Abstract
Ovarian steroid-cell tumors (SCTs) are a rare subgroup of sex-cord tumors of the ovary, accounting for less than 0.1% of all ovarian tumors. Not otherwise specified (NOS) tumors are the most common subtype. More than half of patients with SCTs-NOS show hyperandrogenic symptoms. The primary treatment for SCTs is surgery, as most cases are early-staged and benign. Because of the low incidence of metastatic disease, there is insufficient reliable information on the role of adjuvant therapy and the most effective treatment regimen. In this report, a rare case of a recurrent SCT-NOS in a 36-year-old female patient without endocrine symptoms is presented, highlighting the significance of appropriate pathological evaluation and immunohistochemical testing for the accurate diagnosis of this malignancy, particularly in the case of hormonally “silent” tumors. The metastatic tumor described here showed no response to four courses of adjuvant chemotherapy after several debulking surgeries. Based on the clinical findings, the neoplastic etiology should always be considered during the resection of ovarian tumors to prevent possible disease dissemination due to inappropriate surgical techniques.
Keywords: Adjuvant chemotherapy, chemotherapy failure, debulking surgery, neoplastic etiology, not otherwise specified tumor, recurrent ovarian steroid-cell tumor
Introduction
Ovarian steroid-cell tumors (SCTs) are a rare subgroup of sex-cord tumors of the ovary that account for less than 0.1% of all ovarian tumors. They are classified into three categories based on the cell origin: stromal luteoma, Leydig-cell tumor, and not otherwise specified (NOS). NOS tumors are the most common subtype and comprise the largest proportion of cases (60%), whereas stromal luteoma and Leydig-cell tumors each account for ∼20% of cases.1–3
A SCT of the ovary was first described in 1943 as a “virilizing lipoid cell tumor” by Gemma Barzilai in the Atlas of Ovarian Pathology.4 Later, the term “lipoid cell tumor” was replaced by “steroid-cell tumor.” This change was based on the argument that although all SCTs can produce steroid hormones, up to 25% of them contain little or no lipids.4 In 1979, the term “steroid-cell tumor, not otherwise specified,” was coined by Scully2 to indicate that the cell lineage from which the tumor arises is unknown. SCTs-NOS usually develop in reproductive-aged women with an average age of 43 years. The major symptoms are hirsutism and virilization caused by high levels of testosterone. The cornerstone of SCT-NOS treatment is surgery. However, as most tumors are diagnosed in the early clinical stage and do not recur or metastasize, there is limited information on their response to chemotherapy.1
In this report, a rare case of a recurrent SCT-NOS in a patient without endocrine symptoms is presented. The tumor showed no response to adjuvant chemotherapy after several debulking surgeries.
Case presentation
A 36-year-old Caucasian female patient, gravida 2 para 2, underwent laparoscopic right salpingo-oophorectomy in 2015 at a secondary-level hospital after the identification of a right ovarian tumor (the tumor was fragmented within the abdominal cavity, but it was unclear if it was within the endobag). The post-surgical pathological report revealed a Sertoli–Leydig-cell tumor. The patient was not referred to a clinical oncologist because radical removal was expected. Her past medical and surgical history was unremarkable, and she had no relevant family history. No signs of virilization or hirsutism were observed.
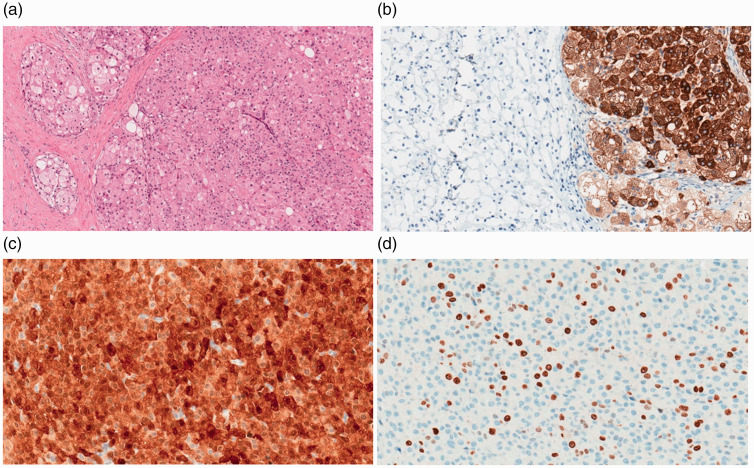
After 6 months, the patient was referred to the Gynecologic Department of our tertiary hospital because of metastases. During laparoscopy, multiple metastatic lesions were found in the pelvic peritoneum, on the diaphragm, and at the site of the former trocar; the left ovary was solid with suspected tumor spread. Metastatic lesions appeared as 3- to 4-mm subperitoneal fatty nodules, which were biopsied. Washings from the peritoneal cavity were negative. Unexpectedly, the histopathological diagnosis was a SCT-NOS. Laboratory analysis revealed normal values of follicle-stimulating hormone, luteinizing hormone, prolactin, estradiol, progesterone, testosterone, dehydroepiandrosterone sulfate (DHEA-S), and cortisol. No hormonal assays were performed prior to surgery, as there were no clinical manifestations of any excessive hormone secretion. The ovarian tumor markers, including human epididymis protein 4, β-human chorionic gonadotropin, and α-fetoprotein, were within the normal range. Cancer antigen 125 (Ca125) was elevated to 48.8 kU/L (normal value up to 35 kU/L), but after 3 weeks, the level returned to normal (24.8 kU/L). Ten months after the first surgery, the patient underwent a total abdominal hysterectomy, left salpingo-oophorectomy, omentectomy, pelvic and diaphragmatic peritonectomy, and pelvic lymphadenectomy. Optimal cytoreductive surgery was performed. Microscopic findings exhibited diffuse tumor cells with abundant eosinophilic granular cytoplasm and vacuolization but no signs of nuclear atypia and a mitotic count of 5 per 10 high-power fields. No Reinke crystals, which are usually observed in Sertoli–Leydig-cell tumors, were identified. Immunohistochemistry revealed positive expression of inhibin A, calretinin, and synaptophysin but negative expression of pan-cytokeratin, CD68, epithelial membrane antigen, chromogranin A, and estrogen receptor/androgen receptor. The Ki-67 labeling index was up to 15% (Figure 1). The histopathologic features supported the diagnosis of a SCT-NOS.
Figure 1.
Microscopic appearance of the SCT-NOS (H&E) in a 36-year-old Caucasian female patient (a) and positive staining of the tumor cells for inhibin A (b), calretinin (c), and Ki-67 (d) (20×)
H&E: hematoxylin and eosin.
After the surgery, the patient received six cycles of adjuvant carboplatin-paclitaxel chemotherapy. Ca125 was measured several times during the treatment period; its levels were within the normal range. At the end of the six courses of chemotherapy, a control computed tomography (CT) scan was performed, revealing the absence of disease progression.
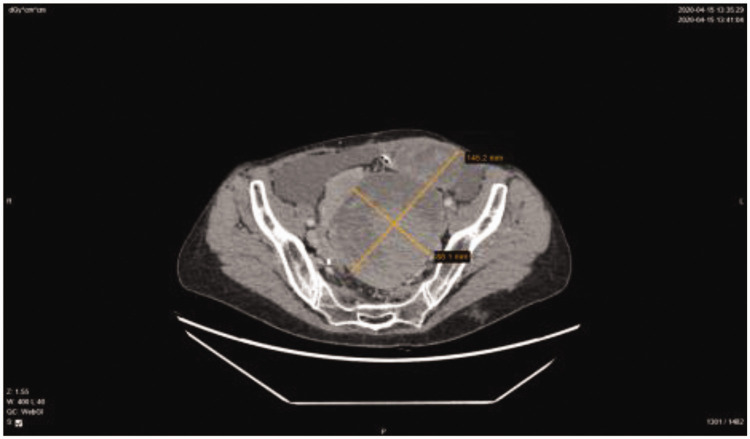
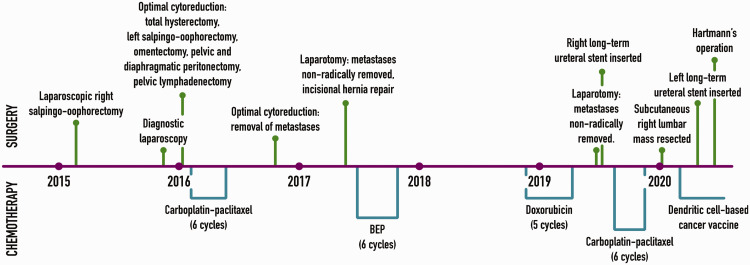
After 3 months of follow-up, the CT scan showed disease progression in the left lateral abdominal flank; hence, we decided to perform secondary cytoreductive surgery. McBurney’s laparotomy was performed. During abdominal exploration, an infiltrative growing tumor was found at the site of the right former trocar that had grown in all layers of the abdomen and spread into the right flank. The laparotomy was extended in the cephalad direction. Metastases were also observed in the left superior diaphragmatic space and the previous left trocar area. Secondary optimal cytoreduction was performed to remove metastases from the right iliac region and abdominal lateral canals. The analysis of frozen sections demonstrated SCT metastases in fibrous adipose tissue. The patient experienced disease-free survival for only 6 months, after which the follow-up CT scan showed disease progression and a large incisional hernia at the incision site of the previous McBurney’s laparotomy. Serum tumor markers and hormone levels were within the normal range. During the subsequent laparotomy, micrometastases were observed on the aponeurosis with multiple small metastases on the small and large intestines, which could not be radically removed. The excision of injured subcutaneous tissue and incisional hernia repair with mesh were performed. The histological diagnosis was SCT metastasis to soft tissues. The patient was subjected to six cycles of second-line bleomycin, etoposide, and cisplatin (BEP) chemotherapy. On account of a dry cough, bleomycin has not been prescribed after the second cycle due to suspicion of pulmonary toxicity. The patient was put under surveillance every 3 months after the completion of chemotherapy. An abdominopelvic CT scan revealed the absence of disease progression, and no metastases were observed on the right side of the anterior abdominal wall or the bowel. Serum tumor markers and hormone levels were normal. After 1 year, disease progression was observed on the CT scan, which revealed increased foci in the anterior and lateral abdominal wall (>50%) and a small amount of ascites in the lateral flanks. Systemic treatment was recommended, and five cycles of liposomal doxorubicin were administered. Despite undergoing treatment, radiologic disease progression was observed after 4 months, as there was an ∼40% increase in tumor mass above the vaginal cuff on the left side with an increase in fluid volume in the pelvis. On the right abdominal wall, supra-umbilically, most small tumor foci had increased in size. The patient required re-operation. On the anterior abdominal wall, several necrotic metastases were found. In the abdominal cavity, there was a 10-cm ruptured tumor between the sigmoid colon and the vaginal cuff. Multiple 1- to 4-cm retroperitoneal metastases were found on the right internal iliac artery, right ureter, and pelvic veins. The right ureter was approximately 2 cm in diameter, with metastases completely fixed to the pelvic bones/fasciae. Few small metastases were noted on the peritoneum and mesentery. Because of the infiltration of the lateral pelvic walls, the tumors could not be radically resected; sigmoid colon resection was rendered ineffective because of the presence of residual tumor masses. Only bleeding tumors and metastases on the peritoneum and mesentery were non-radically removed. Histopathological verification revealed metastases of the SCT-NOS. At the pain management center, the patient was advised to start treatment with transdermal fentanyl patches for the treatment of intense abdominal pain. The patient was referred to a urologist because of right hydronephrosis, and a long-term ureteral stent was inserted. The multidisciplinary team decided to repeat the previous regimen of chemotherapy based on carboplatin and paclitaxel (six cycles). After completion of the chemotherapy, a CT scan was performed. The pelvic, vaginal cuff, and sigmoid colon masses were enlarged, indicating disease progression. Only palliative treatment was recommended. After 5 months, another CT scan was performed, and significant disease progression and noticeable bilateral hydronephrosis were detected, with the metastases enlarged by 80% (Figure 2). To manage the pain, the subcutaneous right lumbar mass was resected under regional anesthesia. The right ureteral stent was replaced, and a long-term stent was also inserted into the left ureter. Currently, the hormone levels and cancer biomarkers are within the normal reference ranges. Over the last 2 months, the patient was administered dendritic cell-based cancer vaccines; however, no effect has been reported. Because of the severe pain caused by the large-volume pelvic tumor, laparotomy was performed with palliative intention, revealing an approximately 15-cm metastasis on the rectosigmoid mesentery, a 3-cm metastasis on the sigmoid colon, and multiple 1- to 2-mm implants on the small intestines. All lesions were removed, and Hartmann’s operation was performed because of heavy bleeding from the mesorectum and rectosigmoid mesentery. The patient was discharged from the hospital on the fourth uneventful postoperative day and is currently receiving palliative care. The course of the disease is shown in Figure 3.
Figure 2.
CT scan of the patient reported in this case showing 14.5 × 8.8-cm metastatic masses within the pelvis.
CT: computed tomography.
Figure 3.
Treatment course of the patient described in this report.
BEP: bleomycin, etoposide, and cisplatin.
Discussion
Ovarian SCTs-NOS occur at any age (2.5–93 years), but they are usually observed in reproductive-aged women, with an average age of 43 years.5,6 More than half (56%–77%) of patients with an SCT-NOS show hyperandrogenic symptoms and signs of virilization, such as hirsutism, acne, deepened voice, clitoromegaly, amenorrhea, and infertility.1,7–9 Additionally, it can present with estrogenic manifestations (6%–23%), such as menorrhagia or postmenopausal bleeding. Some patients also develop endometrial cancer.10 Ovarian SCTs can secrete steroid hormones, such as progesterone, cortisol, and aldosterone, which may cause corresponding clinical symptoms. Hyperandrogenic tumors may also be associated with paraneoplastic manifestations, such as hypercalcemia, erythrocytosis, or ascites.11 However, about 25% of patients with SCTs may have atypical presentations, without any symptoms of virilization. In these cases, the diagnosis is usually made postoperatively after histopathological verification.12 In the patient reported in this case, no virilization symptoms were noted, and the tumor was hormonally “silent.” Additional symptoms in some patients may include abdominal distension due to ascites and palpable abdominal masses.13 After tumor dissemination, the patient reported here manifested severe abdominal pain with palpable abdominal masses.
Serum laboratory analyses typically show elevated levels of testosterone and androstenedione, indicating an ovarian origin of androgen release and normal DHEA-S levels, thereby excluding adrenal causes of hyperandrogenism.1 In the reported case, the hormone levels were within the reference range, even after considerable disease dissemination. There is no known specific tumor marker established for the preoperative diagnosis of SCTs-NOS. Tumor markers, such as CA-125 and α-fetoprotein, are generally within the normal range, and the data from the literature do not indicate whether elevated levels signify malignant potential. Some studies even claim that these markers could facilitate the differential diagnosis of ovarian adenocarcinoma.11,14 This correlates with the described case, as even after disease relapse, tumor markers remained within the normal range.
SCTs-NOS are unilateral in 94% of cases, large at diagnosis (range from 1.2 to 45 cm in the greatest dimension, with an average reported size of 8.4 cm), and typically solid and well-circumscribed. SCTs-NOS should be distinguished from other ovarian tumors and SCTs, in which the proliferation of steroid hormone-producing cells occurs as a secondary event. These include stromal luteomas, Leydig-cell tumors, luteinized thecomas, and pregnancy luteomas.15 SCTs-NOS are different from Leydig-cell tumors in terms of their deficiency in cytoplasmic Reinke crystals. In addition, Leydig-cell tumors are usually situated in hilar locations, and they are commonly associated with Leydig-cell hyperplasia.16,17 Stromal luteomas are confined to the ovarian stroma and frequently occur in association with stromal hyperthecosis.12 SCTs-NOS might have a fibromatous component, similar to that of thecomas, but this component accounts for only less than 10% of tumors.18 Pregnancy luteomas are more commonly multifocal (bilateral in one-third of cases), usually discovered at the time of cesarean section, and regress spontaneously after pregnancy.12
In addition to the microscopic features, immunohistochemistry is particularly helpful for proper diagnosis. The sensitivity of positive calretinin is 60% to 90%, whereas the sensitivity of inhibin reactivity ranges from 5% to 90%.3,19 SCTs-NOS are also commonly vimentin, Melan-A, and CD99 positive and variably AE1/3, CAM5.2, HMB45, and S100 positive.3 Currently, techniques are being developed to define SCTs-NOS pathologically by immunohistochemical staining of steroidogenic enzymes. In the future, validated enzymes could serve as markers for the differential diagnosis of hyperandrogenic ovarian conditions.20 It is worth noting that differential diagnosis is challenging, as the primary histopathological diagnosis of this case from the previous hospital indicated a Sertoli–Leydig-cell tumor. However, no Reinke crystals were found after careful evaluation of the metastases resected in our hospital. In this patient, the tumor showed strong and diffuse positivity for inhibin and calretinin.
Usually, these tumors are benign; however, 25% to 43% of SCTs are malignant, with 20% of cases found to exhibit metastases beyond the ovary. Metastatic lesions usually occur within the peritoneal cavity and rarely occur at distant sites.15 In one study, the clinical and pathological features of 63 SCTs-NOS were reviewed. Follow-up data ranging from 1 to 19 years (average 5.2 years) in duration were available for 50 patients. In 24 cases, the tumor was designated as probably benign (no evidence of spread beyond the ovary within 3 or more years postoperatively). In 18 patients, the tumor was malignant. Despite the various chemotherapy and radiation regimens, 12 patients experienced recurrences, and 14 patients died from the disease, suggesting a poor prognosis if the tumor is at an advanced stage, large, or recurring. Five pathological features are considered the best correlates of malignant behavior: the presence of two or more mitotic figures per 10 high-power fields (92% malignant), necrosis (86% malignant), a diameter of 7 cm or greater (78% malignant), hemorrhage (77% malignant), and grade 2 or 3 nuclear atypia (64% malignant).6 In our patient, we were unable to evaluate these features because the primary tumor was not resected at our hospital. We assume that the rapid spread was related to possible tumor fragmentation within the abdominal cavity, as we are uncertain of the use of an endobag.
Because the incidence of SCTs-NOS is low, there are currently no established treatment protocols. Therefore, the tumors are treated similarly to stromal tumors depending on several factors, including the surgical stage, histological type, patient’s age, and history of childbirth. The primary treatment for SCTs is surgery. Ovarian SCTs are generally considered benign because they are often detected at an early clinical stage. Therefore, unilateral salpingo-oophorectomy or tumor removal is acceptable in reproductive-aged women.1 However, regular follow-up with measurement of serum testosterone levels is mandatory. As there is limited information on the mechanisms of these tumors, the optimal length of follow-up is yet to be determined.15 In addition, there have already been reported cases of spontaneous pregnancies after tumor removal, probably because of the decline in testosterone levels.21,22
Surgical treatments using total abdominal hysterectomy and bilateral salpingo-oophorectomy are an appropriate management option for postmenopausal patients and those who have completed childbearing. Endometrial sampling should be performed when fertility-sparing surgery is planned because many of these patients may have coexisting endometrial hyperplasia or even uterine adenocarcinoma that might affect the decision for performing a hysterectomy.1
Because of the limited incidence of metastatic disease, there is a lack of reliable information on the role of adjuvant therapy in SCTs-NOS. The adjuvant chemotherapy regimens currently recommended for treatment are as follows: BEP; cisplatin, doxorubicin, and cyclophosphamide; taxane and platinum; and bleomycin, vinblastine, and cisplatin.23–27 It was reported that intraperitoneal dissemination and liver metastases were completely removed with debulking surgery, radiofrequency ablation of the liver metastasis, and adjuvant BEP (follow-up, 43 months) in a patient with a recurrent SCT-NOS occurring 5 years after the initial surgery.28 In another study, the treatment protocol consisted of docetaxel and nedaplatin, and the patient survived for 2 years with multiple bone metastases.29 Another SCT-NOS case with progressive disease after surgical debulking was reported. Treatment with multi-agent chemotherapy failed, but the patient subsequently showed a robust response to gonadotropin-releasing hormone agonist (GnRHa) therapy. Therefore, although typically treated with surgery alone, GnRHa may be required when abnormal serum hormone levels persist, and there is suspicion of residual tumors, recurrences, or metastases. It is suggested that GnRHa treatment be considered prior to cytotoxic chemotherapy in cases with SCTs-NOS.30 In the case described above, a chemotherapeutic regimen with BEP was administered for the recurrent SCT-NOS. After three cycles of BEP, the chemotherapeutic agent was changed to paclitaxel and carboplatin following a stable disease course. After eight cycles of paclitaxel and carboplatin, chemotherapy was discontinued because of prolonged neutropenia and peripheral neuropathy. The CT scan showed clinically stable disease. As the immunohistochemical analysis of the primary tumor revealed positive cytoplasmic staining for the GnRH receptor, GnRHa therapy was attempted based on reference to previous case reports and the lack of other effective alternatives. After six cycles of GnRHa therapy, the CT scan confirmed significant tumor size reduction. Adverse events were not observed, and the patient’s hirsutism and virilization had improved. Therefore, the treatment was discontinued after the administration of six cycles. However, the testosterone level was increased 2 months after GnRHa discontinuation, and the CT scan revealed an increase in tumor size. Subsequently, GnRHa was re-administered, resulting in immediate normalization of the serum testosterone level and shrinkage of the recurrent tumor. Thus, GnRHa therapy was continued (22 months after the first GnRHa treatment with the absence of symptoms).31
In our patient, the SCT-NOS was completely insensitive to chemotherapy, despite undergoing several debulking surgeries. Removal of the multi-metastases resulted in only short-term improvement. Unfortunately, the dendritic cell-based vaccine recently administered for 2 months did not yield a positive outcome.
Conclusions
Based on our clinical experience of this case, we highlight the importance of ovarian tumor resection without direct fragmentation within the abdominal cavity, as the neoplastic etiology should always be taken into account. Pathological evaluation is essential for the diagnosis of this malignancy, and immunohistochemical testing also aids in the formulation of an accurate diagnosis, especially in a patient with an apparent absence of endocrine manifestations. Surgery is the main treatment method for SCTs-NOS, as most cases are benign, and there are no generalized conclusions on the response of malignant cases to therapies, such as chemotherapy or radiation therapy. According to recent studies, GnRHa therapy could serve as a feasible treatment method and is worthy of further research with large patient groups.
Acknowledgment
We acknowledge oncologists from the Department of Oncology and Chemotherapy of Vilnius University Hospital Santaros Clinics for their considerable contribution to the treatment of the patient.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Ethics statement: In our country, it is not mandatory to receive written consent from the local Ethics Committee for a case report, as it does not strictly meet the criteria of research because it does not require investigation and is intended to develop information to be shared for medical or educational purposes. Written informed consent was obtained from the patient for the publication of this report and the accompanying images.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Danuta Vasilevska https://orcid.org/0000-0002-3136-7281
Ugnius Mickys https://orcid.org/0000-0001-7805-0727
Andrzej Semczuk https://orcid.org/0000-0001-9344-9437
References
- 1.Yuan X, Sun Y, Jin Y, et al. Ovarian steroid cell tumor, not otherwise specified, treated with surgery: a case report and review of literature. Int J Clin Exp Pathol 2019; 12: 1434–1438. [PMC free article] [PubMed] [Google Scholar]
- 2.Seles M, Revathy M, Kanchana MP. Steroid cell tumour of the ovary: a case report with review of literature. Int J Reprod Contracept Gynecol 2018; 7: 3425–3428. [Google Scholar]
- 3.Tan EC, Khong CC, Bhutia K. A rare case of steroid cell tumor, not otherwise specified (NOS), of the ovary in a young woman. Case Rep Obstet Gynecol 2019; 2019: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones MW, Harri R, Dabbs DJ, et al. Immunohistochemical profile of steroid cell tumor of the ovary: a study of 14 cases and a review of the literature. Int J Gynecol Pathol 2010; 29: 315–320. [DOI] [PubMed] [Google Scholar]
- 5.Powell JL, Dulaney DP, Shiro BC. Androgen-secreting steroid cell tumor of the ovary. South Med J 2000, 93: 1201–1204. [PubMed] [Google Scholar]
- 6.Hayes MC, Scully RE. Ovarian steroid cell tumors (not otherwise specified). A clinicopathological analysis of 63 cases. Am J Surg Pathol 1987; 11: 835–845. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda S, Yamaguchi Y, Kaseki H, et al. Case of ovarian steroid cell tumor diagnosed after presenting acute heart failure. J Obst Gynaecol Res 2020; 46: 1211–1215. [DOI] [PubMed] [Google Scholar]
- 8.Wong FCK, Chan AZ, Wong WS, et al. Hyperandrogenism, elevated 17-hydroxyprogesterone and its urinary metabolites in a young woman with ovarian steroid cell tumor, Not Otherwise Specified: case report and review of the literature. Case Rep Endocrinol 2019; 2019: 9237459. DOI: 10.1155/2019/9237459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szeliga A, Zysnarska A, Podfigurna A, et al. Ovarian steroid cell tumor as an example of severe hyperandrogenism in 45-year-old woman. Gynecol Endocrinol 2020; 36: 303–307. [DOI] [PubMed] [Google Scholar]
- 10.Chung DH, Lee SH, Lee KB. A case of ovarian steroid cell tumor, not otherwise specified, treated with surgery and gonadotropin releasing hormone agonist. J Menopausal Med 2014; 20: 39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faten H, Dorra G, Slim C, et al. Ovarian steroid cell tumor (not otherwise specified): a case report of ovarian hyperandrogenism. Case Rep Oncol Med 2020; 2020: 6970823. DOI: 10.1155/2020/6970823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhagat R, Bodal VK, Gupta N, et al. Steroid cell tumour of ovary – a rare case report. J Clin Diagn Res 2016; 10: ED06–ED07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YT, Kim SW, Yoon BS, et al. An ovarian steroid cell tumor causing virilization and massive ascites. Yonsei Med J 2007; 48: 142–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chun YJ, Choi HJ, Lee HN, et al. An asymptomatic ovarian steroid cell tumor with complete cystic morphology: A case report. Obstet Gynecol Sci 2013; 56: 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alves P, Sá I, Brito M, et al. An early diagnosis of an ovarian steroid cell tumor not otherwise specified in a woman. Case Rep Obstet Gynecol 2019; 2019: 2537480. DOI: 10.1155/2019/2537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsukawa J, Takahashi T, Hada Y, et al. Successful laparoscopic resection of virilizing ovarian steroid cell tumor, not otherwise specified, in a 22-year-old woman: a case report and evaluation of the steroidogenic pathway. Fukushima J Med Sci 2019; 65: 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanley KZ, Mosunjac MB. Practical review of ovarian sex cord–stromal tumors. Surg Path 2019; 12: 587–620. [DOI] [PubMed] [Google Scholar]
- 18.Murhekar K, Louis R, Majhi U. A rare occurrence of a steroid cell tumor of the pelvic mesentery: a case report. J Med Case Rep 2011; 5: 517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnuckle EM, Williamson A, Carpentieri D, et al. Ovarian sex cord stromal tumor, steroid cell, NOS in an adolescent: a case report. J Pediatr Adolesc Gynecol 2020: S1083-3188(20)30295-3. DOI: 10.1016/j.jpag.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Scheker EV, Kathuria A, Esnakula A, et al. Expression of key androgen-activating enzymes in ovarian steroid cell tumor, not otherwise specified. J Investig Med High Impact Case Rep 2020; 8: 23247096-20933416. DOI: 10.1177/2324709620933416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sielert L, Liu C, Nagarathinam R, et al. Androgen-producing steroid cell ovarian tumor in a young woman and subsequent spontaneous pregnancy. J Assist Reprod Genet 2013; 30: 1157–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ben Haj Hassine MA, Msakni I, Siala H, et al . Laparoscopic management of an ovarian steroid cell tumor, not otherwise specified causing virilization and amenorrhea: a case report. Case Rep Clin Pathol 2015; 3: 10–13. [Google Scholar]
- 23.Gershenson DM, Copeland LJ, Kavanagh JJ, et al. Treatment of metastatic stromal tumors of the ovary with cisplatin, doxorubicin, and cyclophosphamide. Obstet Gynecol 1987; 70: 765–769. [PubMed] [Google Scholar]
- 24.Homesley HD, Bundy BN, Hurteau JA, et al. Bleomycin, etoposide, and cisplatin combination therapy of ovarian granulosa cell tumors and other stromal malignancies: A Gynecologic Oncology Group study. Gynecol Oncol 1999; 72: 131–137. [DOI] [PubMed] [Google Scholar]
- 25.Pecorelli S, Wagenaar HC, Vergote IB, et al. Cisplatin (P), vinblastine (V) and bleomycin (B) combination chemotherapy in recurrent or advanced granulosa(-theca) cell tumours of the ovary. An EORTC Gynaecological Cancer Cooperative Group study. Eur J Cancer 1990; 35: 1331–1337. [DOI] [PubMed] [Google Scholar]
- 26.Brown J, Shvartsman HS, Deavers MT, et al. The activity of taxanes compared with bleomycin, etoposide, and cisplatin in the treatment of sex cord-stromal ovarian tumors. Gynecol Oncol 2005; 97: 489–496. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimatsu T, Nagai K, Miyawaki R, et al. Malignant ovarian steroid cell tumor, not otherwise specified, causes virilization in a 4-year-old girl: a case report and literature review. Case Rep Oncol 2020; 13: 358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JS, Park SN, Kim BR. Recurrent ovarian steroid cell tumor, not otherwise specified managed with debulking surgery, radiofrequency ablation, and adjuvant chemotherapy. Obstet Gynecol Sci 2014; 57: 534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li K, Zhu F, Xiong J, et al. A rare occurrence of a malignant ovarian steroid cell tumor not otherwise specified: A case report and literature review. Oncol Lett 2014; 8: 770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brewer CA, Shevlin D. Encouraging response of an advanced steroid-cell tumor to GnRH agonist therapy. Obstet Gynecol 1998; 92: 661–663. [DOI] [PubMed] [Google Scholar]
- 31.Nakasone T, Nakamoto T, Matsuzaki A, et al. Direct evidence on the efficacy of GnRH agonist in recurrent steroid cell tumor-not otherwise specified. Gynecol Oncol Rep 2019; 29: 73–75. [DOI] [PMC free article] [PubMed] [Google Scholar]