Abstract
Objective
This study was performed to evaluate the role of posterior suspension of the laminae–ossification of the ligamentum flavum complex combined with miniplate fixation (modified expansive thoracic laminoplasty) in treating thoracic ossification of the ligamentum flavum (TOLF).
Methods
Eight patients with TOLF treated by modified expansive thoracic laminoplasty were retrospectively analyzed. Their general information, operative time, intraoperative blood loss, and postoperative complications were recorded. Neurological functional recovery was evaluated by the modified Japanese Orthopaedic Association (mJOA) score and Hirabayashi recovery rate preoperatively, postoperatively, and at the final follow-up. Preoperative and postoperative imaging was performed, and the decompression range and internal fixation positioning were evaluated.
Results
The mJOA score significantly improved from 4.63 points preoperatively to 9.0 points at the final follow-up (Hirabayashi recovery rate of 77.75%). Postoperative computed tomography and magnetic resonance imaging revealed sufficient decompression of the surgical segment. At the final follow-up, the internal implants were well-placed, the lamina–ligamentum flavum complex showed no significant displacement, and neurological functional recovery was satisfactory.
Conclusion
Surgical treatment of TOLF is complicated and high-risk. Characterized by simplicity and sufficient decompression, modified expansive thoracic laminoplasty can reduce the risk of cerebrospinal fluid leakage and nerve injury with satisfactory neurological functional recovery.
Keywords: Thoracic ossification of the ligamentum flavum, spinal cord compression, expansive laminoplasty, decompression, neurological functional recovery, diagnostic imaging
Introduction
Ossification of the ligamentum flavum (OLF) is the leading pathological factor of thoracic spinal stenosis and has been recognized for nearly a century. Because of its latent onset and various clinical symptoms, OLF is easily confused with diseases such as cervical spondylosis and lumbar spinal stenosis; additionally, its low morbidity usually results in a delayed diagnosis, and patients therefore usually have serious clinical symptoms at the time of diagnosis. Surgical treatment is the primary therapeutic option for patients with symptoms and aggravating spinal dysfunction because conservative treatment is not effective in such cases. Because of the unique anatomical characteristics of the thoracic spine and the insufficient blood supply of the thoracic spinal cord, the occurrence of operative complications after thoracic decompression is much higher than that after cervical and lumbar decompression. Cerebrospinal fluid (CSF) leakage and nerve injury are the most common and clinically significant risks.1,2 Some authors have adopted new operative plans or modified conventional surgical procedures to reduce the occurrence of these complications.3 In the present study, we retrospectively analyzed patients with thoracic OLF (TOLF) who underwent posterior suspension of the laminae–OLF complex (LOC) combined with miniplate fixation (modified expansive thoracic laminoplasty) to share our practices in treating TOLF with this procedure and evaluate its efficacy and safety.
Materials and methods
General information
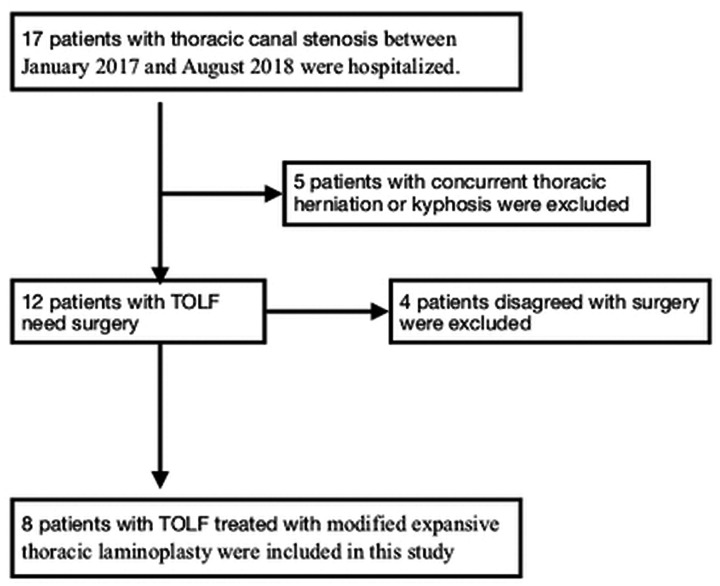
Patients with TOLF who underwent modified expansive thoracic laminoplasty from January 2017 to August 2018 were retrospectively analyzed. The inclusion criteria were thoracic canal stenosis caused by OLF accompanied by aggravating muscle weakness in the lower extremities, gait disturbance, and sphincter dysfunction. The exclusion criterion was thoracic herniation or kyphosis. A flowchart of patient selection is shown in Figure 1.
Figure 1.
Flowchart of patient selection.
TOLF, thoracic ossification of the ligamentum flavum.
Preoperatively, all patients underwent a careful medical history inquiry and physical examination and were diagnosed using X-ray, computed tomography (CT), and magnetic resonance imaging (MRI) scans. Their demographic information is summarized in Table 1.
Table 1.
Demographics and clinical data of eight patients with thoracic ossification of the ligamentum flavum.
| Sex/age(years) | Duration of symptom (months) | Involved segment | Operation duration (minutes) | Blood loss (mL) | Follow-up duration (months) | Preoperative mJOA score | Three-month postoperative mJOA score | mJOA score at last postoperative follow-up | Hirabayashi recovery rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| M/39 | 1 | T11–12 | 140 | 500 | 12 | 4 | 6 | 9 | 71.6 |
| M/59 | 0.5 | T10–12 | 180 | 800 | 28 | 4 | 7 | 10 | 85.6 |
| M/61 | 3 | T6–7 | 135 | 350 | 23 | 6 | 7 | 11 | 100 |
| M/71 | 31 | T8–10 | 170 | 700 | 24 | 2 | 5 | 7 | 55.6 |
| M/66 | 11 | T11–12 | 150 | 600 | 14 | 5 | 6 | 8 | 66.7 |
| F/61 | 4 | T9–11 | 120 | 450 | 12 | 3 | 6 | 6 | 62.5 |
| F/55 | 3 | T7–8 | 170 | 750 | 17 | 7 | 8 | 11 | 100 |
| F/47 | 24 | T8–9 | 145 | 650 | 16 | 6 | 7 | 10 | 80 |
mJOA: modified Japanese Orthopaedic Association.
During hospitalization, all patients provided written informed consent to undergo treatment, including the surgical procedures herein described. This study was approved by the ethics committee of our institution.
Outcome measures
The operative time, intraoperative blood loss, and perioperative complications were recorded. Neurological functional recovery was evaluated by the modified Japanese Orthopaedic Association (mJOA) score (Table 2) and the Hirabayashi recovery rate. The Hirabayashi recovery rate was calculated using the following formula: recovery rate = (postoperative mJOA score − preoperative mJOA score)/ (11 − preoperative mJOA score) × 100%. Neurological functional recovery was rated as “excellent” (75%–100%), “good” (50%–74%), “fair” (25%–49%), “no improvement” (0%–24%), or “deteriorative.” All patients underwent X-ray examinations, CT + sagittal reconstruction, and MRI scans. Preoperative X-rays were used to evaluate the sagittal alignment of the thoracic spine and guide the intraoperative localization, and preoperative CT and MRI scans were used to diagnose and analyze the severity of ligament ossification and spinal cord compression and the presence of spinal cord degeneration. Postoperative X-rays were used to evaluate the location of the internal fixation, and postoperative CT and MRI scans were used to determine the extent of spinal canal enlargement and spinal cord decompression.
Table 2.
Modified Japanese Orthopedic Association scoring system for evaluation of thoracic myelopathy.
| Neurological status | Score |
|---|---|
| Lower limb motor dysfunction | |
| Unable to walk | 0 |
| Able to walk on flat floor with walking aid | 1 |
| Able to walk upstairs and downstairs with handrail | 2 |
| Lack of stability and smooth reciprocation of gait | 3 |
| No dysfunction | 4 |
| Lower limb sensory deficit | |
| Severe sensory loss or pain | 0 |
| Mild sensory deficit | 1 |
| No deficit | 2 |
| Trunk sensory deficit | |
| Severe sensory loss or pain | 0 |
| Mild sensory deficit | 1 |
| No deficit | 2 |
| Sphincter dysfunction | |
| Unable to void | 0 |
| Marked difficulty in micturition | 1 |
| Minor difficulty in micturition | 2 |
| No dysfunction | 3 |
Surgical technique
Patient preparation
A patient with OLF at the T11–12 level, for example, was placed in the prone position after induction of anesthesia. An epidural puncture needle was inserted into the subarachnoid space at the L3–4 or L4–5 interspace. Next, 15 mL of iohexol was slowly injected into the subarachnoid space, and the operating table was adjusted to the Trendelenburg position, helping the contrast medium to flow into the subarachnoid space. The diseased segment was then confirmed under C-arm imaging, and the needle was fixed in situ.
Decompression
After adequate exposure of the bony structure from the spinous process to the base of the transverse process (Figure 2(a)), the range of decompression was confirmed from the superior border of the T11 lamina to the middle segment of the L1 lamina. Using a bone drill, slotting decompression was conducted from both lateral borders of the T11–L1 laminae, extending to half of the articular process and the medial wall of the pedicles. The ligamentum flavum was cut between the T10–T11 and T12–L1 laminae. The T11 and T12 laminae were isolated en bloc, enabling slow posterior suspension of the LOC (Figure 2(b), (c)). A nerve dissector was used to remove adhesions and check the decompression effect. Iohexol was then injected via the epidural puncture needle, and the C-arm was used to observe whether the contrast agent smoothly passed through the compressed segment. Failure of iohexol to pass through the compressed segment suggested insufficient spinal cord decompression and the need to perform further posterior suspension of the laminae. When the contrast agent passed through the decompressive segment and the dura showed no significant filling defect, sufficient decompression was obtained.
Figure 2.
Schematic diagram of the surgery. (a) Adequate exposure of the bony structure from the spinous process to the base of the transverse process. (b), (c) The T11 and T12 laminae were isolated en bloc, enabling slow posterior suspension of the laminae–ossification of the ligamentum flavum complex (LOC). (d) The miniplate was bent and placed at the gap between the transverse process and laminae, and the suspended LOC was then secured to the transverse processes bilaterally with the miniplate and screws.
Stabilization
A miniplate (Weigao Orthopaedic Device Co., Ltd., Weihai City, China) and screws were used to stabilize the LOC. The miniplate was bent and placed at the gap between the transverse process and laminae, and the suspended LOC was secured to the transverse processes bilaterally with the miniplate and screws (Figure 2(d)). After stable fixation, autogenous bone grafts were grafted into the bilateral slots.
Postoperative management
Prophylactic antibiotics were administered 24 hours postoperatively. In-bed lumbar/dorsal muscle and straight leg raise exercises began on the first day postoperatively, and the drainage tube was extubated on the second day postoperatively. Ambulation with a waist support device was allowed 3 days postoperatively. Normal activities were gradually resumed 6 weeks later.
Results
Eight patients with TOLF (total of 11 segments) were included in this study. All patients underwent a successful operation, and no severe complications such as nerve injury or CSF leakage occurred. The mean operation time was 148.75 minutes (range, 120–180 minutes). The mean intraoperative blood loss was 600 mL (range, 350–800 mL).
Each patient underwent complete follow-up for at least 6 months. At the final follow-up, both the mJOA score and Hirabayashi recovery rate had significantly improved compared with the preoperative values. The preoperative, 3-month postoperative, and final follow-up results are shown in Table 1.
X-ray imaging during the postoperative re-examinations and at the final follow-up revealed that the internal fixation devices were well-placed and that the Cobb angles of the surgical segments remained unchanged. The postoperative CT scans showed LOC retropositioning, partial defects in the medial walls of the pedicles, and evidently expanded spinal canal volumes. The CT scans at the final follow-up showed no evident displacement of the LOC, adequate internal fixation in situ, and good bone graft fusion between the transverse processes and laminae. The postoperative MRI scans revealed expanded vertebral canal volumes of the decompressive segments, no evident spinal cord compression, and unobstructed CSF circulation.
Representative case
A 66-year-old man was admitted with a 2-year history of zonesthesia and unstable gait with aggravation during the past 7 months. Preoperative imaging suggested OLF at the T11–12 level, spinal canal stenosis, and spinal cord compression and degeneration in the corresponding plane. The patient’s preoperative mJOA score was 6. After admission, he successfully underwent modified expansive thoracic laminoplasty. Postoperative imaging suggested good spinal canal decompression (Figure 3). His mJOA score was 10 at the final follow-up (16 months postoperatively), and his Hirabayashi recovery rate was 80%.
Figure 3.
(a) Axial and (b) sagittal computed tomography demonstrated the ossified ligamentum flavum protruding into the spinal canal at the level of T11–12, resulting in severe canal stenosis. (c) Axial and (d) sagittal magnetic resonance imaging showed low-signal masses behind the spinal cord in the spinal canal with compression and degeneration of the spinal cord. Postoperative (e) axial and (f) sagittal computed tomography demonstrated retropositioning of the laminae–ossification of the ligamentum flavum complex, a partial defect in the medial wall of the pedicle, and an evidently expanded vertebral canal volume. At the last follow-up (14 months postoperatively), thoracic (g) anteroposterior and (h) lateral X-rays demonstrated good positioning of the miniplates. (i) Axial and (j) sagittal magnetic resonance imaging revealed an expanded vertebral canal volume of the decompressive segments, no evident spinal cord compression, and unobstructed ventral and dorsal cerebral spinal fluid flow.
Discussion
TOLF has been recognized for nearly a century. The morbidity of TOLF was historically believed to be low, and the condition was only reported sporadically in Asian areas, especially in Japan, South Korea, and China.4–6 With the development of new imaging techniques such as CT and MRI, increasingly more cases are being reported and our understanding of the disease is deepening.7 OLF is now believed to be the leading etiology of thoracic spinal stenosis and is associated with a variety of symptoms.8 Surgical treatment is the only effective therapeutic option for thoracic spinal stenosis. Surgical treatment is recommended for patients with severe neurologic symptoms.
Reported surgical techniques include posterior total laminectomy decompression, semi-laminectomy decompression, lamina fenestration, and expansive laminoplasty.9 In recent years, with the extensive application of spinal endoscopy in the lumbar and cervical vertebrae, some researchers have already attempted to perform operations in a minimally invasive manner.10 Despite the increasing number of surgical techniques, the operative outcomes remain unclear, and the occurrence of complications is far higher after thoracic operations than after cervical and lumbar operations. Clinically, the most common operative risks are postoperative neurological deterioration and CSF leakage.1,2 Compared with other parts of the spine, the thoracic spine has a bulky spinal cord, and the volume of the thoracic spinal canal is relatively small; moreover, the blood supply of the thoracic spinal cord is relatively poor. Therefore, decompression of the thoracic spinal canal increases the risk of neurological deterioration. OLF usually has a long course, ossific masses usually adhere to the dura mater, and dural ossification may even occur. The incidence of concomitant dural ossification may be high, reportedly reaching 62% to 65%.11 Intraoperative separation of ossific masses increases the probability of injury to the periosteum of the vertebral canal, which is often accompanied by CSF leakage; delayed incisional healing, deep infection, and even central nervous system infection may also occur.
In conventional operations, spinal canal decompression requires resection of the posterior structure of the spinal canal, and the posterior ligament complex is inevitably damaged. Kyphosis may occur in the long term, potentially leading to local spinal cord traction and resultant neurological deterioration. In addition, local scar tissue ingrowth after laminectomy may result in spinal cord compression. Expansive laminoplasty has a long history in the treatment of cervical diseases. Its benefits are self-evident, including retention of the posterior ligament complex, avoidance of postoperative hematoma formation, and prevention of long-term kyphosis and intraspinal scar tissue ingrowth. In recent years, some authors have begun to apply this technique to thoracic and lumbar diseases, and a variety of surgical techniques have been derived with satisfactory clinical results. Nie et al.12 treated TOLF with posterior lamina osteotomy and replantation with miniplate fixation. They reported that lamina replantation could help to avoid intraspinal hematoma formation and scar tissue ingrowth as well as preserve the attachment points of the posterior muscles and ligaments, thus avoiding kyphotic deformity and ensuring the long-term effects of the operation. Because laminectomy was needed intraoperatively, trimming the laminae and removing the calcified ligamentum flavum caused patients with dural adhesion and ossification to be susceptible to dural tears and CSF leakage (which occurred in 4 of 18 patients); this increased the occurrence of complications, including postoperative infections and wound disruptions. Sun et al.13 performed anterior controllable anti-displacement and fusion of the vertebrae–ossification of the posterior longitudinal ligament complex to treat myelopathy caused by ossification of the posterior longitudinal ligament and achieved an excellent outcome. Anterior displacement of the complex can lead to moderate elevation of the dural sac and restoration of the CSF band, favoring recovery of the morphology of the spinal cord. Without direct removal of the ossification, the risk of dural and spinal cord injuries could be lowered. To reduce the risk of dural tears during treatment of TOLF, Sun et al.3 proposed the “bridge crane” technique. First, they used a bone drill to isolate the LOC en bloc and then suspended it over the sway bracing of a pedicle screw fixation system with a polypropylene stay suture. They reported that this technique was safe and controllable because posterior suspension of the lamina with a stay suture expanded the vertebral canal volume, decompressed the spinal cord, and reduced the risk of CSF leakage and spinal cord injury. We treated TOLF with posterior suspension of the LOC combined with miniplate fixation to improve the operative effect and further reduce operative complications, including nerve injury and CSF leakage. The inner walls of the pedicles were also removed with a high-speed bone drill during intraoperative decompression. We believe that this procedure not only reduces the risk of incidental damage to the dural sac and nerve root but also effectively expands the vertebral canal volume. Because it differs from conventional expansive laminoplasty, we call it modified expansive thoracic laminoplasty. The clinical follow-up of the eight patients in the present study showed that this technique improved their neurologic symptoms and reduced the operative risk of CSF leakage and nerve injury.
Spinal canal decompression is a prerequisite for neurological functional recovery. Whether spinal cord compression can be completely relieved intraoperatively is directly related to postoperative neurological functional recovery. In general, the longitudinal range of decompression covers two normal laminae above and below the diseased segment, while the transverse range covers a lamina, half of the bilateral zygapophyseal joints, and the ossified ligament anterior to the articular process. In our study, we further abraded the medial wall of the pedicle in the diseased segment on this basis; this not only expanded the spinal canal volume but also enabled safer decompression and reduced the risk of accidental damage to the nerve roots and dura mater. Conventional decompression methods allow the surgeon to check the decompression by direct vision; myelography can reportedly be used for surgical techniques in which decompression cannot be checked by direct vision. Pao and Wang14 used intraoperative myelography to evaluate the extent of minimally invasive decompression for degenerative lumbar spinal stenosis. With the use of myelography before and after decompression, they precisely localized the stenosis, evaluated the decompression, and obtained good clinical results. Tomčovčík et al.15 used myelography in posterior surgery for fractures of the thoracolumbar spine to determine the reduction of the intraspinal fracture fragments, thereby reducing the risk of surgical trauma and nerve injury. Yang et al.16 used intraoperative myelography to determine the effects of indirect spinal canal decompression during transpsoas lateral lumbar interbody fusion for degenerative lumbar spinal stenosis and performed one-stage minimally invasive endoscopic decompression on patients with insufficient decompression discovered through myelography, and both of these procedures resulted in significant improvements in neurological symptoms and pain. In our study, myelography was performed before and after decompression, and although the decompression could not be checked by direct vision, we were able to observe the thoroughness of the decompression more intuitively through myelography. With its simple technique and lack of interference with the dural sac, myelography is an alternative way to evaluate the decompression.
A variety of factors influence postoperative neurological functional recovery.17 Preoperative factors include the patient’s age, duration and severity of symptoms, preoperative mJOA score, involved segment, imaging classification of the OLF, severity of spinal invasion, whether intramedullary hyperintensity can be seen on MRI, and whether concomitant dural ossification is present or absent. Surgical factors include intraoperative blood loss, the mean arterial pressure, the operation plan, and the sufficiency of decompression. Factors influencing the long-term postoperative outcome include postoperative kyphosis and intraspinal scar tissue ingrowth. To improve the clinical efficacy of TOLF, clinicians must collect a detailed history, perform a thorough physical examination, and, if necessary, perform a CT or MRI scan to enable early detection and diagnosis. For patients requiring an operation, the surgical treatment plan should be established as early as possible according to the patient’s general condition and imaging findings.
Limitations
This study has several limitations. First, the study involved a limited number of patients and a short follow-up period. Thus, more cases and a longer follow-up period will be needed to verify the safety and effectiveness of this technique. Second, iohexol was used in myelography to determine the spinal canal decompression. This contrast agent is associated with a risk of allergy, impaired hepatic and renal function, and puncture needle-induced nerve injury. Third, unlike pedicle screws, which are fixed to three columns, miniplate fixation cannot correct kyphosis simultaneously with the laminae. Other operative techniques may be necessary for patients with concomitant kyphosis. Fourth, because both the spinal canal and dural sac are not exposed during decompression, electric coagulation hemostasis at the bleeding point may fail when massive bleeding occurs in the internal vertebral plexus. Finally, the OLF was preserved intraoperatively. Immature OLF is associated with a risk of continuous growth of the ossification and subsequent spinal canal restenosis.
Conclusion
TOLF is difficult to treat and has a high operative risk and incidence of complications. Posterior suspension of the LOC combined with miniplate fixation is characterized by simplicity, sufficient decompression, and satisfactory neurological functional recovery. It can also reduce interference with the dural sac and may lower the probability of CSF leakage and nerve injury. However, larger surgical samples with continuous follow-up are still needed to evaluate the clinical efficacy and safety of this technique.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Chao Ma https://orcid.org/0000-0001-8694-3015
Wei-Xiang Dai https://orcid.org/0000-0001-6808-4966
References
- 1.Sun X, Sun C, Liu X, et al. The frequency and treatment of dural tears and cerebrospinal fluid leakage in 266 patients with thoracic myelopathy caused by ossification of the ligamentum flavum. Spine (Phila Pa 1976) 2012; 37: E702–E707. doi: 10.1097/BRS.0b013e31824586a8. [DOI] [PubMed] [Google Scholar]
- 2.He B, Yan L, Xu Z, et al. Treatment strategies for the surgical complications of thoracic spinal stenosis: a retrospective analysis of two hundred and eighty three cases. Int Orthop 2014; 38: 117–122. doi: 10.1007/s00264-013-2103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun J, Sun K, Shi J, et al. The bridge crane technique for the treatment of the severe thoracic ossification of the ligamentum flavum with myelopathy. Eur Spine J 2018; 27: 1846–1855. doi: 10.1007/s00586-018-5683-0. [DOI] [PubMed] [Google Scholar]
- 4.Kang KC, Lee CS, Shin SK, et al. Ossification of the ligamentum flavum of the thoracic spine in the Korean population. J Neurosurg Spine 2011; 14: 513–519. doi: 10.3171/2010.11.SPINE10405. [DOI] [PubMed] [Google Scholar]
- 5.Liao CC, Chen TY, Jung SM, et al. Surgical experience with symptomatic thoracic ossification of the ligamentum flavum. J Neurosurg Spine 2005; 2: 34–39. doi: 10.3171/spi.2005.2.1.0034. [DOI] [PubMed] [Google Scholar]
- 6.Aizawa T, Sato T, Sasaki H, et al. Thoracic myelopathy caused by ossification of the ligamentum flavum: clinical features and surgical results in the Japanese population. J Neurosurg Spine 2006; 5: 514–519. doi: 10.3171/spi.2006.5.6.514. [DOI] [PubMed] [Google Scholar]
- 7.Guo JJ, Luk KD, Karppinen J, et al. Prevalence, distribution, and morphology of ossification of the ligamentum flavum: a population study of one thousand seven hundred thirty-six magnetic resonance imaging scans. Spine (Phila Pa 1976) 2010; 35: 51–56. doi: 10.1097/BRS.0b013e3181b3f779. [DOI] [PubMed] [Google Scholar]
- 8.Feng FB, Sun CG, Chen ZQ. Progress on clinical characteristics and identification of location of thoracic ossification of the ligamentum flavum. Orthop Surg 2015; 7: 87–96. doi: 10.1111/os.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang T, Yin C, Wang D, et al. Surgical technique for decompression of severe thoracic myelopathy due to tuberous ossification of ligamentum flavum. Clin Spine Surg 2017; 30: E7–E12. doi: 10.1097/BSD.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 10.Zhao XB, Li XC, Zhou HG, et al. “U” route transforaminal percutaneous endoscopic thoracic discectomy as a new treatment for thoracic spinal stenosis. Int Orthop 2019; 43: 825–832. doi: 10.1007/s00264-018-4145-y. [DOI] [PubMed] [Google Scholar]
- 11.Sato T, Kokubun S, Tanaka Y, et al. Thoracic myelopathy in the Japanese: epidemiological and clinical observations on the cases in Miyagi Prefecture. Tohoku J Exp Med 1998; 184: 1–11. doi: 10.1620/tjem.184.1. [DOI] [PubMed] [Google Scholar]
- 12.Nie ZH, Liu FJ, Shen Y, et al. Lamina osteotomy and replantation with miniplate fixation for thoracic myelopathy due to ossification of the ligamentum flavum. Orthopedics 2013; 36: e353–e359. doi: 10.3928/01477447-20130222-26. [DOI] [PubMed] [Google Scholar]
- 13.Sun J, Shi J, Xu X, et al. Anterior controllable antidisplacement and fusion surgery for the treatment of multilevel severe ossification of the posterior longitudinal ligament with myelopathy: preliminary clinical results of a novel technique. Eur Spine J 2018; 27: 1469–1478. doi: 10.1007/s00586-017-5437-4. [DOI] [PubMed] [Google Scholar]
- 14.Pao JL, Wang JL. Intraoperative myelography in minimally invasive decompression for degenerative lumbar spinal stenosis. J Spinal Disord Tech 2012; 25: E117–E124. doi: 10.1097/BSD.0b013e31825bfdac. [DOI] [PubMed] [Google Scholar]
- 15.Tomčovčík L, Cuha R, Raši R. Peroperačná perimyelografia pri liečbe zlomenín torakolumbálnej chrbtice [Intra-operative myelography in treatment of fractures of thoracolumbar spine]. Acta Chir Orthop Traumatol Cech 2010; 77: 320–326. Slovak. [PubMed] [Google Scholar]
- 16.Yang Y, Zhang L, Dong J, et al. Intraoperative myelography in transpsoas lateral lumbar interbody fusion for degenerative lumbar spinal stenosis: a preliminary prospective study. Biomed Res Int 2017; 2017: 3742182. doi: 10.1155/2017/3742182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyakoshi N, Shimada Y, Suzuki T, et al. Factors related to long-term outcome after decompressive surgery for ossification of the ligamentum flavum of the thoracic spine. J Neurosurg 2003; 99: 251–256. doi: 10.3171/spi.2003.99.3.0251. [DOI] [PubMed] [Google Scholar]