Abstract
Mitochondrial damage is a critical driver in myocardial ischemia-reperfusion (I/R) injury and can be alleviated via the mitochondrial transplantation. The efficiency of mitochondrial transplantation is determined by mitochondrial vitality. Because aldehyde dehydrogenase 2 (ALDH2) has a key role in regulating mitochondrial homeostasis, we aimed to investigate its potential therapeutic effects on mitochondrial transplantation via the use of ALDH2 activator, Alda-1. Our present study demonstrated that time-dependent internalization of exogenous mitochondria by cardiomyocytes along with ATP production were significantly increased in response to mitochondrial transplantation. Furthermore, Alda-1 treatment remarkably promoted the oxygen consumption rate and baseline mechanical function of cardiomyocytes caused by mitochondrial transplantation. Mitochondrial transplantation inhibited cardiomyocyte apoptosis induced by the hypoxia-reoxygenation exposure, independent of Alda-1 treatment. However, promotion of the mechanical function of cardiomyocytes exposed to hypoxia-reoxygenation treatment was only observed after mitochondrial Alda-1 treatment and transplantation. By using a myocardial I/R mouse model, our results revealed that transplantation of Alda-1-treated mitochondria into mouse myocardial tissues limited the infarction size after I/R injury, which was at least in part due to increased mitochondrial potential-mediated fusion. In conclusion, ALDH2 activation in mitochondrial transplantation shows great potential for the treatment of myocardial I/R injury.
Keywords: Ischemia-reperfusion, Mitochondrial transfer, ALDH2 activation, Myocardial injury
Graphical abstract
Highlights
-
•
Internalization of exogenous mitochondria in cardiomyocytes occurrs in a time-dependent manner.
-
•
Mitochondrial Alda1 treatment and transplantation enhances the respiration-mediated mechanical function of cardiomyocytes.
-
•
Transplantation of Alda1 treated mitochondria ameliorates I/R injury in mice.
-
•
The cardioprotective role of ALDH2-activated mitochondrial transplantation is promoted by mitochondrial fusion.
1. Introduction
Mitochondria are essential for cell survival, especially in cardiomyocytes because of their high energy requirements. It is known that 30% of cell volume is composed of mitochondria, which produce a shocking 30 kg of ATP per day to maintain normal cardiac mechanical functions [1]. Thus, mitochondria have a pivotal role in myocardial injury and recovery in response to ischemia-reperfusion (I/R). Cardiac ischemia is caused by restricted blood flow to the heart, which can result in extensive damage to the myocardium. Timely clinical intervention and restoration of blood flow via reperfusion can improve the left ventricular contractile function of the heart after myocardial infarction. However, I/R injury remains an important complication duo to additional myocyte death occurs during reperfusion. To promote the myocardial repair and regeneration, some related studied were focused on 3D bioprinting technology [2], Long Noncoding RNA regulation [3] or the release of VEGF and BMP9 from injectable alginate based composite hydrogel [4]. These treatments could be efficient to restore the cardiac function after injury. In fact, I/R injury causes amounts of mitochondrial damage, which has deleterious effects on cellular processes and ultimately results in cardiomyocyte death [[5], [6], [7]]. Therefore, strategies to restore functional mitochondria after I/R injury may counteract these detrimental effects on cardiomyocytes and have therapeutic potential in cardiac I/R injury.
Mitochondrial transplantation to replace damaged mitochondria has been explored in animal studies. Transplantation of healthy mitochondria into the myocardium has been proven to be safe and effective for promoting the contractile function and viability of injured myocardial tissue [8,9]. Furthermore, mitochondrial transplantation has been shown to ameliorate acute limb ischemia [10] and enhance murine lung viability and recovery after I/R injury [11]. Similarly, transplantation of mitochondria from astrocytes to neurons contributed to endogenous recovery after stroke [12]. Replacement of damaged mitochondrial DNA via mitochondrial transfer has restored cellular function without eliciting an immune or auto-immune response [13]. Although the application of mitochondrial transplantation is promising, many challenges remain to improve the quality and therapeutic efficacy of the transplanted mitochondria. The release of mitochondrial components into the extracellular space could induce significant immune consequences. These components including mitochondrial proteins such as TFAM, together with other mitochondrial damage-associated molecular patterns (DAMPs) like N-formyl peptides, mtDNA, cardiolipin or extracellular ATP [[14], [15], [16]]. The prerequisite of performing successful mitochondrial transplantation therefore lies in keeping high integrity of candidate mitochondria. Furthermore, viable and respiration competent mitochondria could be taken up by cells [17,18]. Since the aim of mitochondrial transplantation was to improve myocardial energetics dependent cardiac contractility. The mitochondria with high and complete respiration activity are therefore required for transplantation.
Our previous studies have demonstrated that mitochondrial aldehyde dehydrogenase 2 (ALDH2) plays an important role in the regulation of mitochondrial metabolism and function [19,20]. ALDH2 activity is responsible for moderating the generation of aldehydes, which contribute to oxidative stress [21]. Additionally, the inhibitory effect of ALDH2 on the formation of cytotoxic aldehydes can efficiently limit damage and infarction size during cardiac ischemia [19,22]. Furthermore, the protective effects of ischemic preconditioning highly depend on the activation of ALDH2 [23,24]. Up-regulation of cellular ALDH2 activity by pre-treatment with lipoic acid decreases cellular apoptosis and reduces production of reactive oxygen species (ROS), 4-hydroxynonenal(4-HNE), and malondialdehyde (MDA), which can be reversed by inhibition of ALDH2 [25]. Down-regulation of mitochondrial ALDH2 elevates the generation of 4-HNE, resulting in apoptosis of cardiomyocytes [26]. Also, ALDH2 activity has been shown to promote the viability and function of cardiomyocytes and protect against cardiomyopathy and post-ischemic heart failure by inhibiting 4-HNE and autophagy [20,27,28].
Alda-1 is an agonist and chemical chaperone for ALDH2, which can result in as high as an 11-fold increase in its activation [19]. Therefore, we used Alda-1 treatment to increase ALDH2 activation in mitochondria prior to transplantation in hopes to enhance the overall therapeutic effect and efficacy of mitochondrial transplantation for I/R injury. Our present study revealed that cellular internalization of exogenous mitochondria occurred in a time-dependent manner. Additionally, ALDH2-activated mitochondrial transplantation enhanced the respiration-mediated mechanical function of cardiomyocytes, which inhibited apoptosis after hypoxia-reoxygenation exposure. Transplantation of Alda-1-treated mitochondria also exhibited a cardioprotective effect by limiting the infarction size and ameliorating I/R injury in mice. We further revealed that the therapeutic effect of ALDH2-activated mitochondrial transplantation was via the promotion of mitochondrial membrane potential-induced fusion, Taken together, our findings suggest that ALDH2 activation is a promising approach to enhance the therapeutic effects of mitochondrial transfer for the treatment of myocardial I/R injury and that further study is warranted.
2. Materials and methods
2.1. Animals
Male C57BL/6 mice (6–8 weeks old; weighing 20–22 g) were provided by Cavens Biogle Model Animal Research Co., Ltd. (Suzhou, China). All animal studies were performed according to the guidelines evaluated and approved by the Animal Ethics Committee of Fudan University.
2.2. Cell culture
H9c2 rat cardiomyoblast cells were purchased from the Chinese Academy of Science Cell Bank (Shanghai, China) and cultured in Dulbecco's modified Eagle medium containing 10% fetal bovine serum (Gibco, Grand Island, NY, USA). Adult primary cardiomyocytes were isolated from 8-week-old male mice using the Langendorff-free method [29]. Cardiomyocytes were subsequently resuspended in pre-warmed plating medium and plated into a laminin-precoated culture dish (5 μg/mL). After 1 h, the plating medium was replaced with pre-warmed culture medium. All cells were maintained in a humidified culture incubator (Thermo Fisher Scientific, Waltham, MA, USA) under 5% CO2 at 37 °C.
2.3. Mitochondria isolation
Mitochondria were isolated from cardiomyocytes and H9c2 cells using a cell mitochondria isolation kit (Beyotime Biotechnology, China) according to the manufacturer's protocol. The isolated mitochondria were then counted by a Beckman counter.
2.4. In vitro mitochondrial transplantation
MitoTracker red and green (Cell Signaling Technology, Danvers, MA, USA) were used to label the mitochondria of recipient and donor cells, respectively. To determine the ability and dynamic internalization of transplanted mitochondria, MitoTracker green-labeled mitochondria were isolated and then co-cultured with recipient cells. For recipient H9c2 cells, the fluorescence intensity was detected by flow cytometry (BD Biosciences, San Jose, CA, USA) at different time points (0 h, 1 h, 3 h, 6 h, 12 h). For cardiomyocytes, the fluorescence intensity was examined by the Lionheart FX living cell imaging analysis system (BioTek, Winooski, VT, USA). Localization of green fluorescence-labeled mitochondria from donor cells into recipient cells with red fluorescence-labeled mitochondria was imaged by a confocal microscope (Thermo Fisher Scientific) after co-culture for 3 h.
2.5. ATP detection
The ATP production of cardiomyocytes after different treatments was analyzed by an enhanced ATP assay kit (Beyotime Biotechnology, China) according to the manufacturer's protocol. The results were measured by a micro-plate reader (BioTek).
2.6. ALDH2 activity assay after Alda-1 treatment
To activate ALDH2, cardiomyocytes were treated with Alda-1 (20 μM, Sigma-Aldrich, St. Louis, MO, USA) for 12 h [19,30,31]. ALDH2 activity was detected by the assay kit from GENMED SCIENTIFICS. Normal and ALDH2-activated mitochondria were collected for further analyses.
2.7. Cell shortening/relengthening assay
Mechanical properties of cardiomyocytes were assessed using a SOFTEDGE. MYOCAM system (IonOptix Corporation, Milton, MA, USA). In brief, cardiomyocytes were seed on the 24 × 24 mm microscope cover glass and cultured with Costar 6-well plates. The mitochondria transferred cardiomyocytes were translocated into a Warner chamber mounted on the stage of an inverted microscope (Olympus, IX-70) and superfused with 0.5 mL no BDM culture medium. Cells were stimulated by 0.5 Hz frequency using a pair of platinum wires connected to a FHC stimulator (Brunswick, NE, USA). The shorthening and relengthening were recorded by IonOptix MyoCam camera. IONOPTIX SOFTEDGE software. The characteristics of shorthening and relengthening in cardiomyocytes were analyzed and demonstrated as the following indices: bl-resting cell length, dep v-maximal velocity of shortening (-dl/dt), dep vt-time to peak shortening (TPS-ms), bl % peak h-peaking shortening (% cell lengthening), ret v:maximal velocity of relengthening (+dl/dt), t to bl 90.0%:Time to 90% relengthening (TR90-ms).
2.8. Hypoxia-reoxygenation treatment of cardiomyocytes
To mimic myocardial I/R injury, isolated cardiomyocytes were cultured with ischemic buffer (118 mmol/L NaCl, 24 mmol/L NaHCO3, 1 mmol/L NaH2PO4, 2.5 mmol/L CaCl2–2H2O, 1.2 mmol/L MgCl2, 20 mmol/L sodium lactate, 16 mmol/L KCl, and 10 mmol/L 2-deoxyglucose, pH adjusted to 6.2) [32] and placed in a 0.5% hypoxic incubator for 45 min. Then, reoxygenation was performed by replacing the ischemic buffer with culture medium for 3 h.
2.9. Bioenergetics assay
The oxygen consumption rates (OCRs) of cardiomyocytes in different treatment groups were analyzed using an XFe96 extracellular flux analyzer (Seahorse Bioscience, Billerica, MA, USA) as described previously [33]. The metabolic OCR profiles were detected by adding oligomycin A (1 μM), 1 μM FCCP, antimycin A (1 μM), and rotenone (1 μM). The basal respiration was defined as OCRpre-Olig minus OCRpost-AntA.
2.10. Myocardial ischemia-reperfusion (I/R) injury
To establish the I/R injury mouse model, 8-week-old mice was anesthetized with 2% isoflurane gas inhalation without intubation. Myocardial ischemia was produced by temporarily exteriorizing the heart through a left thoracic incision and ligating the distal 1/3 portion of the left anterior descending artery with a 6–0 silk suture slipknot [34]. In brief, the heart was manually exposed. After ligating the left anterior descending coronary artery with a 6-0 silk suture slipknot, the heart was carefully returned to the chest cavity with the help of a mosquito clamp. After ligation for 45 min, the slipknot was released to induce reperfusion. To transplant the mitochondria, the heart was re-exposed using the same method. Quantitative mitochondria (100 μL/105) were injected into different 4 points at the myocardium of the left ventricle. For sham-operated mice, the ligature around the left anterior descending coronary artery was not tied. For placebo-treated ischemic reperfusion mice, the myocardium of left ventricle was injected by 100 μL placebo at different 4 points.
2.11. In vivo mitochondrial transplantation
To evaluate the therapeutic effect of ALDH2 on mitochondrial transplant in mice after I/R injury, mice were divided into four groups, including C (sham), I (I/R injury), M (mitochondrial transplantation), and A (ALDH2-activated mitochondrial transplantation). For mitochondrial transplantation, 100 μL (5 × 104) control or ALDH2-activated mitochondria were injected into the myocardium of the left ventricle during perfusion at 4 different points.
2.12. Echocardiography analysis
To measure the cardiac function, mice were anesthetized by isoflurane (3% for induction and 1.5% for maintenance), and M-mode images were acquired with a Vevo 2100 high-frequency ultrasound system (VisualSonics, Toronto, ON, Canada).
2.13. Evan's blue/triphenyltetrazolium chloride staining
After 24 h of reperfusion, mice were anesthetized with 1% pentobarbital (70 mg/kg). The left anterior descending coronary artery was re-tied, and 1% Evan's blue dye was injected into the right auricle of the heart. Then, the heart was quickly excised and rinsed by placebo saline solution and immediately frozen at ˗80 °C for 30 min. The frozen heart was quickly sliced into 4–5 short-axis sections and incubated in 1% triphenyltetrazolium chloride (TTC) solution at 37 °C for 30 min. Each section was flattened and fixed with 4% paraformaldehyde. The white area that was not stained by Evan's blue dye or TTC represented the myocardial infarction area. The red area that was stained by TTC instead of Evan's blue dye represented the myocardial ischemic area. The blue area that was stained by both Evan's blue dye and TTC represented the non-ischemic area. The area at risk (AAR) included both the ischemic and myocardial infarction areas, whereas the areas not at risk were indicated by phthalocyanine blue staining. Image quantification was performed by segmenting the stained areas of each section using Image J software. The infarction area was expressed as the percentage of the AAR.
2.14. Mitochondrial ROS analysis
MitoSOX Red Superoxide Indicator (Invitrogen, Carlsbad, CA, USA) was used to detect ROS levels. Cardiomyocytes were incubated with a working solution (diluting the MitoSOX Red Superoxide Indicator with cellular culture medium with 1:2000) in a humidified culture incubator under 5% CO2 at 37 °C for 10 min. Images with red fluorescence (λex = 510/λem = 580 nm) were captured by a fluorescence microscope (Olympus, Japan).
2.15. TUNEL assay
Both the cardiomyocytes and formalin-fixed heart tissue sections were stained using One Step TUNEL Apoptosis Assay Kit (Beyotime Biotechnology, China) to detect apoptotic cells. Nuclear counter-staining was performed with 4′,6-diamidino-2-phenylindole (DAPI, Beyotime Biotechnology, China), and the results were imaged by a fluorescence microscope (Olympus, Japan).
2.16. In vivo immunofluorescence assay
Transplanted mitochondria were stained using MitoTracker Deep Red FM (Cell Signaling Technology). To detect the retention of transplanted mitochondria, mouse hearts were imaged by a fluorescence detection system (IVIS Lumina XRMS, USA) after reperfusion for 24 h.
2.17. Western blot analysis
Total protein was extracted from myocardial tissue and cells and separated by 10% and 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), respectively, and then transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Burlington, CA, USA). After blocking with 5% bovine serum albumin (BSA), the membranes were incubated with primary antibodies at 4 °C overnight and then horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. The membranes were imaged using a Bio-Rad detection system (Bio-Rad Laboratories, Hercules, CA, USA). The primary antibody Optic atrophy protein 1 (OPA1) obtained from Cell Signaling, Danvers, MA. Mitofusin 1+ Mitofusin 2 (MFN1/2) and Mitochondrial transcription factor A (TFAM) antibody were obtained from Abcam (MitoSciences-Abcam, Eugene, OR).
2.18. Statistical analysis
All experiments were repeated at least three times. Statistical analysis was performed using GraphPad Prism 6.01 (GraphPad Software Inc., San Diego, CA, USA) software, and data are presented as the mean ± standard error of mean (SEM). One-way ANOVA followed by Bonferroni's post-hoc test was applied for comparisons among multiple groups. Unpaired Student's t-tests were used for comparisons between two groups. A p-value less than 0.05 was considered to be statistically significant.
3. Results
3.1. Exogenous mitochondria were internalized by cardiomyocytes in vitro
Mitochondrial transplantation is an innovative and feasible strategy to enhance cellular viability and resistance to various types of stress. To track the process of mitochondrial transfer, isolated mitochondria with intact bilayer membranes were labeled with green fluorescence and were proportionally transferred into the culture medium of the homologous H9c2 cell line. The absorption rate was dynamically calculated by flow cytometry, which suggested that cellular internalization of the exogenous mitochondria reached peak and plateau after almost 1 h (Fig. 1A and B). To further demonstrate the flexibility and application of mitochondrial transfer, primary cardiomyocytes from adult mice were obtained and co-cultured with green fluorescence-labeled mitochondria isolated from donor cardiomyocytes. Recipient cardiomyocytes were imaged following mitochondrial transplantation (Fig. 1C). Fig. 1D displays the absorption characteristics of H9c2 cells co-cultured with exogenous mitochondria at different time points. Mitochondrial transplantation was further monitored by labeling the mitochondria of both the donor and receipt cardiomyocytes with green and red fluorescence, respectively. Fig. 1E and F show the co-localization of donor and receipt mitochondria as yellow fluorescence after mitochondrial transfer for 3 h. Given that mitochondria are the main source of ATP production, we next evaluated the ATP generation of H9c2 cells after mitochondrial transfer at 0 h, 1 h, 3 h, and 6 h by measuring the ATP levels in both the H9c2 receipt cells and the cellular culture medium. A time-dependent increase in ATP production was observed after mitochondrial transplantation in the recipient H9c2 cells; in contrast, a decrease in ATP levels was observed in the cellular culture medium (Fig. 1G and H).
Fig. 1.
Exogenous mitochondria were dynamically internalized by cardiomyocytes in vitro. A) and B) Flow cytometry analysis for the internalization of MitoTracker green-labeled mitochondria in H9c2 cells. C) and D) Live cell imaging of the dynamic internalization of MitoTracker green-labeled mitochondria by cardiomyocytes from adult mice. Scale bar, 200 μm. E) Fluorescence imaging of the co-localization of transplanted mitochondria (green) and H9c2 cells (red) at 3 h. Scale bar, 25 μm. F) ATP generation in H9c2 cells after mitochondrial transplantation at 0 h, 1 h, 3 h, and 6 h. G) Fluorescence imaging of the co-localization of transplanted mitochondria (green) and cardiomyocytes from adult mice (red) at 3 h. Scale bar, 25 μm. H) ATP detection in the culture medium of H9c2 cells after mitochondrial transplantation at 0 h, 1 h, 3 h, and 6 h. *p<0.05,**p<0.01,***p<0.001.
3.2. Mitochondrial Alda-1 treatment promotes cardiomyocytes respiration
ALDH2 activity regulates mitochondrial homeostasis and function by combating oxidative stress [20,33]. Therefore, we treated mitochondria with ALDH2 activator, Alda-1, to determine the effect on mitochondrial respiration. We found that Alda-1 treatment could increase mitochondrial ALDH2 activation by 3.55fold in H9c2 cells and 3.47 in cardiomyocytes respectively (Fig. 2A and B). We hypothesized that Alda-1 treatment might regulate the respiration capacity of cardiomyocytes. To elaborate the mitochondrial respiration, the oxygen consumption rates (OCRs) was detected in cardiomyocytes with or without Alda-1 treatment. As excepted, the OCR levels were prominently stimulated by Alda-1 treatment (Fig. 2C and D). Because mitochondrial oxidative phosphorylation (OXPHOS) is primarily responsible for ATP generation, the ATP production was also analyzed in our cardiomyocytes. The results demonstrated that the ATP formation was accordingly increased in Alda-1 treated cardiomyocytes (Fig. 2E). Based on the above results, we purposed that the respiration activated mitochondria induced by Alda-1 might regulate the effect of mitochondrial transplantation. Therefore, we treated mitochondria with Alda-1 to determine the effect on mitochondrial transplantation in adult mouse primary cardiomyocytes. Live cell imaging showed that the dynamic internalization of exogenous mitochondria was similar in the presence or absence of Alda-1 treatment (Fig. 2F and G). Cardiomyocytes isolated from adult mice die naturally during in vitro culture, which could be observed by a shift from circle into rod-shape. The rod-shaped cardiomyocyte that represent the living cell was calculated during culture in vitro. The results in Fig. 2H and I demonstrated that mitochondrial transplantation prior Alda-1 treatment could effectively delay the process of cardiomyocytes death.
Fig. 2.
Alda-1 treatment promotes mitochondrial respiration by upregulating ALDH2 activation. A) and B) ALDH2 activity analysis in H9c2 cells and cardiomyocytes with or without Alda-1 treatment. C) OCR changes of control and Alda-1 treated cardiomyocytes. D) Calculation of the basal respiration in cardiomyocytes by OCRpre-Olig minus OCRpost-AntA. E) ATP generation in control and Alda-1 treated cardiomyocytes. F) Representative images of normal and mitochondria (green fluorescence) transplanted cardiomyocytes at different time points. Scale bar, 200 μm. G) Statistical analysis of green fluorescence after mitochondrial transplantation for 6 h. H) Representative images of normal and mitochondria transplanted cardiomyocytes after in vitro culture for 12 h, Scale bar, 100 μm. I) Ratio of rod-shape (survival) cardiomyocytes in control and mitochondria treated groups after in vitro culture for 12 h. *p<0.05,**p<0.01.
3.3. Mitochondrial Alda-1 treatment and transplantation enhanced the respiration-mediated mechanical function of cardiomyocytes
We suggested that the respiration-promoted mitochondria internalization affects cardiomyocytes energetic homeostasis. The ATP production of cardiomyocytes that were transplanted with control or Alda-1-treated mitochondria were therefore firstly measured. Our results indicated that Alda-1 treatment further enhanced the already elevated ATP generation of cardiomyocytes after mitochondrial transplantation for 6 h (Fig. 3A). Next, we assessed OCRs as an indicator of cellular basal respiration. Intriguingly, the results showed that cardiomyocytes had a significantly higher basal respiration after mitochondrial Alda-1 treatment and transplantation for 6 h compared to the control or mitochondrial transplantation alone (without mitochondrial Alda-1 treatment) groups (2.4-fold and 1.4-fold increase, respectively) (Fig. 3B and C). Because the mechanical regulation of cardiac cell contractility relies on a constant energy supply from mitochondria, we next examined the systolic and diastolic abilities of single cardiomyocytes with mitochondrial transplantation with or without prior Alda-1 treatment. Mitochondrial transplantation with high ALDH2 activity was able to more effectively trigger the contractile response of cardiac cells, which was shown via an increased maximal velocity of shortening/re-lengthening (±dl/dt), an elevated peak shortening (% cell lengthening), as well as a decreased time to peak shortening (TPS-ms) (Fig. 3D–I). These results suggest that ALDH2 activation efficiently augments the mitochondrial respiration-dependent mechanical contractility of cardiomyocytes after mitochondrial transplantation.
Fig. 3.
The respiration-mediated mechanical function of cardiomyocytes was enhanced by transplantation with ALDH2-activated mitochondria. A) ATP production of cardiomyocytes after transplantation with control or Alda-1-treated mitochondria for 6 h. B) OCR changes of cardiomyocytes transplanted with different mitochondria for 6 h. C) Calculation of the basal respiration in cardiomyocytes by OCRpre-Olig minus OCRpost-AntA. D) Resting cell length. E) Maximal velocity of shortening (-dl/dt). F) Time to peak shortening (TPS-ms). G) Peak shortening (% cell lengthening). H) Maximal velocity of re-lengthening (+dl/dt). I) Time to 90% re-lengthening (TR90-ms). *p<0.05,**p<0.01,***p<0.001.
3.4. Mitochondrial transplantation alleviated hypoxia-reoxygenation-induced cardiomyocyte injury
After evaluating the effect of ALDH2 activation on mitochondrial transplantation in cardiomyocytes under normal conditions, we established a cellular I/R model via the use of hypoxia-reoxygenation treatment. Cardiomyocytes isolated from adult mice were cultured under hypoxic conditions for 45 min and then reoxygenation for 3 h. TUNEL staining was performed after mitochondrial transplantation with or without prior Alda-1 treatment for ALDH2 activation. Results revealed that cardiomyocyte apoptosis induced by hypoxia-reoxygenation exposure was partly alleviated by mitochondrial transplantation and was completely ameliorated by mitochondrial Alda-1 treatment and transplantation (Fig. 4A and B). Consistent with these results, the expression of cleaved-caspase 3 was significantly upregulated after hypoxia-reoxygenation exposure, whereas its expression was downregulated after mitochondrial transplantation and even further downregulated with ALDH2 activation and mitochondrial transplantation (Fig. 4C). Because mitochondrial ROS production during the process of OXPHOS contributes to cellular damage after hypoxia-reoxygenation exposure [35], we further examined the protective effects of mitochondrial ALDH2 activation and transplantation in cardiomyocytes by measuring the ROS levels using MitoSOX. Increased mitochondrial ROS levels were observed in cardiomyocytes after hypoxia-reoxygenation treatment. However, again consistent with our TUNEL results, mitochondrial transplantation decreased cardiomyocyte ROS levels (Fig. 4D). Besides, to analyze the changes of mitochondrial levels induced by hypoxia-reoxygenation treatment, we stained the transplanted mitochondria and recipient cardiomyocytes with Mitotracker Green and Mitotracker Red respectively. After reoxygenation and mitochondrial transplantation for 3 h, the Mitotracker fluorescence was imaged by confocal. The results indicated that the cardiomyocytes were rounded from rod-shape by hypoxia-reoxygenation treatment, which was inhibited by mitochondrial transplantation. Noteworthily, the cardiomyocytes transferred with Alda-1 treated mitochondria showed clear structure of myofiber and mitochondrial arrangement (Fig. 4E). These results suggest a key role of ALDH2 activity in promoting the efficacy of mitochondrial transplantation in cardiomyocytes.
Fig. 4.
Mitochondrial ALDH2 activation and transplantation ameliorated cardiomyocyte injury induced by hypoxia-reoxygenation exposure. A) Fluorescence imaging of cardiomyocyte apoptosis levels induced by hypoxia(45min)-reoxygenation(3 h) treatment with mitochondrial ALDH2 activation and transplantation or mitochondrial transplantation alone. Cell nuclei were stained by DAPI (blue). Red represents apoptotic cardiomyocytes. Scale bar, 200 μm. B) Statistical difference of cardiomyocyte apoptosis levels. C) Western blot of cleaved-caspase 3 levels in cardiomyocytes after hypoxia-reoxygenation treatment and mitochondrial transplantation with or without prior Alda-1 treatment. D) Representative images of cellular shape (bright field) and mitochondrial ROS level changes (red fluorescence) resulting from mitochondrial transplantation with or without prior Alda-1 treatment after hypoxia-reoxygenation treatment. Scale bar, 200 μm. E) Representative images of mitochondrial levels in cardiomyocytes induced by hypoxia-reoxygenation treatment with mitochondrial ALDH2 activation and transplantation or mitochondrial transplantation alone. Cell nuclei were stained by Hoeschst 33342 (blue). Green represents the transplanted mitochondria. Red represents mitochondria in recipient cardiomyocytes. Scale bar, 20 μm. *p<0.05,**p<0.01.
3.5. Mitochondrial Alda-1 treatment and transplantation enhanced the mechanical response of cardiomyocytes after hypoxia-reoxygenation treatment
Myocardial ischemia causes damage to the electron transport chain (ETC) and subsequently disrupts OXPHOS in cardiomyocytes [36]. Therefore, corresponding OCRs were examined in cardiomyocytes after hypoxia (45min) -reoxygenation (3 h) treatment. Considerably, hypoxia-reoxygenation exposure compromised the OXPHOS capacity of cardiomyocytes, as shown by the significantly decreased basal OCR levels compared with control cardiomyocytes. Our data showed no difference between the OCR levels of hypoxia-reoxygenation exposed cardiomyocytes with or without mitochondrial transplantation. However, OCR levels were overtly enhanced in cardiomyocytes after transplantation with Alda1-treated mitochondria (Fig. 5A and B). Because energy deprivation affects the function of cardiomyocytes, we further evaluated the effect of mitochondrial Alda-1 treatment and transplantation on the contractile function of mouse cardiomyocytes ex vivo. Hypoxia-reoxygenation exposure overtly impaired the mechanical function of cardiomyocytes, which was shown via a decreased maximal velocity of shortening/re-lengthening (±dl/dt), decreased peak shortening (% cell lengthening), and increased time to peak shortening (TPS-ms). Interestingly, transplantation of Alda-1-treated mitochondria reversed the impaired contractile function in the cardiomyocytes (Fig. 5C–H). Taken together, these results further suggest that ALDH2 activation promotes the therapeutic effect of mitochondrial transplantation in cardiomyocytes after hypoxia-reoxygenation-induced injury.
Fig. 5.
ALDH2 activity and mitochondrial transplantation enhanced the mechanical response of cardiomyocytes after hypoxia-reoxygenation exposure. A) OCR changes of cardiomyocytes after hypoxia (45min)-reoxygenation (3 h) exposure and mitochondrial transplantation with or without prior Alda-1 treatment. B) Calculation of the basal respiration in cardiomyocytes. The basal respiration was defined as OCRpre-Olig minus OCRpost-AntA. C) Resting cell length. D) Maximal velocity of shortening (-dl/dt). E) Time to peak shortening (TPS-ms). F) Peak shortening (% cell lengthening). G) Maximal velocity of re-lengthening (+dl/dt). H) Time to 90% re-lengthening (TR90-ms). *p<0.05,**p<0.01,***p<0.001,****p < 0.0001.
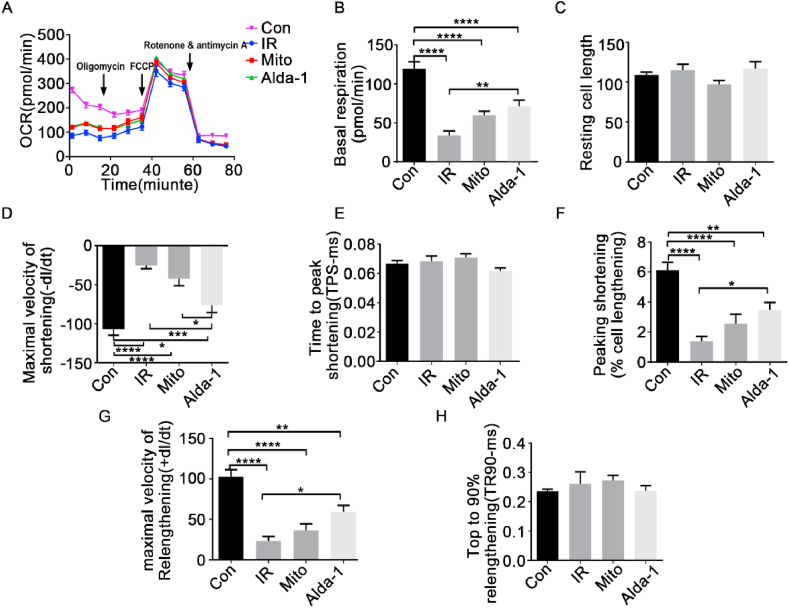
3.6. Mitochondrial Alda-1 treatment and transplantation ameliorated I/R injury in mice
To investigate the therapeutic potential of ALDH2 activation in mitochondrial transfer in vivo, we used an I/R mouse model. I/R injury was mimicked by coronary artery ligation for 45 min, followed by 24 h reperfusion with mitochondrial transplantation. Echocardiography revealed an overtly reduced left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) in mice with I/R injury. However, transplantation of mitochondria with ALDH2 activation via Alda-1 treatment exhibited a significant cardioprotective effect, with a significantly increased LVEF and LVFS (37.56% and 19.05% vs. 57.84% and 27.87%) (Fig. 6A–C). Additionally, the myocardial infarction size was assessed by Evan's blue dye and TTC staining, and a smaller infarction size was observed after transplantation with Alda-1-treated mitochondria, as shown by a reduced ratio of white-to-red area (Fig. 6D and E). Furthermore, the mitochondrial location in the I/R heart was monitored using an imaging system with fluorescence tracking. Transplanted mitochondria were detected in the myocardium by red fluorescence 24 h after transplantation (Fig. 6F). The fluorescence intensity was significantly increased in the mitochondrial Alda-1 treatment and transplantation group compared with the controls (Fig. 6G). Furthermore, cellular apoptosis and cleaved-caspase 3 expression levels were evaluated, and Alda-1 treatment prior to mitochondrial transplantation significantly ameliorated cardiomyocyte apoptosis in response to I/R injury (Fig. 6H and I). To evaluate the long-term effects of mitochondrial transplantation on cardiac functions, the cardiac function and morphology were analyzed in mice of I/R injury after mitochondria transfer with or without Alda-1 treatment for 4 weeks. Echocardiography revealed that transplantation of mitochondria with ALDH2 activation via Alda-1 treatment overtly increased LVEF and LVFS in comparison with mice of I/R injury (Sup. Fig. 1A–C). Accordingly, the fibrosis levels induced by I/R injury was significantly ameliorated by mitochondrial transplantation plus ALDH2 activation with Alda-1 (Sup. Fig. 1D).
Fig. 6.
Transplantation of ALDH2-activated mitochondria ameliorated I/R injury in mice. A)Echocardiography analyses of mice: Con (sham group, n = 5), IR (I/R injury group, n = 9), Mito (I/R injury + mitochondrial transplantation group, n = 10), and Alda-1 (I/R injury + ALDH2-activated mitochondrial transplantation group, n = 11). B) Ejection fraction and C) Fractional shortening mechanism. D) Representative images of Evan's blue dye and TTC staining, mitochondrial retention (pink fluorescence, Scale bar, 100 μm) and TUNEL assay (green fluorescence, Scale bar, 50 μm). E) Changes in infarction size induced by mitochondrial transplantation with or without ALDH2 activation. Area at risk (AAR) shows the percentage of the LV subjected to ischemia. IR, n = 10. Mito, n = 9. Alda-1, n = 7. F) Representative hearts in each group for the retention of transplanted mitochondria in the myocardium (red fluorescence). G) Average radiant efficiency of transplanted mitochondria. IR, n = 3. Mito, n = 4. Alda-1, n = 6. H) and I) Western blot analyses for expression changes of apoptosis protein cleaved-caspase 3. *p<0.05,**p<0.01.
3.7. The cardioprotective effect of Alda-1 treatment in mitochondrial transplantation was mediated by mitochondrial membrane potential-induced fusion
Mitochondria are highly dynamic organelles, and their structure and distribution play a crucial role in the regulation of cellular metabolism, especially in cardiomyocytes. Because we observed a higher myocardial retention of transplanted mitochondria with ALDH2 activation, we speculated that mitochondrial fusion may have an important role in this cardioprotective effect after I/R injury. Optic atrophy protein 1 (OPA1) and mitofusin 1 and 2 (MFN1/2) are the primary regulators of mitochondrial fusion [37]. Therefore, we analyzed active OPA1 and MFN1/2 in cardiomyocytes after mitochondrial transplantation. Upregulated expression levels of OPA1 (Fig. 7A and B) and MFN1/2 (Fig. 7C and D) were detected in the I/R myocardium after ALDH2-activated mitochondrial transplantation. Next, we analyzed the levels of mitochondrial transcription factor A (TFAM) because it is essential for the maintenance of mitochondrial DNA, and its overexpression has been shown to improve cardiac dysfunction after myocardial infarction [38]. Our data exhibited that TFAM expression was upregulated with mitochondrial transplantation after I/R injury, and its expression was further upregulated with mitochondrial ALDH2 activation and transplantation (Fig. 7E and F). It has been reported that loss of mitochondrial membrane potential induces OPA1 proteolysis and inhibits mitochondrial fusion 39-40, which may be responsible for the cardioprotective role of ALDH2 activation in mitochondrial transplantation. Thus, to examine the mitochondrial membrane potentials, JC-1 levels were measured in H9c2 cells and adult mouse primary cardiomyocytes after Alda-1 treatment as well as in the respective controls (Fig. 7G and H). Our results illustrated that high ALDH2 activity with mitochondrial transplantation significantly increased mitochondrial membrane potentials, resulting in increased mitochondrial fusion [[39], [40]].
Fig. 7.
The cardioprotective effect of ALDH2 in mitochondrial transplantation was mediated by mitochondrial membrane potential-induced fusion. A) and B) OPA1 expression regulated by mitochondrial transplantation in mouse myocardial tissue. C) and D) MFN1/2 expression regulated by mitochondrial transplantation in mouse myocardial tissue. E) and F) TFAM expression regulated by mitochondrial transplantation in mouse myocardial tissue. G) JC-1 changes resulting from Alda-1 treatment in H9c2 cells. Scale bar, 100 μm. H) JC-1 changes resulting from Alda-1 treatment in cardiomyocytes. Scale bar, 50 μm. *p<0.05,**p<0.01, ***p < 0.001.
4. General discussion and conclusion
To the best of our knowledge, this is the first study to demonstrate the therapeutic potential of ALDH2 activity to enhance the efficacy of mitochondrial transplantation for myocardial I/R injury. Mitochondrial transplantation with exogenous ALDH2-activated mitochondria to replace damaged or dysfunctional mitochondria in response to I/R injury has exhibited promising results. With this strategy, we observed an improvement in the energy-dependent cardiac and myocardial mechanical functions in mice after I/R injury. Furthermore, we determined that it promoted mitochondrial membrane potential-mediated fusion and thus inhibited cardiomyocyte apoptosis and ROS levels. Our present study demonstrates a key role of ALDH2 activity in regulating the cardioprotective effect of mitochondrial transplantation for treatment of I/R injury.
Cardiac ischemia results from a severe restriction of blood flow that causes extensive damage to the mitochondria of cardiomyocytes, and the resulting dysfunction of cardiomyocytes remains an urgent issue. During ischemia, mitochondria are the primary source of ROS formation [41,42], which leads to abnormal mitochondrial permeation, swelling, and cytochrome c release, ultimately initiating the caspase-mediated cell death program during reperfusion [[43], [44]]. Thus, mitochondrial injury is a major driver of cardiomyocyte impairment and death in response to ischemia. Our results showed significantly enhanced ROS and apoptosis levels in cardiomyocytes after I/R. Therefore, a supply of exogenous mitochondria with high vitality during reperfusion should theoretically facilitate myocardial recovery via the replacement of damaged mitochondria, resulting in increased energy production to sustain the mechanical functions of cardiomyocytes. However, the restored oxygen and selective accumulation of intermediates in mitochondria stimulate mitochondrial ROS formation during reperfusion, which inevitably has adverse effects on both the endogenous and exogenous mitochondria. Therefore, increasing the resistance of transplanted mitochondria to oxidative stress is a fundamental measure. As a mitochondrial enzyme, ALDH2 plays a pivotal role in sustaining the mitochondrial homeostasis of cardiomyocytes [19,20,45]. Our previous studies elucidated that ALDH2 deficiency [20,33] or inhibition [46] stimulated ROS over-production in mitochondria, ultimately resulting in exacerbated cardiomyocyte apoptosis in response to hypoxia. Thus, the close relationship between ALDH2 activity and mitochondrial homeostasis prompted the present study on ALDH2 activation in mitochondrial transplantation. As expected, our results demonstrated that ALDH2 activation in mitochondrial transplantation enhanced the retention of mitochondria in the myocardium and inhibited cardiomyocyte apoptosis, therefore exhibiting a cardioprotective effect in response to I/R injury.
The mitochondrial electron transport chain (ETC) is the major source of energy, and during ischemia it undergoes progressive damage [[47], [48], [49]] that leads to impaired oxidative phosphorylation (OXPHOS), which is indicated by a decreased oxygen consumption rate (OCR) and ATP production. Mitochondrial transplantation can supplement the impaired energy production resulting from I/R injury. Our results showed that mitochondrial transplantation significantly increased ATP production, but not the basal OCR levels, in cardiomyocytes in vitro. However, both the ATP and basal OCR levels were significantly increased in cardiomyocytes with mitochondrial ALDH2 activation and transplantation, suggesting that the therapeutic effect of mitochondrial transplantation highly depends on adequate mitochondrial respiratory function. During hypoxia-reoxygenation-induced cardiomyocyte injury, no significant increases were observed in either the basal OCR levels or the mechanical functions of cardiomyocytes in response to mitochondrial transplantation. However, both were remarkably enhanced by mitochondrial ALDH2 activation and transplantation. Furthermore, the substantial cardioprotective effect of mitochondrial ALDH2 activation and transplantation in mice after I/R injury also indicated that effective mitochondrial respiration is required for recovery. Mechanistically, we discovered that an elevated mitochondrial membrane potential is essential for mitochondrial dynamics as well as an increased energy supply. As expected, increased mitochondrial fusion and energy production were observed in mice with mitochondrial ALDH2 activation and transplantation after I/R injury, as indicated by the up-regulation of mitochondrial fusion proteins and a significantly increased OCR.
The results in Fig. 6F showed higher mitochondrial retention of Alda-1 treatment group in comparison with mitochondria group without Alda-1. These results indicated the Alda-1 treatment might induce higher mitochondrial internalization efficiency. However, we did not observe significantly enhanced internalization of mitochondria stimulated by Alda-1 (Fig. 2F and G). The underlying mechanism remains thus unclear. Our study suggested Alda-1 increased the survival of transplanted mitochondria, and higher retention might result from more intact and respiration competent mitochondria in the Alda-1 treated myocardium. In addition, results presented in Fig. 7 suggested that Alda-1 significantly enhanced mitochondrial membrane potential, which could effectively promote the fusion of exogenous mitochondria with the endogenous ones.
Mitochondria can traverse cell boundaries and thus be incorporated into mammalian cells by simple co-incubation of isolated mitochondria with cells, without any other type of intervention. This phenomenon was firstly discovered in 1982 [50]. Currently the physiological mechanism regarding mitochondrial internalization is still under debate. Studies from Kesner and colleagues suggested that the mitochondrial internalization was involved in macropinocytosis or macropinocytosis-like mechanisms [51]. McCully et al. illustrated that viable and respiration competent mitochondria could be taken up by both ischemia and nonischemic tissue by endocytosis [52,53]. Our study emphasized the enhanced rescue of myocardial IR injury induced by Alda-1 treated mitochondria uptake into cardiomyocytes in vitro and in vivo. However, except for the improvement of mitochondria energetic quality, the further study needs to focus on the identification of the signaling pathways and the proteins controlling of mitochondrial internalization to promote the potential therapeutic application of mitochondrial transplantation.
Our in vivo fluorescence tracking results showed that transplanted mitochondria aggregated in the myocardium and failed to be internalized by cardiomyocytes at distal injection points. Moreover, the injection procedure required exposure of the heart and further direct damage to the myocardium. To avoid these drawbacks, an intracoronary delivery may be a more efficient strategy for the distribution of transplanted mitochondria. McCully et al. [54] reported that intracoronary delivery was safe and that the efficiency depended on the mitochondrial concentration, emphasizing the significance of mitochondrial quantity. Nonetheless, our present results provide evidence that the therapeutic potential of quantitative mitochondrial transplantation is remarkably enhanced by ALDH2 activation for the treatment of I/R injury.
It is noteworthy that 40% of the East Asian population and 8% of the global population carry the mutation of ALDH2, which is caused by the replacement of glutamate with lysine at amino acid 487 and results in only 1–5% of the catalytic activity of the wild-type form of ALDH2 [[55], [56], [57]]. Our present study indicates that the efficacy of mitochondrial transplantation with a deficiency in ALDH2 may be less than satisfactory. As a specific activator of ALDH2, Alda-1 has been shown to increase the activity of a homogenous mutant ALDH2*2/*2 by 11-fold, a heterogenous mutant ALDH2*1/*2 by 2.2-fold, and wild-type ALDH2*1/*1 by 2.1 fold [19]. Therefore, the application of Alda-1 treatment to activate ALDH2 in mitochondria could effectively overcome the current limitations and optimize the clinical application of mitochondrial transplantation.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by grants from the National Science Fund for Distinguished Young Scholars (817200010), the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (81521001), the National Natural Science Foundation of China (81900353), and the China Postdoctoral Science Foundation (2019M651377).
Footnotes
Current address: Shanghai Institute of Cardiovascular Diseases, Zhongshan Hospital, Fudan University, 1609 Xietu Road, Shanghai 200032, China.
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2020.12.024.
Contributor Information
Xiaolei Sun, Email: xlfd1990@163.com.
Aijun Sun, Email: sun.aijun@zs-hospital.sh.cn.
Junbo Ge, Email: jbge@zs-hospital.sh.cn.
Author contributions
Xiaolei Sun, Rifeng Gao and Wenjia Li contributed to the experimental design, data analysis, and manuscript preparation. Yongchao Zhao, Heng Yang, Hang Chen, Hao Jiang and Zhen Dong assisted in operating the mice IR model and completing in vivo experiments. Jingjing Hu and Jin Liu assisted in the isolation of adult cardiomyocytes and in vitro experiments. Yunzeng Zou, Aijun Sun and Junbo Ge supervised these studies and contributed to the experimental design, data analysis, manuscript preparation and editing.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hall A.R., Burke N., Dongworth R.K., Hausenloy D.J. Mitochondrial fusion and fission proteins: novel therapeutic targets for combating cardiovascular disease. Br. J. Pharmacol. 2014;171:1890–1906. doi: 10.1111/bph.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu N., Ye X., Yao B., Zhao M., Wu P., Liu G., Zhuang D., Jiang H., Chen X., He Y., Huang S., Zhu P. Advances in 3D bioprinting technology for cardiac tissue engineering and regeneration. Bioact. Mater. 2021;6:1388–1401. doi: 10.1016/j.bioactmat.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ponnusamy M., Liu F., Zhang Y.H., Li R.B., Zhai M., Liu F., Zhou L.Y., Liu C.Y., Yan K.W., Dong Y.H., Wang M., Qian L.L., Shan C., Xu S., Wang Q., Zhang Y.H., Li P.F., Zhang J., Wang K. Long noncoding RNA CPR (cardiomyocyte proliferation regulator) regulates cardiomyocyte proliferation and cardiac repair. Circulation. 2019;139:2668–2684. doi: 10.1161/CIRCULATIONAHA.118.035832. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y., Chang T., Chen W., Wang X., Li J., Chen Y., Yu Y., Shen Z., Yu Q., Zhang Y. Release of VEGF and BMP9 from injectable alginate based composite hydrogel for treatment of myocardial infarction. Bioact. Mater. 2021;6:520–528. doi: 10.1016/j.bioactmat.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunecki M., Plazak W., Podolec P., Golba K.S. Effects of endogenous cardioprotective mechanisms on ischemia-reperfusion injury. Postepy Hig. Med. Dosw. 2017;71:20–31. doi: 10.5604/17322693.1228267. [DOI] [PubMed] [Google Scholar]
- 6.Chouchani E.T., Pell V.R., Gaude E., Aksentijevic D., Sundier S.Y., Robb E.L., Logan A., Nadtochiy S.M., Ord E., Smith A.C., Eyassu F., Shirley R., Hu C.H., Dare A.J., James A.M., Rogatti S., Hartley R.C., Eaton S., Costa A., Brookes P.S., Davidson S.M., Duchen M.R., Saeb-Parsy K., Shattock M.J., Robinson A.J., Work L.M., Frezza C., Krieg T., Murphy M.P. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy E., Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin B., Saeed M.Y., Esch J.J., Guariento A., Blitzer D., Moskowitzova K., Ramirez-Barbieri G., Orfany A., Thedsanamoorthy J.K., Cowan D.B., Inkster J.A., Snay E.R., Staffa S.J., Packard A.B., Zurakowski D., Del Nido P.J., McCully J.D. A novel biological strategy for myocardial protection by intracoronary delivery of mitochondria: safety and efficacy. JACC (J. Am. Coll. Cardiol.): Basic to Translational Science. 2019;4:871–888. doi: 10.1016/j.jacbts.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guariento A., Blitzer D., Doulamis I., Shin B., Moskowitzova K., Orfany A., Ramirez-Barbieri G., Staffa S.J., Zurakowski D., Del N.P., McCully J.D. Preischemic autologous mitochondrial transplantation by intracoronary injection for myocardial protection. J. Thorac. Cardiovasc. Surg. 2020;160:e15–e29. doi: 10.1016/j.jtcvs.2019.06.111. [DOI] [PubMed] [Google Scholar]
- 10.Orfany A., Arriola C.G., Doulamis I.P., Guariento A., Ramirez-Barbieri G., Moskowitzova K., Shin B., Blitzer D., Rogers C., Del Nido P.J., McCully J.D. Mitochondrial transplantation ameliorates acute limb ischemia. J. Vasc. Surg. 2020;71(3):1014–1026. doi: 10.1016/j.jvs.2019.03.079. Epub 2019 Jul 26. [DOI] [PubMed] [Google Scholar]
- 11.Moskowitzova K., Orfany A., Liu K., Ramirez-Barbieri G., Thedsanamoorthy J.K., Yao R., Guariento A., Doulamis I.P., Blitzer D., Shin B., Snay E.R., Inkster J.A.H., Iken K., Packard A.B., Cowan D.B., Visner G.A., Del Nido P.J., McCully J.D. Mitochondrial transplantation enhances murine lung viability and recovery after ischemia-reperfusion injury. Am. J. Physiol. Lung C. 2020;318:L78–L88. doi: 10.1152/ajplung.00221.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayakawa K., Esposito E., Wang X., Terasaki Y., Liu Y., Xing C., Ji X., Lo E.H. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535:551–555. doi: 10.1038/nature18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masuzawa A., Black K.M., Pacak C.A., Ericsson M., Barnett R.J., Drumm C., Seth P., Bloch D.B., Levitsky S., Cowan D.B., McCully J.D. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am. J. Physiol. Heart C. 2013;304:H966–H982. doi: 10.1152/ajpheart.00883.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q., Raoof M., Chen Y., Sumi Y., Sursal T., Junger W., Brohi K., Itagaki K., Hauser C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galluzzi L., Kepp O., Kroemer G. Mitochondria: master regulators of danger signalling. Nat. Rev. Mol. Cell Biol. 2012;13:780–788. doi: 10.1038/nrm3479. [DOI] [PubMed] [Google Scholar]
- 16.West A.P., Khoury-Hanold W., Staron M., Tal M.C., Pineda C.M., Lang S.M., Bestwick M., Duguay B.A., Raimundo N., MacDuff D.A., Kaech S.M., Smiley J.R., Means R.E., Iwasaki A., Shadel G.S. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–557. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowan D.B., Yao R., Akurathi V., Snay E.R., Thedsanamoorthy J.K., Zurakowski D., Ericsson M., Friehs I., Wu Y., Levitsky S., Del N.P., Packard A.B., McCully J.D. Intracoronary delivery of mitochondria to the ischemic heart for cardioprotection. PloS One. 2016;11 doi: 10.1371/journal.pone.0160889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cowan D.B., Yao R., Thedsanamoorthy J.K., Zurakowski D., Del N.P., McCully J.D. Transit and integration of extracellular mitochondria in human heart cells. Sci. Rep. 2017;7:17450. doi: 10.1038/s41598-017-17813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C., Budas G.R., Churchill E.N., Disatnik M., Hurley T.D., Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun A., Zou Y., Wang P., Xu D., Gong H., Wang S., Qin Y., Zhang P., Chen Y., Harada M., Isse T., Kawamoto T., Fan H., Yang P., Akazawa H., Nagai T., Takano H., Ping P., Komuro I., Ge J. Mitochondrial aldehyde dehydrogenase 2 plays protective roles in heart failure after myocardial infarction via suppression of the cytosolic JNK/p53 pathway in mice. J. Am. Heart Assoc. 2014;3 doi: 10.1161/JAHA.113.000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen C.H., Sun L., Mochly-Rosen D. Mitochondrial aldehyde dehydrogenase and cardiac diseases. Cardiovasc. Res. 2010;88:51–57. doi: 10.1093/cvr/cvq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma H., Guo R., Yu L., Zhang Y., Ren J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur. Heart J. 2011;32:1025–1038. doi: 10.1093/eurheartj/ehq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yellon D.M., Downey J.M. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol. Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 24.Dawn B., Bolli R. Role of nitric oxide in myocardial preconditioning. Ann. N. Y. Acad. Sci. 2002;962:18–41. doi: 10.1111/j.1749-6632.2002.tb04053.x. [DOI] [PubMed] [Google Scholar]
- 25.He L., Liu B., Dai Z., Zhang H.F., Zhang Y.S., Luo X.J., Ma Q.L., Peng J. Alpha lipoic acid protects heart against myocardial ischemia-reperfusion injury through a mechanism involving aldehyde dehydrogenase 2 activation. Eur. J. Pharmacol. 2012;678:32–38. doi: 10.1016/j.ejphar.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 26.Churchill E.N., Disatnik M.H., Mochly-Rosen D. Time-dependent and ethanol-induced cardiac protection from ischemia mediated by mitochondrial translocation of varepsilonPKC and activation of aldehyde dehydrogenase 2. J. Mol. Cell. Cardiol. 2009;46:278–284. doi: 10.1016/j.yjmcc.2008.09.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun A., Cheng Y., Zhang Y., Zhang Q., Wang S., Tian S., Zou Y., Hu K., Ren J., Ge J. Aldehyde dehydrogenase 2 ameliorates doxorubicin-induced myocardial dysfunction through detoxification of 4-HNE and suppression of autophagy. J. Mol. Cell. Cardiol. 2014;71:92–104. doi: 10.1016/j.yjmcc.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y., Ren J. Autophagy in ALDH2-elicited cardioprotection against ischemic heart disease: slayer or savior? Autophagy. 2010;6:1212–1213. doi: 10.4161/auto.6.8.13652. [DOI] [PubMed] [Google Scholar]
- 29.Ackers-Johnson M., Li P.Y., Holmes A.P., O Brien S., Pavlovic D., Foo R.S. A simplified, langendorff-free method for concomitant isolation of viable cardiac myocytes and nonmyocytes from the adult mouse heart. Circ. Res. 2016;119:909–920. doi: 10.1161/CIRCRESAHA.116.309202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez-Miller S., Younus H., Vanam R., Chen C., Mochly-Rosen D., Hurley T.D. Alda-1 is an agonist and chemical chaperone for the common human aldehyde dehydrogenase 2 variant. Nat. Struct. Mol. Biol. 2010;17:159–164. doi: 10.1038/nsmb.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woods C.E., Shang C., Taghavi F., Downey P., Zalewski A., Rubio G.R., Liu J., Homburger J.R., Grunwald Z., Qi W., Bollensdorff C., Thanaporn P., Ali A., Riemer R.K., Kohl P., Mochly-Rosen D., Gerstenfeld E., Large S., Ali Z.A., Ashley E.A. In vivo post–cardiac arrest myocardial dysfunction is supported by Ca2+/Calmodulin-Dependent protein kinase II–mediated calcium long-term potentiation and mitigated by Alda-1, an agonist of aldehyde dehydrogenase type 2. Circulation. 2016;134:961–977. doi: 10.1161/CIRCULATIONAHA.116.021618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie L., Pi X., Wang Z., He J., Willis M.S., Patterson C. Depletion of PHD3 protects heart from ischemia/reperfusion injury by inhibiting cardiomyocyte apoptosis. J. Mol. Cell. Cardiol. 2015;80:156–165. doi: 10.1016/j.yjmcc.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun X., Zhu H., Dong Z., Liu X., Ma X., Han S., Lu F., Wang P., Qian S., Wang C., Shen C., Zhao X., Zou Y., Ge J., Sun A. Mitochondrial aldehyde dehydrogenase-2 deficiency compromises therapeutic effect of ALDH bright cell on peripheral ischemia. Redox Biol. 2017;13:196–206. doi: 10.1016/j.redox.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H., Tao L., Jiao X., Gao E., Lopez B.L., Christopher T.A., Koch W., Ma X.L. Nitrative thioredoxin inactivation as a cause of enhanced myocardial ischemia/reperfusion injury in the aging heart. Free Radic. Biol. Med. 2007;43:39–47. doi: 10.1016/j.freeradbiomed.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shadel G.S., Horvath T.L. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163:560–569. doi: 10.1016/j.cell.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Q., Camara A.K., Stowe D.F., Hoppel C.L., Lesnefsky E.J. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am. J. Physiol. Cell Physiol. 2007;292:C137–C147. doi: 10.1152/ajpcell.00270.2006. [DOI] [PubMed] [Google Scholar]
- 37.Vasquez-Trincado C., Garcia-Carvajal I., Pennanen C., Parra V., Hill J.A., Rothermel B.A., Lavandero S. Mitochondrial dynamics, mitophagy and cardiovascular disease. J. Physiol. 2016;594:509–525. doi: 10.1113/JP271301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang D., Kim S.H., Hamasaki N. Mitochondrial transcription factor A (TFAM): roles in maintenance of mtDNA and cellular functions. Mitochondrion. 2007;7:39–44. doi: 10.1016/j.mito.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 39.Ishihara N., Fujita Y., Oka T., Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 2006;25:2966–2977. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griparic L., Kanazawa T., van der Bliek A.M. Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J. Cell Biol. 2007;178:757–764. doi: 10.1083/jcb.200704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becker L.B., Vanden H.T., Shao Z.H., Li C.Q., Schumacker P.T. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am. J. Physiol. 1999;277:H2240–H2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- 42.Tsutsui H., Kinugawa S., Matsushima S. Mitochondrial oxidative stress and dysfunction in myocardial remodelling. Cardiovasc. Res. 2008;81:449–456. doi: 10.1093/cvr/cvn280. [DOI] [PubMed] [Google Scholar]
- 43.Kuznetsov Javadov, Margreiter Grimm, Hagenbuchner Ausserlechner. The role of mitochondria in the mechanisms of cardiac ischemia-reperfusion injury. Antioxidants. 2019;8:454. doi: 10.3390/antiox8100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borutaite V., Brown G.C. Mitochondria in apoptosis of ischemic heart. FEBS Lett. 2003;541:1–5. doi: 10.1016/s0014-5793(03)00278-3. [DOI] [PubMed] [Google Scholar]
- 45.Ma H., Guo R., Yu L., Zhang Y., Ren J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur. Heart J. 2011;32:1025–1038. doi: 10.1093/eurheartj/ehq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang P., Xu D., Wang S., Fu H., Wang K., Zou Y., Sun A., Ge J. Inhibition of aldehyde dehydrogenase 2 activity enhances antimycin-induced rat cardiomyocytes apoptosis through activation of MAPK signaling pathway. Biomed. Pharmacother. 2011;65:590–593. doi: 10.1016/j.biopha.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Lesnefsky E.J., Slabe T.J., Stoll M.S., Minkler P.E., Hoppel C.L. Myocardial ischemia selectively depletes cardiolipin in rabbit heart subsarcolemmal mitochondria. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2770–H2778. doi: 10.1152/ajpheart.2001.280.6.H2770. [DOI] [PubMed] [Google Scholar]
- 48.Lesnefsky E.J., Tandler B., Ye J., Slabe T.J., Turkaly J., Hoppel C.L. Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. Am. J. Physiol. 1997;273:H1544–H1554. doi: 10.1152/ajpheart.1997.273.3.H1544. [DOI] [PubMed] [Google Scholar]
- 49.Lesnefsky E.J., Gudz T.I., Migita C.T., Ikeda-Saito M., Hassan M.O., Turkaly P.J., Hoppel C.L. Ischemic injury to mitochondrial electron transport in the aging heart: damage to the iron–sulfur protein subunit of electron transport complex III. Arch. Biochem. Biophys. 2001;385:117–128. doi: 10.1006/abbi.2000.2066. [DOI] [PubMed] [Google Scholar]
- 50.Clark M.A., Shay J.W. Mitochondrial transformation of mammalian cells. Nature. 1982;295:605–607. doi: 10.1038/295605a0. [DOI] [PubMed] [Google Scholar]
- 51.Kesner E.E., Saada-Reich A., Lorberboum-Galski H. Characteristics of mitochondrial transformation into human cells. Sci. Rep. 2016;6:26057. doi: 10.1038/srep26057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cowan D.B., Yao R., Akurathi V., Snay E.R., Thedsanamoorthy J.K., Zurakowski D., Ericsson M., Friehs I., Wu Y., Levitsky S., Del N.P., Packard A.B., McCully J.D. Intracoronary delivery of mitochondria to the ischemic heart for cardioprotection. PloS One. 2016;11 doi: 10.1371/journal.pone.0160889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cowan D.B., Yao R., Thedsanamoorthy J.K., Zurakowski D., Del N.P., McCully J.D. Transit and integration of extracellular mitochondria in human heart cells. Sci. Rep. 2017;7:17450. doi: 10.1038/s41598-017-17813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCully J.D., Cowan D.B., Pacak C.A., Toumpoulis I.K., Dayalan H., Levitsky S. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am. J. Physiol. Heart C. 2009;296:H94–H105. doi: 10.1152/ajpheart.00567.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eng M.Y., Luczak S.E., Wall T.L. ALDH2, ADH1B, and ADH1C genotypes in Asians: a literature review. Alcohol Res. Health. 2007;30:22–27. [PMC free article] [PubMed] [Google Scholar]
- 56.Yukawa Y., Muto M., Hori K., Nagayoshi H., Yokoyama A., Chiba T., Matsuda T. Combination of ADH1B*2/ALDH2*2 polymorphisms alters acetaldehyde-derived DNA damage in the blood of Japanese alcoholics. Canc. Sci. 2012;103:1651–1655. doi: 10.1111/j.1349-7006.2012.02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li H., Borinskaya S., Yoshimura K., Kal'Ina N., Marusin A., Stepanov V.A., Qin Z., Khaliq S., Lee M.Y., Yang Y., Mohyuddin A., Gurwitz D., Mehdi S.Q., Rogaev E., Jin L., Yankovsky N.K., Kidd J.R., Kidd K.K. Refined geographic distribution of the oriental ALDH2*504Lys (nee 487Lys) variant. Ann. Hum. Genet. 2009;73:335–345. doi: 10.1111/j.1469-1809.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.