Abstract
Purpose:
To compare the image quality of three-dimensional magnetic resonance cholangiopancreatography (MRCP) acquired with respiratory triggering against breath-hold 3D MRCP with compressed sensing (CS) and parallel imaging (PI) in a clinical setting.
Methods:
This study included 93 patients (45 men, mean age: 69.7 ± 9.3 years), in whom three types of 3D MRCP were performed: 3D breath-hold MRCP with CS and PI reconstruction (BH-CS-MRCP) and PI only reconstruction (BH-PI-MRCP) additionally to 3D respiratory triggered MRCP with navigator echoes (Nav-MRCP). Duct visualization and overall image quality were blindly evaluated on a four-point scale by two independent radiologists. Quantitative analysis was performed by calculating the relative duct-to-periductal contrast (RC) of three main biliary segments. Comparison between the methods was performed using paired t-test.
Results:
Acquisition time was 23 s for both breath-hold MRCP protocols and 1 min 29 s for Nav-MRCP. Mean grading (Nav/CS/PI) for common bile duct (2.74/2.87/2.94), common hepatic duct (2.82/2.92/3.00), central right hepatic duct (2.75/2.85/2.98), central left hepatic duct (2.75/2.85/2.92) and cystic duct (2.22/2.34/2.42) was higher in BH-CS- and BH-PI-MRCP, whereas Nav-MRCP showed higher grading in the peripheral segments (peripheral right hepatic duct: 2.24/2.01/2.12; peripheral left hepatic duct: 2.23/2.02/2.13). Overall image quality of Nav-MRCP (2.91 ± 0.7) was not different from BH-PI-MRCP (2.92 ± 0.6) (P = 0.163), but higher than BH-CS-MRCP (2.80 ± 0.7) (P = 0.031). Quantitative analysis showed lower RC values for CS- and PI-MRCP than Nav-MRCP (P < 0.001).
Conclusion:
Breath-hold 3D MRCP were feasible using PI and CS. Visualization of the greater ductal system was even superior in breath-hold MRCP than in Nav-MRCP by considerably reducing acquisition time. Both breath-hold methods are suitable for revised MRI protocols notably in patients with irregular respiratory cycle.
Keywords: breath holding, magnetic resonance cholangiopancreatography, magnetic resonance imaging
Introduction
For the evaluation of the biliary and pancreatic ductal system magnetic resonance cholangiopancreatography (MRCP) constitutes a valuable non-invasive technique. Stationary or slowly moving fluids, such as bile or pancreatic juice, are depicted hyperintense in heavily T2-weighted images and can thereby be easily distinguished from abdominal tissue. The possibility of 3D imaging upgrades the portrayal of duct anatomy while additionally providing a great volume of coverage and a high signal-to-noise ratio (SNR).1,2 Previous studies have shown that 3D MRCP delivers a higher image quality than other imaging methods, such as endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiography (PTC),3 as well as better visualization of the ductal system than the two dimensional technique.4 However, as standard 3D MRCP relies on a respiratory triggered T2-weighted sequence, it is also characterized by a higher frequency of ghosting and blurring artifacts due to irregular respiratory cycle or body motion of the patients caused by a longer acquisition time. Those lead to image degradation and impedes diagnostic accuracy.5,6 Artifact reduction during image acquisition, therefore, is required in clinical setting.
Data acquisition can be considerably accelerated by parallel imaging (PI) or compressed sensing (CS), which enable image reconstruction with undersampled data sets or reduced/compressed k-space.7,8 CS acquires less data through exploiting image sparsity, incoherent sampling and a reconstruction algorithm that enforces sparsity constrained by data consistency.9,10 Autocalibrating reconstruction for Cartesian imaging is a PI technique based on multi-channel coil sensitivities, which performs correction in k-space before Fourier transformation.
A limited number of previous studies have investigated the application of either PI or CS alone or their combination in MRCP in a clinical setting.5,10–13 Therefore, the aim of this study was to compare the image quality of 3D MRCP acquired with respiratory triggering with navigator echoes against breath-hold 3D MRCP with CS and PI in a patient population.
Materials and Methods
This retrospective, cross-sectional study was approved by the Institutional Review Board of our institution and informed consent was obtained from the patients. The study population included a total of 93 consecutive patients (45 men, 48 women; mean age ± SD: 69.7 ± 9.3 years; range: 39–90 years) receiving three types of MRCP as a clinical workup for known or suspected pancreaticobiliary diseases between February and April 2018: (1) respiratory triggered 3D MRCP with navigator echoes (Nav-MRCP), (2) breath-hold MRCP with PI reconstruction alone (BH-PI-MRCP) and (3) breath-hold MRCP with both PI and CS reconstruction (BH-CS-MRCP). Patients’ characteristics and diagnoses are figured in Table 1.
Table 1.
Demographics of study population
| n | |
|---|---|
| Study population | 93 |
| Age in years (range) | 69.7 ± 9.3 (39–90) |
| Sex | |
| Male | 45 |
| Female | 48 |
| Diagnosis | |
| BD-IPMN | 46 |
| IMPN/IPMC | 2 |
| Pancreatic cyst, SCN | 2 |
| Dilatation of pancreatic duct/bile duct | 4 |
| Autoimmune pancreatitis | 6 |
| Adenomyomatosis | 5 |
| Acute cholecystitis/cholangitis/PBC | 4 |
| Stones | 7 |
| Pancreatic carcinoma/metastasis | 5 |
| Hepatobiliary malignoma | 6 |
| Others | 6 |
| Duct discontinuity (caused by pathology) | 10 |
| Pancreaticobiliary disorders | |
| Cholecystectomy | 13 |
| Gall stones | 15 |
| Bile duct stricture | 5 |
| Cholangiojejunostomy/Child operation | 3 |
BD-IPMN, branch duct-intraductal papillary mucinous neoplasm; IPMC, intraductal papillary mucinous carcinoma; SCN, serous cystadenoma; PBC, primary biliary cholangitis; stones includes common bile duct (CBD) stones, gall stones, intrahepatic stones, pancreaticolithiasis, hepatobiliary malignoma includes gallbladder carcinoma, extrahepatic bile duct cancer, intrahepatic cholangiocarcinoma (CCC), hepatocellular carcinoma (HCC), others includes accessory spleen in the pancreas, chronic pancreatitis, hepatobiliary enzyme abnormalities, Serum IgG4 elevation, hypoechogenic area in the pancreas, non-alcoholic steatohepatitis (NASH).
Magnetic resonance imaging acquisition
All MR examinations were performed at a 3T system (Discovery 750, GE Healthcare, Waukesha, WI, USA) using a 32-channel torso phased-array coil.
Imaging parameters for 3D respiratory triggered MRCP were as follows: TR = variable, depending on respiratory rate, TE = 700 ms, matrix: 320 × 320, flip angle: 90°, echo train length: 140, number of excitations: 0.5, FOV: 320 mm, slice thickness = 1.4 mm, number of slices: 68. PI and CS factors were 2.0 × 1 and 1.5, respectively. The complete sequence was acquired in ~1.5 min under 15 respirations per minute.
Breath-hold 3D MRCP sequences with CS and PI were performed with the following imaging parameters: TR = ~2000, TE = 1186, matrix: 256 × 256, flip angle: 90°, echo train length: 160, number of excitations: 0.5, FOV: 300 mm, slice thickness = 1.6 mm, number of slices: 54, acceleration factor: 2. PI factors were 2.8 × 2.0 for BH-PI-MRCP and 2.0 × 1.0 for BH-CS-MRCP. The complete sequence was acquired in 23 s for both BH-CS- and BH-PI-MRCP. Breath-hold was achieved in all 93 patients for the time of image acquisition.
Image analysis
Qualitative image analysis
Reconstructed coronal maximal intensity projection (MIP) images for the three methods (Nav-MRCP, BH-CS-MRCP and BH-PI-MRCP) were anonymized concerning acquisition technique and randomly presented to two independent radiologists with 11 and 3 years of clinical experience in abdominal MR imaging. 3D reconstructed images and arbitrary projections were available on request. Observers received clinical information concerning pancreaticobiliary diseases for each patient to estimate, whether an occurring duct discontinuity was caused by pathological condition or limitation of the imaging method. Quality evaluation was performed by applying a four-point scoring system regarding duct visualization for common bile duct (CBD), common hepatic duct (CHD), central right and left hepatic duct (cRHD, cLHD), peripheral right and left hepatic duct (pRHD, pLHD), cystic duct and pancreatic duct (1 = poor visualization, limited diagnostic value; 2 = partial (<one and a half) or blurry visualization, decreased diagnostic quality; 3 = clear but partial (>one and a half) or not clear (mild blur) visualization; 4 = complete and clear visualization).10 Additionally, overall image quality [1 = poor (below average); 2 = fair (average); 3 = good; 4 = excellent] and occurrence of image artifacts (0 = severe, non-diagnostic; 1 = major artifacts, still diagnostic quality, 2 = minor or no artifacts, no effect on diagnostic quality) were assessed.
Quantitative image analysis
For quantitative analysis the relative duct-to-periductal contrast ratios (RC) of the CBD, cRHD and cLHD were performed using Picture Archiving Communication System (Synapse, Fujifilm Pharma, Tokyo, Japan). RC instead of common SNR calculation relying solely on background ROI was carried out, as it is difficult to define the noise in the images acquired with PI and CS. One radiologist with 3 years of experience in abdominal MRI placed a ROI within the largest area of each duct (mean area/range: CBD 25.4 mm2/3.1–92.9 mm2; cRHD 9.8 mm2/2.4–28.9 mm2; cLHD 10.4 mm2/2.0–33.9 mm2) as well as a circular 100 mm2 ROI in the adjacent periductal tissue. The derived signal intensities used for calculation of RC values as follows:
with SIDuct equaling the SI of the biliary ducts and SIPeriductal the SI of periductal tissue.14
Statistics
All descriptive data were described as means and standard deviation (categorical and continuous variables). Differences between Nav-MRCP and breath-hold MRCP were analyzed using paired t-test after graphical evaluation of data distribution. A P-value of <0.05 was considered statistically significant. All analyses were performed using Stata 14.1 (Stata Corporation, College Station, TX, USA).
Interobserver reliability between the two readers was calculated by using kappa statistics, with a value below 0.20 defining disagreement, 0.20–0.40 poor agreement, 0.41–0.60 moderate agreement, 0.61–0.80 good agreement and over 0.80 excellent agreement.
Results
Qualitative analysis/comparison of duct visualization
The interobserver agreement for the three imaging methods with regard to duct visualization, image quality and assessment of image artifacts was good to excellent in most cases and is depicted in Table 2.
Table 2.
Interobserver agreement of grading for duct visualization and image quality
| Nav | BH-CS | BH-PI | |
|---|---|---|---|
| CBD | 0.52 | 0.75 | 0.78 |
| Cystic duct | 0.78 | 0.77 | 0.76 |
| CHD | 0.63 | 0.76 | 0.83 |
| cRHD | 0.74 | 0.61 | 0.72 |
| cLHD | 0.60 | 0.60 | 0.71 |
| pRHD | 0.80 | 0.91 | 0.95 |
| pLHD | 0.84 | 0.81 | 0.77 |
| Pancreatic duct | 0.72 | 0.76 | 0.85 |
| Image quality | 0.83 | 0.72 | 0.66 |
| Artifacts | 1.00 | 0.89 | 0.85 |
Numbers in the cells present Cohen’s kappa values. CS, compressed sensing; PI, parallel imaging; CBD, common bile duct; CHD, common hepatic duct; cRHD/cLHD, central right hepatic duct/left hepatic duct; pRHD/pLHD, peripheral right hepatic duct/left hepatic duct.
Means and standard deviation of the scoring values for all three imaging methods are presented in Table 3. The breath-hold MRCP showed higher or at least comparable mean grading compared with Nav-MRCP for the main ducts (CBD, CHD, cRHD and cLHD) and the cystic duct (Figs. 1 and 2). For the peripheral ductal system, however, Nav-MRCP showed higher mean grading than BH-CS- or BH-PI-MRCP for both pRHD and pLHD using CS (P < 0.001), as well as for pRHD (P = 0.040) but not pLHD (P = 0.068) with PI. No significant difference was detected with regard to the pancreatic duct.
Table 3.
Mean grading and standard deviation of duct visualization and image quality for Nav-MRCP, BH-CS- and BH-PI-MRCP
| Nav | BH-CS | P (CS vs Nav) | BH-PI | P (PI vs Nav) | |
|---|---|---|---|---|---|
| CBD | 2.74 ± 0.5 | 2.87 ± 0.5 | 0.015 | 2.94 ± 0.5 | <0.001 |
| Cystic duct | 2.22 ± 0.7 | 2.34 ± 0.7 | 0.014 | 2.42 ± 0.7 | <0.001 |
| CHD | 2.82 ± 0.5 | 2.92 ± 0.4 | 0.050 | 3.00 ± 0.7 | 0.001 |
| cRHD | 2.75 ± 0.6 | 2.85 ± 0.6 | 0.108 | 2.98 ± 0.5 | <0.001 |
| cLHD | 2.75 ± 0.5 | 2.85 ± 0.5 | 0.094 | 2.92 ± 0.4 | 0.003 |
| pRHD | 2.24 ± 0.7 | 2.01 ± 0.7 | <0.001 | 2.12 ± 0.7 | 0.040 |
| pLHD | 2.23 ± 0.7 | 2.02 ± 0.7 | <0.001 | 2.13 ± 0.7 | 0.068 |
| Pancreatic duct | 2.54 ± 0.7 | 2.43 ± 0.7 | 0.137 | 2.53 ± 0.8 | 0.882 |
| Image quality | 2.91 ± 0.7 | 2.80 ± 0.7 | 0.031 | 2.92 ± 0.6 | 0.844 |
| Artifacts | 1.99 ± 0.1 | 1.44 ± 0.5 | <0.001 | 1.66 ± 0.5 | <0.001 |
CS, compressed sensing; PI, parallel imaging; CBD, common bile duct; CHD, common hepatic duct; cRHD/cLHD, central right hepatic duct/left hepatic duct; pRHD/pLHD, peripheral right hepatic duct/left hepatic duct.
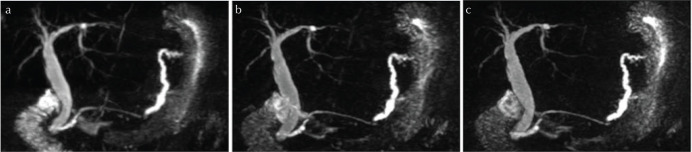
Fig. 1.
MIP images of 3D MRCP in a 68-year old female patient with minimal invasive pancreatic carcinoma and accompanying pancreatic duct dilatation. Comparison of (a) Nav-MRCP, (b) BH-CS-MRCP and (c) BH-PI-MRCP shows improved visualization of the pancreaticobiliary tree in CS and PI, while the respiratory triggered method yields slight blurring. Signal intensities of the ducts compared with the background are the best in Nav-MRCP due to longer acquisition time. Image quality was rated comparably good by both readers for all methods (Note the missing visualization of the cystic duct after cholecystectomy.). MIP, maximal intensity projection; Nav-MRCP, 3D magnetic resonance cholangiopancreatography with navigator echoes; BH-CS-MRCP, breath-hold magnetic resonance cholangiopancreatography with both parallel imaging and compressed sensing reconstruction; BH-PI-MRCP, breath-hold magnetic resonance cholangiopancreatography with parallel imaging reconstruction alone.
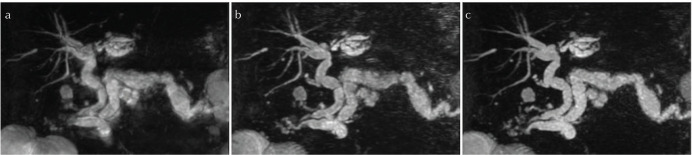
Fig. 2.
3D MRCP coronal MIP reconstruction images in an 80 year-old male patient. Nav-MRCP (a) displays image blurring due to respiratory motion. Image quality was rated higher and the visualization of ductal segments is much clearer in BH-CS-MRCP (b) and BH-PI-MRCP (c). MIP, maximal intensity projection; Nav-MRCP, 3D magnetic resonance cholangiopancreatography with navigator echoes; BH-CS-MRCP, breath-hold magnetic resonance cholangiopancreatography with both parallel imaging and compressed sensing reconstruction; BH-PI-MRCP, breath-hold magnetic resonance cholangiopancreatography with parallel imaging reconstruction alone.
Overall image quality did not differ between Nav-MRCP and BH-PI-MRCP (P = 0.163). However, Nav-MRCP yielded significantly higher image quality than BH-CS-MRCP (P = 0.031). Nav-MRCP showed significantly less imaging artifacts than the breath-hold sequences (P < 0.001).
Quantitative analysis
ROI measurements of the CBD, cRHD and cLHD as well as adjacent tissue showed lower signal intensity values for BH-CS- and BH-PI-MRCP compared with respiratory triggered technique (Table 4). Relative duct-to-periductal contrast ratios were significantly higher for all three biliary segments on Nav-MRCP (mean ± SD: RCCBD = 0.89 ± 0.04; RCcRHD = 0.89 ± 0.04; RCcLHD = 0.89 ± 0.03) compared with BH-CS-MRCP (RCCBD = 0.84 ± 0.05; RCcRHD = 0.85 ± 0.05; RCcLHD = 0.84 ± 0.05) or BH-PI-MRCP (RCCBD = 0.83 ± 0.06; RCcRHD = 0.84 ± 0.06; RCcLHD = 0.83 ± 0.06), respectively (all P-values < 0.001).
Table 4.
Relative contrast (mean ± standard deviation) for CBD, cRHD and cLHD in Nav-MRCP, BH-CS- and BH-PI-MRCP
| Nav | BH-CS | P (CS vs Nav) | BH-PI | P (PI vs Nav) | |
|---|---|---|---|---|---|
| CBD | 0.889 ± 0.039 | 0.845 ± 0.053 | <0.001 | 0.834 ± 0.061 | <0.001 |
| cRHD | 0.894 ± 0.045 | 0.849 ± 0.050 | <0.001 | 0.838 ± 0.058 | <0.001 |
| cLHD | 0.892 ± 0.034 | 0.844 ± 0.052 | <0.001 | 0.833 ± 0.057 | <0.001 |
CS, compressed sensing; PI, parallel imaging; SI, signal intensity; RC, relative duct-to-periductal contrast ratio; CBD, common bile duct; cRHD/cLHD, central right hepatic duct/left hepatic duct.
Discussion
Ensuring a high image quality by accelerating examination time remains a challenging problem of MRI, notably against the background of increasing health care costs, improving workflow and patient convenience. In our study we compare the performance of new breath-hold MRCP methods based on parallel imaging or compressed sensing against respiratory triggered MRCP in a clinical setting using a 3T GE device. In a shorter acquisition time, both breath-hold methods showed comparable or improved image quality and visualization of the main pancreaticobiliary ductal system.
Three-dimensional magnetic resonance cholangiopancreatography is a well-established method, which is able to provide detailed information on the anatomy and pathology of the pancreaticobiliary tree. It has proven to deliver superior image quality compared with two-dimensional MRCP and invasive techniques, such as ERCP or PTC.3,4 In terms of respiratory triggered image acquisition, however, it also bares the disadvantage of long scanning times6 accompanied by motion-related artifacts and patient discomfort. To solve this problem, new imaging techniques to accelerate data acquisition have moved into the center of researcher’s attention. Two such methods – parallel imaging and compressed sensing –, which are based on the process of undersampling, appear to be highly promising approaches and since their development there has been a considerable number of studies evaluating their performance, although clinical data remain scarce so far.
In a study on 30 patients PI was shown to deliver comparable image quality to respiratory triggered MRCP by significantly reduced acquisition time.5 Likewise, Yoon et al. demonstrated that CS-MRCP runs considerably faster but does not impair image quality. On the contrary, the method enabled an even higher image sharpness and visualization of the common bile and pancreatic ducts.10 An improved image quality in a patient cohort by using compressed sensing was confirmed in a study by Kwon et al.,15 who also found a superior accuracy for the diagnosis of common bile duct obstruction. Although this study was carried out using a 3T GE MR scanner (Discovery 750, GE Healthcare, Waukesha, WI, USA) as in our study, image quality and anatomy of only the CBD was evaluated and the cohort comprised solely patients with obstruction of the CBD. Our results, therefore, may contribute to an extended evaluation concerning the diagnostic performance of undersampling imaging methods with a GE device.
Other authors investigated the combination of PI and CS in MRI and found a higher resolution and faster image acquisition than with conventional PI alone.11,16 Seo et al.13 applied this combination to 3D MRCP and found no difference in image quality, thereby concluding it to be feasible in a clinical setting.
Consistent with these previous works we found a considerably decreased acquisition time for MRCP with breath-hold CS or PI (each 23 s), respectively, compared with respiratory triggered MRCP (1 min 29 s). Likewise, in our study qualitative comparison of Nav-MRCP with both undersampling methods demonstrated a significant improvement in the visibility of the main biliary ductal system – CBD, CHD, cRHD and cLHD – and the cystic duct for BH-PI-MRCP, as well as comparable (CHD, cRHD and cLHD) or better visibility (CBD and cystic duct) for BH-CS-MRCP. Imaging of the pancreatic duct was not different between the three methods. However, contrary to previous findings Nav-MRCP appeared to be superior in the peripheral biliary segments. One reason for that might be the already high image quality of MRCP images we observed in our study population compared with other authors,10,15 hampering an equivalent or even improved performance of the new techniques. Apart from that, our data exhibit comparable overall image quality of PI-MRCP and Nav-MRCP, whereas Nav-MRCP showed slightly better results than CS-MRCP.
In terms of quantitative evaluation we found significantly lower RC values in breath-hold CS- and PI-MRCP compared with Nav-MRCP for CBD as well as cRHD and cLHD. One possible reason might be observed differences in effective TE times. Although the lower RC values had no effect on the main biliary ducts, the visualization of the peripheral ductal segments appeared deteriorated in our study population.
Taken together, these findings in our opinion support the clinical application of undersampling methods preferentially in those cases where a pathology is presumed to be situated in the central segments of the pancreaticobiliary system. Especially PI is able to even increase diagnostic quality in cases with low grades in the respiratory triggered method and the accelerated acquisition time does not only lead to a reduction in motion artifacts but also enhances patient convenience and allows examination of persons with restricted ability to breath or otherwise insufficient cooperation.
Our study has several limitations. First, the study design was retrospective, which, however, allowed the inclusion of a higher number of patients compared with previous studies. Furthermore, analysis was undertaken only on the basis of maximal intensity projection images. Evaluation of the source images might improve diagnostic accuracy in some cases. However, that would account for all sequences and might not affect the outcome of comparability of the methods tested. Moreover, our study did not compare diagnostic performance in biliary or pancreatic diseases between the three MRCP methods.
Conclusion
Our study supports the clinical feasibility of BH-CS- and BH-PI-MRCP, especially for depicting the central pancreaticobiliary system, as they considerably shorten acquisition time without deterioration of image quality compared with respiratory triggered MRCP. Both breath-hold methods are promising approaches and suitable for revised MRI protocols notably for patients with poor quality in Nav-MRCP due to motion or irregular respiratory cycle.
Footnotes
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Sodickson A, Mortele KJ, Barish MA, Zou KH, Thibodeau S, Tempany CM. Three-dimensional fast-recovery fast spin-echo MRCP: comparison with two-dimensional single-shot fast spin-echo techniques. Radiology 2006; 238:549–559. [DOI] [PubMed] [Google Scholar]
- 2.Choi JY, Kim MJ, Lee JM, et al. Magnetic resonance cholangiography: comparison of two- and three-dimensional sequences for assessment of malignant biliary obstruction. Eur Radiol 2008; 18:78–86. [DOI] [PubMed] [Google Scholar]
- 3.Choi JY, Lee JM, Lee JY, et al. Navigator-triggered isotropic three-dimensional magnetic resonance cholangiopancreatography in the diagnosis of malignant biliary obstructions: comparison with direct cholangiography. J Magn Reson Imaging 2008; 27:94–101. [DOI] [PubMed] [Google Scholar]
- 4.Palmucci S, Mauro LA, Coppolino M, et al. Evaluation of the biliary and pancreatic system with 2D SSFSE, breathhold 3D FRFSE and respiratory-triggered 3D FRFSE sequences. Radiol Med 2010; 115:467–482. [DOI] [PubMed] [Google Scholar]
- 5.Asbach P, Dewey M, Klessen C, et al. Respiratory-triggered MRCP applying parallel acquisition techniques. J Magn Reson Imaging 2006; 24:1095–1100. [DOI] [PubMed] [Google Scholar]
- 6.Masui T, Katayama M, Kobayashi S, et al. Magnetic resonance cholangiopancreatography: comparison of respiratory-triggered three-dimensional fast-recovery fast spin-echo with parallel imaging technique and breath-hold half-Fourier two-dimensional single-shot fast spin-echo technique. Radiat Med 2006; 24:202–209. [DOI] [PubMed] [Google Scholar]
- 7.Gamper U, Boesiger P, Kozerke S. Compressed sensing in dynamic MRI. Magn Reson Med 2008; 59:365–373. [DOI] [PubMed] [Google Scholar]
- 8.Hollingsworth KG. Reducing acquisition time in clinical MRI by data undersampling and compressed sensing reconstruction. Phys Med Biol 2015; 60:R297–R322. [DOI] [PubMed] [Google Scholar]
- 9.Feng L, Benkert T, Block KT, Sodickson DK, Otazo R, Chandarana H. Compressed sensing for body MRI. J Magn Reson Imaging 2017; 45:966–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon JH, Lee SM, Kang HJ, et al. Clinical feasibility of 3-dimensional magnetic resonance cholangiopancreatography using compressed sensing: comparison of image quality and diagnostic performance. Invest Radiol 2017; 52:612–619. [DOI] [PubMed] [Google Scholar]
- 11.Vasanawala SS, Alley MT, Hargreaves BA, Barth RA, Pauly JM, Lustig M. Improved pediatric MR imaging with compressed sensing. Radiology 2010; 256:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandarana H, Doshi AM, Shanbhogue A, et al. Three-dimensional MR cholangiopancreatography in a breath hold with sparsity-based reconstruction of highly undersampled data. Radiology 2016; 280:585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seo N, Park MS, Han K, et al. Feasibility of 3D navigator-triggered magnetic resonance cholangiopancreatography with combined parallel imaging and compressed sensing reconstruction at 3T. J Magn Reson Imaging 2017; 46:1289–1297. [DOI] [PubMed] [Google Scholar]
- 14.Klessen C, Asbach P, Kroencke TJ, et al. Magnetic resonance imaging of the upper abdomen using a free-breathing T2-weighted turbo spin echo sequence with navigator triggered prospective acquisition correction. J Magn Reson Imaging. 2005; 21:576–582. [DOI] [PubMed] [Google Scholar]
- 15.Kwon H, Reid S, Kim D, Lee S, Cho J, Oh J. Diagnosing common bile duct obstruction: comparison of image quality and diagnostic performance of three-dimensional magnetic resonance cholangiopancreatography with and without compressed sensing. Abdom Radiol (NY) 2018; 43: 2255–2261. [DOI] [PubMed] [Google Scholar]
- 16.Zhang T, Chowdhury S, Lustig M, et al. Clinical performance of contrast enhanced abdominal pediatric MRI with fast combined parallel imaging compressed sensing reconstruction. J Magn Reson Imaging 2014; 40:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]