Abstract
Objective: To review the safety and efficacy of romosozumab (Evenity) in the treatment of osteoporosis in women. Data Sources: An English-language search of PubMed and Medline (1966 to August 2020) was conducted using the keywords romosozumab, sclerostin inhibitor, AMG785, and osteoporosis. Manufacturer prescribing information, abstracts, fda.gov, and ClinicalTrials.gov data were incorporated for additional materials. In addition, a review of bibliographies of retrieved articles was performed to identify additional references. Study Selection/Data Extraction: Articles selected included those that described clinical studies of pharmacokinetics, efficacy, or safety of romosozumab. Data Synthesis: Romosozumab is a human monoclonal antibody that inhibits the action of sclerostin and is the first agent in its class to reach Phase III trials. Significant increases in bone mineral density and decreases in vertebral and hip fractures are demonstrated in Phase III trials. Favorable results led to its marketing approval in several countries. Major adverse cardiac events were observed in one clinical trial. Other adverse effects include arthralgia, headache, and injection site reactions. Place in Therapy: Romosozumab is the first agent to inhibit bone resorption and stimulate bone formation. Romosozumab should be reserved for postmenopausal women at highest risk for fracture and should be followed by an anti-resportive agent to maintain or further increase bone mineral density. This injectable agent should not be considered for women with a history of or at high risk of cardiovascular disease.
Keywords: romosozumab, sclerostin inhibitor, osteoporosis
Introduction
In April 2019, the Food and Drug Administration (FDA) approved romosozumab (Evenity; Amgen Inc), the first sclerostin inhibitor, for the treatment of osteoporosis in postmenopausal women at high risk of fracture. Osteoporosis affects more than 10 million Americans and leads to more than 2 million osteoporosis-related fractures each year.1 In individuals over the age of 65 years, osteoporosis affects women more than men, with 25% of women afflicted in comparison with 5% of men.2
Bone remodeling occurs as a result of continuous osteoclast-controlled bone resorption and osteoblast-controlled bone formation. As individuals age, an imbalance in the bone remodeling process occurs, favoring bone resorption over bone formation.3 Sclerostin, a glycoprotein produced primarily by osteocytes, significantly inhibits bone formation and indirectly stimulates bone resorption. The impetus behind inhibiting sclerostin resulted from evidence in 2 rare genetic disorders, sclerosteosis and van Buchem’s disease, where patients lacking sclerostin suffered from complications associated with bone thickening.3 Targeting sclerostin inhibition is an innovative pathway to stimulate bone formation.
Data Selection
Relevant articles were identified through a comprehensive search of PubMed (1966-August 2020) and International Pharmaceutical Abstracts (1970-August 2020) using the key terms romosozumab, sclerostin inhibitor, Evenity, and AMG785. All relevant English-language articles of studies assessing pharmacokinetics, efficacy, or safety of romosozumab were selected. In addition, a review of bibliographies of retrieved articles was performed to identify additional references. The manufacturer product labeling, Evenity website, and ClinicalTrials.gov also served as resources.
Pharmacology
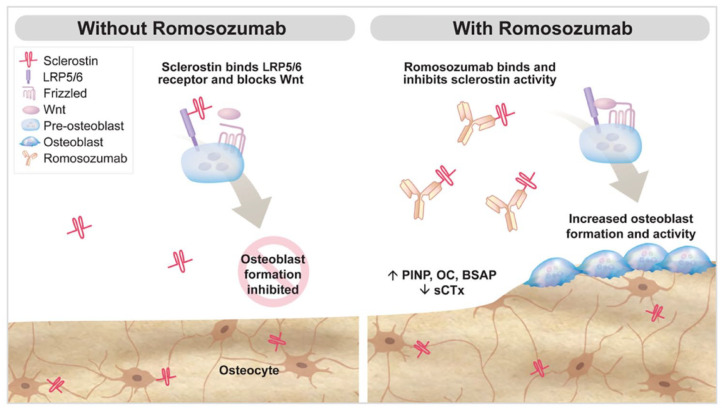
Romosozumab, a sclerostin inhibitor, is a monomeric glycoprotein secreted by osteocytes.4,5 Sclerostin binds to osteoblasts via the low-density lipoprotein receptor protein 5 and 6 and frizzled co-receptor, thereby inhibiting the Wnt signaling pathway. Accordingly, sclerostin decreases osteoblastic formation and has a pivotal role in skeletal development (Figure 1).4,6,7 Romosozumab binds to sclerostin and prevents inhibition of the Wnt signaling pathway, thereby promoting osteogenesis and, to a lesser extent, reducing bone resorption. The result is an increase in bone mass in both cortical and trabecular bone.4 Markers of bone formation return to baseline within 12 months of discontinuation of romosozumab.
Figure 1.
Mechanism of romosozumab. Effects of Wnt signaling and role in bone turnover. Abbreviations: P1NP, procollagen type 1 N-propeptide; OC, osteocalcin; BSAP, bone-specific alkaline phosphatase; sCTx, serum C-telopeptide.
Reprinted from Padhi et al.6 Reprinted with permissions of Oxford University Press.
Pharmacokinetics
Romosozumab is a humanized immunoglobulin G2 monoclonal antibody (romosozumab-aqqg) and is administered monthly via subcutaneous (SQ) injection.4 An overview of the pharmacokinetic parameters of romosozumab is provided in Table 1. Administration of a single 210 mg dose results in a peak plasma concentration (Cmax) of 22.2 ± 5.8 µg/mL and a mean area under the plasma drug concentration-time curve of 389 µg*day/mL.4,8 Steady state concentrations are achieved by month 3. Romosozumab exhibits nonlinear pharmacokinetics. As the dose is increased, clearance of romosozumab decreases and exposure to the drug increases at a greater rate relative to the given dose.
Table 1.
| Absorption | Tmax of 5 days (range 2 to 7 days) |
| Distribution | Estimated volume of distribution at steady state is 3.92 L |
| Metabolism | Not characterized. Expected to be catabolized into amino acids and smaller peptides similar to the metabolic pathway for human immunoglobulin G. |
| Elimination | Estimated systemic clearance of 0.38 mL/kg/h t1/2 of 12.8 days after 3 monthly doses Exhibits nonlinear pharmacokinetics |
During clinical trials, the formation of anti-romosozumab-aqqg antibodies in clinical trials led to decreased romosozumab-aqqg concentrations. No apparent effects on pharmacokinetic or safety were observed. Increased body weight is associated with a decreased exposure to romosozumab-aqqg. However, decreased exposure had minimal impact on bone mineral density (BMD). Dose adjustments are not required based on body weight.4
Clinical Trials
Two pivotal phase I studies evaluated the pharmacokinetics, pharmacodynamics, safety, and tolerability of romosozumab6,8 (Table 2). The first in-human trial, evaluated 72 healthy men and postmenopausal women.8 Subjects were randomized to receive a single dose of romosozumab SQ (0.1 mg, 0.3 mg, 1 mg, 5 mg, or 10 mg/kg), intravenously (1 mg or 5 mg/kg), or placebo. Single dose by either route of administration resulted in increased bone formation markers in a dose-dependent fashion (procollagen type 1 N-propeptide [P1NP], bone-specific alkaline phosphatase [BAP], and osteocalcin). A decrease was observed in serum C-telopeptide (sCTx), a bone resorption marker. The largest increase in BMD occurred with the SQ dose of 10 mg/kg at the lumbar spine (5.3%, P < .01) and at the total hip (2.8%, P < .01), compared with placebo.8
Table 2.
Important Phase I, II, and III Clinical Trials.
| Reference | Design | Subjects | Dose | Duration | Comparator | Outcomes |
|---|---|---|---|---|---|---|
| Padhi et al8 | DB, R, PC, SC, Phase I | 785 men and postmenopausal women | ROMO SQ single dose (0.1, 0.3, 1, 5, or 10 mg/kg) or ROMO IV (1 or 5 mg/kg) | 85 days | Placebo | • Increase P1NP, BAP, osteocalcin • Increase BMD LS (5.3%, P < .01) • Increase BMD TH (2.8%, P < .01) |
| Padhi et al6 | DB, R, PC, MC, Phase I | 48 men and postmenopausal women | Women ROMO SQ 1 or 2 mg/kg Q2W; 2 or 3 mg/kg Q4W Men ROMO SQ 1 mg/kg Q2W; 3 mg/kg Q4W |
24 weeks | Placebo | • Increase BMD LS 2% to 3% • Increase P1NP, decrease in sCTx |
| McClung et al9 | DB, R, MC, Phase II | 419 postmenopausal women | ROMO SQ 70 mg, 140 mg, or 210 mg monthly or 140 mg or 210 mg every 3 months | 12 months | ALEN 70 mg weekly orally or TERI 20 µg daily SQ | • Increase BMD at LS 11.3%a
• Increase BMD at TH 4.1%a • Increase in BMD at FN 3.7%a |
| Ishibashi et al10 | DB, PC, MC, Phase II | 252 postmenopausal women | ROMO SQ 70 mg, 140 mg, or 210 mg monthly | 12 months | Placebo | • Increase BMD at LS 16.9%a,b
• Increase BMD at TH 4.7%a,b • Increase BMD at FN 3.8%a,b |
| Cosman et al11 (FRAME) | OL, DB, R, MC, Phase III | 7180 postmenopausal women | ROMO 210 mg SQ monthly × 12 months; followed by DENO 60 mg Q 6 months × 12 months | 24 months | Placebo; followed by DENO 60 mg Q 6 months × 12 months | New vertebral fractures at 12 months • ROMO 0.5%, PBO 1.8%b • New vertebral fractures at 24 months • ROMO 0.6%, PBO 2.5%b Nonvertebral fractures at 12 months • ROMO 1.6%, 2.1% PBOc • Nonvertebral fracture at 24 months • ROMO 2.7%, 3.6% PBOc |
| Saag et al13 (ARCH) | OL, DB, R, MC, Phase III | 4093 postmenopausal women | ROMO 210 mg SQ monthly; followed by ALEN 70mg weekly × 12 months | 12 months | ALEN 70 mg weekly; followed by ALEN 70 mg weekly × 12 months | New vertebral fractures at 24 months • ROMO/ALEN 6.2%b • ALEN/ALEN 11.9%b Incidence of clinical fractured • ROMO/ALEN 9.7%b • ALEN/ALEN 13.0%b |
| Langdahl et al14 (STRUCTURE) | OL, R, MC, Phase III | 436 postmenopausal women taking alendronate | ROMO 210 mg SQ monthly | 12 months | TERI 20 µg daily SQ | Change in BMD • ROMO +2.6%; TERI −0.6%e |
Abbreviations: DB, double blind; R, randomized; PC, placebo controlled; SC, single center; ROMO, romosozumab; SQ, subcutaneously; IV, intravenous; P1NP, procollagen type 1 N-propeptide; BAP, bone-specific alkaline phosphatase; BMD, bone mineral density; LS, lumbar spine; TH, total hip; MC, multi-center; sCTx, serum C-telopeptide; ALEN, alendronate; TERI, teriparatide; FN, femoral neck; FRAME, Fracture Study in Postmenopausal Women with Osteoporosis; OL, open label; DENO, denosumab; PBO, placebo; ARCH, Active-Controlled Fracture Study in postmenopausal women with osteoporosis at high risk.
Romosozumab 210mg monthly.
P < .001.
Did not reach statistical significance.
Nonvertebral plus symptomatic vertebral at 24 months.
P < .0001.
A subsequent double-blind, placebo-controlled, study randomized 32 postmenopausal women and 16 healthy men with low bone mass for a 12-week treatment period, followed by a 12-week treatment-free period.6 Postmenopausal women received SQ doses of 1 or 2 mg/kg once every 2 weeks; 2 or 3 mg/kg once every 4 weeks; or placebo. Men received 1 mg/kg once every 2 weeks; 3 mg/kg once every 4 weeks; or placebo. Multiple doses increased bone formation markers (P1NP, osteocalcin, BAP) and decreased sCTx levels. Increases in BMD at the total hip (~2% to 3%) were noted for women in the 2 mg/kg every 2 weeks and the 3 mg/kg every 4 weeks groups. Increases persisted through follow-up at 24 weeks.
A phase II trial evaluated the safety and efficacy of romosozumab in 419 postmenopausal women aged 55 to 85 years, over a 12-month period.9 Women with a T-score of −2.0 at the lumbar spine, femoral neck, or total hip and −3.5 or more at each of the 3 sites, were randomized to receive romosozumab monthly (70 mg, 140 mg, or 210 mg SQ), romosozumab every 3 months (140 or 210 mg SQ), placebo SQ, or open-label comparators (oral alendronate 70 mg weekly or teriparatide 20 µg SQ daily). Percent change of BMD from baseline was the primary endpoint. Increases in BMD at the lumbar spine were observed in romosozumab subjects (all doses pooled), compared with placebo (P < .001). The largest gain occurred in the 210 mg monthly cohort. At 12 months, BMD was significantly increased from baseline by 11.3%, 4.1%, and 3.7%, at the lumbar spine, total hip, and femoral neck, respectively. These increments were significantly larger than those observed with alendronate or teriparatide (P < .001 for all comparisons).
Changes in BMD with romosozumab was also evaluated in postmenopausal Japanese women with osteoporosis.10 Women with a T-score <−2.5 were randomized to romosozumab (70 mg, 140 mg, 210 mg) SQ once monthly for 12 months, or placebo. All romosozumab doses increased BMD compared with placebo (P < .01). Similar to the previous trial, the 210 mg dose produced the largest gain from baseline (16.9%, P < .001), when compared with the 70 and 140 mg dose (8.4%, 13.3%, respectively; P < .001). All doses increased bone formation marker P1NP and decreased bone resorption marker sCTx at week 1, compared with placebo (P < .001). The mean age in this study was 67.7 years with average T-scores of −2.7, −1.9, and −2.3 at the lumbar spine, total hip, and femoral neck, respectively. Adverse events were comparable between the groups.
FRAME (Fracture Study in Postmenopausal Women with Osteoporosis) was the first published Phase III trial to evaluate efficacy of romosozumab in vertebral and nonvertebral fracture risk reduction.11 Postmenopausal women (n = 7180) with a T-score of −2.5 to −3.5 at total hip or femoral neck were randomized to receive monthly SQ injections of romosozumab 210 mg or placebo for 12 months. After 1 year, subjects in each group received the antiresorptive treatment denosumab 60 mg SQ every 6 months for 12 months. Women with a history of hip fracture, metabolic bone disease, osteonecrosis of the jaw, low 25-hydroxyvitamin D levels (<20 ng/mL), or current hypocalcemia or hypercalcemia were excluded from the trial. Subjects with a baseline 25-hydroxyvitamin D of <40 ng/mL were given a loading dose of 50 000 to 60 000 IU of vitamin D at trial initiation. All subjects received daily calcium (500-1000 mg) accompanied by vitamin D3 or D2 (600-800 IU).
Primary endpoints in the FRAME trial included the cumulative incidence of new vertebral fractures at 12 and 24 months. Secondary endpoints were the incidence of clinical fracture (composite of symptomatic vertebral fracture and nonvertebral fracture), nonvertebral fracture, new or worsening vertebral fracture, hip fracture, major osteoporotic fracture, and multiple new or worsening vertebral fractures at 12 and 24 months. Romosozumab use revealed a 73% risk reduction in new vertebral fracture, compared with placebo (0.5% incidence in romosozumab vs 1.8% in placebo; risk ratio [RR] = 0.27; 95% confidence interval [CI] = 0.16-0.47). A nonsignificant difference was reported for nonvertebral fractures at 12 months (1.6% in romosozumab vs 2.1% in placebo; hazard ratio = 0.75; 95% CI = 0.53-1.05; P = .10).
Denosumab was added to both groups at 12 months to maintain the gains observed in BMD. The 24-month incidence of new vertebral fractures was 75% lower in the romosozumab group (RR = 0.25; 95% CI = 0.16-0.40; P < .001). An extension to the FRAME study assessed an additional year of SQ denosumab administered every 6 months.12 Significant fracture risk reduction was sustained through 36 months, as well as increases in BMD. Adverse events and serious adverse events were similar between the 2 groups.
Women with a previous fragility fracture were evaluated in the Active-Controlled Fracture Study in Postmenopausal Women with Osteoporosis at High Risk (ARCH).13 Saag and colleagues compared romosozumab as initial therapy transitioning to alendronate, versus alendronate treatment alone. Over 4000 women (mean age 74.3 years) were randomized to receive monthly SQ romosozumab (210 mg) or weekly alendronate (70 mg) for 12 months. After 1 year, all subjects received weekly oral alendronate (70 mg) for an additional year. The exclusion criteria was similar to the FRAME study, with the added exclusion of glomerular filtration rate below 35 mL/min. Subjects also received calcium and vitamin D as described in the FRAME study. The primary end points included incidence of new vertebral fracture at 24 months and incidence of clinical fracture (nonvertebral and symptomatic vertebral fracture). BMD at 12 and 24 months was a secondary end point.
Results of the ARCH trial showed a 48% lower risk of new vertebral fracture in the romosozumab/alendronate group, compared with the alendronate/alendronate group (6.2% [127 of 2046 subjects] vs 11.9% [243 of 2047 subjects]; RR = 0.52; 95% CI = 0.40-0.66; P < .001) and a 19% reduction in nonvertebral fractures (hazard ratio = 0.81; 95% CI = 0.66-0.99; P = .04). In addition, hip fractures occurred in 41 of 2046 subjects in romosozumab/alendronate group compared with 66 of 2047 subjects in the alendronate/alendronate group, a 38% risk reduction (hazard ratio = 0.62; 95% CI = 0.42-0.92; P = .02). Serious cardiovascular (CV) adverse events observed in the first year of the trial included 50 subjects (2.5%) in the romosozumab group and 38 (1.9%) in the alendronate-treated group.
Romosozumab was compared with teriparatide in a smaller international multicenter study in women previously treated with a bisphosphonate for at least 3 years.14 Postmenopausal women with osteoporosis were randomly assigned romosozumab 210 mg SQ monthly (n = 218) or teriparatide 20 µg SQ once daily (n = 218). The primary endpoint was change in BMD. Change from baseline at total hip was a 2.6% increase in the romosozumab arm and a 0.6% decrease in the teriparatide group: a between group of difference 3.2% (95% CI = 2.7-3.8; P < .001). Changes in BMD at the femoral neck and lumbar spine were also significantly greater in the romosozumab group at 6 and 12 months. Frequency of adverse events were similar between the treatment groups. The authors concluded transitioning from a bisphosphonate to romosozumab led to gains in hip BMD, which were not seen with teriparatide.
Safety
Adverse events occurring in >5% of subjects in clinical trials with romosozumab include arthralgia and headache.4 Injection site reactions have been reported in approximately 5% of subjects; however, this adverse event did not appear to recur with subsequent injections.3,4
In 2017, the FDA denied approving romosozumab requesting additional information on CV risk.15 This decision was based, at least in part, on results of the ARCH trial that showed an imbalance between groups in CV-related adverse events.13 In order to gain FDA approval, Amgen Pharmaceuticals included a boxed warning in the product labeling outlining the potential risk of myocardial infarction (MI), stroke, and CV death associated with romosozumab use.4 The boxed warning includes the following recommendations: romosozumab should not be used in patients who have had an MI or stroke in the past year, and in patients with CV risk factors, the benefits versus risks should be evaluated on a per patient basis.4 In addition, a pharmacovigilance plan that includes postmarketing monitoring for drug safety and periodic safety reports to the FDA must be completed by the manufacturer.16
Other serious adverse events, including osteonecrosis of the jaw (ONJ) and atypical femur fractures, are of concern in medications that decrease bone resorption. In the FRAME trial, there were 2 reported cases of ONJ (one in the romosozumab group and one in the romosozumab followed by denosumab group).11 In the ARCH trial, there were no cases of ONJ reported in the first year of treatment. However, 2 cases were reported in the second 12-month period (one in the alendronate/alendronate group and one in the romosozumab/alendronate group).13 As a precaution, the product labeling suggests patients being initiated on romosozumab should receive a routine oral examination prior to drug initiation.4 Atypical femur fracture was reported in one subject in the FRAME trial who received romosozumab, while there were 6 reported cases in the ARCH trial (4 in the alendronate/alendronate group and 2 in the romosozumab/alendronate group).11,13
Drug Interactions
While there are no drug interactions listed in the official product labeling, there is a potential, theoretical interaction between romosozumab and other medications that cause hypocalcemia.4 Therefore, caution should be exercised if romosozumab is used concomitantly in patients who take medications associated with hypocalcemia. If the combination cannot be avoided, serum calcium levels should be monitored and patients should be educated about the signs and symptoms of hypocalcemia.
Dosing and Administration
Romosozumab is administered at a dose of 210 mg SQ monthly for 12 months.4 Each prefilled syringe of romosozumab contains 105 mg; thus, 2 syringes are administered at each dosing interval to achieve a total dose of 210 mg. Romosozumab is administered via injection by a health care professional into the abdomen, thigh, or upper arm; injection sites should be rotated to minimize patient discomfort. In the event of a missed dose, romosozumab should be administered as soon as possible and the new monthly dosing schedule should be based on the date of the most recent injection.4 Use of romosozumab should be limited to 12 months as the anabolic effects diminish after that time.4 Romosozumab should be kept in the refrigerator; however, the medication should be allowed to reach room temperature (approximately 30 minutes) before administration. Patients should be counseled regarding appropriate calcium and vitamin D intake in order to prevent hypocalcemia.
The dose of romosozumab does not require adjustment in patients with renal impairment. Patients with severe impairment and/or those who are on dialysis may be at greater risk for hypocalcemia and should be monitored closely. In addition, these patients should receive adequate calcium and vitamin D supplementation.4
Place in Therapy
Fractures due to osteoporosis lead to reduced mobility, pain, and decreased independence and quality of life. The risk of a subsequent fracture is approximately doubled after a first fracture.17 Treatment of osteoporosis targets either inhibition of bone resorption (bisphosphonates, denosumab, estrogen, raloxifene) or stimulation of bone formation (teriparatide, abaloparatide), in combination with calcium and vitamin D. Romosozumab is the first agent to inhibit bone resorption and stimulate bone formation.18
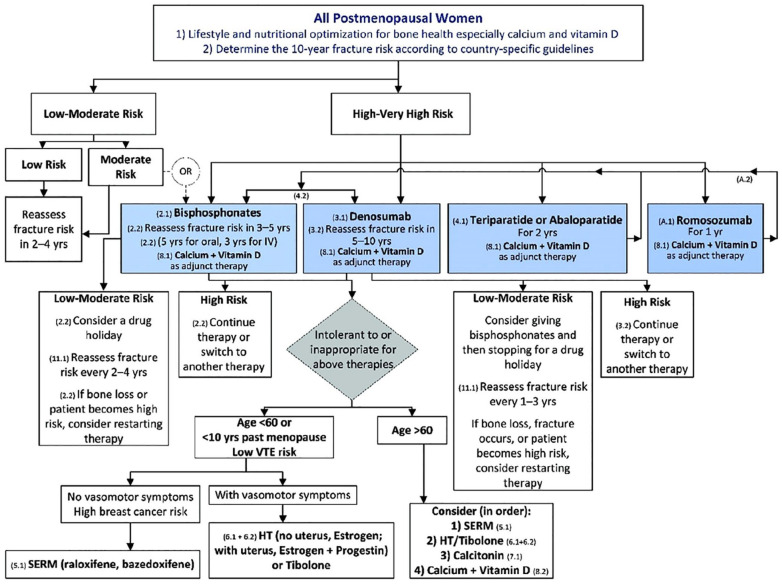
Following the approval of romosozumab, the Endocrine Society updated recommendations to include the addition of romosozumab (Figure 2).18 Bisphosphonate therapy is still recommended as initial treatment for osteoporosis in most women; these agents are low cost and have established clinical experience. Switching to an agent in a different class is often based on patient preference, tolerability, or failure of therapy (defined as loss of BMD >5% in lumbar spine and femoral neck, or 4% in total hip).19 An overview of currently available options is provided in Table 3. Denosumab may be used as an alternative initial treatment in patients who cannot tolerate bisphosphonates or comply with dosing recommendations. The anabolic agents teriparatide, abaloparatide, and now romosozumab are recommended for those at very high risk as defined by multiple vertebral fractures and a BMD T-score at the hip or spine of −2.5 or below. In clinical trials, BMD significantly increased with romosozumab compared with teriparatide suggesting romosozumab may be a more potent agent in its effect on BMD.18 The list price of romosozumab ($1825 per month) may be a barrier to widespread use; however, the manufacturer is offering a discount program. Patients enrolled in Medicare Part B may receive financial assistance after a deductible is met.20
Figure 2.
Treatment algorithm for management of postmenopausal osteoporosis. Categories have been defined based on the fracture risk assessment tool (FRAX). Low risk includes a bone mineral density (BMD) T-score at the hip and spine above −1.0 and a 10-year hip fracture risk <3%, a 10-year risk of major osteoporotic fracture <20%, and no prior hip or spine fracture. Moderate risk includes a BMD T-score at the hip and spine both above −2.5, and a 10-year hip fracture risk <3%, or risk of major osteoporotic fracture <20%, and no prior hip or spine fracture. High risk includes a BMD T-score at the hip and spine above −2.5 or below, or a 10-year hip fracture risk >3%, or risk of major osteoporotic fracture >20%, or a prior hip or spine fracture. Very high risk includes a BMD T-score at the hip and spine above −2.5 or below, and multiple spine fractures. Reprinted from Shoback et al.18 Reprinted with permission of Oxford University Press.
Table 3.
Overview of Available Treatment Options for Postmenopausal Osteoporosis.21
| Class/drug | Adverse events | Relative annual costa (US) |
|---|---|---|
| Oral bisphosphonates Alendronate Ibandronate Risedronate |
>10%: bone pain, hypertension, hypocalcemia, hypophosphatemia Rare: jaw osteonecrosis, atypical thigh fracture, dysphagia |
$ to $$$ |
| Intravenous bisphosphonates Zoledronic acid Ibandronate |
>10%: anemia, bone pain, confusion, constipation, dehydration Rare: jaw osteonecrosis, atypical thigh fracture |
$ to $$ |
| Conjugated estrogen/SERM Conjugated estrogen/bazedoxifene |
>10%: amenorrhea, breakthrough bleeding, mastalgia | $$$ |
| SERM Raloxifene |
>10%: peripheral edema, hot flashes | $$ |
| Calcitonin nasal spray | >10%: antibody formation, rhinitis, nausea | $$ |
| Parathyroid hormone analogs Abaloparatide* Teriparatide^ |
>10%: dizziness, hyperuricemia hypercalciuria*, nausea^, abdominal pain, fatigue, headache, leg cramps^ | $$$$ |
| RANKL inhibitor Denosumab |
>10%: anemia, bone pain, dyspnea, hypocalcemia, hypophosphatemia, peripheral edema | $$$ |
| Sclerostin inhibitor Romosozumab |
>10%: antibody formation, arthralgia | $$$ |
Cost key: $ (<$500); $$ ($500 to $1200); $$$ ($1500 to $3000); $$$$ ($20 000+).
Romosozumab is the first anabolic agent in its class to complete Phase III trials. Two other sclerostin inhibitors are currently being investigated, including blosozumab (LY251546) and setrusumab (BPS804).22,23 Romosozumab is approved for postmenopausal women with osteoporosis at very high risk of fracture defined as history of osteoporotic fracture, multiple risk factors for fracture, or patients who have failed or are intolerant to other therapies.4 Romosozumab has been studied in men, but is not approved to treat osteoporosis in that population.24 Postmenopausal women should receive treatment with romosozumab 210 mg SQ once a month for 1 year, followed by antiresportive therapy to maintain or further increase BMD. Major adverse cardiac events noted in the ARCH trial were not observed in the FRAME study. The overall rate of events was small; however, the boxed warning exists and warrants caution in patients at risk of MI, stroke, or CV death.
Conclusion
Romosozumab is an anti-sclerostin human monoclonal antibody that stimulates bone formation and inhibits bone resorption. Romosozumab is one of the most potent anabolic agents, representing a novel treatment option for osteoporosis. Clinical trials highlight efficacy in decreasing vertebral and hip fractures, as well as substantially increasing BMD in the hip and spine. This agent should be reserved for postmenopausal women who are at very high risk of fractures, and are not at high risk of cardiovascular disease or stroke.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Shannon A. Miller  https://orcid.org/0000-0003-1479-2902
https://orcid.org/0000-0003-1479-2902
Erin Lyn St. Onge  https://orcid.org/0000-0002-5404-2906
https://orcid.org/0000-0002-5404-2906
References
- 1. Camacho PM, Petak SM, Binkley N, et al. AACE/ACE clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2016. Endocr Pract. 2016;22(suppl 4):1-42. doi: 10.4158/EP161435.GL [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Genomics and precision health: does osteoporosis run in your family? Accessed June 2, 2020 https://www.cdc.gov/genomics/disease/osteoporosis.htm?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Ffeatures%2Fosteoporosis%2Findex.html
- 3. Bandeira L, Lewiecki EM, Bilezikian JP. Romosozumab for the treatment of osteoporosis. Expert Opin Biol Ther. 2017;17:255-263. doi: 10.1080/14712598.2017.1280455 [DOI] [PubMed] [Google Scholar]
- 4. Evenity [package insert]. Thousand Oaks, CA: Amgen Inc; 2019. Accessed May 22, 2020 https://www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/evenity/evenity_pi_hcp_english.ashx [Google Scholar]
- 5. Lewiecki EM. Role of sclerostin in bone and cartilage and its potential as a therapeutic target in bone diseases. Ther Adv Musculoskelet Dis. 2014;6:48-57. doi: 10.1177/1759720X13510479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Padhi D, Allison M, Kivitz AJ, et al. Multiple doses of sclerostin antibody romosozumab in healthy men and postmenopausal women with low bone mass: a randomized, double-blind, placebo- controlled study. J Clin Pharmacol. 2014;54:168-178. doi: 10.1002/jcph.239 [DOI] [PubMed] [Google Scholar]
- 7. Maeda K, Kobayashi Y, Koide M, et al. The regulation of bone metabolism and disorders by Wnt signaling. Int J Mol Sci. 2019;20:5525. doi: 10.3390/ijms20225525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26:19-26. doi: 10.1002/jbmr.173 [DOI] [PubMed] [Google Scholar]
- 9. McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412-420. doi: 10.1056/NEJMoa1305224 [DOI] [PubMed] [Google Scholar]
- 10. Ishibashi H, Crittenden DB, Miyauchi A, et al. Romosozumab increases bone mineral density in postmenopausal Japanese women with osteoporosis: a phase 2 study. Bone. 2017;103:209-215. doi: 10.1016/j.bone.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 11. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543. doi: 10.1056/NEJMoa1607948 [DOI] [PubMed] [Google Scholar]
- 12. Lewiecki EM, Dinavahi RV, Lazaretti-Castro M, et al. One year of romosozumab followed by two years of denosumab maintains fracture risk reductions: results of the FRAME extension study. J Bone Miner Res. 2019;34:419-428. doi: 10.1002/jbmr.3622 [DOI] [PubMed] [Google Scholar]
- 13. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427. doi: 10.1056/NEJMoa1708322 [DOI] [PubMed] [Google Scholar]
- 14. Langdahl BL, Libanati C, Crittenden DB, et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet. 2017;390:1585-1594. doi: 10.1016/s0140-6736(17)31613-6 [DOI] [PubMed] [Google Scholar]
- 15. Brooks M. FDA OKs romosozumab (Evenity) for postmenopausal osteoporosis. Medscape. Published April 9, 2019. Accessed August 12, 2020 https://www.medscape.com/viewarticle/911584#:~:text=On%20its%20first%20pass%20for,is%20to%20block%20bone%20formation
- 16. US Food and Drug Administration. Biologics license application for romosozumab. Published January 16, 2019. Accessed August 12, 2020 https://www.fda.gov/media/121255/download
- 17. Johansson H, Siggeirsdóttir K, Harvey NC, et al. Imminent risk of fracture after fracture. Osteoporosis Int. 2017;28:775-780. doi: 10.1007/s00198-016-3868-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shoback D, Rosen C, Black D, Cheung AM, Murad MH, Eastell R. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020;105:dgaa048. doi: 10.1210/clinem/dgaa048 [DOI] [PubMed] [Google Scholar]
- 19. Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622. doi: 10.1210/jc.2019-00221 [DOI] [PubMed] [Google Scholar]
- 20. Evenity.com. Paying for Evenity®. Accessed August 5, 2020 https://www.evenity.com/evenity-support/evenity-cost
- 21. Elsevier. Clinical Pharmacology. Accessed August 6, 2020 http://www.clinicalpharmacology.com
- 22. BioSpace.com. Mereo BioPharma announces additional positive data from phase 2b ASTEROID study of setrusumab in adults with osteogenesis imperfecta and provides update on regulatory progress. Published January 14, 2020. Accessed May 22, 2020 https://www.biospace.com/article/releases/mereo-biopharma-announces-additional-positive-data-from-phase-2b-asteroid-study-of-setrusumab-in-adults-with-osteogenesis-imperfecta-and-provides-update-on-regulatory-progress/
- 23. Recker RR, Benson CT, Matsumoto T, et al. A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density. J Bone Miner Res. 2015;30:216-224. doi: 10.1002/jbmr.2351 [DOI] [PubMed] [Google Scholar]
- 24. Lewiecki EM, Blicharski T, Goemaere S, et al. A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J Clin Endocrinol Metab. 2018;103:3183-3193. doi: 10.1210/jc.2017-02163 [DOI] [PubMed] [Google Scholar]