarism and olfactory dysfunction. The need to spare OARs might hinder the delivery of sufficient dose to the tumours, which together with the dynamic anatomy pose a major challenge in RT of SNC. The standard RT technique is intensity-modulated radiotherapy (IMRT), enabling a high degree of dose conformity to the target and at the same time sparing surrounding tissues. The plan is composed by several highly inhomogeneous fields that altogether form a very homogeneous dose-distribution shaped precisely to the target [14]. Ionising radiation can also be delivered as proton therapy (PT), which carry different physical properties [15], [16]. Protons deliver most of the dose in a specific depth, the Bragg peak, and beyond this point, practically no dose is deposited. This quality enables protons to spare OARs in a higher degree, but at the same time, makes the treatment susceptible to changes in tissue density in the beam path.
In the treatment of SNC with IMRT or PT, accurate dose-deposition is crucial, and strategies for managing different densities in the maxillary sinus through proper robust planning, use of image-guided radiotherapy, repeat CT scanning, and re-planning are important. In order to generate such strategies, a thorough characterisation of aeration changes is essential. A simulation study by Placidi et al. [17] evaluating multiple cancer sites showed that sinonasal filling caused most re-planning scenarios, and concluded that sinonasal filling changed remarkably in a rapid fashion. Only a few studies investigated aeration changes during RT [18], [19], none of them reported daily variations measured in actual patients in CBCTs generated during treatment. This study aims to quantify and characterise the aeration changes in the ipsilateral maxillary sinus during IMRT for SNC and investigate the predictability of aeration changes based on observations during the first week of treatment. In addition, we aim to investigate if quantification of the amount of air could serve as a simple monitoring system for the need of re-planning during RT of SNC with either IMRT or PT.
2. Materials and methods
2.1. Patients
All study patients were treated in a single institution. Eligible patients were identified in the DAHANCA database and in the local clinical treatment system. Inclusion criteria were carcinomas or esthesioneuroblastomas of the nasal cavity or maxillary, sphenoid, ethmoid or frontal sinus, primary or postoperative radiotherapy with a curative intent in 2009–2017, and daily cone beam CT (CBCT) during treatment. Exclusion criteria were tumours located in the nasal vestibule, and conditions hindering evaluation of air and fluid in the maxillary sinus. The study was approved by the Danish Data Protection Agency (1-16-02-676-18).
2.2. Radiotherapy
All patients were treated with IMRT. All treatment plans were generated using CT with additional magnetic resonance imaging or positron emission tomography for target definition. The target was defined by a clinical target volume (CTV). The prescribed dose was 66–68 Gray (Gy) in 33–34 fractions, 5–6 fractions weekly for primary radiotherapy, and 60–66 Gy in 30–33 fractions, 5 fractions per week for postoperative radiotherapy. All radiotherapy was planned and delivered in accordance with current DAHANCA guidelines [20]. Setup was verified with a CBCT for every fraction. Significant non-correctable incoherence triggered re-planning of the patient with the generation of a new planning CT and a new treatment plan adjusted to the new circumstances. The decision of re-planning was made at the treating physician’s discretion based on target coverage, time in the treatment course, anatomical conditions and individual patient factors. Target coverage was expressed as radiation dose delivered to 99% of the CTV (D99%).
2.3. Cone beam CT analysis
During the present study, air in the ipsilateral maxillary sinus was delineated in all CBCTs, i.e. 30–34 delineations per patient. All delineations were performed using Eclipse (v.13.7). For each patient, a plot of air-volume per fraction was generated, and the patients were categorised according to patterns of air variation during the treatment course. The distribution into groups was based on the relative deviation from the mean as well as linear regression models, yielding groups of stable, increasing, decreasing and erratic patterns of aeration change. The patients were defined as stable if air volumes deviated less than 15% from the mean with less than four outliers. As for the rest, if the regression coefficient exceeded 1% of the mean, they were categorised as increasing or decreasing depending on the sign of the slope, meaning that they had increasing or decreasing amounts of air in the ipsilateral maxillary sinus, respectively. Patients whose regression-curve slopes were less than 1% of the mean, but had deviation from the mean >15%, were categorised as erratic. The threshold value of 1% was selected, as it would yield a total increase/decrease of 30% over 30 fractions, and the value of 15% were derived from the 1% per fraction, yielding a threshold of half of the total increase/decrease from the mean. In order to evaluate the ability to predict patterns of volume after the first week of treatment, a distribution in similar groups was performed based on the slopes of fraction 1–5 (F1–5). Patients were defined as stable if the air-volume deviated less than 15% from the mean and increasing/decreasing if the regression coefficient exceeded 6% of the mean, depending on the sign of the regression coefficient. Patients with regression coefficients less than 6% of the mean, but with large deviations >15% were characterised as erratic.
2.4. Treatment planning recalculation
A sample of four patient cases was selected for the illustration of potential dosimetric consequences of aeration changes in the ipsilateral maxillary sinus. One test case was selected from each category based on the largest range of absolute volume change during the treatment course and beam passage through the ipsilateral maxillary sinus. Photon and proton plans were generated for these four patients using Eclipse (v.15.6). The photon plans were calculated using Acuros v 15.6.05 and they were generated as volumetric modulated arc therapy using two planar arcs and two non-coplanar partial arcs. The proton plans were calculated using PCS (Proton Convolution Superposition) v 15.6.05, using pencil beam scanning methods, consisted of 4–5 beams with multiple-field optimization. Photon plans were evaluated using PTV and PRV margins of 3 mm. To evaluate the robustness of the proton plans, they were re-calculated in 14 uncertainty scenarios using combinations of ± 2 mm in all cardinal directions and ±2% range uncertainty. All plans were made in accordance with the DAHANCA guidelines [20]. Synthetic CTs were generated using deformable image registration between the planning CT and the CBCTs using MIM (v. 7.0.2). Structure transfers were verified to be below 1 mm, and the quality of synthetic CTs was verified by dose comparison for photons on CBCTs. OAR delineations were evaluated and corrected in the planning CT if needed and missing delineations contoured manually. The plans were recalculated on the synthetic CTs and the resulting doses were accumulated on the planning CT.
2.5. Statistical analysis
Descriptive statistics were performed to describe the cohort. Linear regression models were used to visualise patterns of overall treatment courses and the initial five fractions. The fraction of correct prediction values were calculated, and the Chi-Square Test was used in the evaluation of predictions. Pearson correlation statistics were used for evaluating the strength of correlations between air volume and target dose. P-values <0.05 were considered statistically significant. All statistical analyses were performed in STATA (v. 15).
3. Results
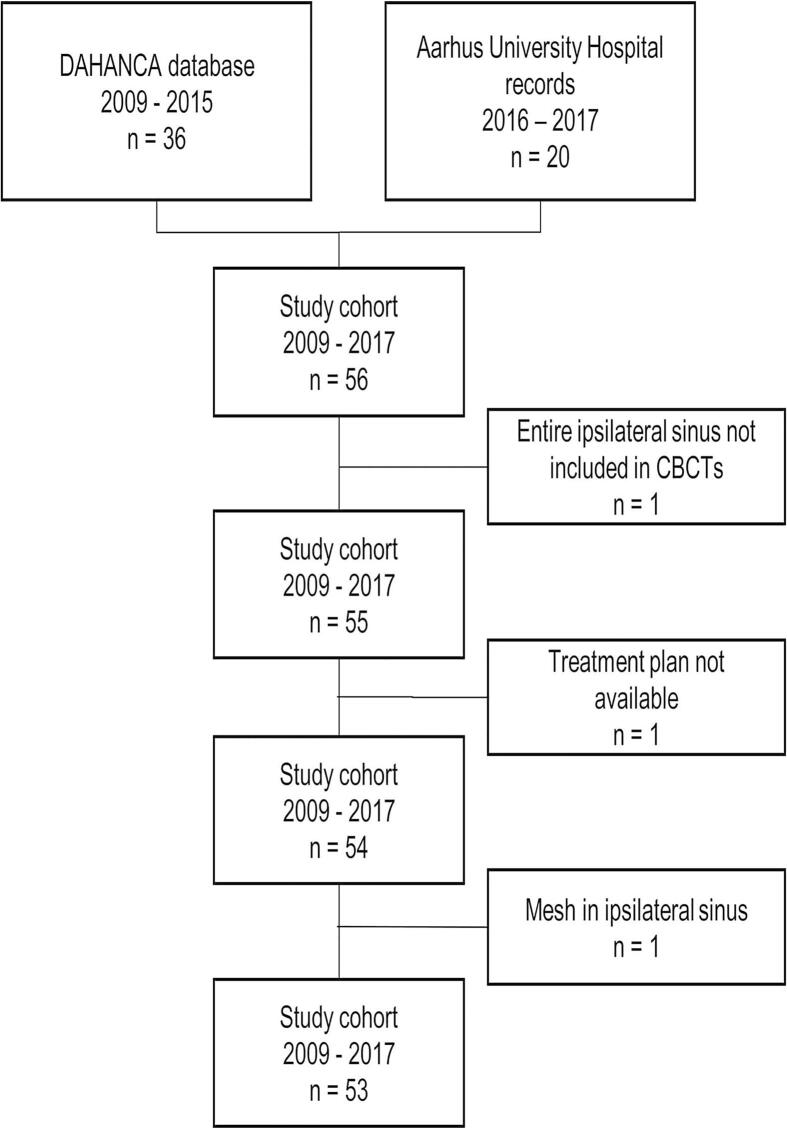
The study population comprised 53 patients with 1578 CBCTs. The identification and collection of the final cohort is described in Fig. 1. Details of disease and treatment of the patients are displayed in Table 1. Two patients did not complete the treatment course with one and nine missing fractions, respectively. They were included in the analysis, and only the completed fractions were evaluated. One patient deviated from the standardised fractionation scheme with a prescribed dose of 54 Gy in 27 fractions, 5 fractions per week. Nine patients were re-planned during treatment, one of whom had the plan revised twice during treatment. A median of eight fractions (range 3–19) were delivered until the first re-planning occurred.
Fig. 1.
Identification and inclusion of patients.
Table 1.
Details regarding disease and treatment of the study patients. NE: Not evaluated, Gy: Gray.
| Characteristic | n = 53 (100%) |
|---|---|
| Gender | |
| Male | 36 (68) |
| Female | 17 (32) |
| Tumour location | |
| Nasal cavity | 28 (53) |
| Maxillary sinus | 18 (34) |
| Sphenoid sinus | 3 (5.5) |
| Ethmoid sinus | 4 (7.5) |
| Frontal sinus | 0 (0) |
| Radiotherapy schedule | |
| Primary | 17 (32) |
| Postoperative | 31 (59) |
| NE | 5 (9) |
| No. of delivered fractions | |
| <30 | 3 (6) |
| 30–31 | 23 (43) |
| 33 | 18 (34) |
| ≥34 | 9 (17) |
| Prescribed dose | |
| 60 Gy | 24 (45) |
| 66 Gy | 20 (38) |
| 68 Gy | 8 (15) |
| Other | 1 (2) |
| Replanning during treatment | |
| Replanning once | 8 (15) |
| Replanning twice | 1 (2) |
| Total | 9 (17) |
3.1. Air volume analysis
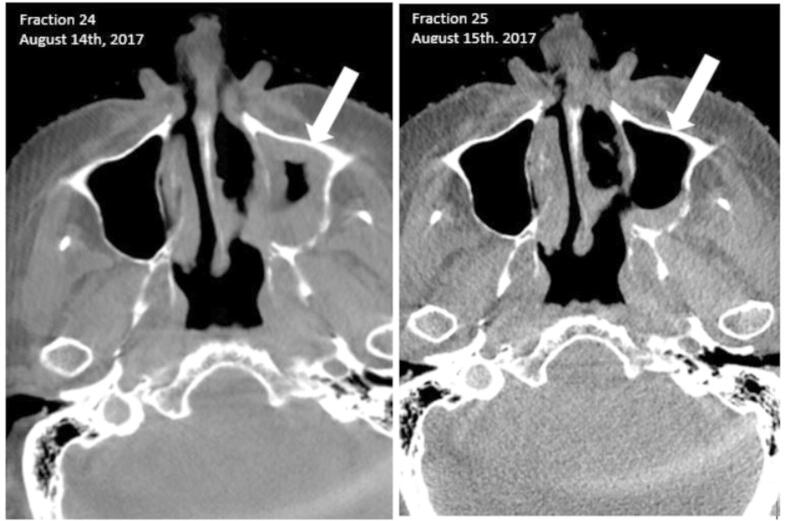
The absolute volume of air in the ipsilateral maxillary sinus during the treatment course displayed large variation, both between patients and within the same patient during the treatment course. Air-volume changes of considerable sizes could occur suddenly with no preceding events. Fig. 2 illustrates the substantial aeration changes in a participant on two consecutive treatment days. Over the entire population, the absolute volume of air ranged from zero to 25.9 cm3 and the absolute volume-range per patient during a treatment course was zero to 18.7 cm3. Seven patients (15%) had no air in the ipsilateral maxillary sinus throughout the treatment course, as tumour tissue invaded the cavity completely.
Fig. 2.
Two consecutive cone beam CT scans from the same patient illustrating the rapidly changing volumes of fluid in the sinus.
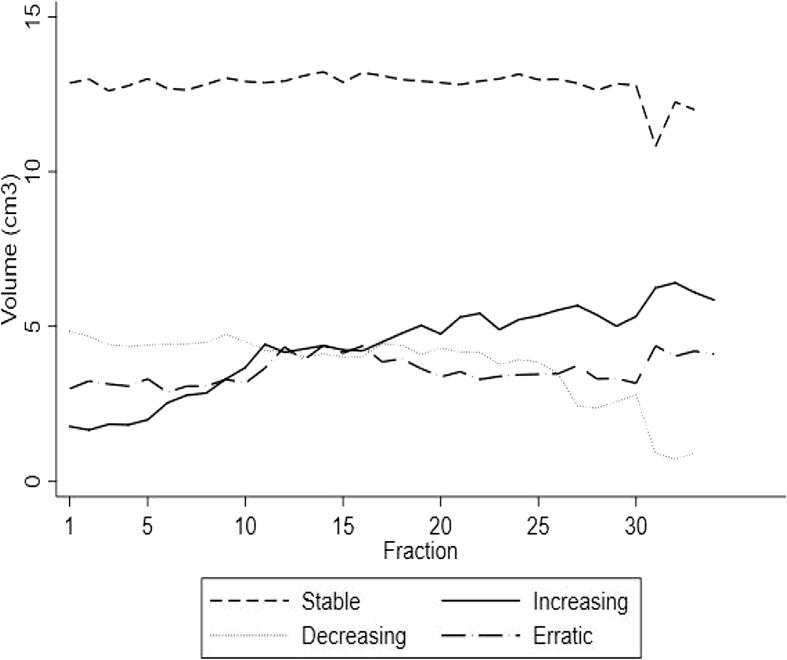
The distribution of the patients into the categories described in the Materials and methods section is shown in Table 2, displaying the distribution across the cohort, while Fig. 3 illustrates the mean of the absolute volumes of air in each category per fraction. Nearly half of the patients (47%) had increasing amounts of air in the sinus during the treatment course, and the remaining patients were equally distributed across the remaining three categories.
Table 2.
The distribution of patients’ air volume changes in different categories of patterns according to the first five fractions and the entire treatment course. Furthermore, the number of true predictions and the positive predictive value is included. The volume of air according to patterns are furthermore displayed for patients who received primary and postoperative radiotherapy along with the number of true predictions in each category (prediction) and the fraction of true predictions. F1–5: Fraction 1–5.
| Entire cohort |
Primary radiotherapy |
Postoperative radiotherapy |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All fractions n |
F1–5 n |
Prediction n |
Correct prediction | All fractions n |
F1–5 n |
Prediction n |
Correct prediction | All fractions n |
F1–5 n |
Prediction n |
Correct prediction | |
| Stable | 8 | 22 | 8 | 36% | 4 | 6 | 4 | 67% | 4 | 16 | 4 | 25% |
| Increasing | 25 | 9 | 7 | 78% | 7 | 2 | 1 | 50% | 18 | 7 | 6 | 86% |
| Decreasing | 7 | 5 | 0 | 0% | 0 | 1 | 0 | 0% | 7 | 4 | 0 | 0% |
| Erratic | 6 | 3 | 0 | 0% | 2 | 0 | 0 | 0% | 4 | 3 | 0 | 0% |
| No air | 7 | 14 | 7 | 50% | 4 | 8 | 4 | 50% | 3 | 6 | 3 | 50% |
| Total | 53 | 53 | 22 | 17 | 17 | 9 | 33 | 36 | 13 | |||
Fig. 3.
The mean volume of air in the ipsilateral maxillary sinus in 53 patients of the study, distributed in each of the four categories per fraction.
3.2. Patterns and the prediction of patterns
The distribution in categories of patterns based on all fractions and F1–5 is presented in Table 2. The ability to predict patterns of air variability, expressed as how well the distribution based on F1–5 matched the distribution based on the entire treatment course, was 22/53 patients (41.5%) over the entire cohort. The fraction of patients predicted correctly varied largely with different categories, and were highest for patients categorised as increasing (78%), meaning that patients who were categorised as having increasing aeration in F1–5 were likely to remain in the category when evaluating all fractions (Table 2). No correct predictions were made for the categories decreasing and erratic. Significantly more patients were not predicted correctly than the number that had correct predictions (p < 0.01). Fourteen patients had no air in the maxillary sinus through F1–5, seven of those remained filled throughout the treatment course, and the remaining seven were categorised as increasing in the evaluation of all fractions. When dividing the cohort into patients receiving primary or postoperative radiotherapy, the results were similar, with the pattern of a high prediction for patients categorised as increasing most pronounced for the patients receiving postoperative radiotherapy (PPV 86%).
3.3. Dosimetric impact
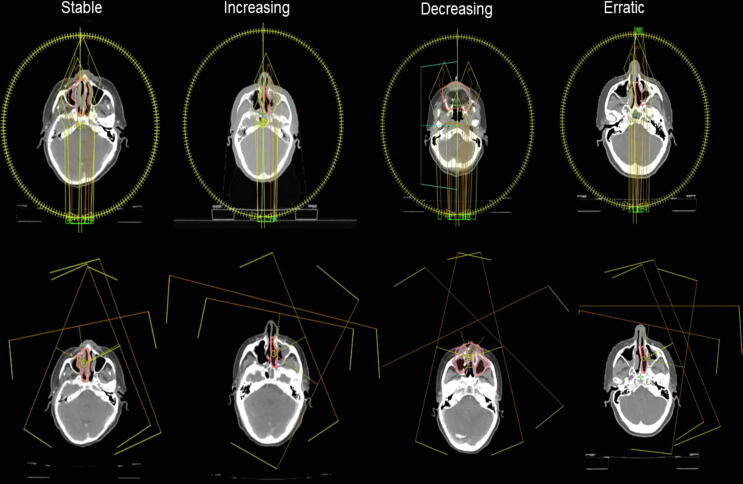
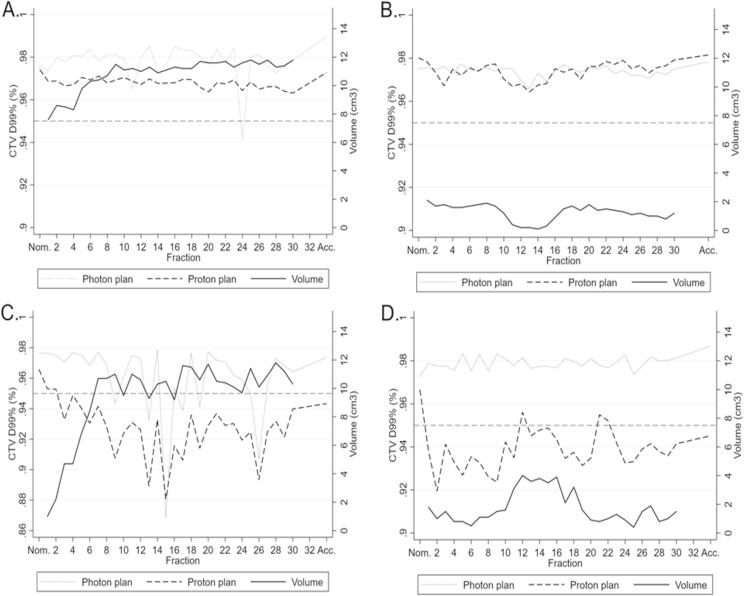
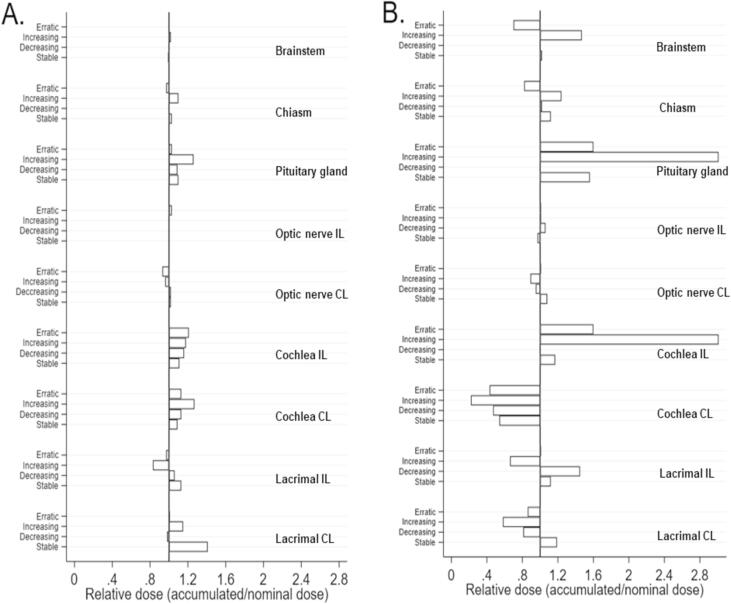
Photon and proton plans for the four cases are presented in Fig. 4. Fig. 5 shows CTV D99% and volume per fraction for the sample of four patients from each of the four categories, illustrating examples of volume changes and target coverage interplay. For the recalculated plans on the synthetic CTs, photon plans were robust; for PT, target coverage fell below 95% during treatment for 2/4 patients. Target coverage was not consistently affected by changes in air volume fluctuations in the ipsilateral maxillary sinus for neither photon nor proton therapy. Pearson correlation coefficients between CTV D99% and air volume for the four photon and four proton scenarios varied widely in the range of −0.48 to 0.69. Three of the four photon scenarios had low numeric correlation coefficients of 0.03–0.23, whereas the corresponding three proton scenarios had numeric correlation coefficients of 0.26–0.48, indicating a tendency toward a better correlation for PT. OARs may likewise be subject to altered dosage during a RT treatment course. Radiation doses to the majority of OARs were reduced with PT, as compared to photon therapy. Doses for the majority of OARs were stable when evaluating accumulated doses (Fig. 6). The doses to organs of small volumes (the pituitary gland and cochlea) varied considerably during the PT treatment courses; yet, they remained within the limits of dose constraints. Thus, when accumulated over the treatment course, serious overdosage of OARs due to displacement of the Bragg peak was not present in this sample of cases selected with the most extreme air volume variations.
Fig. 4.
The nominal dose plans for photons (top) and protons (bottom) for each of the four sample patients, illustrating the clinical target volume (pink delineation) and the radiation fields. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
Each plot illustrates a treatment course for one participant. The distribution in categories are A) stable, B) decreasing, C) increasing, D) erratic). They show the volume (solid line) as well as the CTV D99% for photons (dotted line) and protons (dash line). The dotted line at D99% = 0.95 indicates the lower threshold for target coverage according to the current guidelines. D99%: Dose delivered to 99% of the CTV. Nom: Nominal dose. Acc: Accumulated dose.
Fig. 6.
Relative doses to organs at risk illustrating the difference between nominal dose and accumulated dose to organs at risk, i.e. a measure of the degree of dose variation to each OAR during a treatment course. Each spike represent one patient. (A) Illustrates photon therapy and (B) proton therapy. IL: Ipsilateral, CL: Contralateral.
4. Discussion
The current analysis of aeration variations in the ipsilateral maxillary sinus of 53 patients and 1578 CBCTs, revealed that aeration changes were unpredictable and varied substantially with a large range of absolute volumes. Generally, it was not possible to predict patterns of air and fluid changes over the entire treatment course based on F1–5. The dosimetric evaluation of four patients with large volume changes indicated that anatomical changes seemed to have very little impact on accumulated dose to the target and OAR doses for either photon or proton therapy. With PT the target dose varied during the treatment course. Air volume was unpredictable and rapidly changing and seemed a poor measure for evaluation of target coverage. Evaluation of the treatment during the course of RT should therefore be based on image guided RT with dosimetric rather than anatomical evaluation.
Only a few studies have investigated air and fluid changes in the maxillary sinus and the subsequent potential impact in the planning of RT. Shusharina et al. (2018) [19] evaluated changes in aeration, tumour shrinkage and target coverage between the initial treatment plan and re-planning CTs in 14 patients treated with passively scattered PT. Similar to our study, they found a wide range of aeration change across the cohort (1.7–18.0%), with a significant correlation with tumour shrinkage. No significant decrease in doses to the GTV or the CTV were detected. The study was an example of real-life scenarios with one evaluation during treatment, and their conclusion, similar to the current study, emphasised the need for adaptive strategies with PT of SNC. Fukumitsu et al. (2014) [18] aimed to investigate the target coverage of 20 patients who received passive scattering proton therapy. They performed a simulation study, assuming that the primary treatment plan had been continued throughout the treatment course, and investigated the aeration of the maxillary sinus. They found considerable variations in aeration, ranging from −8% to 67% between the planning CT and the last CT, which is in good agreement with the current series. Furthermore, they found unacceptable simulated dose-levels to OARs, namely the brainstem in three patients and the chiasm in ten patients. The authors concluded that predicting alterations in aeration was difficult, but suggested that patients with large quantities of fluid at baseline should be checked more often. In the current study, large variation in aeration was detected as well, but our sample series indicated that even substantial air and fluid changes did not necessarily lead to overdosage of OARs, but could deteriorate target coverage, and the anatomical parameters were not able to predict dosimetric shortcomings.
A strength of the current study was the inclusion of measurements of air volume changes at every fraction in a relatively large cohort of patients treated in actual clinical scenarios. Other studies have used estimations based on a few observations, e.g. a single repeat CT scan. The daily air-volume variations in the current series were large and unpredictable, underlining the relevance of understanding the dosimetric consequences as well as the need for adaptive protocols with image monitoring of SNC patients during treatment. With the current study’s evaluation of patterns in F1–5, we sought to illuminate a pattern of anatomical changes, and thereby aid the anatomy-based monitoring of the patients for the remaining treatment course. Even though tendencies of patterns for aeration changes were present for the group of patients with increasing air in the maxillary sinus in F1–5, anatomy-based evaluation was shown not to be a successful strategy, and there is a need for development of other adaptive strategies. The need for adaption and re-planning is highlighted by a study by Evans et al. [21], who evaluated the influence of regular quality assurance CT on the frequency of re-planning during a proton treatment course. They found the sinonasal disease site to be a significant predictor for re-planning, and concluded that the quality assurance strategy affected the adaptive strategies. Nine patients of the current cohort had repeated planning during their radiation treatment course. Re-planning is prescribed by the treating physician, based on current target coverage, location of the tumour, anatomical factors, and the number of fractions left in the treatment course. Therefore, the fraction of patients who had re-plannings does not indicate neither the quality nor the robustness of the treatment plans. This is supported by a study by Deiter et al. (2020) [22], investigating factors affecting the frequency of re-planning, finding no correlation between re-planning and robustness.
Limitations of the study were the small number of patients with dosimetric evaluation, uncertainties regarding deformable image registration, and inclusion of only the ipsilateral maxillary sinus. The aim of the study was the investigation of aeration changes, the ability to predict aeration changes, and the ability to generate adaptive strategies based on anatomical changes. With four patients constituting worst-case scenarios, this proved not to be a feasible approach. No certain correlation between aeration and target coverage was obtained; had an association been evident with these patients, the need for validation with a larger number of patients would be necessary. One could argue, however, that other factors other than the variation in air volumes could theoretically influence target coverage, namely the anatomical distribution of fluid and air, set-up uncertainties, margins, and beam arrangement. Regarding uncertainties of image registration, they are inevitable to some degree, as well as uncertainties regarding the generation of synthetic CTs and re-calculation of the dose plans; however, meticulous quality assurance in all processes leading to the final recalculations diminished those uncertainties as much as possible. Only the ipsilateral sinus was delineated. It is a limitation, however, with the aim of investigating patterns and predictability and the generation of adaptive strategies based on anatomical conditions, evaluation of the ipsilateral sinus was sufficient. Furthermore, a relatively large fraction of the cohort underwent sinonasal surgery, meaning that ipsilateral changes of aeration patterns were potentially different compared with the contralateral maxillary sinus. As dose plans of both IMRT and PT used fields entering through both the ipsi and the contralateral sinus, the anatomical conditions were, however accounted for in the dose-calculations.
In conclusion, we found that the variation in aeration of the ipsilateral maxillary sinus was unpredictable and very variable in absolute size. The anatomical variations did not clearly affect the accumulated radiation dose to the clinical target or OARs. However, further dosimetric studies are required to investigate the proper treatment strategy in terms of adaptive strategy and prospects of novel treatment planning methods.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was funded by The Danish Cancer Society (grant R167-A10968), Aarhus University, the Danish Cancer Research Foundation and the Health Research Fund of Central Denmark Region.
References
- 1.Manji A., Faucher J., Resnik R.R., Suzuki J.B. Prevalence of maxillary sinus pathology in patients considered for sinus augmentation procedures for dental implants. Implant Dent. 2013;22:428–435. doi: 10.1097/ID.0b013e31829d1a20. [DOI] [PubMed] [Google Scholar]
- 2.Wang J.H., Lee J.H., Han J.H., Lee B.J., Jang Y.J. Contralateral maxillary sinus lesions in patients with nasal cavity and/or paranasal sinus carcinoma: analysis of computed tomography findings. Ann Otol Rhinol Laryngol. 2008;117:909–913. doi: 10.1177/000348940811701208. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y.W. A paradigm for evaluation and management of the maxillary sinus before dental implantation. Laryngoscope. 2018;128:1261–1267. doi: 10.1002/lary.26856. [DOI] [PubMed] [Google Scholar]
- 4.Rege I.C.C., Sousa T.O., Leles C.R., Mendonça E.F. Occurrence of maxillary sinus abnormalities detected by cone beam CT in asymptomatic patients. BMC Oral Health. 2012;12 doi: 10.1186/1472-6831-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ovesen T., von Buchwald C. Ear-nose-throat diseases and head and neck surgery. 2012. Rhinology; pp. 154–173. [Google Scholar]
- 6.Lawson W., Patel Z.M., Lin F.Y. The development and pathologic processes that influence maxillary sinus pneumatization. Anat Rec. 2008;291:1554–1563. doi: 10.1002/ar.20774. [DOI] [PubMed] [Google Scholar]
- 7.Birgi S.D., Teo M., Dyker K.E., Sen M., Prestwich R.J.D. Definitive and adjuvant radiotherapy for sinonasal squamous cell carcinomas: a single institutional experience. Radiat Oncol. 2015;10:190. doi: 10.1186/s13014-015-0496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robin T.P. A comprehensive comparative analysis of treatment modalities for sinonasal malignancies: NCDB analysis of sinonasal malignancies. Cancer. 2017;123:3040–3049. doi: 10.1002/cncr.30686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorup C. Carcinoma of the nasal cavity and paranasal sinuses in Denmark 1995–2004. Acta Oncol. 2010;49:389–394. doi: 10.3109/02841860903428176. [DOI] [PubMed] [Google Scholar]
- 10.Camp S. Long-term follow-up of 123 patients with adenocarcinoma of the sinonasal tract treated with endoscopic resection and postoperative radiation therapy: adenocarcinoma of the sinonasal tract. Head Neck. 2016;38:294–300. doi: 10.1002/hed.23900. [DOI] [PubMed] [Google Scholar]
- 11.Mendenhall W.M. Carcinoma of the nasal cavity and paranasal sinuses: nasal carcinoma. Laryngoscope. 2009;119:899–906. doi: 10.1002/lary.20196. [DOI] [PubMed] [Google Scholar]
- 12.Sharma M.B. A multidimensional cohort study of late toxicity after intensity modulated radiotherapy for sinonasal cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2020;151:58–65. doi: 10.1016/j.radonc.2020.07.029. [DOI] [PubMed] [Google Scholar]
- 13.Sharma M.B. Late toxicity in the brain after radiotherapy for sinonasal cancer: neurocognitive functioning, MRI of the brain and quality of life. Clin Transl Radiat Oncol. 2020;25:52–60. doi: 10.1016/j.ctro.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salimi M. Assessment and comparison of homogeneity and conformity indexes in step-and-shoot and compensator-based intensity modulated radiation therapy (IMRT) and three-dimensional conformal radiation therapy (3D CRT) in prostate cancer. J Med Signals Sens. 2017;7:102–107. [PMC free article] [PubMed] [Google Scholar]
- 15.Engelsman M., Schwarz M., Dong L. Physics controversies in proton therapy. Semin Radiat Oncol. 2013;23:88–96. doi: 10.1016/j.semradonc.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 16.van de Water S. Anatomical robust optimization to account for nasal cavity filling variation during intensity-modulated proton therapy: a comparison with conventional and adaptive planning strategies. Phys Med Biol. 2018;63 doi: 10.1088/1361-6560/aa9c1c. [DOI] [PubMed] [Google Scholar]
- 17.Placidi L. Effect of anatomic changes on pencil beam scanned proton dose distributions for cranial and extracranial tumors. Radiat Oncol Biol. 2018;97:616–623. doi: 10.1016/j.ijrobp.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Fukumitsu N. Dose distribution resulting from changes in aeration of nasal cavity or paranasal sinus cancer in the proton therapy. Radiother Oncol. 2014;113:72–76. doi: 10.1016/j.radonc.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 19.Shusharina N., Fullerton B., Adams J.A., Sharp G.C., Chan A.W. Impact of aeration change and beam arrangement on the robustness of proton plans. J Appl Clin Med Phys. 2019:14–21. doi: 10.1002/acm2.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DAHANCA . 2013. Danish head and neck cancer group 2013 radiotherapy guidelines. [DOI] [PubMed] [Google Scholar]
- 21.Evans J.D. The importance of verification CT-QA scans in patients treated with IMPT for head and neck cancers. Int J Part Ther. 2020:1–13. doi: 10.14338/ijpt-20-00006.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deiter N. Evaluation of replanning in intensity-modulated proton therapy for oropharyngeal cancer: factors influencing plan robustness. Med Dosim. 2020;45:384–392. doi: 10.1016/j.meddos.2020.06.002. [DOI] [PubMed] [Google Scholar]