Abstract
Pseudaliid lungworm (Metastrongyloidea) infections and associated secondary bacterial infections may severely affect the health status of harbour porpoises (Phocoena phocoena) in German waters. The presented retrospective analysis including data from 259 harbour porpoises stranded between 2006 and 2018 on the German federal state of Schleswig-Holstein's North Sea coast showed that 118 (46%) of these stranded individuals harboured a lungworm infection. During this 13-year period, a significant difference in annual lungworm prevalence was only observed between the years 2006 and 2016. Lungworm coinfections of bronchi and pulmonary blood vessels were observed in 85.6% of positive cases. Mild infection levels were detected in 22.9% of infected animals and were most common in the age class of immature individuals (74.1%). Moderate and severe infections were present in 38.1% and 39.0% of the lungworm positive animals, respectively. Their distribution in immatures (51.1% and 54.3%) and adults (48.9% and 43.4%) did not show significant differences. In stranded animals, lungworm diagnosis can be easily obtained via necropsy, while reliable lungworm diagnosis in living porpoises requires invasive bronchoscopy or faecal examination, which is difficult to obtain in cetaceans. To overcome this issue, an enzyme-linked immunosorbent assay (ELISA) and immunoblot based on recombinant major sperm protein (MSP) of the cattle lungworm were evaluated as potential diagnostic tools in harbour porpoises. However, in contrast to hitherto other investigated host species, no reliable antibody response pattern was detectable in harbour porpoise serum/plasma or whole blood samples. Thus, MSP-based serological tests are considered unsuitable for lungworm diagnosis in harbour porpoises.
Keywords: Harbour porpoise, Lungworms, Nematodes, Animal health, Serology, Antibodies
Graphical abstract
Highlights
-
•
Overall lungworm prevalence in North Sea harbour porpoises was 46%.
-
•
Co-infection of bronchial tree and pulmonary vessels in 85.5% of positive animals.
-
•
Most harbour porpoises suffered from moderate (38%) or severe infection (39%).
-
•
Both MSP-ELISA and immunoblot did not reveal reliable results.
1. Introduction
The harbour porpoise (Phocoena phocoena) is one of the world's smallest odontocetes, widely distributed throughout northern oceans and the only cetacean native to German waters (Hammond et al., 2002; 2013; Siebert et al., 2006a; ICES 2020). It is protected under several national and international agreements, including the EU Habitats Directive (EEC 1992), the Agreement on the Conservation of Small Cetaceans of the Baltic, North East Atlantic, Irish and North Seas (ASCOBANS), the Convention on the Conservation of Migratory Species of Wild Animals (CMS), and the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES).
Harbour porpoises prey on small schooling fish, cephalopods and polychaetes (Santos and Pierce, 2003; Leopold, 2015) and can live up to 24 years, whereas the average life span is only eight to ten years (Siebert et al., 2001; Kesselring et al., 2017; Bjørge and Tolley, 2018). Porpoises wean their offspring between eight and ten months, while the calves already start supplemental feeding on solid prey at a few months of age (Camphuysen and Krop, 2011; Bjørge and Tolley, 2018). As some prey fish species act as intermediate hosts in pseudaliid lungworm life cycles (Dailey, 1970; Bergeron et al., 1997; Houde et al., 2003; Lehnert et al., 2010), harbour porpoises most likely acquire infections as soon as they start feeding on prey.
Several species of the nematode family Pseudaliidae (Metastrongyloidea) parasitise the respiratory tract of their definitive hosts and can cause pathological lesions within the pulmonary structures (Dougherty, 1944; Testi and Pilleri, 1969; Stockdale, 1976; Bolt et al., 1994; Measures, 2018). Three pseudaliid nematode species infecting harbour porpoises in German waters are Pseudalius inflexus (Pseudaliinae, Rudolphi, 1808), Torynurus convolutus (Stenurinae, Kuhn 1829) and Halocercus invaginatus (Halocercinae, Quekett 1841), all residing in the lungs (Delyamure, 1955; Arnold and Gaskin, 1975; Lehnert et al., 2010). These nematode species often co-occur (Balbuena et al., 1994; Lehnert et al., 2005), but inhabit different niches within the respiratory tract. While P. inflexus and T. convolutus reside in the bronchi, bronchioles and blood vessels, H. invaginatus is found in the pulmonary parenchyma, often forming encapsulated nodules (Measures, 2001; Siebert et al., 2001), and has also been observed in the blood vessels of Greenlandic porpoises (Lehnert et al., 2014). Lungworms are known to negatively affect marine mammal health (e.g. Arnold and Gaskin, 1975; Dailey and Stroud, 1978; Bishop, 1979; Baker and Martin, 1992). They can cause respiratory distress, influence foraging abilities and weight gain, and instigate secondary bacterial infections, which can lead to severe and often fatal bronchopneumonia (Jepson et al., 2000; Wünschmann et al., 2001; Siebert et al., 2001, 2006b, 2020; Jauniaux et al., 2002; Lehnert et al., 2005). However, clinical symptoms due to lungworms are sparse, difficult to observe in free-ranging cetaceans and can be non-specific (Measures, 2001; van Elk et al., 2019). Hence, clinical signs may seldom be indicative of lungworm infections in cetaceans. Furthermore, intra-vitam lungworm diagnosis is generally restricted to highly invasive bronchoscopies or examination of faecal or sputum samples, which are logistically challenging to obtain in aquatic wildlife (Kastelein et al., 1990; Hunt et al., 2013; Kleinertz et al., 2014). Additionally, the sensitivity of faecal detection appears to be suboptimal (Fauquier et al., 2009), while bronchoscopy can only evaluate the larger bronchi for nematode presence. Consequently, available information on lungworm infections in harbour porpoises mostly relies on stranded, by-caught or rehabilitated individuals.
Within the framework of the German federal state of Schleswig-Holstein's stranding network and the ongoing marine mammal population health monitoring projects in the German Wadden Sea, cumulative data on lungworm burden and the resulting health impairments of stranded harbour porpoises are being collected (Benke et al., 1998; Siebert et al. 2001, Siebert et al., 2006a, Siebert et al., 2006b). Here, a retrospective necropsy data analysis of individuals found dead between 2006 and 2018 along Schleswig-Holstein's North Sea coast was conducted to allow a comprehensive prevalence assessment of parasitic bronchopneumonia in harbour porpoises during this period. A further aim of the study was to evaluate serological methods to detect lungworm infections in harbour porpoises. An enzyme-linked immunosorbent assay (ELISA) using recombinant bovine lungworm major sperm protein (MSP) fused to glutathione-S-transferase (GST) of the trematode Schistosoma japonicum as diagnostic antigen was developed for lungworm detection in cattle (von Holtum et al., 2008). MSP is a nematode specific male sperm protein that is highly conserved among different genera (Schnieder, 1992; Hojas and Post, 2000; Strube et al., 2009; Ulrich et al., 2015; Zottler et al., 2017). Recently, this ELISA was successfully adapted for lungworm antibody detection in cats (Felis catus) (Zottler et al., 2017) as well as harbour seals (Phoca vitulina) and grey seals (Halichoerus grypus) (Ulrich et al., 2015). So far, no serological tests or comparable minimally invasive diagnostic methods for lung parasites in living porpoises have been established. Thus, it appeared promising to evaluate whether this MSP-ELISA or an MSP-immunoblot may serve as suitable tools for lungworm serodetection in harbour porpoises.
2. Materials and methods
2.1. Retrospective data analysis of harbour porpoise lungworm infections
In the German federal state of Schleswig-Holstein, marine mammal necropsies are routinely performed by the Institute for Terrestrial and Aquatic Wildlife Research (ITAW), University of Veterinary Medicine Hannover, Foundation, as part of the federal state's long-standing stranding network (Siebert et al., 2001, 2006a, 2020). The Institute's database contains comprehensive marine mammal records and samples from Schleswig-Holstein's coasts. To assess the prevalence and level of lungworm infections, a retrospective data analysis was performed for carcasses found along the North Sea coast between 2006 and 2018, whose preservation status allowed respiratory tract assessment. According to their length (Siebert et al., 2001, 2006a) or the tooth growth layer group counts (Lockyer, 1995), animals were classified as immatures (≤100 cm in length/≤0.5 years = neonates or calves; 101–130 cm in length/>0.5–4 years = juveniles) or adults (>130 cm in length/>4 years). The levels of lungworm infections were diagnosed macroscopically by experienced veterinary pathologists during necropsy, rated semiquantitatively as mild, moderate or severe, and their location in pulmonary blood vessels and/or bronchi recorded in necropsy protocols (Siebert et al., 2001; IJsseldijk, Brownlow & Mazzariol, 2019). Lungworm presence was additionally confirmed by histopathological investigations.
Pairwise Fisher's exact tests with subsequent Bonferroni correction of P values were used to compare prevalences between all the study years. Additionally, a possible temporal relationship in lungworm prevalence was investigated using a Spearman's rank correlation of year with percent prevalence. To determine whether the distribution of infection levels (mild, moderate or severe) and lungworm localisation (bronchi, pulmonary blood vessels or both) were significantly different between sexes or age classes, χ2 contingency tests were used. All tests were conducted using R software (version 3.5.2, R Core Team, 2018). A (corrected) P-value ≤0.05 was considered statistically significant.
2.2. Samples for serological analysis
To evaluate the suitability of serological tests for lungworm diagnosis, both whole blood and serum/plasma samples were utilised. Samples originated from Germany, the Netherlands, Denmark and Canada, and included various sample types. Sample import adhered to CITES regulations (permit numbers 17CA04156/CWHQ and 19CA00048/49FONHQ). Post-mortem samples from the ITAW's biobank mainly derived from harbour porpoises collected in different preservation conditions between 1996 and 2018 along the Schleswig-Holstein coasts. Since most animals had been frozen prior to necropsy, the majority were haemolytic whole blood samples in different qualities depending on the carcass condition at time of collection and interim thawing for previous studies on other topics. Samples from lungworm-positive post-mortem cases were subcategorised into mild, moderate and severe infections according to the rating described above (Section 2.1). Samples from live harbour porpoises dated back to 1994 and included sera or plasma collected during routine clinical examinations of harbour porpoises in rehabilitation facilities or permanent human care. Some of these individuals were sampled repeatedly over a longer period. However, for the majority of animals in rehabilitation, two samples were available, one on admission and one prior to being discharged. At the Dolfinarium Harderwijk (the Netherlands), rehabilitation of live stranded harbour porpoises (SOS Dolfijn permit FF/75/2012/036) was performed in two 70 m3 salted freshwater indoor pools, while permanently held animals are kept in an outdoor enclosure consisting of two connected pools (185 m3 and 285 m3, 300 m2 total surface area, 2.6m max. depth, 18 °C max. water temperature). The harbour porpoises are mostly fed herring (Clupea harengus), sprat (Sprattus sprattus) and sometimes mackerel (Scomber scombrus), which have been frozen at −20 °C for a few months up to a maximum of two years before thawing for feeding. The Vancouver Aquarium (Canada) has outside filtered seawater pools, and feeds locally sourced herring (Clupea pallasii), capelin (Mallotus villosus socialis), sardines (Sardinops sagax) and squid that have been frozen at −20 °C for a minimum of 45 days prior to feeding. A total of 494 samples from different age classes were available for the study, assigned to the following categories: (1) lungworm negative (animals born in human care and exclusively fed with previously frozen fish); (2) presumed lungworm negative (negative direct lungworm detection during diagnostic examinations or necropsy and histopathology); (3) lungworm positive (direct lungworm detection during bronchoscopy, necropsy or histopathology); (4) consecutive samples (successive samples covering months or several years from the same individuals in rehabilitation or permanent human care, regardless of infection status); (5) unknown (wild caught animals without clinical history, animals in human care without diagnostic examination or antiparasitically treated animals after treatment date). All samples were stored at −20 °C until serological analyses, while the Vancouver samples were archived at −70 °C prior to shipment.
All samples from living animals in the present study were taken for diagnostic purposes for the benefit of the animals. Thus, no ethical approval was required.
2.3. ELISA for the detection of anti-lungworm antibodies
To adapt and evaluate the MSP-ELISA (von Holtum et al., 2008) for harbour porpoises, a subset of pre-determined positive and negative whole blood and serum samples were analysed. For predefinition of the best-suitable dilutions, five positive and five negative samples were each trialled at dilutions of 1:40 and 1:100 in phosphate buffered saline (PBS; Carl Roth GmbH & Co. KG, Karlsruhe, Germany) containing 0.05% Tween-20™ (Carl Roth GmbH & Co. KG, Karlsruhe, Germany). Following the protocol for lungworm antibody detection in seals established by Ulrich et al. (2015), Nunc® Immobilizer™ Amino-plates were coated with 0.25 μg recombinant GST-MSP/well. 100 μL volume of the respective sample dilutions was added to the wells in duplicate, and plates were incubated and washed as previously described. As secondary antibody, 100 μL recombinant horseradish peroxidase (HRP)-conjugated protein A (Pierce® #32400; Thermo Fisher Scientific GmbH, Dreieich, Germany) diluted 1:5,000, 1:10,000, 1:50,000 and 1:100,000 in PBS-Tween was used. For signal development, 50 μL substrate solution (0.4 mg/mL o-phenylenediamine dihydrochloride in 25 mM citrate [Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany]/50 mM phosphate buffer comprising 0.04% of a 30% hydrogen peroxide solution [Carl Roth GmbH & Co. KG, Karlsruhe, Germany]) was added and incubated in the dark for 10 min at room temperature. The enzymatic reaction was stopped by adding 50 μL 2.5 M sulphuric acid (Carl Roth GmbH & Co. KG, Karlsruhe, Germany) to each well and optical density (OD) was measured at a wavelength of 490 nm with an ELx800 ELISA Reader (BioTek Instruments GmbH, Bad Friedrichshall, Germany). For OD ratios, the OD arithmetic mean of the duplicates was calculated and corrected for the blank OD.
Sample subset analysis revealed a sample dilution of 1:100 and secondary antibody dilution of 1:5000 as most promising for discriminating positive and negative samples, which were hence applied in analyses of a subset of 245 samples, comprising 97 serum/plasma samples (14 negative, 30 presumed negative and 53 positive including 23 consecutive samples) and 148 whole blood samples (48 presumed negative and 100 positive samples).
2.4. Immunoblotting for the detection of anti-lungworm antibodies
To visualise binding of harbour porpoise anti-lungworm serum antibodies to recombinant cattle lungworm (Dictyocaulus viviparus) MSP and to evaluate its suitability as a diagnostic tool, SDS-PAGE and subsequent immunoblotting were performed in accordance with the protocol of Ulrich et al. (2015). Thrombin cleavage of the fusion protein, resulting in a mixture of pure MSP and GST as well as uncleaved fusion protein, was loaded on a 12% gel. Proteins were separated at 150 V for 70 min, transferred onto nitrocellulose membranes (Porablot NCL, 0.45 μm pore size, Macherey-Nagel, Dueren, GmbH & Co. KG, Germany) for 1 h at 100 mA, and washed and blocked in accordance with Ulrich et al. (2015). The blots were then cut into stripes and incubated with negative (n = 17), positive (n = 44) and consecutive (n = 30) porpoise samples diluted at 1:100 in Tris-buffered saline (TBS; Carl Roth GmbH & Co. KG, Karlsruhe, Germany) supplemented with 0.05% Tween-20™ (Carl Roth GmbH & Co. KG, Karlsruhe, Germany) for 1 h at room temperature. As secondary antibody, protein A conjugated to alkaline phosphatase (AP) (Calbiochem® #539251; EMD Millipore/Merck Chemicals GmbH, Darmstadt, Germany) diluted 1:5000 in TBS-Tween was used, and strips were again incubated for 1 h followed by washing in accordance with Ulrich et al. (2015). A positive control using D. viviparus positive cattle serum and a monoclonal anti-bovine IgG antibody (clone BG-18; Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) was run with each blot. For visualising antibody binding to MSP and/or GST, the AP-specific substrate BCIP/NBT (5-bromo-4-chloro-3-indolyl phosphate dipotassium/nitrotetrazolium blue chloride; Carl Roth GmbH & Co. KG, Karlsruhe, Germany) was used. Signal development was allowed for 5 min for each immunoblot.
Since the bands resulting at 1:100 dilution were barely visible, immunoblotting with the total of 494 available harbour porpoise samples (172 positive, 123 negative, 92 unknown and 107 consecutive) was conducted at 1:50 and 1:20 dilution, respectively. The 1:20 dilution was chosen for final evaluation of the test results.
Additionally, protein G (Calbiochem® #539305; EMD Millipore/Merck Chemicals GmbH, Darmstadt, Germany) at a 1:5000 dilution was tested as secondary antibody with a subset of nine harbour porpoise samples (three negative neonate samples with high, moderate and low MSP-ELISA OD values as well as six juvenile individuals positive in bronchoscopy or necropsy; 1:20 dilution) in direct comparison with protein A.
3. Results
3.1. Retrospective data analysis of harbour porpoise lungworm infections
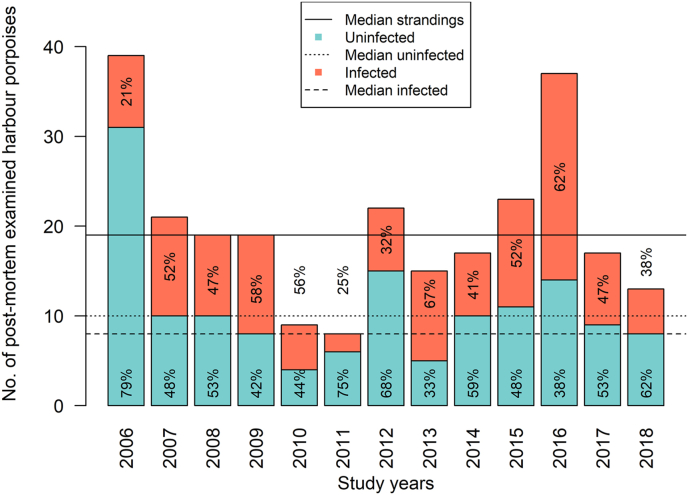
The retrospective evaluation of harbour porpoise necropsy data from 2006 to 2018 included a total of 259 individuals from the Schleswig-Holstein North Sea coast, whose preservation status allowed respiratory tract assessment. Lungworm infection was diagnosed in 45.6% (118/259) of the investigated animals. The annual course of lungworm prevalence is depicted in Fig. 1. Prevalence comparison between all years showed a significantly lower prevalence in 2006 than in 2016 (Fisher's exact test, Bonferroni-corrected P = 0.03). No significant decline or increase in the number of infected individuals over the years was observed (Spearman's rank correlation, P = 0.79).
Fig. 1.
Annual comparison of lungworm prevalence in harbour porpoises stranded along the North Sea coast of the German federal state Schleswig-Holstein between 2006 and 2018. The median of total deaths (19 ± 9.23, black line), uninfected cases (10 ± 6.83, dotted line) and positive cases (8 ± 5.04, dashed line) are additionally depicted.
With 50% each, the sexes (n = 58 males, 59 females) were equally represented among positive individuals. Age distribution among positive animals ranged from neonates to 18-year-old individuals. Age analysis showed that 41.5% (49/118) of the positives were adults, 53.4% (63/118) juveniles and 4.2% (5/118) neonates/calves, while in one infected animal (0.9%), age and sex could not be determined due to advanced decomposition. Hence, 57.6% (68/118) of the lungworm-positive stranded harbour porpoises in the investigated 13-year period were immature.
Analysis of infection level showed similar percentages of severe (39.0%; 46/118) and moderate (38.1%; 45/118) infections, while mild infections were only diagnosed in 22.9% (27/118) of the animals (Table 1). Immature and adult animals contributed similarly to severe (54.3% [25/46] vs. 43.4% [20/46]) and moderate (51.1% [23/45] vs. 48.9% [22/45]) infections, while the majority of mild infections were detected in immatures (74.1% [20/27]). No significant differences in the distribution of infection severity levels were found between sexes (χ2 = 2.681, P = 0.26) or different age classes (χ2 = 3.853, P = 0.15).
Table 1.
Lungworm infection intensity in harbour porpoises stranded along the Schleswig-Holstein North Sea coast between 2006 and 2018 (n = 118), sorted by level of infection in relation to age class and sex. Age and sex of one severely infected individual could not be determined due to decomposition status.
| Total no. (%) | No. of females (%) | No. of males (%) | |
|---|---|---|---|
| Mild Infection | 27 (22.9%) | 12 (44.4%) | 15 (55.6%) |
| Neonates/calves | 2 (7.4%) | 1 (50.0%) | 1 (50.0%) |
| Juveniles | 18 (66.7%) | 8 (44.4%) | 10 (55.6%) |
| Adults |
7 (25.9%) |
3 (42.9%) |
4 (57.1%) |
| Moderate Infection | 45 (38.1%) | 20 (44.4%) | 25 (55.6%) |
| Neonates/calves | 2 (4.4%) | – | 2 (100%) |
| Juveniles | 21 (46.7%) | 10 (47.6%) | 11 (52.4%) |
| Adults |
22 (48.9%) |
10 (45.5%) |
12 (54.5%) |
| Severe Infection | 46 (39.0%) | 27 (58.7%) | 18 (39.1%) |
| Neonates/calves | 1 (2.2%) | 1 (100%) | – |
| Juveniles | 24 (52.2%) | 14 (58.3%) | 10 (41.7%) |
| Adults | 20 (43.4%) | 12 (60.0%) | 8 (40.0%) |
| Unknown | 1 (2.2%) | n.a. | n.a. |
n.a. = not available.
Of all infected individuals, 85.6% (101/118) revealed a mixed infection of the bronchial tree and pulmonary vessels, while 11.9% (14/118) showed parasites only in the bronchial tree and 2.5% (3/118) only in the pulmonary vessels. There were no significant differences in the proportion of animals with lungworms in bronchi or pulmonary vessels only, or in both localisations according to age (χ2 = 4.295, P = 0.12; χ2 = 0.737, P = 0.69) or sex (χ2 = 0.876, P = 0.65; χ2 = 3.071, P = 0.22).
3.2. Samples for serological analysis
A total of 494 samples were available for serological analysis, divided into 302 serum/plasma and 192 whole blood samples. The assigned lungworm infection status is given in Table 2. Of the 125 lungworm-positive whole blood samples, 22 originated from severe, 49 from moderate and 54 from mild lungworm infections, while six serum samples could be categorised into three severe and three moderate infections.
Table 2.
Overview of the composition of the 494 samples available for serological analyses.
| Infection status | No. of serum/plasma samples (%) | No. of whole blood samples (%) | No. of total samples (%) |
|---|---|---|---|
| Lungworm negative | 20 (6.6%) | – | 20 (4.0%) |
| Presumed lungworm negative | 52 (17.2%) | 51 (26.6%) | 103 (20.9%) |
| Lungworm positive | 47 (15.6%) | 125 (65.1%) | 172 (34.8%) |
| Consecutive samples | 107 (35.4%) | – | 107 (21.7%) |
| Unknown |
76 (25.2%) |
16 (8.3%) |
92 (18.6%) |
| Total | 302 (61.1%) | 192 (38.9%) | 494 (100%) |
3.3. ELISA for the detection of anti-lungworm antibodies
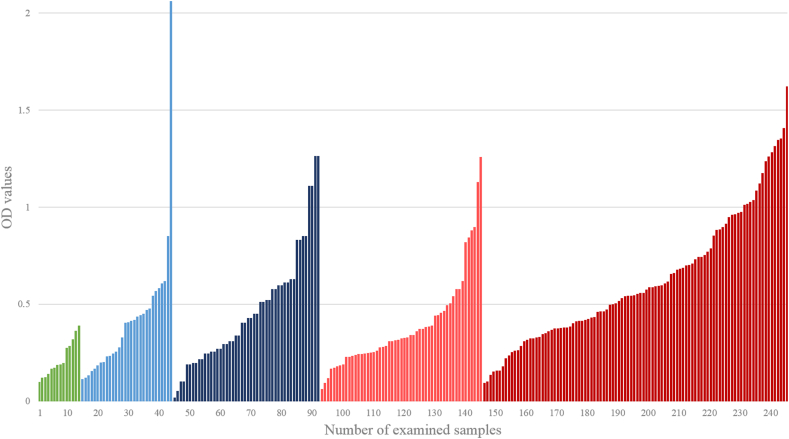
MSP-ELISA analysis of the 245 samples from harbour porpoises assignable to a positive or (presumed) negative lungworm infection status resulted in the following mean OD values: 0.211 OD for lungworm negative sera/plasma from animals born in human care (range: 0.094–0.385 OD), 0.415 OD for presumed lungworm negative sera/plasma (range: 0.108–2.056 OD), 0.463 OD for presumed lungworm negative whole blood (range: 0.013–1.257 OD), 0.387 OD for lungworm positive sera/plasma (range: 0.056–1.253 OD) and 0.615 OD for lungworm positive whole blood (range: 0.088–1.616 OD). Detailed OD patterns are pictured in Fig. 2. Since a multitude of positive samples covered the OD range of the negatives, no reliable cut-off value could be determined, and further ELISA analysis of the remaining samples was omitted.
Fig. 2.
MSP-ELISA results of the 245 samples from harbour porpoises assignable to a specific infection status. Green = lungworm negative (born in captivity); light blue = presumed lungworm negative sera (negative direct detection); dark blue = presumed lungworm negative whole blood (negative direct detection); light red = lungworm positive sera (direct lungworm detection); dark red = lungworm positive whole blood (direct lungworm detection). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4. Immunoblotting for the detection of anti-lungworm antibodies
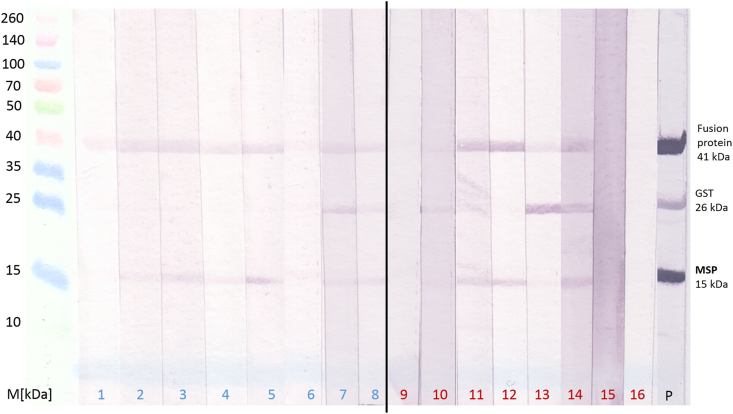
Electrophoretic separation of the thrombin-cleaved GST-MSP fusion protein produced bands of approximately 40 kDa (fused GST-MSP), 26 kDa (GST) and 14 kDa (MSP), respectively. However, immunoblot analysis of the 295 (presumed) lungworm negative or positive harbour porpoise samples did not show a specific band pattern within the different infection groups, which are exemplarily presented in Fig. 3. To investigate whether the choice of the sample type (serum/plasma or whole blood) could improve results, the band pattern of 11 animals with both sample types were compared. Whole blood results were often less intense but showed a similar pattern to the respective sera (Table 3).
Fig. 3.
Immunoblot pattern of (presumed) lungworm negative (lane numbers indicated in blue: 1–5 = animals born in human care; 2–5 sampled over three consecutive years; 6–8 = no infection detected) as well as lungworm positive (lane numbers indicated in red: lane 9 = moderate infection; lane 10 = severe infection; lanes 11–16 = direct lungworm detection) harbour porpoise sera. Lane 17 = D. viviparus positive control serum, M = Spectra™ Multicolour Broad Range Protein Ladder (Thermo Fisher Scientific GmbH, Dreieich, Germany). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 3.
Immunoblot results of the 11 animals of which both serum and whole blood (or a blood clot) was available. Band intensity was rated as follows: 0 = not visible, (1) = faint, 1 = weak, 2 = moderate, 3 = strong.
| Lungworm infection status | Sample | Blot result |
Comments | Sampling year | ||
|---|---|---|---|---|---|---|
| MSP | GST-MSP | GST | ||||
| Presumed negative | serum | (1) | (1) | (1) | neonate, no infection diagnosed at necropsy | 2000 |
| whole blood | (1) | (1) | (1) | |||
| Presumed negative | serum | 0 | (1) | 0 | neonate, no infection diagnosed at necropsy | 2002 |
| whole blood | (1) | (1) | 0 | |||
| Presumed negative | serum | 0 | 1 | 2 | neonate, no infection diagnosed at necropsy | 2016 |
| whole blood | 0 | (1) | 1 | |||
| Presumed negative | serum | (1) | 2 | 1 | neonate, no infection diagnosed at necropsy | 2018 |
| whole blood | 0 | (1) | 2 | |||
| Presumed negative | serum | 0 | 0 | 0 | juvenile, no infection diagnosed, biannual antiparasitic treatment | 1999 |
| whole blood | 0 | 0 | 0 | |||
| Presumed negative | serum | 1 | 2 | 1 | juvenile, no infection diagnosed | 2013 |
| whole blood | 0 | 1 | 1 | |||
| Positive | serum | 1 | 2 | 2 | adult, positive bronchoscopy | 2013 |
| whole blood | 1 | 1 | 1 | |||
| Positive | serum | 0 | (1) | 0 | adult, mild infection at necropsy | 2000 |
| whole blood | 0 | (1) | 0 | |||
| Positive | serum | 1 | (1) | 2 | adult, moderate infection at necropsy | 2016 |
| whole blood | (1) | (1) | 1 | |||
| Positive | serum | 1 | 0 | 1 | adult, moderate infection at necropsy | 2017 |
| blood clot | 0 | (1) | 0 | |||
| Unknown | serum | (1) | (1) | (1) | juvenile, no lung infection at necropsy, mild infection of peribullar sinuses | 2000 |
| blood clot | 0 | (1) | 0 | |||
Overall, immunoblotting did not allow differentiation between negative and positive samples, as the recombinant diagnostic antigen (pure MSP or the GST-MSP fusion protein) was also recognised by (presumed) negative samples and band intensities were not consistently weaker than in positive samples. Additionally, many lungworm positive sera did not react with the diagnostic antigen, neither as pure nor fused MSP. Thus, reliable assignment of the 92 unknown samples failed. A summary of the band pattern analysis of the 494 samples is given in Table 4.
Table 4.
Overview of the band recognition pattern in immunoblot analyses.
| Lungworm infection status | No. of total samples | No. recognising MSP (%) | No. recognising GST-MSP (%) | No. recognising GST (%) |
|---|---|---|---|---|
| Negative | 20 | 5 (25.0%) | 13 (65.0%) | 2 (10.0%) |
| Presumed negative | 103 | 33 (32.0%) | 78 (75.7%) | 77 (63%) |
| Neonates/calves | 48 | 13 (27.1%) | 35 (72.9%) | 34 (70.8%) |
| Juveniles | 45 | 18 (40.0%) | 36 (80.0%) | 36 (80.0%) |
| Adults | 10 | 2 (20.0%) | 7 (70.0%) | 5 (50.0%) |
| Positive | 172 | 95 (55.2%) | 142 (82.6%) | 140 (81.4%) |
| Neonates/calves | 6 | 2 (33.3%) | 3 (50.0%) | 5 (83.3%) |
| Juveniles | 124 | 73 (58.9%) | 106 (85.5%) | 105 (84.7%) |
| Adults | 42 | 20 (47.6%) | 33 (78.6%) | 30 (71.4%) |
| Consecutive samples | 107 | 21 (19.6%) | 46 (43.0%) | 24 (22.4%) |
| Neonates/calves | 2 | - | - | - |
| Juveniles | 53 | 10 (18.9%) | 29 (54.7%) | 19 (35.8%) |
| Adults | 52 | 11 (21.2%) | 17 (32.7%) | 2 (3.8%) |
| Unknown | 92 | 31 (33.7%) | 65 (70.7%) | 52 (56.5%) |
| Neonates/calves | 5 | 1 (20.0%) | 3 (60.0%) | 2 (40.0%) |
| Juveniles | 42 | 12 (28.6%) | 31 (73.8%) | 25 (59.5%) |
| Adults | 8 | 3 (37.5%) | 4 (50.0%) | 3 (37.5%) |
| Unknown |
37 |
15 (40.5%) |
27 (73.0%) |
22 (59.5%) |
| Total | 494 | 185 (37.4%) | 344 (69.6%) | 293 (59.3%) |
Notably, using protein G as secondary antibody in a subset of nine (three negatives, six positives) samples did not result in any bands, while six of the samples produced bands when protein A was the secondary antibody.
4. Discussion
Lungworm infections and associated secondary bronchopneumonia are a major cause of harbour porpoise mortality in the North Sea (Siebert et al., 2001; 2006a; Jauniaux et al., 2002; Lehnert et al., 2005; ten Doeschate et al., 2017; van Elk et al., 2019). Here, the lungworm prevalence in North Sea harbour porpoises was retrospectively analysed over a 13-year period based on necropsies of stranded individuals, resulting in a mean prevalence of 45.6%. Annual prevalences ranged from 21% in 2006 to 62% in 2016, which were the only years with a significant difference in prevalence during this study period. Additionally, both 2006 and 2016 were outlier years, clearly deviating from the median of 19 stranded animals with a higher stranding occurrence than usual, while in 2010 and 2011, fewer stranded animals than usual were found. The general prevalence of 45.6% (n = 118) in harbour porpoises from the German North Sea in the present study is similar to the value of 40% (n = 126) found in harbour porpoises from the German Baltic Sea between 1990 and 2015 (Siebert et al., 2020), while 62.4% (n = 83) of harbour porpoises from both German coasts were lungworm infected between 1991 and 1996 (Siebert et al., 2001). Prevalences of 68% described for the Netherlands (ten Doeschate et al., 2017), 58% for Poland (Siebert et al., 2020) and 57%–60% described for Denmark (Lockyer and Kinze, 2003; Siebert et al., 2020) were slightly higher than those observed in the present study, probably influenced by the age structure. Further northwards, markedly higher prevalences, but mostly only mild infections with mild associated lesions, were determined, with values ranging from 80% to 98% in Norway (Balbuena et al., 1994; Siebert et al., 2006b), 83% in Iceland (Siebert et al., 2006b) and 95% in West Greenland (Lehnert et al., 2014).
Additionally, an opposing overall distribution between infection levels can be observed: mild infections were detected in 22.9% of positive animals in the present study, while between 59% and 68% of Norwegian, Icelandic and West Greenlandic animals showed only mild lesions (Siebert et al., 2006b; Lehnert et al., 2014). Furthermore, animals from Norway and Iceland did not exhibit severe levels of lungworm infection (Lehnert et al., 2005; Siebert et al., 2006b), while this was the most common finding in 39% of animals in the present analysis. The reasons for this clear discrepancy may be influenced by the consumption of different prey and/or intermediate host species in the different geographical regions with their respective oceanographic conditions (Lehnert et al., 2014), but more likely reflects the impaired health status of harbour porpoises in the Baltic and North Sea (Siebert et al., 2001, 2006a, 2020; Lehnert et al., 2005). Similarly, the discrepancy between roughly 46% infected and 54% uninfected harbour porpoises in the present study might be explained with a different age structure, varying prey species and underlying health conditions, though all animals share the same environment.
Infection of all harbour porpoise age classes as previously observed (e.g. Jepson et al., 2000; Siebert et al., 2001; 2006b; 2020; Jauniaux et al., 2002; van Elk et al., 2019) was confirmed by the investigated dataset, with 57.6% of the positive individuals being immature, i.e. under four years of age, and 41.5% being adults. The regular finding of positive adults suggests that harbour porpoises do not develop protective immunity against lungworms, enabling lifelong infection as proposed previously (Lehnert et al., 2014; ten Doeschate et al., 2017). However, it remains unclear if observed infections in adult harbour porpoises represent persistent infections acquired as immatures and not cleared by the immune system, while with advanced age, they might be protected against reinfections by immune trapping of newly invading lungworm larvae. Moreover, it is uncertain whether they represent (cumulative) reinfections during adulthood due to insufficient or lacking immunity. Similar to terrestrial mammals, cetacean immune response against invading pathogens is regulated by innate and adaptive immunity. Nonetheless, specific data on antibody-based immunity of harbour porpoises is currently not available (Levin, 2018; Di Guardo et al., 2018, 2019). However, age distribution of infection levels may indicate that adult harbour porpoises suffer from cumulative reinfections, as mild infections occurred generally more often in immatures than in adults.
One of the factors influencing lungworm pathogenicity is their location within the lungs and perhaps species-specific differences in pathogenicity. Due to their size, P. inflexus (females: 120–160 mm long, 1.4 mm wide) and T. convolutus (females: 31–67 mm long, 0.4 mm wide) can cause airway occlusion, endocarditis and thrombosis (Gibson et al., 1998; Jepson et al., 2000; Siebert et al., 2001, 2020). In contrast, lungworms residing within the pulmonary parenchyma like H. invaginatus might be more pathogenic due to their potential to cause abscessation and granulomatous reactions (Measures, 2001, 2018). However, very few studies assessed the different lungworm species separately, and it is mostly unclear whether all species were recorded during these previous investigations. In the present study, the vast majority of 85.6% of the examined animals harboured lungworms in both the bronchi and the pulmonary vessels. High levels of lungworm co-occurrence has also been previously described (Balbuena et al., 1994; Lehnert et al., 2005).
Lungworm infections are commonly associated with secondary bacteriosis (Jepson et al., 2000; Siebert et al., 2001, 2009; Lehnert et al., 2005, 2014), which may compromise the animals to varying degrees, since impaired lung function can cause systemic effects due to respiratory stress and increasing metabolic costs (Agustí et al., 2003). In view of the importance of lungworm infections in harbour porpoises, a further aim of this study was to evaluate serodiagnosis as a sensitive and minimally invasive method for general health monitoring or rehabilitation management. Faecal detection of lungworm larvae is a non-invasive technique. Faecal collection, however, remains logistically challenging and test sensitivity is questionable, since larval excretion in bottlenose dolphins (Tursiops truncatus) appeared to be sporadic (Fauquier et al., 2009), concordant to shedding patterns in terrestrial carnivores (Ribeiro and Lima, 2001; Al-Sabi et al., 2010; Elsheikha et al., 2016). Thus, we aimed to adapt an ELISA developed for detecting anti-lungworm antibodies in cattle (von Holtum et al., 2008) to harbour porpoises. As the nematode-specific male major sperm protein is highly conserved among different genera (Schnieder, 1992; Hojas and Post, 2000; Strube et al., 2009; Ulrich et al., 2015; Zottler et al., 2017), D. viviparus MSP as antigen has been successfully validated for metastrongylid lungworm detection in cats as well as in harbour and grey seals (Ulrich et al., 2015; Zottler et al., 2017) and suitability of whole blood samples was proven in the adapted seal MSP-ELISA (Ulrich et al., 2015), it seemed reasonable to assume that the MSP-ELISA would be a suitable tool for detecting antibodies against metastrongyloid/pseudaliid (Metastrongyloidea) harbour porpoise lungworms.
However, the MSP-ELISA was not able to reliably discriminate between predefined lungworm negative and positive harbour porpoise samples, as a larger number of positive serum/plasma as well as whole blood samples showed OD values in the negative range. Furthermore, OD distribution between positive and presumed negative samples was similar. Even though one might explain the low OD in positive samples with prepatent infections or low immune responders, and the high OD of presumed negative samples with persisting antibodies after elimination of the parasites or a misdiagnosed actual infection, it appeared most likely that reliable discrimination failed due to undesired cross-reactions.
To clarify whether the ELISA results reflect antibody binding to MSP or non-specific binding, e.g. to the N-terminal GST-tag, which allows easy and efficient purification of the recombinants, or co-purified proteins of the Escherichia coli expression system, immunoblot analyses were performed. Additionally, we were confident that in case of failure of the ELISA due to non-specific binding, immunoblot analysis could be a suitable alternative serodiagnostic test by assessing the MSP band, which should be clearly visible or strong in lungworm positive, but missing or very faint in negative harbour porpoises. Contrary to our expectations, however, only seven positive but two negative samples showed striking MSP band intensity, and only 55% of the lungworm positive samples recognised the MSP band. This percentage is too low to be explained by prepatency as it is unlikely that nearly half of the harbour porpoises did not harbour adult lungworms, of which male individuals express MSP as a sperm component, restricting the MSP-ELISA to the detection of patent infections.
Additionally, 25% of the samples from animals born in human care and raised with frozen fish, i.e. lungworm negatives, as well as a number of consecutive samples of these negatives showed undesired MSP binding. Also, about one third of the presumed lungworm negative samples recognised MSP. Persisting antibodies, maternal antibody transmission or misdiagnosed infections may account for this finding. However, it is currently unknown whether harbour porpoises even produce viable anti-lungworm antibodies, hence it remains questionable if maternal transmission of these antibodies occurs. Nonetheless, also potential cross-reactions with other nematode infections cannot be ruled out, even though ELISA evaluation in cattle and seals did not show MSP recognition of animals infected with nematodes other than lungworms (von Holtum et al., 2008; Ulrich et al., 2015). Since no blood samples were available from individuals with single-species infections, it was not possible to evaluate if different lungworm species could have induced different humoral immune responses against the used antigen.
Notably, in all categories, the percentage of samples binding to GST was substantially higher than that binding to MSP. Logically, the highest percentages bound to the MSP-GST fusion protein. This reaction pattern could be due to cross-reactions of antibodies raised against helminth GST. However, as observed for MSP, even (consecutive) samples from animals born and raised in captivity showed reactions to GST, indicating non-specific reactions. Here, low sample dilution (1:20) and/or the fairly long signal development time required to visualise protein bands might account for non-specific binding of the protein A conjugate and visualisation of false positives. Additionally, potential influencing factors like varying sample quality from stranded animals in a more advanced state of decomposition or samples that had undergone freeze-thaw cycles over the years for other studies cannot explain the comprehensive picture of irregular band recognition pattern of the entire sample set.
Overall, the obtained ELISA and immunoblot data suggest that harbour porpoise lungworm MSP is not exposed to or not sufficiently recognised by the host's immune system, contrary to other hitherto examined species (von Holtum et al., 2008; Ulrich et al., 2015; Zottler et al., 2017). Hence, both MSP-ELISA and immunoblot presented in this study cannot be recommended as diagnostic tools for detecting lungworm infection in harbour porpoises. The failure of both tests might mirror the special characteristics of lungworm infection in harbour porpoises, i.e. an incapacity to develop an effective immune response against lungworms and to clear infections as proposed by ten Doeschate et al. (2017). Nevertheless, future attempts to develop serological tests for lungworm detection in harbour porpoises, either detecting anti-lungworm antibodies or lungworm antigens are desirable to provide a sensitive, minimally invasive tool for health monitoring purposes.
5. Conclusions
This study confirmed that pseudaliid lungworm infection regularly occurs in stranded German North Sea harbour porpoises. Retrospective data analysis over a 13-year period showed an annual median of 19 stranded harbour porpoises, with a total of 45.6% of all investigated animals being lungworm infected, 85.6% of which had coinfections of bronchi and pulmonary blood vessels. In contrast to previous studies, severe infections were frequently detected in the investigated harbour porpoises. Serological tests would constitute a valuable tool to reveal epidemiological dynamics of lungworm infections in wild harbour porpoises and additionally aid in diagnostics in animals in human care. However, both the evaluated MSP-ELISA and MSP-immunoblot proved unsuitable as lungworm serodiagnostic tests.
Declarations of interest
All authors declare that they have no competing interests.
Acknowledgements
The authors wish to thank Stichting SOS Dolfijn, especially Annemarie van den Berg and Jolanda Meerbeek, the Dolfinarium Harderwijk, as well as Sion Cahoon from Vancouver Aquarium for their help with samples and background data. The excellent technical assistance of Kornelia Wolff-Schmidt, Miriam Hillmann, Ursula Küttler and Daniela Jordan is gratefully acknowledged. This publication was supported by Deutsche Forschungsgemeinschaft and the University of Veterinary Medicine Hannover, Foundation within the funding programme Open Access Publishing.
References
- Agustí A.G.N., Noguera A., Sauleda J., Sala E., Pons J., Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur. Respir. J. 2003;21:347–360. doi: 10.1183/09031936.03.00405703. [DOI] [PubMed] [Google Scholar]
- Al-Sabi M.N.S., Deplazes P., Webster P., Willesen J.L., Davidson R.K., Kapel C.M.O. PCR detection of Angiostrongylus vasorum in faecal samples of dogs and foxes. Parasitol. Res. 2010;107:135–140. doi: 10.1007/s00436-010-1847-5. [DOI] [PubMed] [Google Scholar]
- Arnold P.W., Gaskin D.E. Lungworms (Metastrongyloidea: Pseudaliidae) of harbor porpoise phocoena phocoena (L. 1758) Can. J. Zool. 1975;53:713–735. doi: 10.1139/z75-087. [DOI] [PubMed] [Google Scholar]
- Baker J.R., Martin A.R. Causes of mortality and parasites and incidental lesions in harbour porpoises (Phocoena phocoena) from British waters. Vet. Rec. 1992;130:554–558. doi: 10.1136/vr.130.25.554. [DOI] [PubMed] [Google Scholar]
- Balbuena J.A., Aspholm P.E., Andersen K.I., Bjørge A. Lung-worms (Nematoda: Pseudaliidae) of harbour porpoises (Phocoena phocoena) in Norwegian waters: patterns of colonization. Parasitology. 1994;108(3):343–349. doi: 10.1017/S0031182000076186. [DOI] [PubMed] [Google Scholar]
- Benke H., Siebert U., Lick R., Bandomir B., Weiss R. The current status of harbour porpoises (Phocoena phocoena) in German waters. Arch. Fish. Mar. Res. 1998;46:97–123. [Google Scholar]
- Bergeron E., Huot J., Measures L.N. Experimental transmission of Otostrongylus circumlitus (Railliet, 1899) (Metastrongyloidea: Crenosomatidae), a lungworm of seals in eastern arctic Canada. Can. J. Zool. 1997;75:1364–1371. doi: 10.1139/z97-762. [DOI] [Google Scholar]
- Bishop L. Parasite-related lesions in a bearded seal. J. Wildl. Dis. 1979;15:285–293. doi: 10.7589/0090-3558-15.2.285. [DOI] [PubMed] [Google Scholar]
- Bjørge A., Tolley K.A. Harbor porpoise: phocoena phocoena. In: Perrin W.F., Würsig B., Thewissen J.G.M., editors. Encyclopedia of Marine Mammals. Elsevier; Amsterdam: 2018. pp. 448–451. [DOI] [Google Scholar]
- Bolt G., Monrad J., Koch J., Jensen A. Canine angiostrongylosis: a review. Vet. Rec. 1994;135:447–452. doi: 10.1136/vr.135.19.447. [DOI] [PubMed] [Google Scholar]
- Camphuysen C.J., Krop A. Maternal care, calf-training and site fidelity in a wild harbour porpoise in the North Sea. Lutra. 2011;54:123–126. [Google Scholar]
- Dailey M., Stroud R. Parasites and associated pathology observed in cetaceans stranded along the Oregon coast. J. Wildl. Dis. 1978;14:503–511. doi: 10.7589/0090-3558-14.4.503. [DOI] [PubMed] [Google Scholar]
- Dailey M.D. Proc. Helminthol. Soc. Wash. 1970. The transmission of Parafilaroides decorus (Nematoda: Metastrongyloidea) in the California sea lion (Zalophus californianus) pp. 215–222. [Google Scholar]
- Delyamure S.L. In: Helminthofauna of Marine Mammals; Ecology and Phylogeny. (Gelʹmintofauna Morskikh Mlekopitayushchikh V Svete Ikh Ekologii I Filogenii), 1968th. Academy of Science of the USSR, editor. Laboratory of Helminthology; 1955. [Google Scholar]
- Di Guardo G., Centelleghe C., Mazzariol S. Cetacean host-pathogen interaction(s): critical knowledge gaps. Front. Immunol. 2018;9:2815. doi: 10.3389/fimmu.2018.02815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Guardo G., Criscitiello M.F., Sierra E., Mazzariol S. Frontiers Media SA; Lausanne: 2019. Comparative Immunology of Marine Mammals. Frontiers Research Topics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty E. The lungworms (Nematoda: Pseudaliidae) of the Odontoceti. Part I. Parasitology. 1944;36:80–94. doi: 10.1017/S0031182000012014. [DOI] [Google Scholar]
- Elsheikha H.M., Schnyder M., Traversa D., Di Cesare A., Wright I., Lacher D.W. Updates on feline aelurostrongylosis and research priorities for the next decade. Parasites Vectors. 2016;9:389. doi: 10.1186/s13071-016-1671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauquier D.A., Kinsel M.J., Dailey M.D., Sutton G.E., Stolen M.K., Wells R.S., Gulland F.M.D. Prevalence and pathology of lungworm infection in bottlenose dolphins Tursiops truncatus from southwest Florida. Dis. Aquat. Org. 2009;88:85–90. doi: 10.3354/dao02095. [DOI] [PubMed] [Google Scholar]
- Gibson D.I., Harris E.A., Bray R.A., Jepson P.D., Kuiken T., Baker J.R., Simpson V.R. A survey of the helminth parasites of cetaceans stranded on the coast of England and Wales during the period 1990–1994. J. Zool. 1998;244:563–574. doi: 10.1111/j.1469-7998.1998.tb00061.x. [DOI] [Google Scholar]
- Hammond P.S., Berggren P., Benke H., Borchers D.L., Collet A., Heide-Jørgensen M.P., Heimlich S., Hiby A.R., Leopold M.F., Øien N. Abundance of harbour porpoise and other cetaceans in the North Sea and adjacent waters. J. Appl. Ecol. 2002;39:361–376. doi: 10.1046/j.1365-2664.2002.00713.x. [DOI] [Google Scholar]
- Hammond P.S., Macleod K., Berggren P., Borchers D.L., Burt L., Cañadas A., Desportes G., Donovan G.P., Gilles A., Gillespie D., Gordon J., Hiby L., Kuklik I., Leaper R., Lehnert K., Leopold M., Lovell P., Øien N., Paxton C.G.M., Ridoux V., Rogan E., Samarra F., Scheidat M., Sequeira M., Siebert U., Skov H., Swift R., Tasker M.L., Teilmann J., Van Canneyt O., Vázquez J.A. Cetacean abundance and distribution in European Atlantic shelf waters to inform conservation and management. Biol. Conserv. 2013;164:107–122. doi: 10.1016/j.biocon.2013.04.010. [DOI] [Google Scholar]
- Hojas R.M., Post R.J. Regional genetic variation in the major sperm protein genes of Onchocerca volvulus and Mansonella ozzardi (Nematoda: Filarioidea) Int. J. Parasitol. 2000;30:1459–1465. doi: 10.1016/S0020-7519(00)00117-X. [DOI] [PubMed] [Google Scholar]
- Houde M., Measures L.N., Huot J. Experimental transmission of Pharurus pallasii (Nematoda: Metastrongyloidea), a lungworm of the cranial sinuses of the beluga whale (Delphinapterus leucas), to fish. Can. J. Zool. 2003;81:364–370. doi: 10.1139/z03-016. [DOI] [Google Scholar]
- Hunt K.E., Moore M.J., Rolland R.M., Kellar N.M., Hall A.J., Kershaw J., Raverty S.A., Davis C.E., Yeates L.C., Fauquier D.A., Rowles T.K., Kraus S.D. Overcoming the challenges of studying conservation physiology in large whales: a review of available methods. Conserv. Physiol. 2013;1:cot006. doi: 10.1093/conphys/cot006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICES . International Council for the Exploration of the Sea; Barcelona, Spain: 2020. Working Group on Marine Mammal Ecology (WGMME), ICES Scientific Reports. [DOI] [Google Scholar]
- IJsseldijk L.L., Brownlow A.C., Mazzariol S., editors. Best Practice on Cetacean Post Mortem Investigation and Tissue Sampling. Principality of Monaco: ASCOBANS/ACCOBAMS; Bonn, Germany: 2019. [DOI] [Google Scholar]
- Jauniaux T., Petitjean D., Brenez C., Borrens M., Brosens L., Haelters J., Tavernier T., Coignoul F. Post-mortem findings and causes of death of harbour porpoises (Phocoena phocoena) stranded from 1990 to 2000 along the coastlines of Belgium and Northern France. J. Comp. Pathol. 2002;126:243–253. doi: 10.1053/jcpa.2001.0547. [DOI] [PubMed] [Google Scholar]
- Jepson P.D., Kuiken T., Bennett P.M., Baker J.R., Simpson V.R., Kennedy S. Pulmonary pathology of harbour porpoises (Phocoena phocoena) stranded in England and Wales between 1990 and 1996. Vet. Rec. 2000;146:721–728. doi: 10.1136/vr.146.25.721. [DOI] [PubMed] [Google Scholar]
- Kastelein R.A., Bakker M.J., Dokter T. The medical treatment of 3 stranded Harbour porpoises (Phocoena phocoena) Aquat. Mamm. 1990;15:181–202. [Google Scholar]
- Kesselring T., Viquerat S., Brehm R., Siebert U. Coming of age: do female harbour porpoises (Phocoena phocoena) from the North Sea and Baltic Sea have sufficient time to reproduce in a human influenced environment? PloS One. 2017;12 doi: 10.1371/journal.pone.0186951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinertz S., Hermosilla C., Ziltener A., Kreicker S., Hirzmann J., Abdel-Ghaffar F., Taubert A. Gastrointestinal parasites of free-living Indo-Pacific bottlenose dolphins (Tursiops aduncus) in the Northern Red Sea, Egypt. Parasitol. Res. 2014;113:1405–1415. doi: 10.1007/s00436-014-3781-4. [DOI] [PubMed] [Google Scholar]
- Lehnert K., Raga J., Siebert U. Macroparasites in stranded and bycaught harbour porpoises from German and Norwegian waters. Dis. Aquat. Org. 2005;64:265–269. doi: 10.3354/dao064265. [DOI] [PubMed] [Google Scholar]
- Lehnert K., Seibel H., Hasselmeier I., Wohlsein P., Iversen M., Nielsen N.H., Heide-Jørgensen M.P., Prenger-Berninghoff E., Siebert U. Increase in parasite burden and associated pathology in harbour porpoises (Phocoena phocoena) in West Greenland. Polar Biol. 2014;37:321–331. doi: 10.1007/s00300-013-1433-2. [DOI] [Google Scholar]
- Lehnert K., von Samson-Himmelstjerna G., Schaudien D., Bleidorn C., Wohlsein P., Siebert U. Transmission of lungworms of harbour porpoises and harbour seals: Molecular tools determine potential vertebrate intermediate hosts. Int. J. Parasitol. 2010;40:845–853. doi: 10.1016/j.ijpara.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Leopold M.F. Wageningen University; 2015. Eat and Be Eaten − Porpoise Diet Studies.https://www.wur.nl/en/activity/Eat-and-be-eaten-porpoise-diet-studies-1.htm Doctoral thesis. [Google Scholar]
- Levin M. Marine mammal immunology. In: Gulland F.M.D., Dierauf L.A., Whitman K.L., editors. CRC Handbook of Marine Mammal Medicine. third ed. CRC Press; Boca Raton: 2018. pp. 209–230. [Google Scholar]
- Lockyer C. A review of factors involved in zonation in odontocete teeth, and an investigation of the likely impact of environmental factors and major life events on harbour porpoise tooth structure. In: Bjørge A., Donovan G.P., editors. The Biology of the Phocoenids. Rep. Int. Whal. Commn. Special issue 16; Cambridge, UK: 1995. pp. 511–529. [Google Scholar]
- Lockyer C., Kinze C. Status, ecology and life history of harbour porpoise (Phocoena phocoena), in Danish waters. NAMMCO Sci. Publ. 2003;5:143–175. doi: 10.7557/3.2745. [DOI] [Google Scholar]
- Measures L.N. Lungworms of marine mammals. In: Samuel W.M., Pybus M.J., Kocan A.A., editors. Parasitic Diseases of Wild Mammals. Iowa State University Press; Ames: 2001. pp. 279–300. [DOI] [Google Scholar]
- Measures L.N. Helminths and parasitic arthropods. In: Gulland F.M.D., Dierauf L.A., Whitman K.L., editors. CRC Handbook of Marine Mammal Medicine. third ed. CRC Press; Boca Raton: 2018. pp. 471–497. [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2018. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- Ribeiro V.M., Lima W.S. Larval production of cats infected and re-infected with Aelurostrongylus abstrusus (Nematoda: Protostrongylidae) Rev. Med. Vet. 2001;152:815–820. [Google Scholar]
- Santos M., Pierce G. The diet of harbour porpoise (Phocoena phocoena) in the northeast Atlantic. In: Gibson R.N., Atkinson R.J.A., editors. vol. 41. Taylor and Francis; London and New York: 2003. pp. 355–390. (Oceanography and Marine Biology: an Annual Review). [Google Scholar]
- Schnieder T. Use of a recombinant Dictyocaulus viviparus antigen in an enzyme-linked immunosorbent assay for immunodiagnosis of bovine dictyocaulosis. Parasitol. Res. 1992;78:298–302. doi: 10.1007/BF00937087. [DOI] [PubMed] [Google Scholar]
- Siebert U., Gilles A., Lucke K., Ludwig M., Benke H., Kock K.-H., Scheidat M. A decade of harbour porpoise occurrence in German waters - analyses of aerial surveys, incidental sightings and strandings. J. Sea Res. 2006;56:65–80. doi: 10.1016/j.seares.2006.01.003. [DOI] [Google Scholar]
- Siebert U., Pawliczka I., Benke H., von Vietinghoff V., Wolf P., Pilāts V., Kesselring T., Lehnert K., Prenger-Berninghoff E., Galatius A., Anker Kyhn L., Teilmann J., Hansen M.S., Sonne C., Wohlsein P. Health assessment of harbour porpoises (PHOCOENA PHOCOENA) from Baltic area of Denmark, Germany, Poland and Latvia. Environ. Int. 2020;143:105904. doi: 10.1016/j.envint.2020.105904. [DOI] [PubMed] [Google Scholar]
- Siebert U., Prenger-Berninghoff E., Weiss R. Regional differences in bacterial flora in harbour porpoises from the North Atlantic: environmental effects? J. Appl. Microbiol. 2009;106:329–337. doi: 10.1111/j.1365-2672.2008.04006.x. [DOI] [PubMed] [Google Scholar]
- Siebert U., Tolley K., Vikingsson G.A., Ólafsdóttir D., Lehnert K., Weiss R., Baumgärtner W. Pathological findings in harbour porpoises (Phocoena phocoena) from Norwegian and Icelandic waters. J. Comp. Pathol. 2006;134:134–142. doi: 10.1016/j.jcpa.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Siebert U., Wünschmann A., Weiss R., Frank H., Benke H., Frese K. Post-mortem findings in harbour porpoises (phocoena phocoena) from the German North and baltic Seas. J. Comp. Pathol. 2001;124:102–114. doi: 10.1053/jcpa.2000.0436. [DOI] [PubMed] [Google Scholar]
- Stockdale P.H.G. Pulmonary pathology associated with metastrongyloid infections. Br. Vet. J. 1976;132:595–608. doi: 10.1016/S0007-1935(17)34536-0. [DOI] [PubMed] [Google Scholar]
- Strube C., Buschbaum S., Schnieder T. Molecular characterization and real-time PCR transcriptional analysis of Dictyocaulus viviparus major sperm proteins. Parasitol. Res. 2009;104:543–551. doi: 10.1007/s00436-008-1228-5. [DOI] [PubMed] [Google Scholar]
- ten Doeschate M., IJsseldijk L., Hiemstra S., de Jong E., Strijkstra A., Gröne A., Begeman L. Quantifying parasite presence in relation to biological parameters of harbour porpoises Phocoena phocoena stranded on the Dutch coast. Dis. Aquat. Org. 2017;127:49–56. doi: 10.3354/dao03182. [DOI] [PubMed] [Google Scholar]
- Testi F., Pilleri G. Verminous pulmonitis induced by Nematoda (Halocerus, Pseudaliidae) in the dolphin (Delphinus delphis L.) Investig. Cetacea. 1969;1:179–188. [Google Scholar]
- Ulrich S.A., Lehnert K., Siebert U., Strube C. A recombinant antigen-based enzyme-linked immunosorbent assay (ELISA) for lungworm detection in seals. Parasites Vectors. 2015;8:443. doi: 10.1186/s13071-015-1054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elk C.E., van de Bildt M.W.G., van Run P.R.W.A., Bunskoek P., Meerbeek J., Foster G., Osterhaus A.D.M.E., Kuiken T. Clinical, pathological, and laboratory diagnoses of diseases of harbour porpoises (Phocoena phocoena), live stranded on the Dutch and adjacent coasts from 2003 to 2016. Vet. Res. 2019;50:88. doi: 10.1186/s13567-019-0706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Holtum C., Strube C., Schnieder T., von Samson-Himmelstjerna G. Development and evaluation of a recombinant antigen-based ELISA for serodiagnosis of cattle lungworm. Vet. Parasitol. 2008;151:218–226. doi: 10.1016/j.vetpar.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Wünschmann A., Siebert U., Frese K., Weiss R., Lockyer C., Heide-Jørgensen M.P., Müller G., Baumgärtner W. Evidence of infectious diseases in harbour porpoises (Phocoena phocoena) hunted in the waters of Greenland and by-caught in the German North Sea and Baltic Sea. Vet. Rec. 2001;148:715–720. doi: 10.1136/vr.148.23.715. [DOI] [PubMed] [Google Scholar]
- Zottler E.-M., Strube C., Schnyder M. Detection of specific antibodies in cats infected with the lung nematode Aelurostrongylus abstrusus. Vet. Parasitol. 2017;235:75–82. doi: 10.1016/j.vetpar.2017.01.015. [DOI] [PubMed] [Google Scholar]