Highlights
-
•
Cortisol detection based on surface plasmon resonance (SPR) using an unclad plastic optical fiber (POF).
-
•
High sensitivity and low limit of detection (LOD).
-
•
Selectivity of the proposed sensor for stress hormone was performed showing high specificity.
-
•
Optical sensor solution with relatively low-cost interrogation method, straightforward signal processing.
-
•
Interesting working range (0.005−10 ng/mL) for different biological samples from human or marine life.
Keywords: Plastic optical fiber, Unclad POF, Biosensor, SPR, Stress monitoring, Well-being monitoring
Abstract
This paper presents the development and feasibility tests of a cortisol immunosensor. The sensor is based on surface plasmon resonance (SPR) using an unclad plastic optical fiber (POF) in which the SPR is used as sensitivity enhancer, promoted by a gold/palladium (AuPd) alloy coating. The AuPd coated fibers were functionalized with an anti-cortisol antibody and passivated with bovine serum albumin (BSA) to be tested in the presence of cortisol as target analyte. The antibody-antigen binding reaction caused a variation of the refractive index on the surface of the AuPd coating, which leads to a shift of the SPR signature wavelength. The sensor was tested for different cortisol concentrations, ranging from 0.005 to 10 ng/mL. The reported biosensor presented a total wavelength shift of 15 nm for the testing range, putting in evidence a high sensitivity. Control tests for selectivity assessment were also performed. Concentrations as high as 10 ng/mL of cortisol, in a sensor functionalized with anti-hCG antibodies, only resulted in 1 nm variation of the resonance wavelength, 15 times lower than the one functionalized with the anti-cortisol antibodies, which indicates a high selectivity for the proposed approach. For this sensing approach the limit of detection (LOD) was determined to be 1 pg/mL. The proposed SPR based POF sensor has a low-cost interrogation method, high sensitivity and low LOD, straightforward signal processing and find important applications in different biological fields.
1. Introduction
All biological systems had to adapt to physical and psychological stress, developing effective responses to maintain physiologic integrity even in the most demanding of circumstances. In response to an imminent danger, physical injury, perceived threat or stressful events, the body triggers a multi-dynamic set of processes to restore homeostasis [1]. The stress response is regulated by the hypothalamic-pituitary-adrenal (HPA) axis, aiming at maintain the homeostatic processes, whose end product, cortisol, is secreted in a pulsatile pattern respecting with the circadian cycle and situations perceived as stressful [2]. In this process, cortisol triggers a set of responses at the cardiovascular, immunologic, and metabolic levels [3].
The rise of cortisol levels, as response to stress, is normal and promote beneficial physiological responses, enabling the body to restore homeostasis [3]. However, chronic elevation and nonphysiological patterns of cortisol are a maladaptation, which can lead to a broad range of problems, including metabolic syndromes, mental health disorders, cardiovascular diseases and decrease of the immune function [2]. In a systematic review and meta-analysis, Adam el al. showed that a continuous high level of diurnal cortisol slopes is associated with health problems, with strong effects on inflammation and immune system, and that can be translated in subsequent effects on multiple aspects of behavior and health [4].
Currently, cortisol levels quantification still depends on conventional laboratory testing of blood or urine with the associated disadvantages, such as long time to conclusive result, patient’s discomfort, difficult to perform distinct measures through the day, among others [5]. Thus, efforts have been gathered for the development of rapid and user-friendly point-of-care (POC) monitoring tools, for instance to perform saliva and sweat analysis, where the concentration ranges are 1–8 ng/mL and 8 ng/mL to 140 ng/mL, respectively [6,7]. These solutions also present the advantages of eliminating additional source of stress related to laboratory visits and blood/urine collection.
Besides biomedical applications, there is also a very special need for this type of biosensors in aquatic biology applications such as in farmed fish, an efficient source of protein to overcome the expectation that the world population will increase by 2 billion persons in the next 30 years, from 7.7 billion currently to 9.7 billion in 2050 [8]. In this way, aquaculture is an industry that is set to continue growing and needs to do so in a sustainable manner as reported by United Nations [9]. In the aquaculture field, this hormone has proven to be also a reliable indicator of stress in fish [10] causing large mortality and consequently industry profit losses. Assessing the stress levels of fish in aquaculture, directly in the water, is of outmost importance in order to understand its interaction with growth, reproduction, immune system and the fitness of the fish in the environment as well as their welfare [11]. The cortisol concentration range in water fish tanks is roughly between 0.007 and 5 ng/mL, depending the stocking density [10]. However, there are no commercial solutions in the market and this industry rarely measures this critical parameter due to the lack of probes available for installation in water tanks. In addition, way to measure cortisol in fish is through blood sampling and analysis of fish plasma (in conventional laboratory testing), an invasive and time-consuming method (results only available long after the sampling) where the results may be doubtful from the stress caused by the sampling method.
Different strategies have been employed in cortisol biosensing, especially electrochemical [[12], [13], [14]] and optical [[15], [16], [17]], with limits of detection (LOD) ranging from 1 pg/mL to 0.2 μg/mL. Despite all these approaches being promising, efforts must continue to achieve low-cost and easy to use sensors in different environments, including aquatic industry, that effectively allow accurate, rapid and repeatable cortisol assessment in everyday life [7,11].
Surface plasmon resonance (SPR) is an optical sensing technique increasingly exploited in biosensing due to its high sensitivity to surrounding refractive index (SRI). SPR relies on a metal-dielectric interface supporting p-polarized electromagnetic waves, namely surface plasmon waves (SPW), which propagates along the interface [18]. When p-polarized light hits this interface in such a way that the propagation constant and energy of the resultant evanescent wave is equal to the one of the SPW, a strong absorption of light occurs. The sensor spectrum consequently shows a sharp dip at a particular wavelength known as resonance wavelength. The resonance effect depends on the wavelength and SRI of the metal layer [19]. Even though firstly developed for planar approaches, SPR biosensing has been applied to different waveguides surface, especially optical fibers. Due to their small size, biocompatibility, good sensitivity and fast response, they are promising solutions for biosensing assays [20].
Plastic optical fibers (POFs) are particularly attractive, especially due to their flexibility, easiness of handling, high numerical aperture, and associated low-cost instrumentation [19]. POF biosensors, particularly applying SPR for sensitivity enhancement, had been investigated in different configurations, such as tapered [21], uncladded [22], U-bend [23], and D shaped [24].
This work aims to develop and test the feasibility of an unclad POF cortisol immunosensor, using the SPR effect, promoted by gold/palladium (AuPd) alloy. Due to the strong physico-chemical interaction of gold and sulphur [25], the AuPd coating was firstly functionalized with a thiol derivative – cysteamine. Then, the anti-cortisol antibody was covalently attached to this linker using N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochlorine and N-hydroxysuccinimide (EDC/NHS).
The preliminary cortisol detection study included the monitoring of the functionalization steps and the final sensor testing to different cortisol concentrations, ranging from 0.005 to 10 ng/mL, achieving a LOD of 1 pg/mL. Control tests for selectivity assessment were also performed in a sensor functionalized with antibodies for human chorionic gonadotropin (anti-hCG antibodies), only resulted in 1 nm variation of the resonance wavelength.
2. Materials and methods
2.1. Chemicals and AuPd coated POF production
Cortisol (hydrocortisone solution 50 μM), D-(+)-glucose (≥99.5) and cysteamine hydrochloride (≥98 %) were purchased from Sigma-Aldrich. Deionized water was purchased from a Milli-Q water purification system. EDC and NHS (98 %) were acquired from Merck whereas anti-cortisol polyclonal antibody was acquired from antibodies-online GmbH. Bovine serum albumin (BSA) was obtained from Alfa Aesar and phosphate buffer saline (PBS) tablets (pH = 7.4, 10 mM) were purchased from Fisher Bioreagent. All reagents were used as received.
For the optical sensor platform production was used a POF (ESKA Mitsubishi, Japan) with a diameter of 1 mm, divided into a core of polymethyl methacrylate (PMMA) with 980 μm diameter and a cladding of fluorinated polymer with 10 μm thickness. First, the cladding was removed using an abrasive process in which the region for the cladding removal was rotated against different sandpapers, starting with 30 μm grit size for the material removal with sequential polishing steps using the 5 μm and 1 μm grit size sandpapers. The fiber samples were cleaned with deionized water between the steps. The uncladded POF samples were coated with a nanolayer (∼40 nm) of AuPd using the sputtering technique. For the coating process, first the fiber is cleaned with isopropanol and then placed in the sputtering chamber (SEM coating Unit E5000 mounted with a sputter target composed of 20 % Pd and 80 % Au). After the first deposition, the fiber was rotated 180° for a second deposition to ensure total 360° covering. The AuPd thickness was estimated through the control of the deposition time. In addition, tests were made before regarding to the thickness of the nanolayer for this target via a scanning electron microscope, where the film thickness for different deposition times was assessed. Subsequently, the AuPd-coated POFs were annealed during 2 h at ∼50 °C to strengthen the AuPd adhesion on the POFs surface.
2.2. AuPd coated POF funcionalization and cortisol monitoring
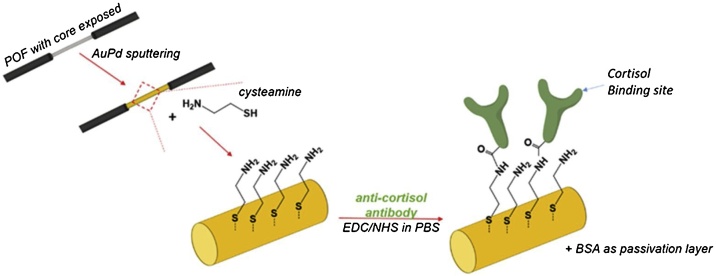
Firstly, each AuPd coated POF was cleaned by immersing the fiber in a deionized water bath during few minutes, follow by immersing in an aqueous solution of cysteamine (20 mM, 400 μL) overnight, for the preparation of the amine-terminated fiber. Then, the fiber was washed three times with deionized water in order to remove the unbounded cysteamine. The fiber was placed in PBS solution, and then, functionalized with the anti-cortisol antibody (ac-AB) by immersing it in a fresh mixture of 200 μL of ac-AB (500 μg/mL), 100 μL of EDC (0.2 M) and 100 μL NHS (0.5 M), prepared in PBS, and let to react during 2 h. Thereafter, the fiber was washed three times with PBS, and the surface was passivated using a solution of BSA (100 μg/mL, 500 μL) during 4 h. After this process, the biofunctionalized fiber was again washed 3 times with PBS. The functionalization steps, schematically represented in Fig. 1, were performed and monitored using the setup presented in Fig. 2, recording the optical spectra in the wavelength range of 500−700 nm.
Fig. 1.
Optical fiber functionalization steps.
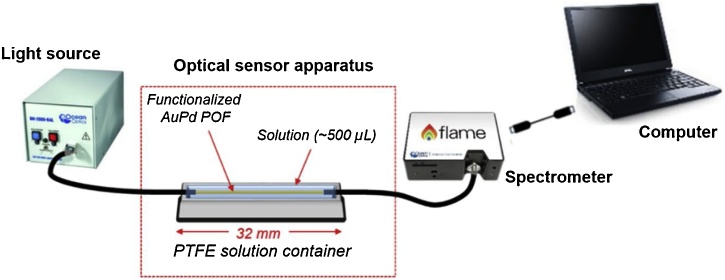
Fig. 2.
Schematic representation of the experimental setup.
The concentration range of biological interest between 0.005 and 10 ng/mL was used to collect and analyze the biosensor response, specifically 0.005, 0.01, 0.1, 0.5, 1, 2.5, 5, 7.5 and 10 ng/mL. The functionalized sensor described above was kept in the container in contact with each cortisol solution during 30 min to guarantee the formation of the antibody-antigen complex. Next, by aid of a microsyringe the cortisol solution was removed, following the washing (3 times) with PBS solution before the transmission optical spectrum be acquired with the sensor sample immersed in PBS solution. This procedure was repeated using the same sample fiber for each cortisol solution in an increasing concentration order.
2.3. Instrumentation
The experimental setup for the sensor characterization is schematically represented in Fig. 2. The biosensor was placed in a small polytetrafluoroethylene (PTFE) container with the dimensions of 32 × 5 × 3 mm3. The optical sensor system was completed with a small size, simple and low-cost equipment, composed by a halogen lamp and a spectrometer connected to a laptop. The white light source (halogen lamp, DH-2000-S-DUV-TTL, manufactured by Ocean Optics, USA) has an emission range from 183 nm to 890 nm, whereas the spectrometer (FLAME-T-UV–vis, manufactured by Ocean Optics, USA) has a detection range from 180 nm to 890 nm. The AuPd POF sensor was connected to the equipment, light source and spectrometer by two removable SMA connectors with additional 3D printed customized adapters to position the POF on the SMA connectors of light source and spectrometer. The SPR signatures along with data values were displayed on-line on the laptop screen and saved with the help of a software provided by Ocean Optics, setting the integration time at 500 μs and the averaging of scans at 100. The optical transmission spectra were normalized to the reference spectrum (air as the surrounding medium). All experiments were conducted in temperature-controlled room at 21 °C.
3. Results and discussion
3.1. Sensor’s biofunctionalization monitoring
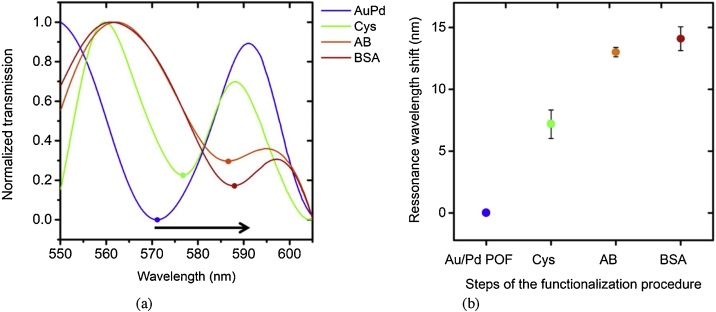
AuPd POF sensors were immersed in deionized water for cleaning before the sensor’s biofunctionalization procedure. As regards the first step, the fiber was immersed in the cysteamine solution overnight. During this period, a cysteamine monolayer is formed and self-organized on the fiber surface leading to a refractive index (RI) variation of the surrounding medium, and, consequently, to light scattering differences. This phenomenon is detectable by a red shift of the SPR signature. Fig. 3 shows the spectra of the AuPd POF in PBS as well as the spectrum for each functionalization step. All the optical spectra were acquired after washing the fiber with PBS, in order to mitigate the possibility of unlinked molecules interference in the surrounding RI.
Fig. 3.
a) Spectra of the POF coated with AuPd before and after each biofunctionalization process; b) resonance wavelength shifts acquired in buffer solution (PBS) after the different biofunctionalization steps (results for triplicates are presented).
Fig. 3 puts in evidence a large red shift of the SPR signature after each functionalization step. The biofunctionalization steps caused a total change in the SPR signature wavelength of 15 nm. In particular, the immobilization of the anti-cortisol antibody (ac-AB) on the sensor surface is confirmed by the SPR results: the transmission spectra, reported in Fig. 3a), show an increase of the resonance wavelength, before and after the biofunctionalization procedure, even though in the presence of the same bulk refractive index. This wavelength shift is due to an increase of the surrounding RI of the AuPd surface and indicates the ac-AB immobilization on the AuPd surface. Fig. 3b) shows the resonance shift value, after this biofunctionalization step, of about 13 nm and a shift of 15 nm after the final step (surface passivation with BSA).
3.2. Cortisol sensing – detection range
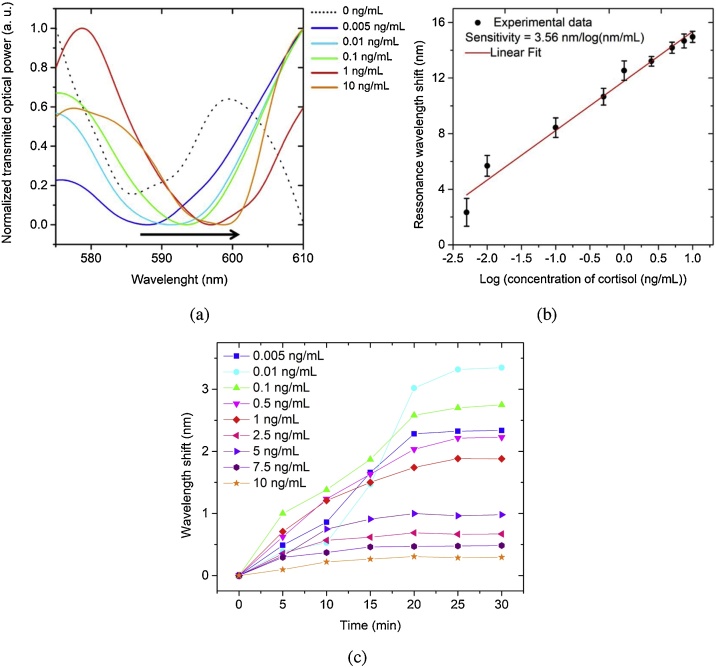
The SPR wavelength shift of the AuPd POF was monitored for the different cortisol concentrations and the respective spectra is displayed in Fig. 4a. A naked eye red shift can be seen, which indicates the high sensitivity of the sensor for this concentrations range. The wavelength shift was calculated as the SPR wavelength at a given cortisol concentration minus the SPR wavelength at zero ng/mL, being this wavelength considered the minimum of the sinusoidal curve reported in Fig. 4a). The presented spectra were smoothed by an FFT lowpass filter (cutoff at 0.19 Hz) and the peak minimum was manually identified as the value with the immediate left and right points with a higher transmitted optical power. Fig. 4b shows that this sensor configuration exhibits an exponential response (R2 = 0.977) to the cortisol concentration range from 0.005 to 10 ng/mL. The resonance wavelength shift for this concentration range was of around 14.9 nm. Sensitivity, defined as the shift of the resonance wavelength per unit change in the logarithm concentration of cortisol, is calculated to be 3.56 ± 0.20 nm/log(ng/mL). The experiments were performed on 3 different sensors and the standard deviation in resonance wavelength was obtained for each concentration. Fig. 4c) also shows the spectra recorded every 5 min during the formation of the antibody-antigen complex, where it can be seen that the resonance wavelength position was substantially stable after at least 20 min. It can be considered that, at the end of this time, the equilibrium of the cortisol – ac-AB reaction was reached.
Fig. 4.
a) Optical spectra of the functionalized AuPd POF, acquired in PBS, as a response for cortisol solutions in a concentration range from 0.005 to 10 ng/mL, after incubation during 30 min, (for sake of clarity just some of the response spectra are displayed); b) SPR signature wavelength variation as a function of the logarithm of the cortisol concentration (results for triplicates are presented), showing the respective linear fit (R2 = 0.9975); c) wavelength shift over time measured for each different concentration of cortisol solution during incubation and the antigen-antibody complex formation (points represent the recorded data and lines are for a better guidance in the kinetic response).
3.3. Control test and LOD
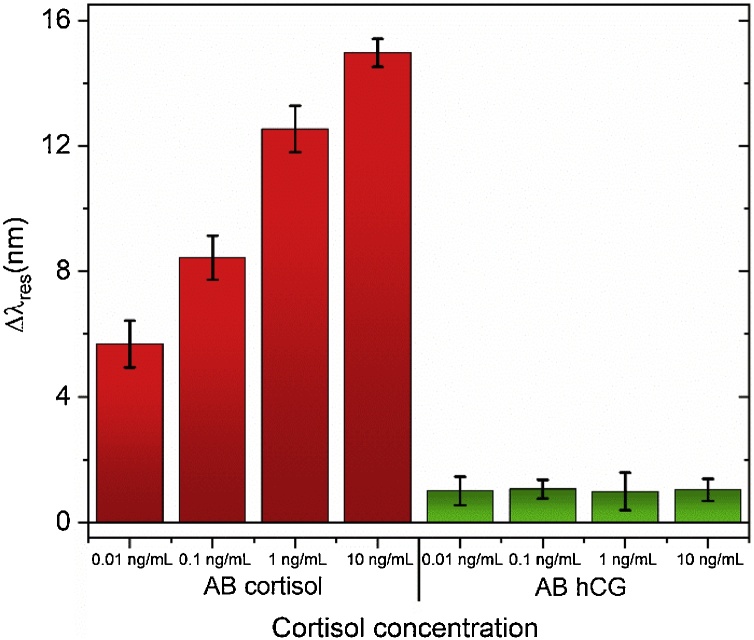
In order to ascertain if the analyte tested (cortisol) do not interact with the sensor surface by non-specific interactions, a control test was performed. The fiber was functionalized with anti-hCG (antibody for human chorionic gonadotropin), which does not have affinity for the target bioanalyte, cortisol. In this way, an AuPd POF sensor was biofunctionalized with anti-hCG AB instead of ac-AB (following the same procedure as described in section 2.3). Then, the sensor was tested in different cortisol concentrations (0, 0.01, 0.1, 1, 10 ng/mL) during 30 min, and the spectra were collected for each concentration. Fig. 5 shows the comparison of resonance wavelength shift as a function of cortisol concentration using the fiber functionalized with anti-cortisol and anti-hCG antibodies. It can be observed that, in this case, the sensor does not respond to the different cortisol solutions, proving that its response is not due to non-specific interactions. The observed variation in resonance wavelength is around 15 nm when the anti-cortisol AB is used, (see the left results of the histogram in the Fig. 5 for ac-AB) and only around 1 nm for anti-hCG, for the concentrations tested. These results demonstrate that the anti-cortisol antibody has high specificity to cortisol and when the antibody-antigen complex is formed occurs a slightly change in the surrounding refractive index which is translated in a shift of resonance wavelength. Unlike, in the control test a different antibody was used with no specificity for the target analyte. Thus, no antibody-antigen was formed and no significant shift in resonance wavelength has been observed. The reason behind the very small shift observed could be assigned to some non-specific interactions promoted by some physical-adsorption of the cortisol onto the fiber surface.
Fig. 5.
Histogram comparison of the shift in resonance wavelength between when is used ac-AB (left) and anti-hCG AB (right), for cortisol concentrations from 0.01 ng/mL to 10 ng/mL.
Applying a conservative approach, the LOD of the sensor was calculated as the 3σ(blank), being the blank considered the standard deviation of the first control cortisol concentration (0.01 ng/mL), which resulted in a LOD of 1 pg/mL. The achieved LOD matches the lowest value found in the literature of cortisol detection [14].
Considering the features of the AuPd POF biosensor, namely low-cost approach, easiness of interrogation, low LOD, high sensitivity and response range, it is envisioned that the sensor will be well suitable for monitoring cortisol levels in saliva [6] and fish stress levels in aquaculture by determining the cortisol concentration at the water fish tanks [26].
4. Conclusions
A new approach for cortisol monitoring using SPR and POF technology based biosensing was investigated aiming to achieve a highly sensitive, low sample volume and low cost immunosensor. The biofunctionalization with the anti-cortisol antibodies was made via cysteamine and the final sensor was passivated with BSA. This design along with a simple and low-cost interrogation method, resulted in a highly sensitive and low LOD biosensor (1 pg/mL). The achieved detection range matches the concentration range of different biomedical and environmental samples, such as human saliva or sweat and water from aquaculture tanks to monitor stress of fish. The fiber optics technology would be of great interest, especially in the latter, since a simple and low-cost interrogation system (as presented in this work) could be in the controlling office, with only the optical fiber probes in the water fish tanks to be monitored. Moreover, there is the possibility of sensors be reusable by applying a regeneration procedure, thus allowing to be used more than once and lowering the manufacture costs [27].
Funding
This research was funded by Fundação para a Ciência e a Tecnologia (FCT) through the CEECIND/00034/2018 (iFISH project) and this work was developed within the scope of the project i3N, UIDB/50025/2020 & UIDP/50025/2020, financed by national funds through the FCT/MEC. A. Leal-Junior was funded by FAPES (2020-F057G). C. Leitão and S. O. Pereira thanks i3N for the BPD grants BPD/UI96/5142/2017 and BPD/UI96/5808/2017, respectively.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
The authors would like to thank Tiago Silva, from Department of Materials and Ceramic Engineering (DEMaC), University of Aveiro, for his valuable support during the AuPd thin layer deposition.
References
- 1.Radley J.J., Kabbaj M., Jacobson L., Heydendael W., Yehuda R., Herman J.P. “Stress risk factors and stress-related pathology: neuroplasticity, epigenetics and endophenotypes,”. Stress. 2011;14(September (5)):481–497. doi: 10.3109/10253890.2011.604751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell G., Lightman S. “The human stress response,”. Nat. Rev. Endocrinol. 2019;15(September 01 (9)):525–534. doi: 10.1038/s41574-019-0228-0. Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- 3.Burford N.G., Webster N.A., Cruz-Topete D. “Hypothalamic-pituitary-adrenal axis modulation of glucocorticoids in the cardiovascular system,”. Int. J. Mol. Sci. 2017;18(10):1–16. doi: 10.3390/ijms18102150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adam E.K., Quinn M.E., Tavernier R., McQuillan M.T., Dahlke K.A., Gilbert K.E. “Diurnal cortisol slopes and mental and physical health outcomes: a systematic review and meta-analysis,”. Psychoneuroendocrinology. 2017;83:25–41. doi: 10.1016/j.psyneuen.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steckl A.J., Ray P. “Stress biomarkers in biological fluids and their point-of-use detection,”. ACS Sens. 2018;3(10):2025–2044. doi: 10.1021/acssensors.8b00726. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi M. 2012. “Immunosensor with Fluid Control Mechanism for Salivary Cortisol Analysis,”. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogenelst K., Soeter M., Kallen V. “Ambulatory measurement of cortisol: where do we stand, and which way to follow?,”. Sens. Bio-Sensing Res. 2019;22(July 2018):100249. doi: 10.1016/j.sbsr.2018.100249. [DOI] [Google Scholar]
- 8.United Nations “Growing at a slower pace, world population is expected to reach 9.7 billion in 2050 and could peak at nearly 11 billion around 2100 UN DESA | United Nations Department of Economic and Social Affairs.” https://www.un.org/development/desa/en/news/population/world-population-prospects-2019.html (accessed Oct. 23, 2020).
- 9.United Nations “Sustainable consumption and production – United Nations Sustainable Development.” https://www.un.org/sustainabledevelopment/sustainable-consumption-production/ (accessed Oct. 23, 2020).
- 10.Mota V.C., Martins C.I.M., Edinga E.H., Canário A.V.M., Verretha J.A.J. “Steroids accumulate in the rearing water of commercial recirculating aquaculture systems,”. Aquac. Eng. 2014;62(September (1)):9–16. doi: 10.1016/j.aquaeng.2014.07.004. [DOI] [Google Scholar]
- 11.Sadoul B., Geffroy B. “Measuring cortisol, the major stress hormone in fishes,”. J. Fish Biol. 2019;94(Apr. 01(4)):540–555. doi: 10.1111/jfb.13904. Blackwell Publishing Ltd. [DOI] [PubMed] [Google Scholar]
- 12.Jang H.J. “Electronic cortisol detection using an antibody-embedded polymer coupled to a field-effect transistor,”. ACS Appl. Mater. Interfaces. 2018;10(May (19)):16233–16237. doi: 10.1021/acsami.7b18855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuteja S.K., Ormsby C., Neethirajan S. “Noninvasive label-free detection of cortisol and lactate using graphene embedded screen-printed electrode,”. Nano-Micro Lett. 2018;10(3):1–10. doi: 10.1007/s40820-018-0193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice P., Upasham S., Jagannath B., Manuel R., Pali M., Prasad S. “CortiWatch: Watch-based cortisol tracker,”. Futur. Sci. OA. 2019;5(9) doi: 10.2144/fsoa-2019-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens R.C., Soelberg S.D., Near S., Furlong C.E. “Detection of cortisol in saliva with a flow-filtered, portable surface plasmon resonance biosensor system,”. Anal. Chem. 2008;80(September (17)):6747–6751. doi: 10.1021/ac800892h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalirirad S., Steckl A.J. “Aptamer-based lateral flow assay for point of care cortisol detection in sweat,”. Sens. Actuators, B Chem. 2019;283(November 2018):79–86. doi: 10.1016/j.snb.2018.11.161. [DOI] [Google Scholar]
- 17.Ray P., Steckl A.J. “Label-Free optical detection of multiple biomarkers in sweat, plasma, urine, and saliva,”. ACS Sens. 2019;4(May (5)):1346–1357. doi: 10.1021/acssensors.9b00301. [DOI] [PubMed] [Google Scholar]
- 18.Sharma A.K., Jha R., Gupta B.D. “Fiber-optic sensors based on surface plasmon resonance: a comprehensive review,”. IEEE Sens. J. 2007;7(8):1118–1129. doi: 10.1109/JSEN.2007.897946. [DOI] [Google Scholar]
- 19.Cennamo N., Massarotti D., Conte L., Zeni L. “Low cost sensors based on SPR in a plastic optical fiber forbiosensor implementation,”. Sensors. 2011;11(December (12)):11752–11760. doi: 10.3390/s111211752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mowbray S.E., Amiri A.M. “A brief overview of medical fiber optic biosensors and techniques in the modification for enhanced sensing ability,”. Diagnostics. 2019;9(1) doi: 10.3390/diagnostics9010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cennamo N., Arcadio F., Minardo A., Montemurro D., Zeni L. “Experimental characterization of plasmonic sensors based on lab-built tapered plastic optical fibers,”. Appl. Sci. 2020;10(June (12)):4389. doi: 10.3390/app10124389. [DOI] [Google Scholar]
- 22.Verma R., Gupta B.D. “Fiber optic SPR sensor for the detection of 3-pyridinecarboxamide (vitamin B3) using molecularly imprinted hydrogel,”. Sens. Actuators B Chem. 2013;177:279–285. doi: 10.1016/j.snb.2012.10.135. [DOI] [Google Scholar]
- 23.Christopher C., Subrahmanyam A., Sai V.V.R. “Gold sputtered U-Bent plastic optical fiber probes as SPR- and LSPR-based compact plasmonic sensors,”. Plasmonics. 2018;13(April (2)):493–502. doi: 10.1007/s11468-017-0535-z. [DOI] [Google Scholar]
- 24.Gong Z. “Wearable fiber optic technology based on smart textile: a review,”. Mater. (Basel). 2019;12(20) doi: 10.3390/ma12203311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y., Li Y., Wu W., Jiang Y., Hu B. “Chitosan coated on the layers’ glucose oxidase immobilized on cysteamine/Au electrode for use as glucose biosensor,”. Biosens. Bioelectron. 2014;60(October):271–276. doi: 10.1016/j.bios.2014.04.035. [DOI] [PubMed] [Google Scholar]
- 26.Ruane N.M., Komen H. “Measuring cortisol in the water as an indicator of stress caused by increased loading density in common carp (Cyprinus carpio),”. Aquaculture. 2003;218(March (1–4)):685–693. doi: 10.1016/S0044-8486(02)00422-2. [DOI] [Google Scholar]
- 27.Zhou L. “Universal and reusable hapten/antibody-mediated portable optofluidic immunosensing platform for rapid on-site detection of pathogens,”. Chemosphere. 2018;210(November):10–18. doi: 10.1016/j.chemosphere.2018.06.159. [DOI] [PubMed] [Google Scholar]