Abstract
Dental pulp contains multipotent mesenchymal stem cells that improve outcomes when administered early after temporary middle cerebral artery occlusion in rats. To further assess the therapeutic potential of these cells, we tested whether functional recovery following stroke induced by photothrombosis could be modified by a delayed treatment that was initiated after the infarct attained maximal volume. Photothrombosis induces permanent focal ischemia resulting in tissue changes that better reflect key aspects of the many human strokes in which early restoration of blood flow does not occur. Human dental pulp stem cells (approximately 400 × 103 viable cells) or vehicle were injected into the infarct and adjacent brain tissue of Sprague-Dawley rats at 3 days after the induction of unilateral photothrombotic stroke in the sensorimotor cortex. Forepaw function was tested up to 28 days after stroke. Cellular changes in peri-infarct tissue at 28 days were assessed using immunohistochemistry. Rats treated with the stem cells showed faster recovery compared with vehicle-treated animals in a test of forelimb placing in response to vibrissae stimulation and in first attempt success in a skilled forelimb reaching test. Total success in the skilled reaching test and forepaw use during exploration in a Perspex cylinder were not significantly different between the 2 groups. At 28 days after stroke, rats treated with the stem cells showed decreased immunolabeling for glial fibrillary acidic protein in tissue up to 1 mm from the infarct, suggesting decreased reactive astrogliosis. Synaptophysin, a marker of synapses, and collagen IV, a marker of capillaries, were not significantly altered at this time by the stem-cell treatment. These results indicate that dental pulp stem cells can accelerate recovery without modifying initial infarct formation. Decreases in reactive astrogliosis in peri-infarct tissue could have contributed to the change by promoting adaptive responses in neighboring neurons.
Keywords: stroke, focal ischemia, dental pulp stem cells, functional recovery, peri-infarct, astrocytes
Introduction
Stroke is a major cause of adult disability. Ischemic stroke, the predominant form of this disorder, usually results from occlusion of a cerebral artery by a thrombus or embolus. This produces a region of markedly impaired blood flow in the brain. Tissue infarction, involving the death of essentially all cells, develops in the affected tissue if the occlusion is not rapidly reversed. Each year, this form of stroke accounts for almost 50 million disability-adjusted life years1, a parameter that measures time lost to premature death and disability.
Neurological function improves over the initial few months in most survivors of ischemic stroke2,3. These improvements apparently result from a combination of adaptive changes in neurons in the “peri-infarct” tissue immediately surrounding the area of major cell loss as well as alterations in the function of neurons at other sites in the central nervous system2–4. There is currently considerable interest in identifying interventions that can promote favorable elements of these responses and improve recovery3,5,6.
In animal models of stroke, multipotent and pluripotent stem cells from various sources have been found to improve functional recovery6,7. Phase I and some phase II clinical trials, mostly using mesenchymal cells from bone marrow and umbilical cord blood cells, have supported the safety and feasibility of this approach for treating stroke, although unequivocal evidence of efficacy has not yet been obtained6–9. Future trials would benefit from a better understanding of factors influencing outcomes, including the timing of the treatment and the types of stem cells that are used.
A population of stem cells in the dental pulp of adult teeth10 provides a potential source of human material that is suitable for autologous transplantation11. These multipotent cells are derived, at least partly, from Schwann cells or Schwann cell precursors in peripheral nerves12 and have a high proliferative capacity in vitro 10. They normally generate pulp cells and odontoblasts but can be induced to generate neurons and astrocytes13–15.
The ability of dental pulp stem cells (DPSCs) to promote recovery in animal models of stroke has been much less investigated compared with some other stem cell populations, particularly mesenchymal cells prepared from bone marrow6,7. Nonetheless, intracerebral injections of DPSCs from humans15 and pigs16, as well as an intravenous injection of human cells17, have been found to improve recovery of neurological function in rats treated one day after temporary middle cerebral artery occlusion. Interestingly, the functional improvement was similar in animals treated with either DPSCs or mesenchymal cells from bone marrow, but there were substantial differences between the 2 treatments in the changes induced in some cellular markers and the pattern of gene expression in the brain tissue and cerebrospinal fluid17.
The previous investigations indicate that DPSCs are promising candidates for improving outcomes following stroke in humans. However, the use of a short period of arterial occlusion in these studies potentially reduces the relevance of the findings for many cases of stroke in humans. Current interventions for acute stroke involving the use of thrombolysis and clot retrieval have increased the numbers of patients with early reperfusion of the affected tissue, but the majority of those affected by this disease still do not receive such treatments3. Although reperfusion commonly develops without intervention, this usually does not happen for many hours or days. Analysis of spontaneous reperfusion using angiography in patients not receiving treatments to remove clots indicated that at least 75% of arterial occlusions persisted at 6 h with approximately 50% still occluded at 4 days18. Thus, blood flow remains severely impaired in most patients until local tissue damage is well advanced or fully developed.
In animal models of stroke, early reperfusion alters the pattern of cellular changes that result in infarct development and reduces infarct volume when compared with the responses to longer periods of occlusion or permanent ischemia19–21. These differences are also likely to influence adaptive responses of cells in the peri-infarct tissue, which are important contributors to functional recovery. Furthermore, treatment with DPSCs at 1 day after temporary middle cerebral artery occlusion directly reduced infarct volume in some studies16,17, raising the complication that the reduced tissue damage was a likely contributor to improved functional recovery.
To help address these limitations and further assess the therapeutic potential of DPSCs, we have tested whether delayed treatment with these cells can improve neurological recovery following stroke induced by photothrombosis in rats. Photothrombotic stroke can be targeted to specific cortical regions to reproducibly generate small infarcts22, mimicking the situation in humans that is associated with better functional recovery2,23. This model has been widely employed to characterize neuronal changes contributing to the recovery of neurological function following stroke2,24. Furthermore, the blockage of cerebral vessels by photothrombosis is usually permanent. The pattern of tissue damage and potential for recovery following photothrombosis provides a better model than short-term middle cerebral artery occlusion for those cases of human stroke in which the occlusion persists for many hours or days.
Photothrombosis leads to a more rapid development of the mature infarct than is seen in models based on occlusion of a major artery. Infarct size peaks within 1 to 3 days and then contracts25,26. In the present study, the DPSCs were injected 3 days after stroke to ensure that the volume of the infarct was maximal prior to treatment. Recovery of neurological function compared with a vehicle-treated control group was monitored over 28 days. The brains were then analyzed for cellular changes indicative of reactive astrogliosis, angiogenesis, and synaptogenesis in peri-infarct tissue that could potentially contribute to treatment-induced modifications of recovery.
Materials and Methods
Experimental Design
Male Sprague-Dawley rats were obtained from Laboratory Animal Services (University of Adelaide, Adelaide, Australia). All studies involving the rats were conducted according to the “Australian code for the care and use of animals for scientific purposes, 2013” published by the National Health and Medical Research Council, Australia. Ethical approval for these studies was obtained from the Animal Welfare Committee of Flinders University, Adelaide, South Australia (Project number 869/13). Ethical approval for the use of human DPSCs in these investigations was from the Southern Adelaide Clinical Human Research Committee (Application 205.14-HREC/14/SAC/202).
The rats were kept in a temperature-controlled and humidity-controlled room with a 12 h /12 h light/dark cycle. Stroke was induced in rats weighing between 295 and 331 g (DPSC-treated: 310 ± 11 g; vehicle-treated: 315 ± 9 g; mean ± SD)
Rats were randomly preassigned to treatment with either DPSCs or vehicle (n = 9 per group). Forepaw function was assessed at multiple time points up to 28 days. Brain tissue was then perfusion fixed for immunohistochemical analysis of cell markers in peri-infarct tissue.
Functional assessment using the single-pellet skilled reaching test and cylinder test, as well as imaging and data analyses, was performed by blinded investigators.
Induction of Focal Ischemia by Photothrombosis
An infarct was produced in the forelimb motor cortex using a minor modification26 of previously described procedures22,27. The rat was anesthetized with an intraperitoneal injection of 100 mg/kg ketamine and 10 mg/kg xylazine. A cannula was inserted into the femoral vein and used to maintain anesthesia with additional injections of ketamine as required. The rat was placed in a stereotaxic frame, the scalp retracted, and the surface of the skull cleared of periosteum and blood. To limit light exposure to tissue in the region of the forelimb motor cortex, a brass shim stencil with a rectangular window (3 × 5 mm) was positioned at 1.5 mm anterior to bregma and 2.5 mm lateral to the midline. The stencil was placed on the side contralateral to the limb that was used preferentially by the rat in a single pellet retrieval task (see below).
The skull was transilluminated for stroke induction using a 150 W light source (Intralux 5000; Volpi AG, Schlieren, Switzerland) equipped with a green filter (532.5 to 587.5 nm passband) and attached fiber-optic cable (6 mm diameter). Prior to each procedure, the light intensity was measured at the point of emission from the fiber-optic cable to ensure that it was between 160,000 and 170,000 lux. The fiber-optic cable was positioned directly over the window of the stencil and as close as possible to the skull without contacting it. Rose Bengal (10 mg/ml, 13 mg/kg) was injected via the femoral vein cannula at 200 µL/min. The light was illuminated for 15 min immediately after the injection was initiated.
Preparation of Human DPSCs
DPSCs were isolated from an impacted third molar from a 20-year-old person by digestion of the pulp tissue as previously described10,15. Cells were initially grown in culture to passage 4, cryoprotected in 10% dimethyl sulfoxide in fetal calf serum (Thermo Fisher Scientific, Waltham, MA, USA), and stored frozen in aliquots in liquid nitrogen. The cultured cells were shown to be free of mycoplasma contamination. For the present study, an aliquot of cells was thawed and expanded by culturing for 2 additional passages in α-modified Eagle’s medium (Sigma-Aldrich, St Louis MO, USA) supplemented with 20% fetal calf serum, 100 μM l-ascorbic acid 2-phosphate, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37 °C15. Aliquots of these cells were again frozen in 10% dimethyl sulfoxide in fetal calf serum. When required for injection, cells were thawed and grown in culture to approximately 75% confluence. Immediately prior to injection, the cells (now passage 7) were harvested following dissociation with trypsin and resuspended in α-modified Eagle’s medium without supplements. The suspensions used for injection contained 104 ± 9 × 103 cells / μL. Cell viability assessed by trypan blue exclusion in samples prepared for injection was 91.0% ± 6.6%.
Intracerebral Injections
At 3 days after induction of the stroke, rats (9 per treatment group) were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine intraperitoneally and placed in a stereotaxic frame. The DPSCs in α-modified Eagle’s medium or vehicle were injected (2 µL at each site) into the infarct (anteroposterior [AP] +1.5 mm, mediolateral [ML] ±2.5 mm, dorsoventral [DV] +2.4 mm) and into cortical tissue 1.5 mm caudal to the infarct (AP −2.5 mm, ML ±2.5 mm, DV +2.4 mm). All rats received a daily subcutaneous injection of 10 mg/kg cyclosporin A between day 2 after stroke induction (i.e., 1 day before the intracerebral injections) and day 27.
Assessment of Forelimb Function
The forelimb function of rats was assessed up to 28 days following stroke induction using 4 measures: a forelimb placing test, 2 components of a single pellet skilled reaching test, and a cylinder test. Baseline measures of function were obtained within the 4 days prior to stroke induction. One vehicle-treated rat died on day 25 after stroke without prior signs of illness or obvious abnormalities in the prior pattern of recovery of neurological function. Thus, values reported for 28 days were obtained from 8 rats in the vehicle-treated group and 9 rats in the DPSC-treated group.
Forelimb Placing Test
A minor modification26 of the forelimb placing response, as described by Schallert and Woodlee28, was used as a criterion for inclusion in the study and as the primary measure of recovery of forelimb motor response after stroke. No specific training was needed for this task but rats were familiarized with the procedure before stroke induction. Each rat was handled by an investigator for at least 10 min per day on 3 separate days leading up to the induction. In these sessions, rats were placed on the testing platform for a few minutes and were given approximately 5 trials of each variant of the forelimb placing task.
Three variants of the test were used for subsequent assessments of forelimb placing26. In the first, the rat was moved toward the platform so that both sets of vibrissae were stimulated. If the rat placed the forelimb on the platform, the trial was scored as a success. Stimulation of the vibrissae ipsilateral and then contralateral to the limb being assessed was used in subsequent tests. Each forelimb was tested 10 times with each of the 3 stimuli and a total success score out of 30 was calculated. Rats were tested at 3 h after induction of the stroke. To reduce variability in functional assessment, rats with a score greater than 5 at this time were not used for the study26. Only 1 rat that underwent surgery was not subsequently used because of this exclusion criterion. Rats were tested again at 1, 7, 14, 21, and 28 days.
Single Pellet Skilled Reaching Test
Rats were trained to retrieve a pellet through a narrow slit in a single pellet reaching box and tested after stroke for total success and first attempt success in retrievals using the forelimb contralateral to the infarct, as described previously27,29. To facilitate training, rats were food restricted so that their body weight gradually reached 90% to 95% of that expected if they had free access to food. Rats were trained in daily 10-min sessions over 2 to 3 weeks up to the day prior to stroke induction. Initial training allowed the paw preference for this task to be identified. Baseline performance was assessed on a 20-trial test within the 4 days prior to stroke induction. At this time, the median value for total success in each group was above 50%.
In a previous study, recovery on this task was highly variable if rats were only tested at weekly intervals26. To improve the reproducibility of the findings and potentially promote recovery in this study, rats were tested on day 1 and then on 10 separate occasions between days 7 and 28 (days 7, 8 or 9, 10 or 11, 14, 15 or 16, 17 or 18, 21, 22 or 23, 24 or 25, and 28). Reported values were determined from the average of the scores on 2 consecutive tests. The values reported for days 7, 14, and 21 are the average of the scores for the test on this day and the subsequent test, which was completed within the following 2 days. The 28-day values represent the average of the scores from the final 2 tests. Rats showed limited interest in pellet retrieval on day 1 and most completed 50% or less of the 20 trials with little, if any, success when trials were terminated at 15 min. Because of the low number of trials completed by many of the rats, results for this time point have not been reported.
Cylinder Test for Forelimb Asymmetry
Forelimb use for weight support during rearing, while rats were engaged in exploration in a Perspex cylinder (20 cm diameter; 30 cm height) was evaluated as previously described26,27,30. The exploratory behavior of rats was recorded with an Otek high definition digital video camera (Otek Corporation, Tucson, AZ, USA). Rats were tested within 4 days prior to stroke induction and then at 1, 7, 14, 21, and 28 days after stroke. The recording on each occasion was continued until an observer detected at least 90 separate wall touches. Subsequent analysis of video recordings showed median values between 122 and 139 total touches for the different time points with individual rat values ranging between 95 and 166 touches. The percentage of affected limb usage was calculated as 100 × (the number of touches of the forepaw contralateral to the infarct + half the number of simultaneous placements)/total paw placements.
Brain Fixation
At the conclusion of functional testing on day 28, rats were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine injected intraperitoneally. They were transcardially perfused with approximately 180 ml of 10 g/L NaNO2 in 0.01 M sodium phosphate buffer (pH 7.4) and then with 200 ml of 40 g/L paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.4). The brains were incubated in the same paraformaldehyde solution overnight at 4 °C. They were then cryoprotected by phased transfer into 300 g/L sucrose in phosphate-buffered saline (PBS, pH 7.4) containing 0.2 g/L sodium azide and frozen in O.C.T. compound (Tissue-Tek, Sakura Finetek USA Inc., CA, USA). Coronal sections (20 µm) were prepared using a cryo-microtome (Leica Systems, NSW, Australia).
Immunohistochemistry
The antibody used to detect the glial fibrillary acidic protein (GFAP; rabbit polyclonal; catalog number G4546) was from Sigma-Aldrich, those for collagen IV (rabbit polyclonal; ab6586), synaptophysin (rabbit polyclonal; ab1492), and human mitochondrial protein (mouse monoclonal; ab92824) were from Abcam (Cambridge, UK) and for NeuN (mouse monoclonal; clone A60; MAB377) was from Millipore-Chemicon (Merck, Darmstadt, Germany). Alexa-fluor-conjugated secondary antibodies were from Thermo Fisher Scientific. Coronal sections from 6 rat brains for each of the treatment groups were investigated in this part of the study. However, the immunolabeling with GFAP and with synaptophysin for 1 vehicle-treated rat showed variability in intensity across the sections and could not be analyzed.
For each rat, separate sets of duplicate free-floating brain sections separated by at least 500 µm were immunolabeled to detect GFAP, collagen IV, and synaptophysin. Each section was also immunolabeled for NeuN to identify the infarct and assist in defining tissue for analysis (see below). For the collagen IV labeling, the sections were initially incubated in 10 mM citrate buffer (pH 8.5) for 10 min at 85 °C. All sections were then blocked and permeabilized in 0.3% Triton-X 100, 5% donkey serum in PBS, pH 7.4 (blocking buffer) for 2 h at room temperature. The sections were incubated overnight at 4 °C in primary antibodies diluted in blocking buffer (collagen IV and human mitochondrial protein: 1/500; NeuN and GFAP: 1/400; synaptophysin: 1/250). After washing, secondary antibodies (1/2000) and Hoechst 33258 (1/5000; Thermo Fisher Scientific) diluted in PBS were added, and the sections incubated for 2 h at room temperature on an orbital shaker. They were then washed before being mounted on glass slides in Prolong Gold antifade mountant (Thermo Fisher Scientific).
Whole immunolabeled sections were batch-scanned using the Pannoramic 250 Flash II slide scanner (3DHistech; Budapest, Hungary). Images from the peri-infarct and equivalent contralateral regions were then extracted using the CaseViewer software (ver 2.1; 3DHistech) into TIFF files for analysis in ImageJ31. GFAP immunolabeling was assessed using a modification of the approach of Lopez-Valdes et al.32. Preliminary analysis of coronal sections indicated that elevated GFAP immunolabeling extended at least 1 mm into cortical tissue from the lateral edge of the infarct. The area fraction of GFAP in this tissue was assessed in 4 adjoining 250-µm wide rectangular regions of interest (ROIs). The medial edge of the first ROI was positioned approximately parallel to the lateral edge of the infarct at the point at which NeuN immunoreactivity was similar to that in contralateral tissue. The ROIs extended through the full depth of cortical gray matter excluding any parts of the tissue in which NeuN labeling was reduced. Corresponding ROIs from the contralateral cortex were also analyzed.
Initial examination of sections immunolabeled for collagen IV revealed increases in immunolabeling in peri-infarct tissue relative to that in the contralateral hemisphere that were smaller and more restricted in distribution than those for GFAP. Thus, the labeling of collagen IV and of synaptophysin was analyzed in 2 adjacent rectangular ROIs extending 500 µm perpendicular to the lateral edge of the infarct. As for GFAP, the ROIs included the full depth of cortical tissue in which NeuN labeling was preserved.
For analysis of GFAP immunolabeling, the ROIs within the contralateral region were thresholded using the “Triangle method” in ImageJ and the same thresholding level was applied to the ROIs within the peri-infarct region. The area fraction of GFAP immunolabeling was determined using the “Measure” function. For collagen IV labeling, the ROIs within the peri-infarct tissue and equivalent contralateral regions were thresholded using the “Li method”. The area fraction of collagen IV immunolabeling within each ROI was determined using the “Analyze Particle” function with the particle size set to a lower limit of 1000 pixels (to exclude debris). For synaptophysin, the mean fluorescence intensity within each ROI was determined using the “Measure” function. For each protein marker, the analysis from 2 coronal brain sections was averaged in obtaining the final results.
Statistical Analysis
Data from tests of neurological function are presented as box plots. All other data are shown as mean ± SD.
Behavioral responses were modeled using linear-mixed effects models using Stata software (StataCorp, USA; version 15). Linear mixed-effects models are suitable for continuous data in which there are repeated measurements performed over time. They include both “random” effects and “fixed” effects and account for the correlation within each experimental unit across time by way of a variance-covariance matrix for the random effects, which allows adjustment of the SEs obtained from standard linear regression models. The random effects (i.e., random intercepts and random slopes) allow the modeling of variation of each experimental unit’s trajectory over time around the overall mean effect for each group. An additional strength of the method is to ensure that all recorded information is used, thereby ensuring optimal power and that missing data points borrow strength from surrounding information rather than using imputation techniques. Data that is skewed (nonparametric) but measured repeatedly over time will often still meet the assumptions of the model which are the normality of the random effects (intercepts and slopes) and normality of the (level 1) residuals, since experimental units tend to measure consistently over time, for example, consistently higher or consistently lower, which results in the normality of both the random effects and residuals.
For each analysis, the mixed models included both fixed-effect and random-effect terms. The fixed-effect terms consisted of the day, which was treated as a categorical variable (e.g., day 1, day 7, day 14, day 21, and day 28) and a time × treatment interaction term, in order to test whether the recovery curves differed between treatments across time. The random-effects component consisted of a random intercept for each rat, a random slope for the day, and a random slope for the treatment × day term. The covariance matrix for each of these random effects was an identity matrix (i.e., 1’s on the diagonal and 0’s on the off-diagonal terms) and the within rat variability (i.e., model residuals) was modeled using an independence matrix (i.e., within-rat variance’s on the diagonal terms and 0’s on the off-diagonal terms). Each of the models thus accounted for variability around each of the overall fixed effects for each of the individual rats (both the overall mean response at each time point and the difference in response depending on treatment group) and also accounted for the correlation across time for each rat. When testing for the significance of the time × treatment interaction, we first tested for global significance, and when significant, then assessed at which of the time points the difference in treatment effects occurred using the separate treatment × time interaction terms estimated from the model. For each analysis in this study, the normality of the level 1 and level 2 residuals were confirmed using histograms of these residuals.
Statistical analysis of data derived from images of immunolabeled brain sections was performed using the SPSS software package (IBM SPSS Statistics for Windows. Version 23.0; IBM Corp., Armonk, NY, USA). The effects of DPSC treatment and of distance from the infarct (or linear distance from an equivalent starting point within the contralateral region) were analyzed using a two-way analysis of variance in the 2 hemispheres. For all analyses, differences with P < 0.05 were considered statistically significant.
Results
Recovery of Forepaw Function Following Stroke
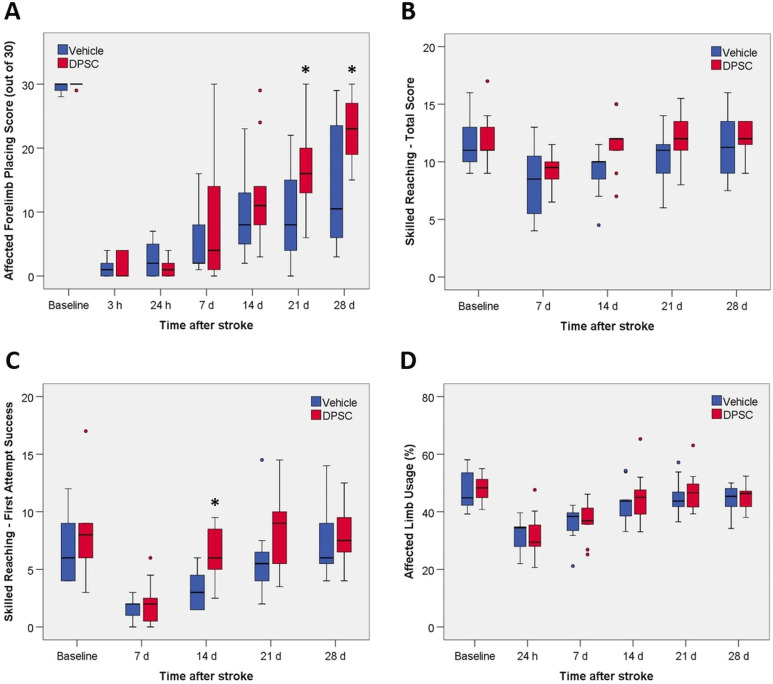
At 3 h after stroke, forelimb placing in response to vibrissae stimulation on the side contralateral to the infarct was almost completely impaired (Fig. 1A). These severe deficits largely persisted at 24 h. Performance on this task then recovered slowly but remained substantially below pre-stroke values in most vehicle-treated rats at 28 days. The overall rate of recovery was faster in rats treated with DPSCs compared with vehicle, with significant differences in performance detected at 21 and 28 days (Fig. 1A). Testing of the forelimb ipsilateral to the stroke showed that function was essentially fully preserved, with median values of 29 or 30 at all time points in both treatment groups.
Fig. 1.
The effect of DPSC treatment on forelimb function following stroke. (A) Forelimb placing test. (B) Single pellet skilled reaching test: total success from 20 trials. (C) Single pellet skilled reaching test: first attempt success from 20 trials. (D) Cylinder test. Values are for 9 rats per group except for the vehicle-treated group on day 28 where n = 8 because 1 rat was lost to the study on day 25. Outliers are shown as filled circles. There was a statistically significant overall effect of treatment on recovery in the forelimb placing test and first attempt success in the skilled reaching test. The asterisk identifies individual days showing a significant difference between the 2 treatments (P < 0.05).
For the skilled reaching test involving retrieval of a pellet through a slit in a Perspex chamber, both total success and success on the first attempt were analyzed (Fig. 1B, C). At 7 days, the ability to retrieve pellets on the first attempt remained greatly impaired in both groups. Improvement over the next 3 weeks led to a return to near pre-stroke performance by 28 days (Fig. 1C). Recovery in this aspect of the test was faster in the DPSC-treated rats, with a significant difference in performance seen on day 14. Total success in retrieving pellets (irrespective of the number of attempts) was less impaired at 7 days relative to the baseline values (Fig. 1B). The rate of recovery of total success did not differ significantly between the 2 groups.
The other test of forelimb function examined the use of the affected forelimb during rearing while exploring in a Perspex cylinder (Fig. 1D). This measure showed substantial decreases at 1 and 7 days but near complete restoration of function in many animals at later time points. There was no difference in recovery between the DPSC-treated and vehicle-treated rats up to 28 days.
Analysis of Cellular Responses in Peri-Infarct Tissue
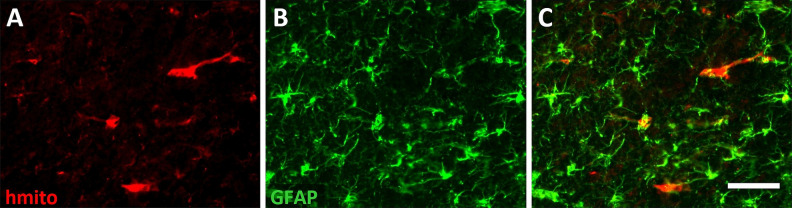
Coronal sections prepared from fixed brain tissue removed at the conclusion of the functional testing on day 28 were immunolabeled and analyzed to identify cellular changes that could have contributed to the faster functional recovery in DPSC-treated rats. To assess whether DPSCs or their progeny remained in the brain, sections near the site of injection posterior to the infarct were immunolabeled for human mitochondrial protein. Occasional immunopositive cells were observed in the cortical tissue (Fig. 2). Immunolabeling for GFAP in the same sections suggested this protein was also expressed in several cells that were positive for human mitochondrial protein (Fig. 2).
Fig. 2.
Human mitochondrial protein was detected in brain tissue at 28 days after stroke. Micrographs showing immunolabeling for (A) human mitochondrial protein (hmito) to identify cells derived from the injected dental pulp stem cells, (B) glial fibrillary acidic protein, and (C) the merged image. Scale bar = 50 μm.
At 4 weeks after stroke, GFAP was dramatically increased in cells immediately adjacent to the infarct (Fig. 3A), consistent with advanced development of an astroglial scar at this time33,34. Increases in GFAP, albeit smaller, were also still readily detectible within the peri-infarct tissue that surrounds this developing scar and in which the numbers of neurons are essentially preserved. These GFAP changes, which were the focus of our analysis, are indicative of reactive astrogliosis that can influence adaptive responses in neighboring surviving neurons. Within the first 250 µm of this tissue, the area fraction of GFAP immunolabeling was increased more than 5-fold compared with the corresponding contralateral tissue (Fig. 3B). The area fraction decreased progressively with increasing distance from the infarct indicating reduced reactive astrogliosis (Fig. 3A, B). Nonetheless, in tissue between 750 µm and 1 mm from the infarct, the GFAP area fraction was still more than twice that in contralateral tissue.
Fig. 3.
Immunolabeling for GFAP at 28 days after stroke. (A) GFAP immunolabeling in a coronal section showing increased labeling in the tissue surrounding the infarct. The rectangles show the regions in each hemisphere in which area fraction was analyzed. (B and C) Higher power images of (B) the peri-infarct neocortical tissue and (C) equivalent tissue in the contralateral hemisphere showing the 4 adjoining 250 µm wide regions of tissue in which area fraction of immunolabeling was assessed. (B) Area fraction of immunolabeling up to 1 mm from the infarct. There was a statistically significant effect of treatment with DPSCs (P < 0.05) and of distance from the lesion (P < 0.01) in the peri-infarct tissue (two-way analysis of variance; n = 5 to 6 per treatment group). There was no significant interaction between the 2 factors. DPSC treatment produced a statistically significant increase in area fraction in the contralateral hemisphere (P < 0.01), but there was no significant difference across the 4 bands of tissue that were analyzed.
Treatment with DPSCs resulted in a small but consistent decrease in GFAP area fraction across the regions analyzed within the first 1 mm of tissue in which NeuN immunolabeling was essentially preserved (Fig. 3B). Two-way analysis of variance showed a significant effect of distance from the lesion and of the treatment. There was no significant interaction between the 2 factors reflecting the similar effect of treatment across each of the 4 bands of tissue that were investigated. Interestingly, treatment with DPSCs also produced a statistically significant change in GFAP area fraction in corresponding tissue in the contralateral cortex. However, this involved a small increase in area fraction in the DPSC-treated samples contrasting with the decrease that was seen in the affected hemisphere.
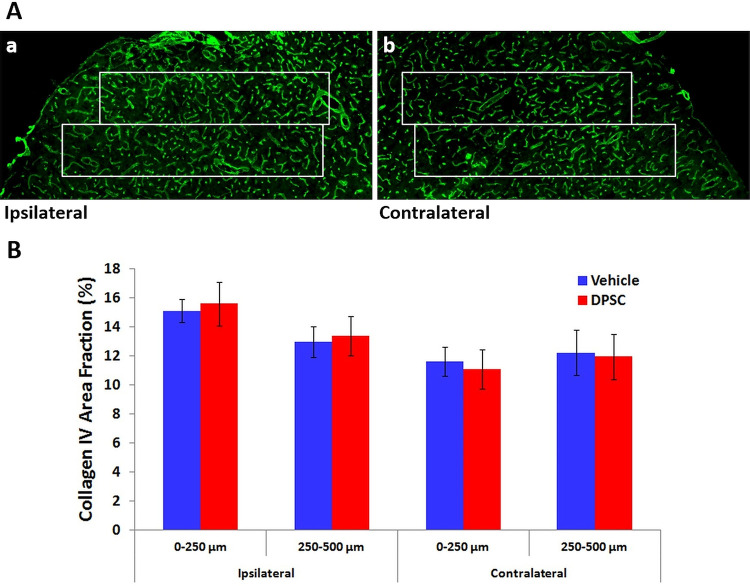
Immunolabeling for collagen IV was used to detect possible changes in capillaries associated with angiogenesis in the peri-infarct tissue. Increases in this labeling compared with contralateral tissue were again most apparent immediately around the infarct (Fig. 4A). However, there were smaller increases relative to equivalent tissue in the contralateral hemisphere than those seen for GFAP, and these were largely restricted to the first 250 µm of peri-infarct tissue in which NeuN immunolabeling was preserved (Fig. 4A). Analysis of the area fraction of collagen IV labeling (Fig. 4B) confirmed a significant effect of distance from the infarct (P < 0.01). However, there was no significant effect of DPSC treatment compared with vehicle treatment.
Fig. 4.
Immunolabeling for collagen IV at 28 days after stroke. (A) Peri-infarct neocortical tissue and (B) equivalent tissue in the contralateral hemisphere. The 2 adjoining 250-µm wide rectangles show the regions in which area fraction of immunolabeling was assessed. (B) Area fraction of immunolabeling up to 500 µm from the infarct. In the peri-infarct tissue, there was a statistically significant effect of distance from the infarct (P < 0.01) but no effect of DPSC treatment (two-way analysis of variance; n = 6 per treatment group). There was no significant interaction between the 2 factors. There was no significant effect of either factor in the contralateral hemisphere.
Immunolabeling for synaptophysin produced a diffuse pattern of immunolabeling across the tissue that is consistent with previous reports35–37 and reflects the localization of this protein mainly in nerve terminals. There was a small increase in the intensity of labeling around the infarct (Fig. 5A). Because the labeling was not associated with identifiable structures in the image, mean pixel intensity was determined rather than area fraction. This measure was not significantly affected by either DPSC treatment or distance from the infarct (Fig. 5B).
Fig. 5.
Immunolabeling for synaptophysin at 28 days after stroke. (A) Peri-infarct neocortical tissue and (B) equivalent tissue in the contralateral hemisphere. The 2 adjoining 250-µm wide rectangles show the regions in which the mean pixel intensity of immunolabeling was assessed. (B) Mean pixel intensity of immunolabeling up to 500 µm from the infarct. There was no significant effect of treatment in either hemisphere and no effect of distance from the lesion (two-way analysis of variance; n = 5 to 6 per treatment group).
Discussion
The present study demonstrates the ability of delayed treatment with DPSCs to accelerate recovery of neurological function following photothrombotic stroke. This treatment also led to altered reactive astrogliosis in peri-infarct tissue that could have contributed to the more rapid restoration of function. The findings extend previous reports of improved functional recovery following treatment with DPSCs15–17. In contrast to the earlier reports, faster recovery in the present study was achieved in a stroke model that does not involve early reperfusion of the ischemic tissue and with a treatment initiated after the infarct attained maximal volume.
Improvements in the rate of recovery were detected with both the forelimb placing test and first attempt success on the skilled reaching test. These tests both assess forelimb function but have very different requirements for training. The forelimb placing task involves a motor response to stimulation of the vibrissae and requires no prior training to achieve consistent responses. For the single pellet skilled reaching task, rats were trained for at least 2 weeks prior to stroke and showed marked improvements in performance during the training period. In contrast to the faster recovery induced in these functional measures, the DPSC treatment did not significantly improve the recovery of either total success on the skilled reaching test or use of the affected limb in the cylinder test, another assessment that does not require prior training.
The apparent differential effects of DPSC treatment in influencing recovery across the 4 measures of forepaw function could potentially arise from differences in the neural circuitry required for each task, although such differences are likely to be small for the 2 components of the skilled reaching test. Differences in the size and pattern of the behavioral changes that were induced by stroke are also likely to have contributed to the ability to detect changes following DPSC treatment. Deficits in forelimb placing and first attempt success in skilled reaching were larger relative to the pre-stroke baseline response than for the other measures, providing more scope for detecting differences following treatment. Furthermore, the forelimb placing response was still impaired at 28 days in most vehicle-treated animals providing a greater opportunity compared with the other measures to detect differences later in the recovery period. Improved recovery in a forelimb placing test but not alternative tests of forelimb function have been seen in previous studies testing other interventions following photothrombotic stroke in rats26,38. These results are again consistent with the possibility that the placing test is more sensitive to treatment or has greater potential for modification in this stroke model.
Occasional cells derived from the injected DPSCs were still present around the injection site at 4 weeks after stroke. This finding is consistent with previous reports indicating that only a few percent of injected DPSCs remained in rat brain at 4 weeks after treatment15 and that many of these cells migrated toward the damaged tissue15,17. Low survival of injected cells has also been reported following treatments with other stem cells7,39. DPSCs can produce neurons under some conditions in vitro 13,14 and have been found to generate cells with either neuronal or astrocytic properties in the brain following intracerebral or intravenous injection15,17. Nonetheless, as concluded from other reports of stem cell treatments7,9,11, the small numbers of surviving cells and the difficulties of incorporating new neurons into functional circuits in the damaged brain suggest that cell replacement is likely to play little, if any, role in the faster recovery in aspects of neurological function. Rather, materials released by these stem cells, including trophic factors and extracellular vesicles, are likely to be important in influencing cellular responses to damage that contribute to recovery, as seen following treatments with mesenchymal stem cells6,7. Alterations that can promote recovery include increases in synaptogenesis and altered neuronal connectivity that are supported by angiogenesis and modifications to reactive astrogliosis in the tissue surrounding the infarct.
Changes potentially involved in the altered outcomes following the DPSC treatment were assessed from the immunolabeling for 3 proteins in peri-infarct tissue: GFAP as a marker for reactive astrogliosis, collagen IV for angiogenesis, and synaptophysin for synaptogenesis.
As seen in Fig. 3, GFAP immunoreactivity at 4 weeks after stroke remained greatly increased on the rim of the infarct where it is associated with reactive astrocytes that are centrally involved in the development of the glial scar. Changes contributing to scar formation include the proliferation of a subpopulation of these astrocytes, realignment and intertwining of astrocytic processes, and deposition of extracellular matrix33,34. GFAP immunoreactivity was also markedly increased at 28 days in tissue extending at least 1 mm from the site of the developing scar in which the neuronal population is largely preserved. The increase in GFAP is again indicative of ongoing reactive astrogliosis, but there are major differences in responses in these astrocytes compared with those on the rim of the infarct33,34. The astrocytes more distant from the lesion generally do not proliferate. Furthermore, based on the evidence primarily derived from other forms of brain injury, these cells largely remain in the same territories that were occupied prior to the stroke and have very limited overlap with the territories of neighboring astrocytes40,41. Our measure of the area fraction of GFAP immunolabeling focused on the population of astrocytes that were more distant from the infarct. The use of immunohistochemistry for this analysis allowed the reproducible identification of the cells of interest within tissue in which neurons were essentially preserved, as determined from NeuN labeling, without contamination of the signal from astrocytes in the developing glial scar. The substantial increases seen in GFAP area fraction are mostly due to major increases in the caliber of astrocytic processes. This change requires increases in GFAP production in the cells and increased distribution of this protein such that it is detectible in parts of the processes that are much further from the cell soma40,41.
DPSC treatment produced a small but significant decrease in GFAP immunolabeling suggesting a reduction in reactive astrogliosis in the peri-infarct tissue beyond the developing scar at 28 days. Decreases in aspects of reactive astrogliosis have been associated with improvements in recovery of function in other studies34,42. In particular, a similar pattern of decreases in GFAP expression in peri-infarct tissue up to 1 mm from the developing glial scar (albeit with larger changes than the present study) was seen together with improved recovery following treatment with memantine, an N-methyl-d-aspartate receptor antagonist32. To our knowledge, comparable measures focusing on astrocytes outside of the developing scar have not been reported in other investigations of stem cell treatments. However, decreases have been found in the thickness of the mature glial scar in response to treatments with mesenchymal stem cells43,44 and in subgroups of GFAP-positive cells on the rim of the lesion following treatment with DPSCs17. These results point to reductions in aspects of reactive astrogliosis in scar-forming cells and, when considered together with our findings, suggest that generalized reductions in reactive astrogliosis might be associated with better outcomes following stem cell treatments.
Angiogenesis assessed from immunolabeling of the capillary marker, collagen IV, was also increased in peri-infarct tissue at 28 days, although the changes were smaller and more restricted in distribution in the tissue than those for GFAP. Small but more diffuse stroke-induced increases were seen in synaptophysin immunolabeling in the peri-infarct tissue suggesting a local increase in synaptogenesis. However, neither collagen IV nor synaptophysin immunolabeling was significantly modified by the DPSC treatment. These findings suggest that either increased angiogenesis or synaptogenesis in peri-infarct tissue were not major contributors to the faster recovery with DPSC treatment or that changes in these properties were transient or small such that significant differences could not be detected at 28 days with the techniques we employed.
Our findings contrast with increases in markers of angiogenesis and synaptogenesis induced by treatments with mesenchymal stem cells derived from bone marrow17,37 and an increase in angiogenesis following DPSC treatment17 when assessed at 28 days after temporary middle cerebral artery occlusion. Differences in the treatment conditions and stroke model are likely to be major contributors to the more moderate effects on recovery and more limited peri-infarct changes we observed. In particular, the use of a model not involving early reperfusion, the generation of a much smaller infarct and the initiation of treatment well after the infarct had attained maximal size all have the potential to reduce the impact of intervention. Nonetheless, our findings indicate that recovery can still be modified even under these experimental conditions and that delayed DPSC treatment can influence this response.
In conclusion, the major finding from this study is that treatment with DPSCs accelerated recovery of forepaw function when initiated at 3 days after induction of a small infarct in the sensorimotor cortex by photothrombosis. This result provides evidence for a direct influence of DPSCs on the recovery process without affecting infarct volume. The faster functional recovery also occurred in the absence of early reperfusion and any downstream effects on tissue damage. A decrease in GFAP immunolabeling was seen in the tissue surrounding the infarct at 28 days after stroke, indicating a moderation of reactive astrogliosis that could influence plasticity and contribute to the more rapid recovery.
Acknowledgments
We acknowledge the excellent care for the animals and other assistance provided by staff in the Animal Facility at Flinders University.
Footnotes
Ethical Approval: Ethical approval for the use of human DPSCs in this study was obtained from the Southern Adelaide Clinical Human Research Committee (Application 205.14-HREC/14/SAC/202). Ethical approval for the animal studies was obtained from the Animal Welfare Committee of Flinders University, Adelaide South Australia (Project number 869/13).
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the protocols approved by Southern Adelaide Clinical Human Research Committee (Application 205.14-HREC/14/SAC/202) and the Animal Welfare Committee of Flinders University, Adelaide South Australia (Project number 869/13). As required by the Animal Welfare Committee, all studies involving rats were conducted according to the “Australian code for the care and use of animals for scientific purposes, 2013” published by the National Health and Medical Research Council, Australia.
Statement of Informed Consent: Informed written consent was obtained from the patient to make use of teeth or dental pulp removed as part of normal dental treatment as the starting material for the preparation of the DPSCs which were used in this investigation.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from the Brain Foundation (Australia) and a project grant from the Faculty of Health Sciences, Flinders University. Purchase of the antibodies recognizing human mitochondrial protein was supported by a National Stroke Foundation small grant.
ORCID iDs: Wai Ping Yew  https://orcid.org/0000-0001-7436-0779
https://orcid.org/0000-0001-7436-0779
Neil R. Sims  https://orcid.org/0000-0003-1830-091X
https://orcid.org/0000-0003-1830-091X
References
- 1. Feigin VL, Krishnamurthi RV, Parmar P, Norrving B, Mensah GA, Bennett DA, Barker-Collo S, Moran AE, Sacco RL, Truelsen T, Davis S, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013: The GBD 2013 Study. Neuroepidemiology. 2015;45(3):161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nature Rev Neurosci. 2009;10(12):861–872. [DOI] [PubMed] [Google Scholar]
- 3. Cramer SC. Treatments to promote neural repair after stroke. J Stroke. 2018;20(1):57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carmichael ST, Kathirvelu B, Schweppe CA, Nie EH. Molecular, cellular and functional events in axonal sprouting after stroke. Exp Neurol. 2017;287(Pt 3):384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hermann DM, Chopp M. Promoting brain remodelling and plasticity for stroke recovery: therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol. 2012;11(4):369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Venkat P, Shen Y, Chopp M, Chen JL. Cell-based and pharmacological neurorestorative therapies for ischemic stroke. Neuropharmacology. 2018;134(Pt B):310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stonesifer C, Corey S, Ghanekar S, Diamandis Z, Acosta SA, Borlongan CV. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol. 2017;158:94–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nagpal A, Choy FC, Howell S, Hillier S, Chan F, Hamilton-Bruce MA, Koblar SA. Safety and effectiveness of stem cell therapies in early-phase clinical trials in stroke: a systematic review and meta-analysis. Stem Cell Res Therapy. 2017;8(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bang OY. Clinical trials of adult stem cell therapy in patients with ischemic stroke. J Clin Neurol. 2016;12(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo . Proc Natl Acad Sci U S A. 2000;97(25):13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nagpal A, Kremer KL, Hamilton-Bruce MA, Kaidonis X, Milton AG, Levi C, Shi S, Carey L, Hillier S, Rose M, Zacest A, et al. TOOTH (The Open study Of dental pulp stem cell Therapy in Humans): Study protocol for evaluating safety and feasibility of autologous human adult dental pulp stem cell therapy in patients with chronic disability after stroke. Int J Stroke. 2016;11(5):575–585. [DOI] [PubMed] [Google Scholar]
- 12. Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A, An ZW, Wang LL, Hultman I, Ahrlund-Richter L, Blom H, et al. Glial origin of mesenchymal stem cells in a tooth model system. Nature. 2014;513(7519):551–554. [DOI] [PubMed] [Google Scholar]
- 13. Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26(7):1787–1795. [DOI] [PubMed] [Google Scholar]
- 14. Kiraly M, Porcsalmy B, Pataki A, Kadar K, Jelitai M, Molnar B, Hermann P, Gera I, Grimm WD, Ganss B, Zsembery A, et al. Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem Int. 2009;55(5):323–332. [DOI] [PubMed] [Google Scholar]
- 15. Leong WK, Henshall TL, Arthur A, Kremer KL, Lewis MD, Helps SC, Field J, Hamilton-Bruce MA, Warming S, Manavis J, Vink R, et al. Human adult dental pulp stem cells enhance poststroke functional recovery through non-neural replacement mechanisms. Stem Cells Transl Med. 2012;1(3):177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sugiyama M, Iohara K, Wakita H, Hattori H, Ueda M, Matsushita K, Nakashima M. Dental pulp-derived CD31(-)/CD146(-) side population stem/progenitor cells enhance recovery of focal cerebral ischemia in rats. Tissue Eng Part A. 2011;17(9–10):1303–1311. [DOI] [PubMed] [Google Scholar]
- 17. Song M, Lee JH, Bae J, Bu Y, Kim EC. Human dental pulp stem cells are more effective than human bone marrow-derived mesenchymal stem cells in cerebral ischemic injury. Cell Transplant. 2017;26(6):1001–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kassem-Moussa H, Graffagnino C. Nonocclusion and spontaneous recanalization rates in acute ischemic stroke - a review of cerebral angiography studies. Arch Neurol. 2002;59(12):1870–1873. [DOI] [PubMed] [Google Scholar]
- 19. Hossmann KA. Pathophysiological basis of translational stroke research. Folia Neuropathol. 2009;47(3):213–227. [PubMed] [Google Scholar]
- 20. Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochimica Biophysica Acta-Mol Basis of Dis. 2010;1802(1):80–91. [DOI] [PubMed] [Google Scholar]
- 21. Puig B, Brenna S, Magnus T. Molecular communication of a dying neuron in stroke. Int J Mol Sci. 2018;19(9):2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol. 1985;17(5):497–504. [DOI] [PubMed] [Google Scholar]
- 23. Carmichael ST. The 3 Rs of stroke biology: radial, relayed, and regenerative. Neurotherapeutics. 2016;13(2):348–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benowitz LI, Carmichael ST. Promoting axonal rewiring to improve outcome after stroke. Neurobiol Dis. 2010;37(2):259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li HL, Zhang NN, Lin HY, Yu Y, Cai QY, Ma LX, Ding SH. Histological, cellular and behavioral assessments of stroke outcomes after photothrombosis-induced ischemia in adult mice. BMC Neurosci. 2014;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yew WP, Djukic ND, Jayaseelan JSP, Walker FR, Roos KAA, Chataway TK, Muyderman H, Sims NR. Early treatment with minocycline following stroke in rats improves functional recovery and differentially modifies responses of peri-infarct microglia and astrocytes. J Neuroinflammation. 2019;16(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alaverdashvili M, Moon SK, Beckman CD, Virag A, Whishaw IQ. Acute but not chronic differences in skilled reaching for food following motor cortex devascularization vs. photothrombotic stroke in the rat. Neuroscience. 2008;157(2):297–308. [DOI] [PubMed] [Google Scholar]
- 28. Schallert T, Woodlee MT. Orienting and placing In: Whishaw IQ, Kolb B, eds. The Behavior of the Laboratory Rat: A Handbook with Tests. Oxford (UK): Oxford University Press; 2005. pp. 129–140. [Google Scholar]
- 29. Metz GA, Kolb B, Whishaw IQ. Neuropsychological tests In: Whishaw IQ, Kolb B, eds. The Behavior of the Laboratory Rat: A Handbook with Tests. Oxford (UK): Oxford University Press; 2005. pp. 475–498. [Google Scholar]
- 30. Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39(5):777–787. [DOI] [PubMed] [Google Scholar]
- 31. Rasband WS. ImageJ. U. S. National Institutes of Health. 1997-2018. https://imagej.nih.gov/ij/ (accessed 17 February 2017).
- 32. Lopez-Valdes HE, Clarkson AN, Ao Y, Charles AC, Carmichael ST, Sofroniew MV, Brennan KC. Memantine enhances recovery from stroke. Stroke. 2014;45(7):2093–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81(2):229–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sims NR, Yew WP. Reactive astrogliosis in stroke: Contributions of astrocytes to recovery of neurological function. Neurochem Int. 2017;107:88–103. [DOI] [PubMed] [Google Scholar]
- 35. Ishrat T, Pillai B, Soliman S, Fouda AY, Kozak A, Johnson MH, Ergul A, Fagan SC. Low-dose candesartan enhances molecular mediators of neuroplasticity and subsequent functional recovery after ischemic stroke in rats. Mol Neurobiol. 2015;51(3):1542–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iaci JF, Parry TJ, Huang ZH, Pavlopoulos E, Finklestein SP, Ren JM, Caggiano A. An optimized dosing regimen of cimaglermin (neuregulin 1 beta 3, glial growth factor 2) enhances molecular markers of neuroplasticity and functional recovery after permanent ischemic stroke in rats. J Neurosci Res. 2016;94(3):253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shen LH, Li Y, Chen J, Zhang J, Vanguri P, Borneman J, Chopp M. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 2006;137(2):393–399. [DOI] [PubMed] [Google Scholar]
- 38. Madinier A, Quattromani MJ, Sjolund C, Ruscher K, Wieloch T. Enriched housing enhances recovery of limb placement ability and reduces aggrecan-containing perineuronal nets in the rat somatosensory cortex after experimental stroke. PLoS One. 2014;9(3):e93121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dailey T, Metcalf C, Mosley YI, Sullivan R, Shinozuka K, Tajiri N, Pabon M, Acosta S, Kaneko Y, van Loveren H, Borlongan CV. An update on translating stem cell therapy for stroke from bench to bedside. J Clin Med. 2013;2(4):220–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pekny M, Pekna M. Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol Rev. 2014;94(4):1077–1098. [DOI] [PubMed] [Google Scholar]
- 41. Wilhelmsson U, Bushongt EA, Price DL, Smarr BL, Phung V, Terada M, Ellisman MH, Pekny M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci U S A. 2006;103(46):17513–17518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu ZW, Chopp M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog Neurobiol. 2016;144:103–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M, et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49(3):407–417. [DOI] [PubMed] [Google Scholar]
- 44. Shen LH, Li Y, Chen JL, Cui YS, Zhang CL, Kapke A, Lu M, Savant-Bhonsale S, Chopp M. One-year follow-up after bone marrow stromal cell treatment in middle-aged female rats with stroke. Stroke. 2007;38(7):2150–2156. [DOI] [PubMed] [Google Scholar]