Highlights
-
•
BBE impairs the ascending reticular activating system, and causes EEG changes.
-
•
Stereotypical EEG changes correlating with the level of consciousness were observed.
-
•
Characteristic “unarousable sleep-like” EEG can be a diagnostic clue for BBE.
Abbreviations: BBE, Bickerstaff’s brainstem encephalitis; ECG, electrocardiogram; EEG, electroencephalography; MFS, Miller Fisher syndrome; ARAS, ascending reticular activating system
Keywords: Altered mental status, Bickerstaff’s brainstem encephalitis, Electroencephalography, Reticular formation, Sleep pattern, Spindle coma
Abstract
Objectives
Bickerstaff’s brainstem encephalitis (BBE) is a rare post-infectious inflammatory disease, which causes impaired consciousness by the dysfunction of the ascending reticular activating system (ARAS). We aimed to clarify EEG changes possibly caused by the dysfunction of the ARAS in BBE.
Methods
We retrospectively investigated 15 EEGs from 5 patients with definite BBE (i.e., the positivity for serum IgG anti-GQ1b antibodies was mandatory for the diagnosis) admitted to our hospital from January 2014 through December 2019, particularly focusing on whether N1 and N2 sleep patterns were maintained.
Results
All of the 10 EEGs recorded when patients had consciousness disturbance were abnormal. Stereotypical EEG changes correlating with their level of consciousness were identified: poorly organized posterior dominant rhythms with maintenance of sleep patterns in patients with mild consciousness disturbance (n = 5); predominant N1 and/or N2 sleep patterns even with external stimuli, including spindle coma pattern, in patients with moderate consciousness disturbance (“unarousable sleep-like” EEG) (n = 4); and generalized slow waves without N1 and N2 sleep patterns in patients with severe consciousness disturbance (n = 1). Among 5 patients, 3 (60%) had “unarousable sleep-like” EEG in their clinical course.
Conclusions
Patients with BBE showed stereotypical EEG changes correlating with their level of consciousness, mostly with maintenance of N1 and N2 sleep patterns, and often exhibited characteristic “unarousable sleep-like” EEG.
Significance
This study revealed characteristic EEG changes possibly caused by the dysfunction of the ARAS, which can be a diagnostic clue for BBE.
1. Introduction
Bickerstaff’s brainstem encephalitis (BBE) is a rare post-infectious inflammatory disease, characterized by an acutely progressive triad of external ophthalmoplegia, ataxia, and impaired consciousness (Al-Din et al., 1982, Bickerstaff, 1957, Bickerstaff and Cloake, 1951, Koga et al., 2012, Odaka et al., 2003). In recent years, it has become increasingly clear that BBE is part of a continuous clinical spectrum with Miller Fisher syndrome (MFS), linked by the presence of anti-GQ1b IgG antibodies (anti-GQ1b antibody syndrome) (Shahrizaila and Yuki, 2013). The dysfunction of the brainstem reticular formation is considered to be the cause of impaired consciousness in BBE (Bickerstaff and Cloake, 1951, Odaka et al., 2003, Shahrizaila and Yuki, 2013), and may potentially be reflected on electroencephalography (EEG) as it is spatial and temporal summation of postsynaptic potentials in cortical neurons, the rhythmicity of which is controlled by the thalamus and brainstem reticular formation (Yamada and Meng, 2018a).
In fact, EEG is a useful tool to evaluate patients with consciousness disturbance due to various causes, particularly supratentorial lesions, and metabolic derangements (Brenner, 2005). For instance, in supratentorial lesions, focal continuous polymorphic delta activity and lateralized periodic discharges indicate the affected side; in metabolic encephalopathies, besides triphasic waves, diffuse slowing of background rhythms correlating with the degree of consciousness disturbance can be observed (Brenner, 2005). In contrast, EEG changes due to infratentorial lesions are relatively unknown, while unremarkable background slowing and occasional sleep potentials (e.g., alpha coma pattern, and spindle coma pattern) have been described (Brenner, 2005, Young, 2000), probably because of intact thalamocortical function.
Importantly, there is no detailed study in the literature that has investigated the EEG findings in BBE. We therefore explored the EEG changes in BBE in relation to the degree of consciousness disturbance, particularly focusing on sleep patterns. This study would contribute to our knowledge of EEG abnormalities possibly caused by the dysfunction of the ascending reticular activating system (ARAS), and might clarify the usefulness of EEG as a diagnostic modality for BBE.
2. Methods
2.1. Study design and participants
We retrospectively investigated patients who were admitted to the Department of Neurology, Kobe City Medical Center General Hospital, from January 2014 through December 2019. Only patients with definite BBE according to the diagnostic criteria by Koga et al. (Koga et al., 2012) were included in this study. Definite BBE satisfied the following 3 criteria: (1) acute progressive external ophthalmoplegia, ataxia, and impaired consciousness by 4 weeks, followed by spontaneous recovery within 12 weeks after onset; (2) positivity for serum anti-GQ1b IgG antibodies; and (3) exclusion of other disorders with laboratory and imaging tests (Koga et al., 2012). Patients for whom EEGs were not performed during hospitalization were excluded. Patients’ clinical data, including age, sex, neurological findings, laboratory and cerebrospinal fluid tests, neurophysiological studies, magnetic resonance imaging, treatments, and outcomes, were obtained by retrospective chart review. Every patient with BBE was managed by more than one neurologist throughout the hospitalization in our hospital; therefore, neurologists recorded detailed abnormal neurological findings, including the degree of consciousness disturbance, every weekday. The institutional review board of Kobe City Medical Center General Hospital approved this study (zn200118) and waived the need for written informed consent due to the retrospective observational study.
2.2. EEG recordings and review
Routine video EEG was performed for 20–30 min during hospitalization, and repeated as clinically needed, using the international 10–20 system with 21 electrodes (Neurofax EEG-1200; Nihon Kohden, Tokyo, Japan). Commands to open and close eyes, and/or auditory stimuli were executed during the recording as a routine procedure. Two board-certified clinical neurophysiologists (H.Y., and M.T.), blinded to the patients’ level of consciousness, independently reviewed all the EEG recordings visually for this study, paying special attention to N1 and N2 sleep patterns. When their judgments were discordant, they discussed to achieve a consensus; if they could not reach a consensus, a third board-certified clinical neurophysiologist (J.I.) made a final judgment. We defined N1 and N2 sleep patterns for clarity as follows (Yamada and Meng, 2018b): N1 sleep pattern was characterized by the disappearance of posterior dominant rhythm and the appearance of low-amplitude theta waves and/or low-amplitude fast waves with or without vertex sharp transients (if posterior dominant rhythm was absent during the recording, vertex sharp transients were mandatory); N2 sleep pattern was characterized by the increase of theta waves and the appearance of sleep spindles (12–16 Hz) in the frontocentral regions with or without vertex sharp transients and K complexes. Spindle coma pattern was defined as a spindle pattern throughout most of a routine recording despite stimuli aimed at arousal in an unarousable patient (Kaplan et al., 2000). EEG was considered reactive when any EEG changes (e.g., the appearance of new waveforms, or change in frequency) were observed after eye opening/closure or external stimuli. EEG findings were correlated with the patients’ level of consciousness at the time they were acquired. Patients’ level of consciousness was divided into 5 categories: awake and oriented; awake but disoriented; somnolent (drowsy but easily arousable by external stimuli); stuporous (not arousable by external stimuli, but obeys simple commands or vocalizes); comatose (not arousable by external stimuli, and some or no motor response to pain stimuli).
3. Results
During the study period, 7 patients with definite BBE were identified; 2 patients were excluded because of the lack of EEG recordings during hospitalization. Therefore, a total of 15 EEG recordings from 5 patients (ranging from 16 to 56 years old) were investigated. Clinical characteristics and laboratory, neurophysiological, and radiological findings of these patients are shown in Table 1 and Table 2, respectively. Only 1 patient (Case 2) was admitted to an intensive care unit because of an upper airway obstruction due to bulbar palsy, and intubated.
Table 1.
Clinical characteristics of patients with Bickerstaff’s brainstem encephalitis.
| Case | Age | Sex | Antecedent infection | Neurological symptoms |
Treatment | Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Triad of BBEa | Consciousness disturbance at worse | Facial weakness | Limb weakness | Sensory disturbance | Deep tendon reflex | Babinski’s sign | Others | ||||||
| 1 | 20 | F | diarrhea | + | Stuporous | + | – | + | Brisk | – | Abnormal movement, NGB | IVIg, Steroid | Complete recovery at 2.5 months |
| 2 | 16 | F | diarrhea | + | Somnolent | + | + | + (including face) | Absent | + | IVIg, Steroid | Complete recovery at 5 months | |
| 3 | 36 | F | URTI | + | Awake but disoriented | + | + | – | Brisk | + | IVIg | Complete recovery at 3 months | |
| 4 | 56 | F | URTI | + | Comatose | + | – | – | Brisk | + | Photophobia | IVIg, Steroid | Complete recovery at 3 months |
| 5 | 42 | F | URTI | + | Somnolent | + | + | + | Normal | + | Photophobia, Hearing loss, NGB | IVIg, Steroid | Minimal deficit at 2 months |
BBE, Bickerstaff’s brainstem encephalitis; F, female; IVIg, high-dose intravenous immunoglobulin; NGB, neurogenic bladder; URTI, upper respiratory tract infection.
External ophthalmoplegia, ataxia, and impaired consciousness.
Table 2.
Laboratory, neurophysiological, and radiological findings of patients with Bickerstaff’s brainstem encephalitis.
| Case | Serum anti-GQ1b IgG Ab | CSF |
NCS | SEP |
ABR | Blink reflex | Brain MRI | ||
|---|---|---|---|---|---|---|---|---|---|
| Cell count | Protein | Upper ext.a | Lower ext.b | ||||||
| 1 | + | Elevated (23/μL) | Normal | Normal | Abnormal | Abnormal | Normal | Abnormal | Normal |
| 2 | + | Normal | Normal | Abnormal | Abnormal | Abnormal | Not performed | Not performed | Normal |
| 3 | + | Normal | Normal | Normal | Normal | Abnormal | Not performed | Abnormal | Normal |
| 4 | + | Elevated (15/μL) | Normal | Normal | Normal | Abnormal | Normal | Not performed | Normal |
| 5 | + | Normal | Normal | Normal | Abnormal | Abnormal | Abnormal | Not performed | Normal |
Ab, antibody; ABR, auditory brainstem response; CSF, cerebrospinal fluid; Lower ext., Lower extremity; MRI, magnetic resonance imaging; NCS, nerve conduction study; SEP, Somatosensory evoked potential; Upper ext., Upper extremity.
SEPs were recorded by median nerve stimulation at the wrist.
SEPs were recorded by tibial nerve stimulation at the ankle.
All of the 15 EEG findings are summarized in Table 3. Representative serial EEG findings of Case 1, Case 4, and Case 5 are shown in Fig. 1, Fig. 2, and Fig. 3, respectively. While there were no epileptiform discharges in these 15 EEG recordings, all of the 10 EEGs recorded when patients had disturbed consciousness, were abnormal. Conversely, out of 5 EEGs recorded when patients were awake and oriented, 4 EEGs were normal.
Table 3.
EEG findings of patients with Bickerstaff’s brainstem encephalitis.
| Recording datea | The level of consciousness | Main findings | Reactivity | PDR |
Sleep stage |
Vertex sharp transient | Sleep spindle | K complex | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lack of α attenuationb | Anterior distribution | N1 | N2 | ||||||||
| Case 1 | Day 3 | Stuporous | N2 sleep pattern (spindle coma)c,d | + | NA | NA | + | + | + | + | + |
| Day 8 | Stuporous | N1 and N2 sleep patternsc,e | + | NA | NA | + | + | + | + | – | |
| Day 14 | Awake but disoriented | Poorly organized PDR (8–9 Hz) intermixed with generalized θ waves | + | + | – | + | – | – | – | – | |
| Day 25 | Awake and oriented | Well organized PDR (10–11 Hz) | + | – | – | + | + | + | + | – | |
| Case 2 | Day 11 | Somnolent | N1 sleep patternc,f | Not assessed | NA | NA | + | + | + | + (once) | – |
| Case 3 | Day 6 | Awake but disoriented | Poorly organized PDR (8–9 Hz) | – | + | + | + | – | – | – | – |
| Day 16 | Awake and oriented | Well organized PDR (9–10 Hz) | + | – | – | – | – | – | – | – | |
| Case 4 | Day 1 | Awake and oriented | Well organized PDR (9–10 Hz) | + | – | – | – | – | – | – | – |
| Day 4 | Stuporous | N1 and N2 sleep patternsc,d | + | NA | NA | + | + | + | + | – | |
| Day 10 | Comatose | Generalized θ waves intermixed with occasional δ waves | Not assessed | NA | NA | – | – | – | – | – | |
| Day 14 | Awake but disoriented | Poorly organized PDR (8–9 Hz) intermixed with generalized θ and δ waves | Not assessed | Not assessed | + | + | – | – | – | – | |
| Day 18 | Awake but disoriented | Poorly organized PDR (8–9 Hz) | + | + | + | + | + | + | + | – | |
| Case 5 | Day 12 | Somnolent | Poorly organized PDR (8–9 Hz) intermixed with generalized θ waves | + | + | + | + | + | + | + | – |
| Day 21 | Awake and oriented | Poorly organized PDR (8–9 Hz) | + | + | – | + | + | + | + | + | |
| Day 32 | Awake and oriented | Well organized PDR (10–11 Hz) | + | – | – | + | – | – | – | – | |
NA, not applicable; PDR, posterior dominant rhythm.
The date of onset of neurological symptoms is defined as Day 1.
Lack of attenuation of PDR by eye opening.
“unarousable sleep-like” EEG.
PDR appears only for short periods of time after auditory stimuli.
PDR does not appear after auditory stimuli.
Although no stimuli were executed during the recording, the patient was somnolent at the time of recording.
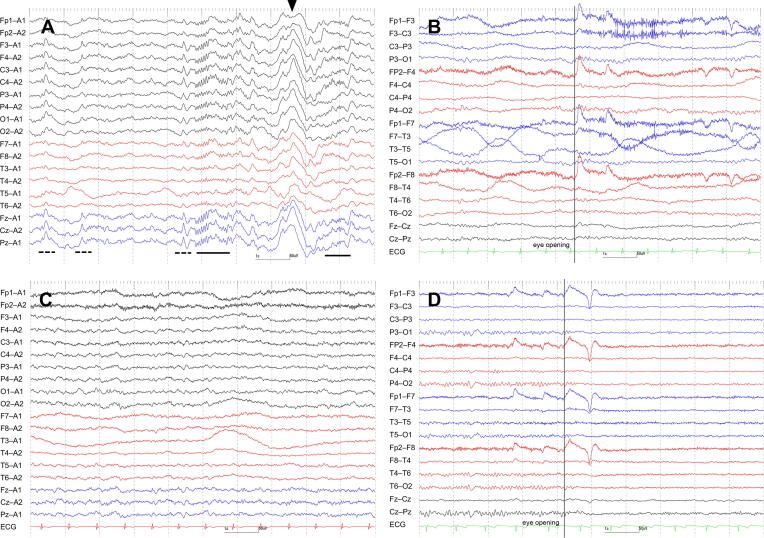
Fig. 1.
Serial EEG findings of Case 1. EEG on Day 3, when the patient was stuporous, exhibited continuous N2 sleep patterns (i.e., massive sleep spindles, K complexes, and vertex sharp transients), namely spindle coma pattern (“unarousable sleep-like EEG”) (A). EEG on Day 14, when she was awake but disoriented, revealed 8–9 Hz poorly organized posterior dominant rhythm (i.e., poor continuity, and lack of attenuation by eye opening) intermixed with generalized theta waves (B) accompanied by N1 sleep pattern (C). EEG on Day 25, when she was awake and oriented, showed 10–11 Hz well organized posterior dominant rhythm (i.e., good continuity, and presence of attenuation by eye opening) (D). Underlines indicate sleep spindles, dotted underlines vertex sharp transients, and arrowheads K complexes. Filter settings: high cut filter, 120 Hz (A), 70 Hz (B, C, D); time constant, 0.3 s (A), 0.1 s (B, C, D); AC filter, ON (A, B, C, D). ECG, electrocardiogram.
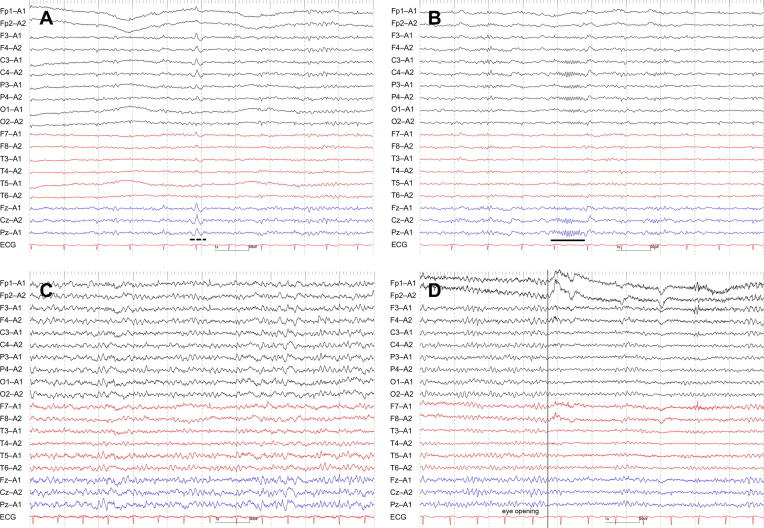
Fig. 2.
Serial EEG findings of Case 4. EEG on Day 4, when the patient was stuporous, exhibited dominant N1 (A) and N2 (B) sleep patterns during the recording (“unarousable sleep-like EEG”). EEG on Day 10, when she was comatose, revealed generalized theta waves intermixed with occasional delta waves without sleep patterns (C). EEG on Day 18, when she was awake but disoriented, showed 8–9 Hz poorly organized posterior dominant rhythm (i.e., incomplete attenuation by eye opening, and anterior distribution) (D) accompanied by N1 and N2 sleep patterns (not shown). The underline indicates a sleep spindle, and the dotted underline a vertex sharp transient. Filter settings: high cut filter, 70 Hz; time constant, 0.1 s; AC filter, ON. ECG, electrocardiogram.
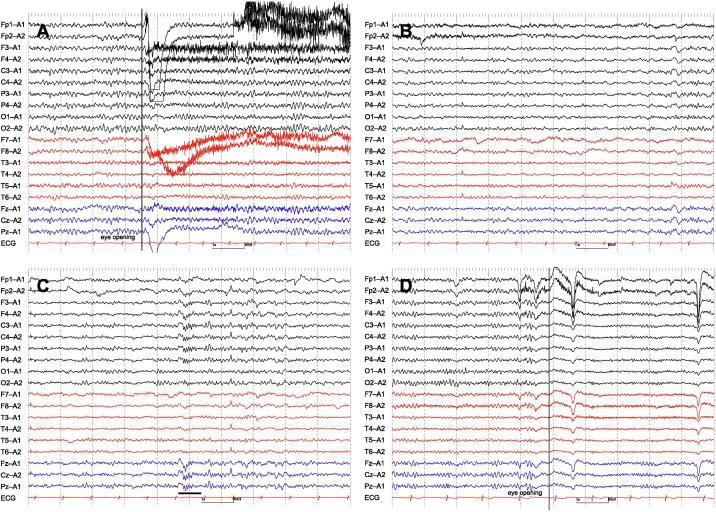
Fig. 3.
Serial EEG findings of Case 5. EEG on Day 12, when the patient was somnolent, exhibited 8–9 Hz poorly organized posterior dominant rhythm (i.e., lack of attenuation by eye opening, and anterior distribution) (A) accompanied by N1 (B) and N2 (C) sleep patterns. EEG on Day 32, when she was awake and oriented, showed 10–11 Hz well organized posterior dominant rhythm (i.e., good continuity, and presence of attenuation by eye opening) (D). The underline indicates a sleep spindle. Filter settings: high cut filter, 70 Hz; time constant, 0.1 s; AC filter, ON. ECG, electrocardiogram.
More importantly, we identified stereotypical EEG changes that correlated with the patients’ level of consciousness. While their sleep patterns were maintained, patients with mild consciousness disturbance (i.e., awake but disoriented, or somnolent) showed poorly organized posterior dominant rhythms (e.g., poor continuity, lack of attenuation by eye opening, or anterior distribution) with or without generalized theta waves (n = 5) (Fig. 1B, 1C, 2D, 3A, 3B, 3C). Patients with moderate consciousness disturbance (i.e., somnolent, or stuporous) exhibited predominant N1 and/or N2 sleep patterns even with auditory stimuli (n = 4) (Fig. 1A, 2A, 2B). We labeled these patterns as “unarousable sleep-like” EEG. While 1 “unarousable sleep-like” EEG (Case 1 on Day 3) qualified as spindle coma pattern, the remaining 3 (Case 1 on Day 8, Case 2 on Day 11, and Case 4 on Day 4) did not, because spindle patterns did not occupy most of the recordings. Patients with severe consciousness disturbance (i.e., comatose) showed generalized slow waves without N1 and N2 sleep patterns (n = 1) (Fig. 2C). It was noteworthy that 3 (60%) out of 5 patients had “unarousable sleep-like” EEG at some point in their clinical course (Case 1, 2, and 4). EEG findings finally normalized in all of the 3 patients (Case 1, 3, and 5), for whom EEGs were repeated until their consciousness disturbance recovered completely (i.e., awake and oriented).
4. Discussion
In the present study, we found that EEGs in patients with BBE exhibited stereotypical changes in correlation with their level of consciousness. Patients with mild consciousness disturbance (i.e., awake but disoriented, or somnolent) showed poorly organized posterior dominant rhythms with or without generalized theta waves, and the maintenance of sleep patterns. Patients with moderate consciousness disturbance (i.e., somnolent, or stuporous) mainly displayed N1 and/or N2 sleep patterns even in the presence of external stimuli. Patients with severe consciousness disturbance (i.e., comatose) showed generalized slow waves without N1 and N2 sleep patterns.
We investigated EEG changes in relation to the degree of consciousness disturbance, presuming that it parallels the degree of dysfunction of the ARAS in BBE. Bickerstaff, in his original articles (Bickerstaff, 1957, Bickerstaff and Cloake, 1951), ascribed impaired consciousness to lesions in the midbrain because typically, “the patients appeared to be asleep, but were easily roused to full co-operation, though if stimulation was relaxed they would quickly slip back into sleep again”. Autopsy studies subsequently confirmed definite inflammatory changes in the brainstem without obvious abnormality in the cerebrum (Hunter et al., 2012, Odaka et al., 2003). In recent years, the anti-GQ1b antibodies are considered to bind to GQ1b antigens probably expressed on the brainstem reticular formation via the site where the blood–brain barrier is relatively permeable, such as the area postrema (Shahrizaila and Yuki, 2013), although this has not been definitely demonstrated. It has been reported that there was no significant difference in antibody reactivities between MFS and BBE (Yoshikawa et al., 2018). It is possible that some factors in sera from BBE patients disrupt blood–brain barrier (Saito et al., 2013).
The most notable EEG findings in this study were predominant N1 and/or N2 sleep patterns even with external stimuli, including spindle coma pattern, in patients with moderate consciousness disturbance. Remarkably, this “unarousable sleep-like” EEG was observed in 3 (60%) out of 5 patients at some point in their clinical course. Although spindle patterns in BBE patients had been described in previous case reports (Odaka et al., 1999, Shimozono et al., 2012), the “unarousable sleep-like” EEG was different from spindle coma patterns in that it also included EEG patterns of relatively few spindles. It was also noteworthy that most of the BBE patients with this EEG feature were unarousable, but obeyed simple commands or vocalized. This characteristic EEG pattern can be explained by the dysfunction of the ARAS (Britt, 1981, Kaplan et al., 2000), which essentially controls the preservation of EEG activation and wakefulness (Yamada and Meng, 2018a). The destruction of the mesencephalic tegmentum in cats resulted in synchronized EEG patterns resembling sleep patterns including spindles (Lindsley et al., 1949). Moreover, the stimulation of the brainstem reticular formation in anesthetized cats replaced synchronized slow discharges with low-voltage desynchronized patterns resembling waking EEG (Moruzzi and Magoun, 1949). The presence of sleep spindles indicates the preservation of thalamocortical circuits, in which the thalamus is the generator of spindles (Britt, 1981, Hirose et al., 1981, Kaplan et al., 2000).
Patients with mild consciousness disturbance showed slight abnormalities on EEG: 8–9 Hz poorly organized posterior dominant rhythms, with or without generalized theta waves, and the maintenance of sleep patterns. These EEG findings were possibly caused by the mild dysfunction of the ARAS, which have not been clearly demonstrated previously, although unremarkable background slowing and occasional sleep potentials have been described in lesions limited to the brainstem (Brenner, 2005, Young, 2000). As one patient with severe consciousness disturbance showed apparent generalized slow waves without sleep patterns, the inflammation might have extended to the diencephalon at the time. This finding, however, should be confirmed in a future study as only one EEG recording was acquired at the time of coma.
All patients in our study showed abnormal EEG findings when they had consciousness disturbance, in contrast to previous studies, in which only 50–57% of patients were reported to have abnormal slow waves (Ito et al., 2008, Koga et al., 2012). Aforementioned slight EEG abnormalities and normal-appearing sleep patterns could have been mistaken as normal in these studies. EEG should be interpreted with the reactivity to eye opening, and auditory and noxious stimuli, and in correlation with the patient’s level of consciousness.
We selected only patients with definite BBE, namely BBE with the positivity for serum anti-GQ1b IgG antibodies, in order to exclude patients with other BBE-like brainstem encephalitides. In a nationwide survey of Japanese patients with BBE, probable BBE with the negativity for serum anti-GQ1b IgG antibodies was shown to be clinically different from definite BBE, and could be caused by other etiologies (Koga et al., 2012). We believe that owing to the strict inclusion criteria, very consistent EEG findings could be obtained from homogeneous patients.
This study has some limitations. First, this is a small retrospective study of 15 EEG recordings from 5 patients. While EEG findings were quite consistent among patients in correlation with their level of consciousness, the accurate frequency of “unarousable sleep-like” EEG in BBE patients should be confirmed in a large prospective study. Second, visual inspection of EEGs could be subjective, and judgment criteria for various EEG patterns could be slightly different among reviewers. Therefore, in this study, 2 clinical neurophysiologists independently reviewed all the EEGs, using specific definitions of characteristic EEG patterns, such as N1 and N2 sleep patterns, and spindle coma pattern. Moreover, most of the slight EEG abnormalities, such as poor organization of posterior dominant rhythms, were reconfirmed by comparing serial EEG recordings in every patient. Third, because the pathophysiology of BBE has not yet been completely clarified, brain areas other than the ARAS might also affect EEG findings.
5. Conclusions
We demonstrated stereotypical EEG changes in correlation with the level of consciousness in patients with BBE, where sleep patterns were mostly maintained, and pathological marked background slowing was rare. Possibly due to the dysfunction of the ARAS, patients were unable to maintain wakefulness, and EEG often showed predominant N1 and/or N2 sleep patterns, even with external stimuli (“unarousable sleep-like” EEG), including spindle coma pattern. Combined with other neurological symptoms, these characteristic EEG findings can be a diagnostic clue for BBE, which poses a diagnostic challenge without a suspicion of this rare disorder and the measurement of serum anti-GQ1b IgG antibodies (Hunter et al., 2012).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Al-Din A.N., Anderson M., Bickerstaff E.R., Harvey I. Brainstem encephalitis and the syndrome of Miller Fisher: a clinical study. Brain. 1982;105:481–495. doi: 10.1093/brain/105.3.481. [DOI] [PubMed] [Google Scholar]
- Bickerstaff E.R. Brain-stem encephalitis: further observations on a grave syndrome with benign prognosis. BMJ. 1957;1(5032):1384–1387. doi: 10.1136/bmj.1.5032.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickerstaff E.R., Cloake P.C.P. Mesencephalitis and rhombencephalitis. BMJ. 1951;2(4723):77–81. doi: 10.1136/bmj.2.4723.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R.P. The interpretation of the EEG in stupor and coma. Neurologist. 2005;11(5):271–284. doi: 10.1097/01.nrl.0000178756.44055.f6. [DOI] [PubMed] [Google Scholar]
- Britt C.W., Jr. Nontraumatic “spindle coma”: clinical, EEG, and prognostic features. Neurology. 1981;31(4):393–397. doi: 10.1212/wnl.31.4.393. [DOI] [PubMed] [Google Scholar]
- Hirose G., Saeki M., Kosoegawa H., Takado M., Yamamoto T., Tada A. Delta waves in the EEGs of patients with intracerebral hemorrhage. Arch Neurol. 1981;38(3):170–175. doi: 10.1001/archneur.1981.00510030064009. [DOI] [PubMed] [Google Scholar]
- Hunter G., Young G.B., Ang L.C. Bickerstaff’s brainstem encephalitis presenting to the ICU. Neurocrit Care. 2012;17(1):102–106. doi: 10.1007/s12028-012-9691-3. [DOI] [PubMed] [Google Scholar]
- Ito M., Kuwabara S., Odaka M., Misawa S., Koga M., Hirata K. Bickerstaff’s brainstem encephalitis and Fisher syndrome form a continuous spectrum: clinical analysis of 581 cases. J Neurol. 2008;255(5):674–682. doi: 10.1007/s00415-008-0775-0. [DOI] [PubMed] [Google Scholar]
- Kaplan P.W., Genoud D., Ho T.W., Jallon P. Clinical correlates and prognosis in early spindle coma. Clin Neurophysiol. 2000;111(4):584–590. doi: 10.1016/s1388-2457(99)00303-x. [DOI] [PubMed] [Google Scholar]
- Koga M., Kusunoki S., Kaida K., Uehara R., Nakamura Y., Kohriyama T. Nationwide survey of patients in Japan with Bickerstaff brainstem encephalitis: epidemiological and clinical characteristics. J Neurol Neurosurg Psychiatry. 2012;83(12):1210–1215. doi: 10.1136/jnnp-2012-303060. [DOI] [PubMed] [Google Scholar]
- Lindsley D.B., Bowden J.W., Magoun H.W. Effect upon the EEG of acute injury to the brain stem activating system. Electroencephalogr Clin Neurophysiol. 1949;1(1-4):475–486. [PubMed] [Google Scholar]
- Moruzzi G., Magoun H.W. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1(4):455–473. [PubMed] [Google Scholar]
- Odaka M., Yuki N., Hirata K. Bilateral ballism in a patient with overlapping Fisher’s and Guillain-Barre syndromes. J Neurol Neurosurg Psychiatry. 1999;67(2):206–208. doi: 10.1136/jnnp.67.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odaka M., Yuki N., Yamada M., Koga M., Takemi T., Hirata K. Bickerstaff's brainstem encephalitis: clinical features of 62 cases and a subgroup associated with Guillain-Barre syndrome. Brain. 2003;126:2279–2290. doi: 10.1093/brain/awg233. [DOI] [PubMed] [Google Scholar]
- Saito K., Shimizu F., Koga M., Sano Y., Tasaki A., Abe M. Blood-brain barrier destruction determines Fisher/Bickerstaff clinical phenotypes: an in vitro study. J Neurol Neurosurg Psychiatry. 2013;84(7):756–765. doi: 10.1136/jnnp-2012-304306. [DOI] [PubMed] [Google Scholar]
- Shahrizaila N., Yuki N. Bickerstaff brainstem encephalitis and Fisher syndrome: anti-GQ1b antibody syndrome. J Neurol Neurosurg Psychiatry. 2013;84(5):576–583. doi: 10.1136/jnnp-2012-302824. [DOI] [PubMed] [Google Scholar]
- Shimozono K., Shimono K., Kusunoki S. Case of Bickerstaff brainstem encephalitis associated with spindle coma and decorticate posture. Rinsho Shinkeigaku. 2012;52(9):656–659. doi: 10.5692/clinicalneurol.52.656. [DOI] [PubMed] [Google Scholar]
- Yamada T., Meng E. Neuroanatomical and neurophysiologic basis of EEG. In: Yamada T., Meng E., editors. Practical Guide for Clinical Neurophysiologic Testing EEG. 2nd ed. Wolters Kluwer; Philadelphia: 2018. pp. 63–85. [Google Scholar]
- Yamada T., Meng E. Characteristics of normal EEG. In: Yamada T., Meng E., editors. Practical guide for Clinical Neurophysiologic Testing EEG. 2nd ed. Wolters Kluwer; Philadelphia: 2018. pp. 101–137. [Google Scholar]
- Yoshikawa K., Kuwahara M., Morikawa M., Fukumoto Y., Yamana M., Yamagishi Y. Varied antibody reactivities and clinical relevance in anti-GQ1b antibody-related diseases. Neurol Neuroimmunol Neuroinflamm. 2018;5(6):e501. doi: 10.1212/NXI.0000000000000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young G.B. The EEG in coma. J Clin Neurophysiol. 2000;17(5):473–485. doi: 10.1097/00004691-200009000-00006. [DOI] [PubMed] [Google Scholar]