Abstract
Discovering the processes through which early adverse experiences affect children’s nervous-system development, health, and behavior is critically important for developing effective interventions. However, advances in our understanding of these processes have been constrained by conceptualizations that rely on categories of adversity that are overlapping, have vague boundaries, and lack consistent biological evidence. Here, we discuss central problems in understanding the link between early-life adversity and children’s brain development. We conclude by suggesting alternative formulations that hold promise for advancing knowledge about the neurobiological mechanisms through which adversity affects human development.
Keywords: early-life stress, adversity, stress neurobiology, child maltreatment
Advances in neuroscience are critical for uncovering causal mechanisms linking exposure to adverse experiences in childhood with impairments in learning, behavior, and both physical and mental health across the life span. Adversity encompasses a range of experiences, from toxin exposure to nutritional restriction to physical abuse to war exposure to limited family resources to lack of nurturance and/or positive parental inputs. The long-term negative consequences of severe and chronic adversity on children’s development are well documented (Cicchetti, 2016; Pechtel & Pizzagalli, 2011; Taylor, Way, & Seeman, 2011). However, there are still gaps in knowledge about critical issues, including the underlying mechanisms or specific pathways that account for particular biobehavioral effects and problems, why individual children may respond differently to adversity, and which types of outcomes (if any) are associated with exposure to different kinds of experiences. Scientists have used a variety of conceptual models of early-life stress/adversity, including chronic strain, cumulative stress, episodic stress, allostatic load, lifetime adversity, and foci on the effects of specific types of adverse events. Yet these various constructs each constrain the possible explanations that data can reveal. Here, we assess the current frameworks used to understand the effects of early-life adversity on brain development and suggest a new approach to advance knowledge about how and why these phenomena affect brain architecture, physiology, and behavioral development. Once uncovered, these neural mechanisms hold promise for serving as targets for future intervention or prevention efforts for children at risk for negative outcomes.
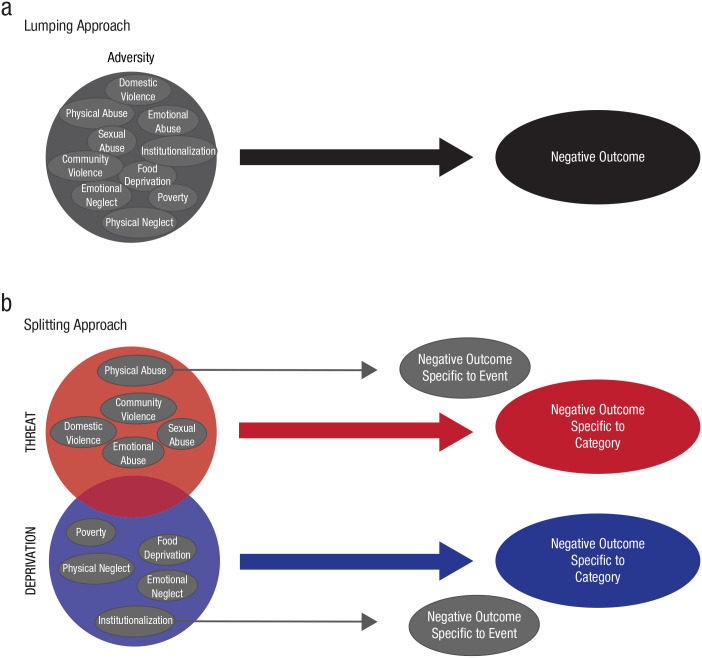
The question of how to best conceptualize early childhood adversity has shifted over time (Fareri & Tottenham, 2016; Palacios-Barrios & Hanson, 2019; Pollak, 2005). The rubric of “lumpers” versus “splitters” that was introduced by George Simpson (1945) provides a simple and useful way to characterize these approaches (Box 1; Fig. 1). In general, lumping means that various types of adversities are treated as a heterogeneous, broad category often labeled as “adversity,” “early-life stress,” or “negative life events” (Burghy et al., 2012; Pechtel & Pizzagalli, 2011; Steele et al., 2016; Steine et al., 2017; Winiarski, Engel, Karnik, & Brennan, 2018). In contrast, splitting perspectives are based on the premise that different types of adversity each confer specific effects, and links to neurobiological or cognitive systems may be masked by heterogeneous samples (Duffy, McLaughlin, & Green, 2018; Humphreys & Zeanah, 2015; Pollak, Cicchetti, Hornung, & Reed, 2000). For example, in this approach, phenomena such as child maltreatment would be examined separately to determine differential effects between exposure to subtypes such as physical abuse, sexual abuse, emotional abuse, physical neglect, and emotional neglect (Heim, Mayberg, Mletzko, Nemeroff, & Pruessner, 2013; Manly, Kim, Rogosch, & Ciccheti, 2001; St Clair et al., 2015).
Box 1. Conceptualizing Adversity—Lumping Versus Splitting.
Lumping and splitting reflect ways of defining categories and characterizing the most common approaches that scientists have adopted for conceptualizing childhood adversity. Lumping is when scientists treat many different types of adversity as all being generally of the same type, or one large category. In these cases, children’s experiences are usually labeled as adversity, life stress, or negative life events (Fig. 1a). In this approach, any exposure to any different kind of event within the broad domain of stress exposure is assumed to have similar effects on the individual’s neurobiology, and it is the exposure to a stressful life event in general, rather than the specific type of stressful event, that has negative repercussions for the individual. In this framework, study samples include participants with a wide variety of early life experiences, with the common denominator of those experiences being predefined as extremely stressful. This approach is illustrated in, for example, Burghy et al. (2012) and Winiarski, Engel, Karnik, and Brennan (2018). An alternative but related variant of the lumping approach uses cumulative measures of multiple exposures to different forms of adversity while still assuming consistency in these additive effects across types of experience. Examples of this approach are Steele et al. (2016) and Steine et al. (2017). In contrast, splitting reflects the view that each specific type of adverse event has a distinct and separable effect (Fig. 1b). In this approach, a study sample might be limited to only children who have experienced physical abuse, or children who purportedly experienced only physical abuse would be compared with children believed to have experienced only neglect. Examples of this type of approach are illustrated in Heim, Mayberg, Mletzko, Nemeroff, and Pruessner (2013) and St Clair et al. (2015). A variation of a splitting approach adopts the strategy of creating slightly broader categories of experience that are based on presumed common features. In this case, groups of some kinds of experiences are assumed to be related to one type of neurobiological effect, whereas groups of other kinds of experiences are assumed to be related to a different set of distinct effects. As an example, exposure to direct threats (physical abuse, sexual abuse, witnessing of violence) is construed as one type of adversity that can be contrasted with a different category reflecting a lack of species-expectant inputs (child neglect, social isolation, deprivation, or impoverishment). Examples of this approach include Busso, McLaughlin, and Sheridan (2017) and Pollak, Cicchetti, Hornung, and Reed (2000). In sum, lumping assigns categories broadly, assuming that differences between events within the category are not important for underlying biology. Splitting assumes that various adverse events differ in important ways and should be classified as such. Lumping makes for a simpler system of classification (and participant recruitment) because it avoids devoting attention to potentially trivial differences. Splitting increases the possibility of discovering interindividual variability that may be important. The problem with both approaches, as highlighted in this article, is when classifications for events are created that are unsound or that distort or mask reality. This is especially true when it is not immediately obvious that these classifications have meaningful impacts on biology. Both lumping and splitting approaches have reported relationships between adversity and neurobiological and behavioral outcomes, but both also lack strong and consistent evidence linked to biologically plausible developmental mechanisms. See Table 1 for examples of how lumping and splitting approaches have been operationalized in recent research.
Fig. 1.
Current models for conceptualizing early adversity. In lumping models (a), types of adverse events are treated similarly and are hypothesized as having similar effects on biology. Splitting models (b) assume that each type of event (gray arrows) or category of events (red and blue arrows) has a distinct effect on biology, resulting in outcomes specific to those types of events. Note that both models approach adversity in terms of the antecedent events that children encounter, and those events are construed as adverse regardless of how they are experienced by an individual (for further explanation of lumping and spitting approaches, see Box 1).
These models have indeed provided a wealth of knowledge surrounding early childhood adversity and its effects on development. Both general and specific models have demonstrated that adversity has strong, consistent negative effects on a wide range of cognitive, emotional, and behavioral processes that place children at greater risk for negative mental and physical health outcomes later in life (Chen & Baram, 2016; Cicchetti, 2016; Hughes et al., 2017). In addition, these models have provided some insight into the mechanisms that may support these effects. These models have found consistent relationships between adversity and disrupted functioning of the hypothalamic-pituitary-adrenal (HPA) axis (Koss & Gunnar, 2017; Strüber, Strüber, & Roth, 2014), autonomic nervous system (Esposito, Koss, Donzella, & Gunnar, 2016; McLaughlin et al., 2015), and immune system (Danese & Lewis, 2017; Kuhlman, Chiang, Horn, & Bower, 2017), along with epigenetic changes, especially in the glucocorticoid receptor gene (Romens, McDonald, Svaren, & Pollak, 2015; Turecki & Meaney, 2016). These alterations appear to be linked to functional and structural changes in the prefrontal cortex (PFC), amygdala, hippocampus, and hypothalamus (Belsky & De Haan, 2011; Palacios-Barrios & Hanson, 2019). These models have also provided some insight into factors that may drive individual differences in children’s responses to adversity, implicating genetic variation, stability within the home and, and a range of psychological factors in how children respond to early negative environments (Belsky & Hartman, 2014; Masten, 2011; McCrory, De Brito, & Viding, 2010; Rutter, 2012). However, there has yet to be a coherent, consistent, and replicable mechanistic brain-behavioral model that conceptualizes experience in such a way that accounts for individual patterns of developmental change after extreme adversity.
Such a model is a tall order. The world is a messy place, and the study of childhood adversity especially does not lend itself to clean, controlled experimental designs. Therefore, some basis of characterizing individuals’ experiences is necessary. The current trend is for scientists to eschew categories that appear too broad. This view is based on the hypothesis that samples of research participants who have had heterogeneous experiences will obscure potential links to discrete biological mechanisms—and the assumption that specific kinds of experiences affect specific aspects of brain function (Kuhlman et al., 2017; McLaughlin & Lambert, 2017; Palacios-Barrios & Hanson, 2019). Although the categories often used in research (threat, abuse, neglect, violence exposure) may well help organize or characterize the range of experiences children might encounter, these are ad hoc distinctions with contemporary social or political meaning, not necessarily biologically meaningful distinctions. Classifying different kinds of human experiences may confer many practical advantages, but reifying these distinctions as reflecting some actual state of reality in nature will likely obfuscate the bigger picture of understanding brain functioning and development (LeDoux, 2015; Sapolsky, 2017). Therefore, the critical issue is determining how to conceptualize experience in a meaningful way such that it is linked to children’s biobehavioral development and allows for an understanding of the mechanisms underlying these associations.
The Ascension of Specificity Models of Adversity
Historically, conceptualizations of early adversity focused on broad, cumulative measures of adversity. In this manner, individuals would be queried about whether they experienced a predefined set of potential adverse events, and their total exposure to events from that list was summed (Evans, Li, & Whipple, 2013). Examples of these methods include variations on the Life Stressors Checklist (Wethington, Brown, & Kessler, 1997) and the Adverse Child Experiences Scale (Felitti et al., 1998; Steele et al., 2016). Although these measures have adequate psychometric properties, many scientists found they conferred limited utility (Evans et al., 2013; Palacios-Barrios & Hanson, 2019). First, the events queried are those that scientists predetermined to be “stressful” for people in general without knowing the circumstances surrounding the adverse event or the individual’s perceptions, construction, meaning, or responses associated with the event. Such lists also exclude idiosyncratic events that most people might not find stressful but could be particularly salient or meaningful to an individual. Second, these methods use time frames that may be limiting. A single epoch represents a larger proportion of a 4-year-old’s lifetime than a 40-year-old’s, and memory for those events may be constructed differently for people at different points in the life span.
Another concern with cumulative models has been that they have historically provided little insight into potential mechanisms linking adverse life events to poor outcomes (Palacios-Barrios & Hanson, 2019). This may be because all types of adversity are weighted equally (Evans et al., 2013) or because these methods often rely on retrospective self-reports, wherein current negative psychological states lead to negatively skewed or inaccurate memories of early childhood (Colman et al., 2016; Reuben et al., 2016). Finally, cumulative approaches rarely focus on issues such as the frequency or length of the impact of adverse life events or the developmental timing of those events. It is instructive to note, however, that many of these criticisms reflect particulars about the methods typically associated with general or cumulative models of life stress, not the validity of construing a range of adverse events as having similar effects on the developing nervous system.
Nonetheless, about 25 years ago, author S. D. Pollak became concerned that general models of cumulative childhood adversity were ill-suited to align with the then burgeoning research on neural plasticity (Sirevaag & Greenough, 1987). In an attempt to more precisely identify the neurobiological mechanisms linking early experiences to development, Pollak sought to test whether there were differences in outcomes for children who had experienced different types of adversity. Specifically, he hypothesized that the developing nervous system would respond differentially to experiences representing direct physical threat (overexposure to negative experiences) compared with the experience of a lack of safety or security (underexposure to positive experiences). These effects were examined in neural systems underlying attention, information processing, and learning (Pollak et al., 2000; Pollak & Tolley-Schell, 2004). Indeed, children who had experienced physical abuse seemed to evince emotion-processing differences compared with children who had experienced physical neglect without direct physical threat (Pollak et al., 2000). These early results suggested that the approach of trying to study samples of children with more homogeneous types of adversity held promise—not only for elucidating the specific neurobiological systems affected by early adversity but also potentially for understanding the wide range of individual differences observed in the sequelae of childhood adversity.
Over the past few decades, many biobehavioral models of early adversity emerged that continued and extended this view. Indeed, it has become the dominant view in the field that examining specific effects of distinct types of stress on development will provide for more mechanistic explanations of the effects of early adversity (Amso & Lynn, 2017; Berens, Jensen, & Nelson, 2017; Carle et al., 2017; Kuhlman et al., 2017; McLaughlin & Sheridan, 2016). Although it is humbling to admit one was wrong, new data and revised theories are how science progresses. In retrospect, the original Pollak et al. (2000) research (and many subsequent independent studies) suffers from a range of important limitations. In the sections that follow, we outline these issues, which indicate the need to reconceptualize early adversity to better understand its effects on biological systems during development.
Conceptual Problems With Specificity Models
Although most current research on early-life adversity attempts to focus on specific types of experiences endured by children, there has also been recognition of problems inherent in this approach (Grant, Compas, Thurm, McMahon, & Gipson, 2004; Hein & Monk, 2017; Messman-Moore & Bhuptani, 2017) and debate about how to characterize the relevant features of children’s environments (Fareri & Tottenham, 2016; Lipina & Evers, 2017; Thomason & Marusak, 2017). Below we present four primary conceptual problems with frameworks attempting to link specific types of adversity with specific neurobiological outcomes.
Problem 1: Subtypes of adverse experiences are fuzzy categories
At first glance, a taxonomy of experiences such as physical abuse versus neglect, or presence of threat versus lack of safety, appears to offer promise of some differentiation of experience. However, categories such as these encompass both multiple and overlapping kinds of experiences (Lipina & Evers, 2017; Thomason & Marusak, 2017). In this manner, subtypes of adversity give the initial impression of being a useful scientific construct but are actually vague and imprecise. This problem is illustrated in Pollak and colleagues’ attempt to distinguish between threat of direct harm to the individual, using physical abuse, versus absence of input or deprivation, using physical neglect (Pollak et al., 2000; Pollak & Tolley-Schell, 2004; Pollak, Vardi, Bechner, & Curtin, 2005). Variants of this approach (e.g., Cameron, Eagleson, Fox, Hensch, & Levitt, 2017; Humphreys & Zeanah, 2015; Sheridan, Peverill, Finn, & McLaughlin, 2017) aim to map threat of direct harm versus lack of species-expectant input with distinct, specific effects on neurobiological systems. To test for specific effects associated with each category of experience, researchers consider in their samples children who purportedly experience threat and children who purportedly experience deprivation as separate groups who have had different experiences.
However, the operational definitions for these categories are many, varied, and applied inconsistently across studies. To demonstrate, threat is often operationally defined as physical abuse or exposure to violence. Many times emotional abuse (a construct that is difficult to operationally define and measure) and sexual abuse are also included in this category (Kuhlman, Geiss, Vargas, & Lopez-Duran, 2018; Platt et al., 2018; Pollak et al., 2005; Shackman, Shackman, & Pollak, 2007; Sheridan et al., 2017). In contrast, deprivation is most often operationalized using neglect, family poverty or low socioeconomic status (which are often incorrectly treated as synonymous), institutional/orphanage rearing, or food insecurity (Dennison et al., 2017; Everaerd et al., 2016; Fox, Levitt, & Nelson, 2010). These groups are assumed to represent different populations that will evince neurobiological and behavioral outcomes distinct to their adversity category.
A critical examination of the available empirical evidence (see Problem 3 below), however, provides minimal support for the idea that children within these categories respond similarly to the events and more similarly than what would be observed among children across these categories. This is because although experiences categorized as deprivation are indeed associated with an absence of expected inputs, these experiences also involve components that are likely to be perceived by individuals as threats to their survival (Fareri & Tottenham, 2016; Hein & Monk, 2017; Lipina & Evers, 2017). When young children are left without culturally appropriate caregiver supervision/protection, they not only lack safety but also are left exposed to and unprotected from a wide range of potential threats in their environment. The deprivation itself also confers threat. Food insecurity refers to a household lacking the ability to provide adequate, regular nutrition (Coleman-Jensen, 2010). In many studies, food insecurity is characterized as neglect or deprivation (Dennison et al., 2017; Platt et al., 2018; Sumner, Colich, Uddin, Armstrong, & McLaughlin, 2019)—this is no doubt true. However, inadequate nutrition may also be experienced by young children as a threat to survival. Similar category overlap is true for experiences categorized as threats. Having a physically or emotionally abusive parent or living in a high-violence neighborhood represents a high threat of extreme physical harm. But these are environmental contexts in which children are likely also deprived of many basic supports, including consistent positive parental feedback and access to material resources. As an example, physically and emotionally abusive parents may withhold access to food as a manipulation technique, or families may be afraid to go to the local store in a high-violence neighborhood.
In sum, most types of deprivation or “lack of input” likely also involve perceived threat, and chronically threatening contexts for young children also involve some aspect of deprivation.1 Thus, subtypes of adversity represent “fuzzy” categories. This means the categories are vague, although not unclear or meaningless altogether (Dietz & Moruzzi, 2009). The problem with fuzzy categories is that they are neither completely true nor completely false, and are, at the same time, partly true and partly false; therefore, the conditions under which they truly make sense can shift across applications (Fara, 2000). Distinctions such as threat versus deprivation are not natural categories likely to map onto the nervous system but imprecise concepts that potentially generate conflicting findings. The issues associated with fuzzy categories are not unique to how we categorize early adversity but are a criticism more broadly with any research that overvalues category distinctions for classifying experience (LeDoux, 2015; Sapolsky, 2017).
Problem 2: Adverse experiences tend to co-occur
Even if, for the sake of argument, these categories or dimensions of adversity represent sound and valid distinct experiences, it is not clear that they are useful. This is because different types of adverse experiences rarely occur in isolation. For example, there is high co-occurrence across all subtypes of child maltreatment (Debowska, Willmott, Boduszek, & Jones, 2017; see Table 1). Witnessing domestic violence is highly comorbid with abuse and neglect (Hamby, Finkelhor, Turner, & Ormrod, 2010) and direct violence (Gonzalez, MacMillan, Tanaka, Jack, & Tonmyr, 2014). Low family socioeconomic status is associated with an increased risk for child abuse (Euser, van IJzendoorn, Prinzie, & Bakermans-Kranenburg, 2011). These examples of high co-occurrence of adverse events illustrate two conceptual problems.
Table 1.
Examples of Participant Sampling in Studies of Child Adversity
| Example | Approach to adversity | Exposure to adverse events |
|---|---|---|
| General/broad/“lumping” | ||
| 1 | “Participants completed a major life event checklist. They reported on whether they had experienced any of 21 events across the domains of family, friends, and school within the past 12 months” (Chiang et al., 2019, p. 702). | Mean number of life events = 3.15 (SD = 2.04) |
| 2 | “The maltreated group consisted of 44 children with maltreatment experiences. . . . All the children had experienced physical, emotional, sexual abuse, and/or neglect early in life prior to coming into care” (Fujisawa et al., 2019, p. 2046). | Mean number of types of maltreatment = 2.4 (SD = 1.0) |
| 3 | “The lifetime adversity section of the Youth Life Stress Interview was used to assess girls’ exposure to negative family events and circumstances during their lifetime (up until the year prior to the interview)” (Stroud, Chen, Doane, and Granger, 2019, p. 512). | Mean number of different types of adverse events = 5.79 (SD = 1.20) |
| 4 | “The mothers completed the Life Events Schedule (LES) interview when the target participants were 12, 18, 30, 42, 48, 54, and 64 months old; in Grades 1, 2, 3, and 6; and 16 and 17 years old. When the target participants were 23, 26, 28, 32, 34, and 37 years old, they completed the LES themselves” (Young et al., 2019, p. 741). | Approximate average life-stress event exposures = 10 events |
| 5 | “Patients were assessed with the short form of the Early Trauma Inventory-Self Report (ETI-SR-SF) questionnaire . . . the number of positive responses (indicating presence of trauma) were summed for the ETI-SR-SF total score with totals for each subcomponent also calculated” (Wittbrodt et al., 2019, p. 50). | Mean exposure for high trauma = 11.8 (SD = 4.2); mean exposure for low trauma = 3.3 (SD = 2.6) |
| Dimensional/specific/“splitting” | ||
| 1 | “Threat-related adversities included six specific adversities including physical abuse, witnessing domestic violence, sexual assault, witnessing or being the victim of violence in the community, and emotional abuse. Deprivation-related adversities included five specific adversities including physical and psychosocial neglect, financial insecurity (i.e., family received money from a government assistant pro- gram), food insecurity, low parental education attainment (less than a high school degree), and household poverty (ratio of household income to poverty level <1.5)” (Colich et al., 2020, p. 2). | Co-occurrence reported between measures of threat and deprivation = 22% |
| 2 | “To quantify extent of neglect the two types of neglect assessed by the MACE (emotional and physical) were summed for each year of childhood. Similarly, the 8 types of abuse assessed (parental non-verbal emotional abuse, parental physical maltreatment, parental verbal abuse, sexual abuse, peer emotional abuse, peer physical abuse, witnessing interparental violence and witnessing violence to siblings) were summed for each year” (Teicher et al., 2018, p. 444–445). | Co-occurrence of the multiple types of adversity assessed = 56% |
| 3 | “In this 50-item paper and pencil questionnaire, the parent marked yes or no to a series of potentially traumatic events including physical abuse (being hit to the point of bruising or injury), sexual abuse (being forced to engage in sexual acts), emotional abuse (persistently being ridiculed or insulted by a caregiver), or non-intentional traumatic events (witnessing an accident, natural disaster)” (Kuhlman, Geiss, Vargas, and Lopez-Duran, 2018, p. 151). | All types of trauma significantly correlated (r > .38, p < .01) |
| 4 | “Early exposure to threat was assessed via four separate measures . . . Child Protective Services (CPS) data to determine the presence and nature of allegations of physical and sexual abuse . . . the CPS narratives that represented cases from each site . . . an expanded version of the child-report Things I Have Seen and Heard Scale . . . to assess exposure to violence and feelings of safety at home, at school, and in the community . . . the caregiver-report Conflict Tactics Scales . . . assessed the extent to which caregivers use reasoning and nonviolent discipline, verbal aggression, or physical aggression in response to their child’s behavior . . . reviews of CPS data were utilized to determine the presence and nature of allegations of neglect” (Milojevich, Norwalk, and Sheridan, 2019, p. 4). | Threat and deprivation indices significantly correlated (r = .10, p < .05) |
| 5 | “Subtype was coded from CPS records . . . Physical abuse reflected a nonaccidental physical injury of a child perpetrated by a caregiver. Neglect reflected failing to meet the child’s minimum needs in terms of failure to provide or a lack of child supervision. Emotional maltreatment reflected failing to meet children’s emotional needs in terms of psychological safety and security, self-esteem, and autonomy” (Lunkenheimer, Busuito, Brown, and Skowron, 2018, p. 214). | Co-occurrence rate of maltreatment subtypes = 51.2% |
Note: Examples of recent research using general (lumping) and specific (splitting) approaches to conceptualizing adversity are shown. Although not an exhaustive review of the literature, the table conveys the variance in how categories of adversity are operationalized. In addition, these examples highlight the common occurrence of multiple exposures to different types of adversity, illustrating how rarely researchers can identify homogeneous samples (many studies focus on a subset of types of adversity and underestimate the overall co-occurrence of different types of adversity).
First, if child physical abuse tends to co-occur with neglect, even if a researcher could identify a group of individuals who were solely exposed to only one of these experiences, it is not clear how representative this sample would be of the general population or the clinical utility of that group. More than 95% of children exposed to child maltreatment experience multiple, co-occurring types of maltreatment (Debowska et al., 2017; Euser et al., 2011; Mennen, Kim, Sang, & Trickett, 2010; Witt et al., 2016). Some scientists have questioned whether children who experience solely one adversity even exist and, if they do, whether they should be considered as outliers in terms of representing human experience (for reviews, see Debowska et al., 2017; Herrenkohl & Herrenkohl, 2009). It is possible that studies reporting children who experienced only one type of maltreatment reflect measurement error, a concern consistent with criticisms of retrospective and prospective measures of childhood experiences (Hardt & Rutter, 2004; Runyan et al., 2005).
Second, there is a lack of conceptual clarity about how to handle the co-occurrence and intersectionality of different types of adverse experiences. Imagine a study with three samples of children. One group hypothetically experienced only threat, a second group experienced only deprivation, and a third group experienced both threat and deprivation. There are a number of options for how a scientist might approach and test the relationship between these groups. In one model, the experiences of threat and deprivation together might be construed as additive experiences. A child exposed to threat plus deprivation may have experienced quantitatively more (or a higher severity of) adversity than a child who has experienced solely threat or deprivation (Koss & Gunnar, 2017; Ouellet-Morin et al., 2019). If this is the case, a cumulative model would likely be most appropriate. An alternative is that experiencing both threat and deprivation concurrently represents a qualitatively different impact on the developing nervous system. In this case, having both experiences is not additive but a different developmental challenge (Witt et al., 2016) for which each type of experience cannot be meaningfully separated. Statistical methods attempting to control for one exposure while testing the effects of the others (e.g., see Duffy et al., 2018; Lawson et al., 2017) would be invalid. Indeed, an increasing amount of research that aims to use latent class analyses to classify children by different profiles of types of co-occurring maltreatment exposures indicates that different profiles of exposures are associated with differential outcomes in children (Berzenski & Yates, 2011; Walsh, Senn, & Carey, 2012).
A third possibility is that two features that have a very high rate of co-occurrence are actually measuring the same underlying phenomenon, which presents several critical statistical problems for models treating them as independent. If measures of threat and deprivation are treated as independent, the resulting data will be imprecise and biased. In statistical analyses, this might be reflected by multicollinearity, in which small changes to the data can lead to exaggerated effects in the models that are tested (Belsley, 1991). The principal danger resulting from redundancy in the data is that regression-type analyses will be overfit, resulting in poor replicability across independent samples.
Nonhuman animal models offer tremendous insight into the mechanisms through which adversity early in life shapes neurobiological systems (Andersen, 2015; Kentner, Cryan, & Brummelte, 2018). But translating the kinds of adversity experienced by animals in the lab or in naturalistic settings to the complexity of human experience is not straightforward. For example, naturally occurring caregiving adversity among nonhuman primates more closely reflects a broad, inclusive category of “insensitive parenting” rather than mapping onto concepts such as abuse versus neglect (Sanchez & Pollak, 2009). However, arguments for specific effects of different types of adversity tend to rely on animal models utilizing single stress exposures, such as foot shock or social isolation (Sheridan & McLaughlin, 2014). Because human children rarely experience only a single type of adversity, animal models involving multiple exposures may translate to human development more readily than animal models that are limited to single stressors.
The potential benefits of animal models of multiple exposures is demonstrated in Perry et al. (2019). This research used a limited-bedding rodent model of scarcity in which rodent mothers are given inadequate bedding to build nests for their pups, which results in variations of parental care. This environment of scarcity resulted in the pups experiencing both deprivation (i.e., decreased time resting in nest and nursing with mother) and threat (i.e., mother transporting pups roughly and stepping on pups). The researchers examined parental care among families living in scarcity, operationalized through poverty, in parallel to the animal study. They found that, like the rodents with limited bedding, living in high poverty was associated with both deprivation through decreased sensitive caregiving behaviors (i.e., decreased responsivity to the infant, cognitive stimulation, and positive regard and increased detachment from the infant) and threat through increased negative caregiving behaviors (i.e., increased intrusiveness and expression of negative affect toward the infant). Moreover, in both rodents and humans, increased scarcity, through changes in maternal behavior, was associated with altered hippocampal-amygdala connectivity. Together these data suggest that different components of adversity co-occur, and models that consider the totality of the individual’s experience may be the most informative about how these experiences shape neurobiological development.
Problem 3: Subtypes of adversity lack consistent evidence for specific effects
It is not clear from extant data that there are consistent and replicable effects associated with different types of early childhood adversities. Although the motivation for dimensional models was to link nonhuman animal research with studies of human children, the translation of this literature often overlooks the complexity of the findings. As an example, threat is often measured in animal studies through foot shock or physical restraint. The application of these methods to rodents is associated with alterations in circuits involved in producing stress responses, including the hippocampus, amygdala, and HPA axis (Eiland & McEwen, 2012; Raineki, Cortés, Belnoue, & Sullivan, 2012). Deprivation in nonhuman animals has been studied using single housing, maternal isolation, and sensory deprivation (i.e., rearing in complete darkness), which result in global cortical changes (Del Arco, Segovia, Garrido, de Blas, & Mora, 2007; Diamond, Lindner, Johnson, Bennett, & Rosenzweig, 1975; Halperin & Healey, 2011). Yet there is evidence for significant overlap in these effects.
Both environmental deprivation and chronic direct stressors are associated with altered hippocampal neurogenesis in rodents (B. S. McEwen & Magarinos, 2001). In addition, global cortical changes are not specific to deprivation but are also observed after experiences of threat (Mychasiuk, Muhammad, & Kolb, 2016; Poletto, Siegford, Steibel, Coussens, & Zanella, 2006; Rodrigues et al., 2015). Moreover, changes to stress-response circuitry are not specific to threat but are also observed after deprivation experiences. To illustrate, chronic stress (e.g., repeated shock or restraint in rodents) is associated with global changes in dendritic branching and synaptic plasticity throughout the PFC, amygdala, and hippocampus—circuitry that has been implicated in alterations in learning, memory, and stress responsivity (Holmes & Wellman, 2009; B. S. McEwen, Nasca, & Gray, 2016; Rodrigues, LeDoux, & Sapolsky, 2009). These changes all appear to be similarly, and at least partially, mediated by corticotropin-releasing hormone (CRH) and glucocorticoids, which are key regulators of the HPA axis (Figueiredo, Bodie, Tauchi, Dolgas, & Herman, 2003; B. S. McEwen & Morrison, 2013; Wang et al., 2011). Comparable effects have also been observed in rodents exposed to abusive maternal behaviors as infants (Ivy et al., 2010; Tsoory, Cohen, & Richter-Levin, 2007). Yet aspects of deprivation such as exposure to prolonged maternal separation also produce these same alterations in the PFC, amygdala, and hippocampus, which are similarly attributable to changes in CRH and altered glucocorticoid function (Barna et al., 2003; Vazquez et al., 2006).
This lack of specificity also applies to humans. Both early child physical abuse and deprivation through early institutionalization have been associated with changes in the amygdala and PFC, as well as altered connectivity between the two regions (Gee et al., 2013; Tottenham & Sheridan, 2009; VanTieghem & Tottenham, 2018). Comparable alterations in the development of the hippocampus are observed in children exposed to a variety of experiences, including abuse, neglect, poverty, and general chronic stress (Gorka, Hanson, Radtke, & Hariri, 2014; Hanson, Nacewicz, et al., 2015; Teicher et al., 2018; Woon & Hedges, 2008). As with animals, there is evidence that alterations in glucocorticoid function mediates the effects of both threat and deprivation on these circuits (Koss & Gunnar, 2017; Turecki & Meaney, 2016), although more research is necessary. Despite there being some suggestion that threat and deprivation differentially effect dopaminergic neural circuits (Dennison et al., 2016, 2017), the bulk of existing evidence indicates that these types of events are similarly associated with reduced responsivity in dopaminergic reward circuits (Birn, Roeber, & Pollak, 2017; Dillon et al., 2009; Gerin et al., 2017; Goff et al., 2013; Hanson, Hariri, & Williamson, 2015).
Another example that illustrates this problem: A recent article concluded—on the basis of a null relationship between early institutionalization and markers of inflammation—that experiences of deprivation may result in differential effects on children’s immune function compared with threat (Slopen et al., 2019). This conclusion was predicated on the observation that children who had been adopted from institutionalized care settings did not evince differences in markers of innate immunity. But it is extremely unlikely that different types of adversity would be reflected in markers of innate immunity. By its nature, the innate immune system functions in a nonspecific manner, producing similar responses to many different types of challenges (Irwin & Cole, 2011; Maier & Watkins, 1998). Indeed, most of the literature reports general effects of adversity on immune reactivity to acute stress and similar levels of immune effects after different types of adversity exposure (Danese et al., 2009; Miller & Chen, 2010; Müller et al., 2019). Evidence of nonspecific patterns of effects based on categories of experience is shown in Table S1 in the Supplemental Material available online.
Overall, various types of adverse early experiences appear to exert important, but similar, effects on the brain and behavior. A recent longitudinal study that followed children from birth to the age of 37 years found that childhood stress interacted with current life stress, regardless of the type of stressor, to predict diurnal cortisol patterns in adulthood (Young et al., 2019). Early childhood adversity, in general, affects neurobiological systems involving the regulation of stress responses and various aspects of learning and behavioral regulation. There is not yet convergent validity for broad patterns of effects based on delineated dimensions of early childhood adversity.
Problem 4: Are distinctions between types of adversity biologically meaningful?
Sociolegal categories are not likely to map onto human biology
One source of misdirection in the field likely stems from initial attempts to seek correlates between biobehavioral measures and grouping variables based on social-service or public-policy-derived categories. Designations such as child neglect or physical abuse reflect legal statutes that are defined and redefined by different communities to reflect changing community standards and the missions of child-welfare and social-service organizations (Dubowitz et al., 2005). What counts as violence or sexual abuse is culturally constructed and has varied over time, reflecting not only changes in power relationships but also in how societies and social scientists have defined and understood these constructs (Muehlenhard & Kimes, 1999). Likewise, constructs such as poverty were developed by economists and policymakers. Measures of poverty are defined and applied differently across time and contexts and were developed to direct and evaluate government aid and services (G. M. Fisher, 1997; Meyer & Sullivan, 2009). Poverty may be relative or absolute and involve aspects of deprivation as well as other stressors. The point here is that ad hoc categories of types of experiences have practical utility, such as providing access to treatment or services or structure to help people communicate about and understand their life experiences. These categories can provide operational criteria to characterize study samples and link scientific research with public policies. However, there is not sufficient evidence that sociolegal constructs are relevant to the human nervous system. That is, the nervous system certainly responds to the environment but is not likely carved at the joints in alignment with modern social designations or constructs.
Stress-response systems are not sensitive to specific types of experiences
The type of stressor to which an organism is exposed does not primarily influence the brain’s stress-response systems (Berntson & Cacioppo, 2004; McEwen, 2012; Sapolsky, 2015). In fact, differences in stress responses are attributable more to individual differences than the type of eliciting event (Korte, Koolhaas, Wingfield, & McEwen, 2005). For example, when rodents are exposed to a large dominant conspecific, some will demonstrate flight behavior whereas others demonstrate freezing (de Boer, van der Vegt, & Koolhaas, 2003). Likewise, in response to the forced swim test, some rodents will display escape behavior and others will demonstrate more passive floating behavior (Veenema, Meijer, de Kloet, & Koolhaas, 2003; Veenema, Meijer, de Kloet, Koolhaas, & Bohus, 2003). Birds also show differential responses to the same stressors, with individuals varying in whether they approach or flee from a novel object or threatening intruder (Carere, Groothuis, Möstl, Daan, & Koolhaas, 2003; Verbeek, De Goede, Drent, & Wiepkema, 1999; Verbeek, Drent, & Wiepkema, 1994). Thus, the consistent findings across species is that an organism’s response to adverse events is a function of the physiology needed to support a behavioral repertoire, not the type of adversity the organism has encountered.
To illustrate, animals that demonstrate fight-or-flight behavior in response to an adverse event have high sympathetic adrenal-medullar activation and hypothalamic gonadal output (testosterone) but low HPA output (glucocorticoids), parasympathetic activation, and pituitary activation (adrenocorticotropic hormone). In contrast, animals who respond to the same type of event with a freezing response demonstrate a very different pattern of physiological responses, characterized by high HPA output, pituitary activation, and parasympathetic activity but low hypothalamic-pituitary-gonadal (HPG) axis output and sympathetic activation (Buwalda, Koolhaas, & Bohus, 1992; Korte et al., 1992; Korte, De Boer, De Kloet, & Bohus, 1995; Veenema, Meijer, de Kloet, & Koolhaas, 2003; Veenema, Meijer, de Kloet, Koolhaas, & Bohus, 2003). In terms of neural activity, animals that engage in freezing behavior show increases in corticotropin-releasing factor expression (mRNA) in the hypothalamus and increased mineralocorticoid receptor expression throughout the hippocampus; however, these changes are not observed in animals that demonstrate fight-or-flight behaviors (Korte et al., 2005; Veenema, Meijer, de Kloet, & Koolhaas, 2003; Veenema, Meijer, de Kloet, Koolhaas, & Bohus, 2003). Similar variability in response to adverse events is observed in humans. Individuals vary considerably in how they respond to the same types of stressors on indices, including cardiac autonomic responsivity (Berntson & Cacioppo, 2004; Berntson et al., 1994), immune reactivity (Cohen & Hamrick, 2003; Manuck, Cohen, Rabin, Muldoon, & Bachen, 1991), cortisol responses (Buchanan, Tranel, & Adolphs, 2006; Roy, Kirschbaum, & Steptoe, 2001; Vedhara, Hyde, Gilchrist, Tytherleigh, & Plummer, 2000), and neural responses to stress in the hippocampus and PFC (Pruessner et al., 2008; Wager et al., 2009). These findings provide further support for the view that the nature or type of adverse experiences is not directly tied to a specific neurobiological response or outcome.
There is some evidence that some types of stressors are associated with differential outcomes—sometimes referred to as primitive specificity—but these stressors tend to be simple events rather than the complex social stressors usually studied in human children. For example, exposure to cold temperature elicits large sympathetic nonoradrenergic responses with relatively little or no HPA and adrenomedullary hormonal responses; in contrast, hypoglycemia elicits little to no sympathetic noradrenergic activity but large HPA and adrenomedullary hormonal responses (Goldstein, 2010; Pacák & Palkovits, 2001). However, as stressor complexity increases, specificity in physiological responses becomes less reflective of the characteristics of the stressor per se and more reflective of the type of behavioral response an animal needs to produce (Goldstein & Kopin, 2008; Korte et al., 2005), and, perhaps, more reflective of genetic or epigenetic factors (B. S. McEwen & Gianaros, 2010) that influence how an organism responds to a stressor.
If it is not the kind of adverse experiences that organisms encounter that triggers different neurobiological responses, what does? One possibility is that the individual’s perception of the event is more influential on neurobiology than the features of the event itself (Brosschot, Verkuil, & Thayer, 2017; Goldstein & McEwen, 2002; Lazarus, 1990; B. S. McEwen, 2019; Peters, McEwen, & Friston, 2017; Sapolsky, 2015). Examples of this include shifts in how organisms perceive the controllability and predictability of a stressor (Bollini, Walker, Hamann, & Kestler, 2004; Henry, 1992; Mormede, Dantzer, Michaud, Kelley, & Le Moal, 1988; Muller, 2012) or rate their own performance in coping with the stressor (Roy et al., 2001). In humans, individual differences in perceptions of control have been linked to differential cortisol responses to acute laboratory stress, differences in brain volume, and differences in brain reactivity to stress in regions, including the hippocampus, amygdala, and PFC (Harnett et al., 2015; Hashimoto et al., 2015; Pruessner et al., 2005). Moreover, perceived adversity, and its associated neurobiological responses, can occur in the absence of any specific identifiable environmental event through rumination over previous experiences or events or anxiety about future events (Hilt & Pollak, 2013; Ottaviani et al., 2016; Paulesu et al., 2010).
It is not simply how a potential stressor is perceived that attenuates or exacerbates physiological responses. Rather, these perceptions trigger different patterns of responses across neural systems. As an illustrative example, how individuals construe adversity elicits distinct autonomic cardiovascular responses (Mendes & Park, 2014; Seery, 2011). If individuals construe their personal resources as sufficient to outweigh a situational demand, they evince increased sympathetic cardiac activation accompanied by increased cardiac output and decreased vascular resistance. In contrast, if individuals perceive that same situation as outweighing their personal resources, their increased sympathetic cardiac activity is accompanied by decreased cardiac efficiency, including minimal change in cardiac output and increased vascular resistance (Mendes & Park, 2014; Quigley, Barrett, & Weinstein, 2002; Sammy et al., 2017). These cardiovascular patterns have been linked to distinct patterns of cortical activity (Koslov, Mendes, Pajtas, & Pizzagalli, 2011) as well as inverse patterns of high versus low HPG and HPA activation (Dickerson & Kemeny, 2004; Mehta, Jones, & Josephs, 2008; Seery, 2011). Other factors that influence how individuals interpret potential stressors include whether individuals perceive themselves to be in a safe or dangerous environment and their perceptions of their coping resources (Blascovich, 2008; Jamieson, Hangen, Lee, & Yeager, 2018; Mendes, Blascovich, Major, & Seery, 2001).
In sum, a biologically meaningful approach to understanding adversity needs to incorporate the factors that shape the individual’s perceptions and interpretations of their events (B. S. McEwen & Gianaros, 2010; C. A. McEwen & McEwen, 2017; Sapolsky, 2015). This makes it improbable that commonly used classes of events such as abuse, deprivation, or poverty themselves will uniquely trigger developmental changes to underlying biology or provide insight into the neurobiological mechanisms through which adversity influences development.
Summary: conceptual problems with specificity models
Although there is a wealth of research linking early adversity to negative outcomes, not much is understood about the mechanisms underlying these effects. Long-term longitudinal studies following individuals from childhood into adulthood suggest that the effects of early adversity are cumulative, nonspecific, and unlikely to be tied to types or categories of adversity (Danese, Pariante, Caspi, Taylor, & Poulton, 2007; Young et al., 2019). The fuzzy nature of adversity subcategories, high prevalence of co-occurring adversity types, and lack of convergent validity for specific effects by category indicate that attempts to link types of adversity with nervous-system responses is unlikely to reveal mechanisms underlying the effects of early adversity on human development. Subsuming all of the issues outlined above is the problem that any a priori taxonomy—abuse versus neglect, threat versus deprivation, socioeconomic status versus poverty, emotional versus physical maltreatment—lacks evidence of biological specificity. In the section that follows, we weigh the potential benefits of a different approach to considering both the diversity of life events and individual difference factors that might inform a next generation of research on the effects of early-life adversity.
Rethinking Conceptualizations of Early Adversity
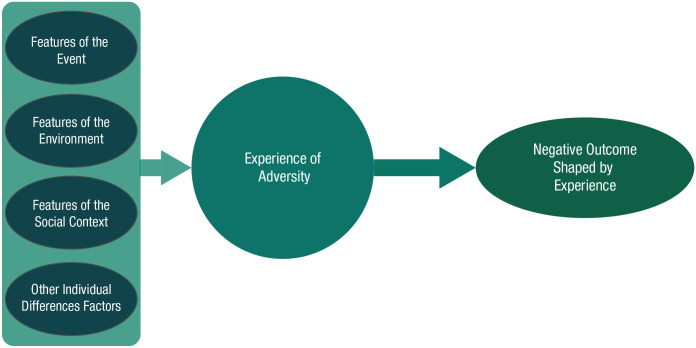
We have highlighted the conceptual and practical limitations of approaching the study of child adversity on the basis of the categories of events that children have encountered. Yet, historically, treating adversity as a broad, heterogeneous category similarly proved unfruitful. An alternative to lumping and splitting construals of child adversity is a “topological” (Fig. 2) approach. In mathematics, topology refers to the fact that an object can undergo dimensional changes such that the object itself can be preserved while taking on different shapes and appearances. In other words, there is an initial structure, but various factors deform that structure along different dimensions. The analogy here is that geometric problems do not depend on the exact initial shape of the object but how the object is put together. In a topological approach, it is not the type or category of initial event a child encounters that is biologically meaningful, but how various factors influence the way events are experienced by the child. Below, we propose, for illustrative purposes, factors that may lead to greater progress in linking children’s experiences with their biology and behavior. These are neither new nor exclusive; others include timing of adversity exposure, genetic variation, temperament, or personality factors. Rather, they are simply a heuristic meant to move away from approaches that focus on the specific types of events a child encountered or simply treat these factors as moderators of events. These types of data allow us to conceptualize children’s experiences through a broader, integrative—and yet biologically plausible—lens.
Fig. 2.
Topological approach for conceptualizing early adversity. Factors or dimensions surrounding the potentially adverse events children are exposed to contribute to the child’s experience, which then activates relevant biobehavioral responses to those circumstances. Meaningful factors might include features of the event (e.g., intensity, chronicity, developmental timing); features of the environment (e.g., predictability, contingency); and features of the child’s social context (e.g., presence of safety, social support), as well as other individual different factors such as genetics, temperament, or previous life history.
Features of the event such as chronicity/developmental timing and intensity
Human and nonhuman animal studies suggest that features such as the intensity, severity, chronicity, and developmental timing of adversity are likely to have greater explanatory power compared with the specific type of event an organism endures (Kuhlman et al., 2017; Teicher et al., 2018; Woodard & Pollak, 2020). In animal research, the precise timing of when during development a stressor occurs can be tightly controlled and have demonstrated strong effects, as described in a number of recent reviews (Andersen, 2015; Kuhlman et al., 2017; Lupien, McEwen, Gunnar, & Heim, 2009; Teicher & Samson, 2016). However, the developmental period in which adversity occurs is tightly intertwined with the chronicity of adversity (i.e., adversity that begins early in a child’s life may be longer-lasting and chronic than adversity that begins later in a child’s life), which also demonstrates profound effects on the variability in responses to stress (Danese & McEwen, 2012; Vyas, Mitra, Rao, & Chattarji, 2002). Compared with acute exposure to stress, chronic exposure is associated with long-term alterations in neural plasticity. This is, in part, a result of heightened activity across a multitude of biological stress-response systems (including heightened HPA and excitatory amino-acid activity) that lead to the overactivation of these systems and ultimately dysregulation (B. S. McEwen, 2019). The effects of chronic adversity include dendritic atrophy in the hippocampus and PFC and increased dendritic branching in the amygdala. These alterations have been linked to poorer learning and memory, increased anxiety and depressive-like behaviors, and hypersensitivity to threat in the environment (Roozendaal, McEwen, & Chattarji, 2009; Vyas et al., 2002; Watanabe, Gould, & McEwen, 1992).
The intensity (or amount) of adversity that an organism endures modulates these responses; increased stressor intensity results in increased reactivity throughout the hippocampus, amygdala, and frontal cortical regions along with increased sympathetic noradrenergic, adrenomedullary, and HPA responses across a variety of types of stressors (Burow, Day, & Campeau, 2005; Campeau & Watson, 1997; Pacák & Palkovits, 2001). In rodents, both behavioral responses (such as freezing) and physiological responses (such as corticosterone levels) are positively related to the intensity of the foot shock the animal receives. Moreover, the intensity of the stressor the animal receives is associated with differential expression of cell-adhesion molecules, which are involved in synaptic plasticity, in the hippocampus (Merino, Cordero, & Sandi, 2000). Likewise, when rodents are exposed to varying intensities of restraint stress, intensity level is associated with increased activity in the hypothalamus and amygdala and decreased activity in the cingulate cortex (Mohammad, Chowdhury, Fujioka, & Nakamura, 2000).
Human adults similarly evince increases in sympathetic noradrenergic, adrenomedullary, and HPA responses for a range of stressors that vary according to the intensity of the stressor (Goldstein, 2010). These effects hold both for intensity of the stimulus (such as temperature for heat pain or milliamps for electric shock) and for how intense individuals perceive a stressor (perceived ratings of pain or stress intensity). Both actual (temperature) and perceived (rated pain) intensity of heat pain are positively associated with activity at a network level in a circuit that includes many of those areas implicated in producing stress responses, including the amygdala, hypothalamus, and PFC (Jepma, Koban, van Doorn, Jones, & Wager, 2018). In addition, actual and perceived intensity of heat pain have been associated with autonomic nervous-system responses (Dildine, Mischkowski, Banker, Atlas, & Palacios-Barrios, 2018; Loggia, Juneau, & Bushnell, 2011; Treister, Kliger, Zuckerman, Aryeh, & Eisenberg, 2012). For a social-speech stressor, individuals’ perceived intensity of the stressor was positively related to their cortisol responses (Campbell & Ehlert, 2012), and stressors perceived as more intense are associated with larger cortisol responses (Skoluda et al., 2015).
Chronicity and intensity of adversity exposure has also been meaningfully related to the effects of adversity on children in ways that parallel observations of nonhuman animals and adults (Lupien et al., 2009). For example, children with high scores on the Life Stress Interview, which quantifies the intensity of children’s adversity exposure, had smaller amygdala and hippocampal volumes than children exposed to less intense levels of early adversity. In contrast, children with reports of child abuse and neglect and those living in poverty all showed similar effects on brain structure (Hanson, Nacewicz, et al., 2015). In addition, the intensity of adversity exposure in children has been associated with altered activation striatal and cortical circuits during value processing (Birn et al., 2017). Retrospectively reported severity of early adversity exposure (recognizing this is subject to the issues with retrospective reports outlined above) in childhood has been associated with increased dorsomedial PFC responses to a social stressor (van Harmelen et al., 2014) and altered global connectivity of the ventrolateral PFC (Cisler et al., 2013).
Both severity and chronicity of maltreatment in children have also been linked to epigenetic changes of the glucocorticoid receptor gene (Perroud et al., 2011), and, as with adults, variations in the intensity of early adversity appears to modulate HPA activity. Retrospectively reported intensity of stress, rather than type of stress, during early childhood has been associated with increased basal levels of CRH in cerebrospinal fluid (Carpenter et al., 2004) and increased cortisol responses to acute social stress (Ouellet-Morin et al., 2019). Children’s rated intensity of adversity interacts with age to also predict cortisol awakening responses (King et al., 2017). In addition, although chronicity and intensity may represent two different dimensions of experience, data from animals suggest that the two interact to influence stress responses. Increasing the intensity of an acute stressor results in an enhancement of adaptive immunity and corticosterone responses, whereas chronic intense stress results in the suppression of adaptive immunity and corticosterone (Dhabhar & McEwen, 1997). However, there is not much data available on how these two dimensions interact in humans to produce differential responses.
Features of the early environment such as predictability and contingency
There is growing recognition that critical features of the early environment such as predictability and contingent responding of caregivers (or, alternatively, chaos and lack of stability) have an important role in shaping young children’s experience and development (Glynn & Baram, 2019; Risbrough et al., 2018; Wismer Fries & Pollak, 2017). Predictability refers to the degree to which accurate predictions can be made about future events on the basis of current ones. Contingency, often characteristic of predictability, refers to the likelihood of one event or action being followed by another (Frankenhuis, 2016; Hasson, 2017). A lack of predictability in the environment leads to perceptions of uncertainty or volatility, which can lead to an extended activation of stress-response systems (Soltani & Izquierdo, 2019). This extended activation alters brain architecture in regions such as the PFC, amygdala, and hippocampus, which undermines adaptive regulation and coping (Peters et al., 2017).
The development of prefrontal-amygdala-hippocampal systems is likely aided by parent-child relationships that are stereotypically repetitive, highly predictable, and marked by contingent parental responses. In normative contexts, adult caregivers reliably respond to infant cries, comfort a child who is hurt, and provide support to a child who is dysregulated (P. A. Fisher, Frenkel, Noll, Berry, & Yockelson, 2016; Hallers-Haalboom et al., 2017). The lack of predictable and contingent input from caregivers affects children’s expectations of the environment, leading to uncertainty and perceptions of vulnerability (Chen & Baram, 2016; Harms, Shannon Bowen, Hanson, & Pollak, 2018). Historically, this same concept has also been a key component of constructs such as healthy parent-child attachment (Stroufe, 1988).
Evidence from nonhuman animals also indicates that parental responsivity and sensitivity plays an important role in shaping learning processes, particularly social learning (Baram et al., 2012; Risbrough et al., 2018; Zajac, Raby, & Dozier, 2019). For example, monkeys reared in isolation demonstrate disruptions in social and reward learning (F. A. Champagne & Curley, 2005; Pryce, Dettling, Spengler, Schnell, & Feldon, 2004). In contrast, if monkeys are removed from the care of their biological mother and reared instead by dogs (referred to as cross-fostering), they demonstrate less severe deficits (Capitanio & Mason, 2000). This is thought to be because infant monkeys raised by the dog foster mother continue to receive contingent responses (e.g., comforting in response to distress cues; Capitanio & Mason, 2000). Similar results have been observed in rodents, in which variations in maternal care, measured via licking and grooming behaviors, have been associated with poorer learning and memory (Barha, Pawluski, & Galea, 2007; Liu, Diorio, Day, Francis, & Meaney, 2000) and altered stress regulation (D. L. Champagne et al., 2008; Weaver et al., 2004). These effects appear to be dependent on changes in hippocampal (D. L. Champagne et al., 2008; Liu et al., 2000), prefrontal (Monroy, Hernández-Torres, Floréz, & Flores, 2010; T. Zhang, Chrétien, Meaney, & Gratton, 2005), and amygdala (Caldji et al., 1998; Fries, Moragues, Caldji, Hellhammer, & Meaney, 2004) synaptic plasticity, areas that together play an important role in both stress responsivity and learning and memory (Eichenbaum, 2017; B. S. McEwen, 2017).
In addition, variations in maternal care have been associated with epigenetic changes in glucocorticoid receptors (Turecki & Meaney, 2016; Weaver et al., 2004) and alterations in both glucocorticoid and mineralocorticoid receptor expression (D. L. Champagne et al., 2008; van Hasselt et al., 2012), suggestive of changes in HPA functioning. Cross-fostering infants of low-licking and grooming mothers with high-licking and grooming mothers appears to reverse some of these changes at the neural and behavioral levels, indicating they are driven by the type of maternal behavior to which pups are exposed (Liu et al., 2000; Weaver et al., 2004).
In humans, the disruption of parental inputs, as characterized by abuse or institutional deprivation, has also been associated with deficits in associative-learning processes (Ironside, Kumar, Kang, & Pizzagalli, 2018; Novick et al., 2018) and altered stress responsivity (Hostinar & Gunnar, 2013; B. S. McEwen & McEwen, 2017). As in rodents, the effects appear to be linked to alterations in the hippocampal, prefrontal, and amygdala circuits (Fan et al., 2014; Gorka et al., 2014; Tottenham & Sheridan, 2009), along with epigenetic changes in glucocorticoid receptors (Turecki & Meaney, 2016; Tyrka, Price, Marsit, Walters, & Carpenter, 2012) and changes in HPA responsivity (Hunter, Minnis, & Wilson, 2011; Koss & Gunnar, 2017). These results have been interpreted as suggesting a role for early predictable inputs in shaping both the development of learning processes and stress-response systems, possibly through alterations in prefrontal-hippocampal-amygdala circuits and glucocorticoid function. However, it is likely that there is a wide range of variance in the unpredictability or, conversely, predictability of experiences of adversity.
Indeed, recent research that has attempted to directly assess the predictability of early inputs in the environment finds that the predictability of parental inputs (measured by calculating the entropy rate for maternal auditory, visual, and tactile inputs during the parent-child interaction) shapes children’s cognitive outcomes above and beyond the type of inputs (measured through coded maternal sensitivity, positive regard, and intrusiveness during a parent-child interaction; Davis et al., 2017). Longitudinal research assessing early influences on adolescents’ externalizing behaviors finds that the unpredictability of the environment during childhood, quantified using changes in maternal employment, changes in residence, and changes in cohabitation, was associated with increased externalizing behaviors in adolescence, whereas the type of adversity (i.e., socioeconomic status) was not (Doom, Vanzomeren-Dohm, & Simpson, 2016). Likewise, research in rodents suggests that these observed effects result from altered functioning in prefrontal-hippocampal-amygdala circuits, finding that unpredictable maternal inputs are associated with altered connectivity between the medial PFC and amygdala (Bolton et al., 2018) as well as decreased dendritic arborization in the hippocampus (Molet et al., 2016) beyond the effects produced by types of maternal inputs. Together, this body of work suggests that a better assessment of the variation in the predictability, stability, and/or degree of contingent responding of adult caregivers to the needs of the developing child will provide insight into developmental alterations in prefrontal cortical and subcortical stress-response circuits.
Features of the social/interpersonal context such as safety and social support
Recent proposals have suggested that flipping the way scientists have historically construed stress from “presence of perceived threat” to “lack of perceived safety” may provide greater insight into individual differences in responses to stress and experiences of adversity (Brosschot et al., 2017; Porges, 2015). This idea is based on findings that psychological factors (such as novelty, withholding reward, and anticipation of punishment) rather than direct physical threat, injury, or actual punishment most potently activates stress-response systems (Mason, 1959, 1975). According to this view, rather than perceptions of threat activating neural threat circuits, these circuits are always active, and cues of perceived safety engage prefrontal circuits that inhibit threat-response circuits. Indeed, the ventromedial PFC (vmPFC) plays an important role in tracking cues of safety (Mobbs et al., 2007; Yao, Qi, Kendrick, & Mobbs, 2018) and fear-extinction learning (Milad et al., 2007; Schiller, Levy, Niv, LeDoux, & Phelps, 2008). Under conditions in which safety is uncertain, subcortical inhibition by the vmPFC is decreased and amygdala activity is enhanced (Grupe & Nitschke, 2013). Although there is likely a role of both perceived safety and threat in shaping stress responses, this perspective of a lack of perceived safety may prove fruitful.
In the context of attachment, early cues of safety have long been recognized as playing an important role in shaping children’s expectations about their caregivers and other adults, with infants expecting adult caregivers to be a source of safety who can be relied on to protect them and respond to their needs (Dykas & Cassidy, 2011). Safety/security in early childhood has been characterized in a variety of different ways, with aspects such as parental presence/adult “buffering,” sensitivity, responsivity, and support thought to be cues of safety and a lack of parental input, through isolation, maternal separation, neglect, or abusive parenting behaviors, being cues of a lack of safety (Callaghan & Tottenham, 2016; Gunnar, Hostinar, Sanchez, Tottenham, & Sullivan, 2015; Sanchez, McCormack, & Howell, 2015). Cues of safety early in development play an important role in engaging the prefrontal circuits that inhibit threat-response circuits, which will have implications for how children perceive and interact with their environment later in life (Porges, 2015). Indeed, evidence from nonhuman primate and rodent models supports this finding. For example, Sanchez et al. (2015) and Sullivan and Opendak (2018) showed that early parental presence plays an important role in inhibiting neurobiological threat-response systems, with both rodent pups and infant primates demonstrating reduced glucocorticoid release and decreased amygdala activation in the presence of the mother. However, in cases of abusive maternal rearing, maternal presence does not appear to exhibit buffering effects. Under these circumstances rodent pups and primate infants demonstrate enhanced glucocorticoid responses to stress (Moriceau, Shionoya, Jakubs, & Sullivan, 2009; Sanchez et al., 2015) as well as alterations in both the structure and function of the amygdala and PFC (Nephew, Huang, Poirier, Payne, & King, 2017; Rincón-Cortés & Sullivan, 2016; Spinelli et al., 2009). From this literature it is clear that parental presence, a salient early cue of safety, is important to supporting the typical development of the neurobiological stress-response systems.
There is some evidence indicative of similar early regulatory effects of parental presence on the development of stress-response systems in humans (for review, see Gunnar et al., 2015; Tottenham, 2015). Parental presence has been demonstrated to dampen both cortisol (Hostinar, Johnson, & Gunnar, 2015; Seltzer, Ziegler, & Pollak, 2010) and amygdala reactivity (Gee et al., 2014) to stress in children, consistent with the rodent and primate literature. The presentation of a parent’s voice during speech stress has been associated with faster poststressor cortisol recovery (Seltzer et al., 2010), suggesting that parental support does not necessarily need to be physical to buffer children’s responses to stress. In addition, recent research suggests that variations in social support better explain problem behaviors in adolescents than traditionally recognized predictors, such as delay of gratification (Michaelson & Munakata, 2020).
There is also evidence that early adversity is associated with altered prefrontal-amygdala connectivity, and these alterations have been linked to children’s risk for psychopathology (Fan et al., 2014; Gee et al., 2013; Herringa et al., 2013). This points to disruptions in the development of these circuits in children lacking early cues of safety that have implications for their behaviors and mental health. However, in cases of adversity in which children still receive high levels of support from their parents, these effects are mitigated. Adolescents living in poverty showed altered connectivity in prefrontal cortical networks involved in executive functioning and emotion regulation, but not if they reported having high levels of parental support (Brody et al., 2019). Children adopted from institutional care who reported high levels of security in the parent-child relationship demonstrated reduced amygdala reactivity to pictures of their parent. This reactivity was similar to that seen in children who had never been in institutional care (Callaghan et al., 2019). In addition, social support may diminish some of the biobehavioral effects of adversity and is associated with a reduced risk of psychopathology in children who experience maltreatment (McLafferty, O’Neill, Armour, Murphy, & Bunting, 2018; van Harmelen et al., 2016). Consistently incorporating an assessment of factors that represents early cues of safety, such as parental support, when studying how children respond to early adversity has the potential to greatly illuminate the neurobiological mechanisms through which negative environments shape development.
Concluding Remarks
There has been significant progress in understanding the role of adversity on child development, but there has not been sufficient progress. Consensus has emerged about what constitutes adversity (or stress, or risk, or strain), and the significant health and behavioral correlates of severe early-life adversity on human development are replicable and well documented. What we still lack, however, is a deep understanding of the neurobiological mechanisms through which adversity exerts those effects and individual differences in those responses. The idea of reducing the construct of adversity to subcomponents or types of experiences seemed a reasonable way to achieve progress in the past but is not likely to be successful. The main problem is two-fold. First, subtypes of adversity (abuse, neglect, threat, deprivation, etc.) are not natural kinds. Natural kinds are categories whose members are sufficiently alike to extrapolate to properties of the categories as a whole. Subtypes of human experiences are too heterogeneous and overlapping to fit this description and function. Yet most current studies (see Table 1) accept a priori that some descriptor of an adverse event designates a natural kind. Being hit is a physical threat, being food-insecure is deprivation, being sexually abused may be something else entirely, and each specific kind or category is presumed to be associated with a corresponding specific neurobiological effect. However, we struggled to find consistent empirical evidence to support this assumption. Splitting types of theories work only if some biological index or behavioral outcome is specific to a particular type of adversity (e.g., neglect or deprivation) and does not generalize to a different form of adversity (e.g., physical abuse, chronic poverty, violence exposure). In fact, most categories of adversity overlap, and most brain and behavioral outcomes are associated with many different aspects of adversity.
Second, research on stress indicates that types or dimensions of events do not determine variability in stress responses. Rather, other factors such as variations in organisms’ perceptions of events drives the specificity in biobehavioral responses. In this regard, both lumping and splitting approaches place primary emphasis on the eliciting physical or sensory event that a child experienced and may miss factors that are more critical in driving children’s biological responses. There is no doubt that actual events in the real world trigger what could become an adverse event for a child. But predicting how the brain will respond to an event requires assessing how children construct their experiences of that event. In other words, an event is not adverse until the child perceives and construes it as such. In this manner, the biobehavioral responses to any given event will depend on a host of factors, including features of the event itself, the child’s environment, the interpersonal context surrounding the event, and preevent individual differences.
This is not to say that the specific events to which a child is exposed hold no explanatory power. Indeed, there are often contexts in which this information is all that is available regarding the child’s experience. However, advancing understanding of the mechanisms through which adversity influences development requires embedding these pieces of information of a child’s experience within more contextual foci. This, along with further elucidating genetic and epigenetic influences, will expand our insight into the neurobiological mechanisms underlying individual differences in children’s responses to early adversity. These may be more difficult things to measure in children’s lives and will require the development of new assessment approaches. However, although more difficult to measure, these factors also represent potential for more targeted prevention and intervention approaches aimed at discrete mechanisms affected by children’s experiences. Expanding our understanding of these dimensions of experience can illuminate the individual differences through which neurobiological systems may shape children’s future health and behavior and provide insight into what types of interventions may be more or less effective for different subsets of individuals.
Supplemental Material
Supplemental material, Smith_Supplemental_Material for Rethinking Concepts and Categories for Understanding the Neurodevelopmental Effects of Childhood Adversity by Karen E. Smith and Seth D. Pollak in Perspectives on Psychological Science
Acknowledgments
We thank both Megan Gunnar and Bruce McEwen for providing very thoughtful and constructive feedback on an earlier version of the manuscript. We also dedicate this article to Bruce McEwen, who died just prior to its acceptance for publication.
One aspect of confusion in the extant literature is a disconnect between theoretical and empirical articles. Although some theoretical articles acknowledge that many adverse experiences involve aspects of both threat and deprivation (see McLaughlin & Sheridan, 2016), many empirical articles gloss over this problem and, in practice, reify these distinctions (see Table 1 and Table S1 in the Supplemental Material available online).
Footnotes
ORCID iDs: Karen E. Smith  https://orcid.org/0000-0002-6689-7346
https://orcid.org/0000-0002-6689-7346
Seth D. Pollak  https://orcid.org/0000-0001-5184-9846
https://orcid.org/0000-0001-5184-9846
Supplemental Material: Additional supporting information can be found at http://journals.sagepub.com/doi/suppl/10.1177/1745691620920725
Transparency
Action Editor: Aina Puce
Editor: Laura A. King
Declaration of Conflicting Interests: The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: This work was supported by National Institute of Mental Health Grants R01-MH61285 (to S. D. Pollak) and T32-MH018931-30 (supporting K. E. Smith), by a James McKeen Cattell Fund Fellowship, and by Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant U54-HD090256 (supporting S. D. Pollak).
References
- Amso D., Lynn A. (2017). Distinctive mechanisms of adversity and socioeconomic inequality in child development: A review and recommendations for evidence-based policy. Policy Insights from the Behavioral and Brain Sciences, 4, 139–146. doi: 10.1177/2372732217721933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S. L. (2015). Exposure to early adversity: Points of cross-species translation that can lead to improved understanding of depression. Development and Psychopathology, 27, 477–491. doi: 10.1017/S0954579415000103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram T. Z., Davis E. P., Obenaus A., Sandman C. A., Small S. L., Solodkin A., Stern H. (2012). Fragmentation and unpredictability of early-life experience in mental disorders. American Journal of Psychiatry, 169, 907–915. doi: 10.1176/appi.ajp.2012.11091347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha C. K., Pawluski J. L., Galea L. A. (2007). Maternal care affects male and female offspring working memory and stress reactivity. Physiology & Behavior, 92, 939–950. doi: 10.1016/J.PHYSBEH.2007.06.022 [DOI] [PubMed] [Google Scholar]
- Barna I., Bálint E., Baranyi J., Bakos N., Makara G. B., Haller J. (2003). Gender-specific effect of maternal deprivation on anxiety and corticotropin-releasing hormone mRNA expression in rats. Brain Research Bulletin, 62(2), 85–91. doi: 10.1016/S0361-9230(03)00216-8 [DOI] [PubMed] [Google Scholar]
- Belsky J., De Haan M. (2011). Annual research review: Parenting and children’s brain development: The end of the beginning. Journal of Child Psychology and Psychiatry and Allied Disciplines, 52, 409–428. doi: 10.1111/j.1469-7610.2010.02281.x [DOI] [PubMed] [Google Scholar]
- Belsky J., Hartman S. (2014). Gene-environment interaction in evolutionary perspective: Differential susceptibility to environmental influences. World Psychiatry, 13, 87–89. doi: 10.1002/wps.20092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsley D. A. (1991). Conditioning diagnostics: Colinearity and weak data in regression. Hoboken, NJ: Wiley. [Google Scholar]
- Berens A. E., Jensen S. K., Nelson C. A. (2017). Biological embedding of childhood adversity: From physiological mechanisms to clinical implications. BMC Medicine, 15(1), Article 135. doi: 10.1186/s12916-017-0895-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson G. G., Cacioppo J. T. (2004). Heart rate variability: Stress and psychiatric conditions. In Malik M., Camm A. J. (Eds.), Dynamic electrocardiography (pp. 57–64). Hoboken, NJ: Wiley. doi: 10.1002/9780470987483.ch7 [DOI] [Google Scholar]
- Berntson G. G., Cacioppo J. T., Binkley P. F., Uchino B. N., Quigley K. S., Fieldstone A. (1994). Autonomic cardiac control. III. Psychological stress and cardiac response in autonomic space as revealed by pharmacological blockades. Psychophysiology, 31, 599–608. doi: 10.1111/j.1469-8986.1994.tb02352.x [DOI] [PubMed] [Google Scholar]
- Berzenski S. R., Yates T. M. (2011). Classes and consequences of multiple maltreatment: A person-centered analysis. Child Maltreatment, 16, 250–261. doi: 10.1177/1077559511428353 [DOI] [PubMed] [Google Scholar]
- Birn R. M., Roeber B. J., Pollak S. D. (2017). Early childhood stress exposure, reward pathways, and adult decision making. Proceedings of the National Academy of Sciences, USA, 114, 13549–13554. doi: 10.1073/pnas.1708791114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blascovich J. (2008). Challenge and threat. In Elliot A. J. (Ed.), Handbook of approach and avoidance motivation (pp. 431–446). New York, NY: Psychology Press. [Google Scholar]
- Bollini A. M., Walker E. F., Hamann S., Kestler L. (2004). The influence of perceived control and locus of control on the cortisol and subjective responses to stress. Biological Psychology, 67, 245–260. [DOI] [PubMed] [Google Scholar]
- Bolton J. L., Molet J., Regev L., Chen Y., Rismanchi N., Haddad E., . . . Baram T. Z. (2018). Anhedonia following early-life adversity involves aberrant interaction of reward and anxiety circuits and is reversed by partial silencing of amygdala corticotropin-releasing hormone gene. Biological Psychiatry, 83, 137–147. doi: 10.1016/J.BIOPSYCH.2017.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G. H., Yu T., Nusslock R., Barton A. W., Miller G. E., Chen E., . . . Sweet L. H. (2019). The protective effects of supportive parenting on the relationship between adolescent poverty and resting-state functional brain connectivity during adulthood. Psychological Science, 30, 1040–1049. doi: 10.1177/0956797619847989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosschot J. F., Verkuil B., Thayer J. F. (2017). Exposed to events that never happen: Generalized unsafety, the default stress response, and prolonged autonomic activity. Neuroscience & Biobehavioral Reviews, 74, 287–296. doi: 10.1016/j.neubiorev.2016.07.019 [DOI] [PubMed] [Google Scholar]
- Buchanan T. W., Tranel D., Adolphs R. (2006). Impaired memory retrieval correlates with individual differences in cortisol response but not autonomic response. Learning & Memory, 13, 382–387. doi: 10.1101/lm.206306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy C. A., Stodola D. E., Ruttle P. L., Molloy E. K., Armstrong J. M., Oler J. A., . . . Birn R. M. (2012). Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience, 15, 1736–1741. doi: 10.1038/nn.3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burow A., Day H. E. W., Campeau S. (2005). A detailed characterization of loud noise stress: Intensity analysis of hypothalamo–pituitary–adrenocortical axis and brain activation. Brain Research, 1062, 63–73. doi: 10.1016/J.BRAINRES.2005.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busso D. S., McLaughlin K. A., Sheridan M. A. (2017). Dimensions of adversity, physiological reactivity, and externalizing psychopathology in adolescence: Deprivation and threat. Psychosomatic Medicine, 79, 162–171. doi: 10.1097/PSY.0000000000000369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwalda B., Koolhaas J. M., Bohus B. (1992). Behavioral and cardiac responses to mild stress in young and aged rats: Effects of amphetamine and vasopressin. Physiology & Behavior, 51, 211–216. doi: 10.1016/0031-9384(92)90133-M [DOI] [PubMed] [Google Scholar]
- Caldji C., Tannenbaum B., Sharma S., Francis D., Plotsky P. M., Meaney M. J. (1998). Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proceedings of the National Academy of Sciences, USA, 95, 5335–5340. doi: 10.1073/PNAS.95.9.5335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B. L., Gee D. G., Gabard-Durnam L., Telzer E. H., Humphreys K. L., Goff B., . . . Tottenham N. (2019). Decreased amygdala reactivity to parent cues protects against anxiety following early adversity: An examination across 3 years. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4, 664–671. doi: 10.1016/j.bpsc.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B. L., Tottenham N. (2016). The neuro-environmental loop of plasticity: A cross-species analysis of parental effects on emotion circuitry development following typical and adverse caregiving. Neuropsychopharmacology, 41, 163–176. doi: 10.1038/npp.2015.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron J. L., Eagleson K. L., Fox N. A., Hensch T. K., Levitt P. (2017). Social origins of developmental risk for mental and physical illness. The Journal of Neuroscience, 37, 10783–10791. doi: 10.1523/JNEUROSCI.1822-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J., Ehlert U. (2012). Acute psychosocial stress: Does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology, 37, 1111–1134. doi: 10.1016/J.PSYNEUEN.2011.12.010 [DOI] [PubMed] [Google Scholar]
- Campeau S., Watson S. J. (1997). Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. Journal of Neuroendocrinology, 9, 577–588. doi: 10.1046/j.1365-2826.1997.00593.x [DOI] [PubMed] [Google Scholar]
- Capitanio J. P., Mason W. A. (2000). Cognitive style: Problem solving by rhesus macaques (Macaca mulatta) reared with living or inanimate substitute mothers. Journal of Comparative Psychology, 114, 115–125. doi: 10.1037/0735-7036.114.2.115 [DOI] [PubMed] [Google Scholar]
- Carere C., Groothuis T. G., Möstl E., Daan S., Koolhaas J. (2003). Fecal corticosteroids in a territorial bird selected for different personalities: Daily rhythm and the response to social stress. Hormones and Behavior, 43, 540–548. doi: 10.1016/S0018-506X(03)00065-5 [DOI] [PubMed] [Google Scholar]
- Carle A., Hudziak J., Gombojav N., Powers K., Wade R., Braveman P. (2017). Methods to assess adverse childhood experiences of children and families: Toward approaches to promote child well-being in policy and practice. Academic Pediatrics, 17(7), S51–S69. doi: 10.1016/J.ACAP.2017.04.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter L. L., Tyrka A. R., McDougle C. J., Malison R. T., Owens M. J., Nemeroff C. B., Price L. H. (2004). Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology, 29, 777–784. doi: 10.1038/sj.npp.1300375 [DOI] [PubMed] [Google Scholar]
- Champagne D. L., Bagot R. C., Hasselt F., van Ramakers G., Meaney M. J., Kloet E. R., . . . de Krugers H. (2008). Maternal care and hippocampal plasticity: Evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. The Journal of Neuroscience, 28, 6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F. A., Curley J. P. (2005). How social experiences influence the brain. Current Opinion in Neurobiology, 15, 704–709. doi: 10.1016/J.CONB.2005.10.001 [DOI] [PubMed] [Google Scholar]
- Chen Y., Baram T. Z. (2016). Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology, 41, 197–206. doi: 10.1038/npp.2015.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang J. J., Ko A., Bower J. E., Taylor S. E., Irwin M. R., Fuligni A. J. (2019). Stress, psychological resources, and HPA and inflammatory reactivity during late adolescence. Development and Psychopathology, 31, 699–712. doi: 10.1017/S0954579418000287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D. (2016). Socioemotional, personality, and biological development: Illustrations from a multilevel developmental psychopathology perspective on child maltreatment. Annual Review of Psychology, 67, 187–211. doi: 10.1146/annurev-psych-122414-033259 [DOI] [PubMed] [Google Scholar]
- Cisler J. M., James G. A., Tripathi S., Mletzko T., Heim C., Hu X. P., . . . Kilts C. D. (2013). Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychological Medicine, 43, 507–518. doi: 10.1017/S0033291712001390 [DOI] [PubMed] [Google Scholar]
- Cohen S., Hamrick N. (2003). Stable individual differences in physiological response to stressors: Implications for stress-elicited changes in immune related health. Brain, Behavior, and Immunity, 17, 407–414. doi: 10.1016/S0889-1591(03)00110-7 [DOI] [PubMed] [Google Scholar]
- Coleman-Jensen A. J. (2010). U.S. food insecurity status: Toward a refined definition. Social Indicators Research, 95, 215–230. doi: 10.1007/s11205-009-9455-4 [DOI] [Google Scholar]
- Colich N. L., Platt J. M., Keyes K. M., Sumner J. A., Allen N. B., McLaughlin K. A. (2020). Earlier age at menarche as a transdiagnostic mechanism linking childhood trauma with multiple forms of psychopathology in adolescent girls. Psychological Medicine, 50, 1090–1098. doi: 10.1017/S0033291719000953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman I., Kingsbury M., Garad Y., Zeng Y., Naicker K., Patten S., . . . Thompson A. H. (2016). Consistency in adult reporting of adverse childhood experiences. Psychological Medicine, 46, 543–549. doi: 10.1017/S0033291715002032 [DOI] [PubMed] [Google Scholar]
- Danese A., Lewis S. (2017). Psychoneuroimmunology of early-life stress: The hidden wounds of childhood trauma? Neuropsychopharmacology, 42, 99–114. doi: 10.1038/npp.2016.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A., McEwen B.S. (2012). Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior, 106, 29–39. [DOI] [PubMed] [Google Scholar]
- Danese A., Moffitt T., Harrington H., Milne B.J., Polanczyk G., Pariante C., . . . Caspi A. (2009) Adverse childhood experiences and adult risk factors for age-related disease. Archives of Pediatrics & Adolescent Medicine, 163, 1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A., Pariante C. M., Caspi A., Taylor A., Poulton R. (2007). Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences, USA, 104, 1319–1324. doi: 10.1073/pnas.0610362104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E. P., Stout S. A., Molet J., Vegetabile B., Glynn L. M., Sandman C. A., . . . Baram T. Z. (2017). Exposure to unpredictable maternal sensory signals influences cognitive development across species. Proceedings of the National Academy of Sciences, USA, 114, 10390–10395. doi: 10.1073/pnas.1703444114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer S. F., van der Vegt B. J., Koolhaas J. M. (2003). Individual variation in aggression of feral rodent strains: A standard for the genetics of aggression and violence? Behavior Genetics, 33, 485–501. doi: 10.1023/A:1025766415159 [DOI] [PubMed] [Google Scholar]
- Debowska A., Willmott D., Boduszek D., Jones A. D. (2017). What do we know about child abuse and neglect patterns of co-occurrence? A systematic review of profiling studies and recommendations for future research. Child Abuse & Neglect, 70, 100–111. doi: 10.1016/J.CHIABU.2017.06.014 [DOI] [PubMed] [Google Scholar]
- Del Arco A., Segovia G., Garrido P., de Blas M., Mora F. (2007). Stress, prefrontal cortex and environmental enrichment: Studies on dopamine and acetylcholine release and working memory performance in rats. Behavioural Brain Research, 176, 267–273. doi: 10.1016/J.BBR.2006.10.006 [DOI] [PubMed] [Google Scholar]
- Dennison M. J., Rosen M. L., Sambrook K. A., Jenness J. L., Sheridan M. A., McLaughlin K. A. (2017). Differential associations of distinct forms of childhood adversity with neurobehavioral measures of reward processing: A developmental pathway to depression. Child Development, 90, 96–113. doi: 10.1111/cdev.13011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison M. J., Sheridan M. A., Busso D. S., Jenness J. L., Peverill M., Rosen M. L., . . . Al D. E. T. (2016). Neurobehavioral markers of resilience to depression amongst adolescents exposed to child abuse. Journal of Abnormal Psychology, 126, 136–136. doi: 10.1037/abn0000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar F. S., McEwen B. S. (1997). Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: A potential role for leukocyte trafficking. Brain, Behavior, and Immunity, 11, 286–306. doi: 10.1006/brbi.1997.0508 [DOI] [PubMed] [Google Scholar]
- Diamond M. C., Lindner B., Johnson R., Bennett E. L., Rosenzweig M. R. (1975). Difference in occipital cortical synapses from environmentally enriched, impoverished, and standard colony rats. Journal of Neuroscience Research, 1, 109–119. doi: 10.1002/jnr.490010203 [DOI] [PubMed] [Google Scholar]
- Dickerson S. S., Kemeny M. E. (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130, 355–391. doi: 10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- Dietz R., Moruzzi S. (2009). Cuts and clouds: Vagueness, its nature, and its logic. London, England: Oxford University Press. [Google Scholar]
- Dildine T. C., Mischkowski D., Banker L., Atlas L. Y., Palacios-Barrios E. E. (2018). Pain or nociception? Subjective experience mediates the effects of acute noxious heat on autonomic responses. Pain, 159, 699–711. doi: 10.1097/j.pain.0000000000001132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon D. G., Holmes A. J., Birk J. L., Brooks N., Lyons-Ruth K., Pizzagalli D. A. (2009). Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological Psychiatry, 66, 206–213. doi: 10.1016/j.biopsych.2009.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doom J. R., Vanzomeren-Dohm A. A., Simpson J. A. (2016). Early unpredictability predicts increased adolescent externalizing behaviors and substance use: A life history perspective. Development and Psychopathology, 28, 1505–1516. doi: 10.1017/S0954579415001169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowitz H., Pitts S. C., Litrownik A. J., Cox C. E., Runyan D. K., Black M. M. (2005). Defining child neglect based on child protective services data. Child Abuse and Neglect, 29, 493–511. doi: 10.1016/j.chiabu.2003.09.024 [DOI] [PubMed] [Google Scholar]
- Duffy K. A., McLaughlin K. A., Green P. A. (2018). Early life adversity and health-risk behaviors: Proposed psychological and neural mechanisms. Annals of the New York Academy of Sciences, 1428, 151–169. doi: 10.1111/nyas.13928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykas M. J., Cassidy J. (2011). Attachment and the processing of social information across the life span: Theory and evidence. Psychological Bulletin, 137, 19–46. doi: 10.1037/a0021367 [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. (2017). Prefrontal–hippocampal interactions in episodic memory. Nature Reviews Neuroscience, 18, 547–558. doi: 10.1038/nrn.2017.74 [DOI] [PubMed] [Google Scholar]
- Eiland L., McEwen B. S. (2012). Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus, 22, 82–91. doi: 10.1002/hipo.20862 [DOI] [PubMed] [Google Scholar]
- Esposito E. A., Koss K. J., Donzella B., Gunnar M. R. (2016). Early deprivation and autonomic nervous system functioning in post-institutionalized children. Developmental Psychobiology, 58, 328–340. doi: 10.1002/dev.21373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euser E. M., van IJzendoorn M. H., Prinzie P., Bakermans-Kranenburg M. J. (2011). Elevated child maltreatment rates in immigrant families and the role of socioeconomic differences. Child Maltreatment, 16, 63–73. doi: 10.1177/1077559510385842 [DOI] [PubMed] [Google Scholar]
- Evans G. W., Li D., Whipple S. S. (2013). Cumulative risk and child development. Psychological Bulletin, 139, 1342–1396. doi: 10.1037/a0031808 [DOI] [PubMed] [Google Scholar]
- Everaerd D., Klumpers F., Zwiers M., Guadalupe T., Franke B., Van Oostrom I., . . . Tendolkar I. (2016). Childhood abuse and deprivation are associated with distinct sex-dependent differences in brain morphology. Neuropsychopharmacology, 41, 1716–1723. doi: 10.1038/npp.2015.344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Herrera-Melendez A. L., Pestke K., Feeser M., Aust S., Otte C., . . . Grimm S. (2014). Early life stress modulates amygdala-prefrontal functional connectivity: Implications for oxytocin effects. Human Brain Mapping, 35, 5328–5339. doi: 10.1002/hbm.22553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fara D. G. (2000). Shifting sands: An interest-relative theory of vagueness. Philosophical Topics, 28(1), 45–81. [Google Scholar]
- Fareri D. S., Tottenham N. (2016). Effects of early life stress on amygdala and striatal development. Developmental Cognitive Neuroscience, 19, 233–247. doi: 10.1016/j.dcn.2016.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti V. J., Anda R. F., Nordenberg D., Williamson D. F., Spitz A., Edwards V. J., . . . Marks J. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. American Journal of Preventive Medicine, 14, 245–258. [DOI] [PubMed] [Google Scholar]
- Figueiredo H. F., Bodie B. L., Tauchi M., Dolgas C. M., Herman J. P. (2003). Stress integration after acute and chronic predator stress: Differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology, 144, 5249–5258. doi: 10.1210/en.2003-0713 [DOI] [PubMed] [Google Scholar]
- Fisher G. M. (1997). The development and history of the U.S. poverty thresholds—a brief overview. Retrieved from https://aspe.hhs.gov/history-poverty-thresholds
- Fisher P. A., Frenkel T. I., Noll L. K., Berry M., Yockelson M. (2016). Promoting healthy child development via a two-generation translational neuroscience framework: The filming interactions to nurture development video coaching program. Child Development Perspectives, 10, 251–256. doi: 10.1111/cdep.12195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S. E., Levitt P., Nelson I. (2010). How the timing and quality of early experiences influence the development of brain architecture. Child Development, 81, 28–40. doi: 10.1111/j.1467-8624.2009.01380.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenhuis W. E. (2016). Environmental predictability. In Shackelford T. K., Weekes-Shackelford V. A. (Eds.), Encyclopedia of evolutionary psychological science. Cham, Switzerland: Springer. [Google Scholar]
- Fries A., Moragues N., Caldji C., Hellhammer D. H., Meaney M. J. (2004). Preliminary evidence of altered sensitivity to benzodiazepines as a function of maternal care in the rat. Annals of the New York Academy of Sciences, 1032, 320–323. doi: 10.1196/annals.1314.051 [DOI] [PubMed] [Google Scholar]
- Fujisawa T. X., Nishitani S., Takiguchi S., Shimada K., Smith A. K., Tomoda A. (2019). Oxytocin receptor DNA methylation and alterations of brain volumes in maltreated children. Neuropsychopharmacology, 44, 2045–2053. doi: 10.1038/s41386-019-0414-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D. G., Gabard-Durnam L., Telzer E. H., Humphreys K. L., Goff B., Shapiro M., . . . Tottenham N. (2014). Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychological Science, 25, 2067–2078. doi: 10.1177/0956797614550878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D. G., Gabard-Durnam L. J., Flannery J., Goff B., Humphreys K. L., Telzer E. H., . . . Tottenham N. (2013). Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences, USA, 110, 15638–15643. doi: 10.1073/pnas.1307893110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerin M. I., Puetz V. B., Blair R. J. R., White S., Sethi A., Hoffmann F., . . . McCrory E. J. (2017). A neurocomputational investigation of reinforcement-based decision making as a candidate latent vulnerability mechanism in maltreated children. Development and Psychopathology, 29, 1689–1705. doi: 10.1017/S095457941700133X [DOI] [PubMed] [Google Scholar]
- Glynn L. M., Baram T. Z. (2019). The influence of unpredictable, fragmented parental signals on the developing brain. Frontiers in Neuroendocrinology, 53, Article 100736. doi: 10.1016/J.YFRNE.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff B., Gee D. G., Telzer E. H., Humphreys K. L., Gabard-Durnam L., Flannery J., Tottenham N. (2013). Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience, 249, 129–138. doi: 10.1016/J.NEUROSCIENCE.2012.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. S. (2010). Adrenal responses to stress. Cellular and Molecular Neurobiology, 30, 1433–1440. doi: 10.1007/s10571-010-9606-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. S., Kopin I. J. (2008). Adrenomedullary, adrenocortical, and sympathoneural responses to stressors: A meta-analysis. Endocrine Regulations, 42, 111–119. [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. S., McEwen B. S. (2002). Allostasis, homeostasis, and the nature of stress. Stress, 5, 55–58. doi: 10.1080/10253890290012345 [DOI] [PubMed] [Google Scholar]
- Gonzalez A., MacMillan H., Tanaka M., Jack S. M., Tonmyr L. (2014). Subtypes of exposure to intimate partner violence within a Canadian child welfare sample: Associated risks and child maladjustment. Child Abuse & Neglect, 38, 1934–1944. doi: 10.1016/J.CHIABU.2014.10.007 [DOI] [PubMed] [Google Scholar]
- Gorka A. X., Hanson J. L., Radtke S. R., Hariri A. R. (2014). Reduced hippocampal and medial prefrontal gray matter mediate the association between reported childhood maltreatment and trait anxiety in adulthood and predict sensitivity to future life stress. Biology of Mood & Anxiety Disorders, 4(1), Article 12. doi: 10.1186/2045-5380-4-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant K. E., Compas B. E., Thurm A. E., McMahon S. D., Gipson P. Y. (2004). Stressors and child and adolescent psychopathology: Measurement issues and prospective effects. Journal of Clinical Child and Adolescent Psychology, 33, 412–425. doi: 10.1207/s15374424jccp3302_23 [DOI] [PubMed] [Google Scholar]
- Grupe D. W., Nitschke J. B. (2013). Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nature Reviews Neuroscience, 14, 488–501. doi: 10.1038/nrn3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M. R., Hostinar C. E., Sanchez M. M., Tottenham N., Sullivan R. M. (2015). Parental buffering of fear and stress neurobiology: Reviewing parallels across rodent, monkey, and human models. Social Neuroscience, 10, 474–478. doi: 10.1080/17470919.2015.1070198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallers-Haalboom E. T., Groeneveld M. G., van Berkel S. R., Endendijk J. J., van der Pol L. D., Linting M., . . . Mesman J. (2017). Mothers’ and fathers’ sensitivity with their two children: A longitudinal study from infancy to early childhood. Developmental Psychology, 53, 860–872. doi: 10.1037/dev0000293 [DOI] [PubMed] [Google Scholar]
- Halperin J. M., Healey D. M. (2011). The influences of environmental enrichment, cognitive enhancement, and physical exercise on brain development: Can we alter the developmental trajectory of ADHD? Neuroscience & Biobehavioral Reviews, 35, 621–634. doi: 10.1016/J.NEUBIOREV.2010.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamby S., Finkelhor D., Turner H., Ormrod R. (2010). The overlap of witnessing partner violence with child maltreatment and other victimizations in a nationally representative survey of youth. Child Abuse & Neglect, 34, 734–741. doi: 10.1016/J.CHIABU.2010.03.001 [DOI] [PubMed] [Google Scholar]
- Hanson J. L., Hariri A. R., Williamson D. E. (2015). Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biological Psychiatry, 78, 598–605. doi: 10.1016/j.biopsych.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J. L., Nacewicz B. M., Sutterer M. J., Cayo A. A., Schaefer S. M., Rudolph K. D., . . . Davidson R. J. (2015). Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biological Psychiatry, 77, 314–323. doi: 10.1016/j.biopsych.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt J., Rutter M. (2004). Validity of adult retrospective reports of adverse childhood experiences: Review of the evidence. Journal of Child Psychology and Psychiatry and Allied Disciplines, 45, 260–273. [DOI] [PubMed] [Google Scholar]
- Harms M. B., Shannon Bowen K. E., Hanson J. L., Pollak S. D. (2018). Instrumental learning and cognitive flexibility processes are impaired in children exposed to early life stress. Developmental Science, 21(4), Article e12596. doi: 10.1111/desc.12596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett N. G., Wheelock M. D., Wood K. H., Ladnier J. C., Mrug S., Knight D. C. (2015). Affective state and locus of control modulate the neural response to threat. NeuroImage, 121, 217–226. doi: 10.1016/J.NEUROIMAGE.2015.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Takeuchi H., Taki Y., Sekiguchi A., Nouchi R., Kotozaki Y., . . . Kawashima R. (2015). Neuroanatomical correlates of the sense of control: Gray and white matter volumes associated with an internal locus of control. NeuroImage, 119, 146–151. doi: 10.1016/J.NEUROIMAGE.2015.06.061 [DOI] [PubMed] [Google Scholar]
- Hasson U. (2017). The neurobiology of uncertainty: Implications for statistical learning. Philosophical Transactions of the Royal Society B: Biological Sciences, 372, Article 20160048. doi: 10.1098/rstb.2016.0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C. M., Mayberg H. S., Mletzko T., Nemeroff C. B., Pruessner J. C. (2013). Decreased cortical representation of genital somatosensory field after childhood sexual abuse. American Journal of Psychiatry, 170, 616–623. doi: 10.1176/appi.ajp.2013.12070950 [DOI] [PubMed] [Google Scholar]
- Hein T. C., Monk C. S. (2017). Research Review: Neural response to threat in children, adolescents, and adults after child maltreatment—A quantitative meta-analysis. Journal of Child Psychology and Psychiatry, 58, 222–230. doi: 10.1111/jcpp.12651 [DOI] [PubMed] [Google Scholar]
- Henry J. (1992). Biological basis of the stress response. Integrative Physiological and Behavioral Science, 27, 66–83. doi: 10.1007/BF02691093 [DOI] [PubMed] [Google Scholar]
- Herrenkohl R. C., Herrenkohl T. I. (2009). Assessing a child’s experience of multiple maltreatment types: Some unfinished business. Journal of Family Violence, 24, 485–496. doi: 10.1007/s10896-009-9247-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa R. J., Birn R. M., Ruttle P. L., Burghy C. A., Stodola D. E., Davidson R. J., Essex M. J. (2013). Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proceedings of the National Academy of Sciences, USA, 110, 19119–19124. doi: 10.1073/pnas.1310766110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilt L. M., Pollak S. D. (2013). Characterizing the ruminative process in young adolescents. Journal of Clinical Child & Adolescent Psychology, 42, 519–530. doi: 10.1080/15374416.2013.764825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A., Wellman C. L. (2009). Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neuroscience & Biobehavioral Reviews, 33, 773–783. doi: 10.1016/J.NEUBIOREV.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar C. E., Gunnar M. R. (2013). The developmental effects of early life stress. Current Directions in Psychological Science, 22, 400–406. doi: 10.1177/0963721413488889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar C. E., Johnson A. E., Gunnar M. R. (2015). Early social deprivation and the social buffering of cortisol stress responses in late childhood: An experimental study. Developmental Psychology, 51, 1597–1608. doi: 10.1037/dev0000029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K., Bellis M. A., Hardcastle K. A., Sethi D., Butchart A., Mikton C., . . . Dunne M. P. (2017). The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. The Lancet Public Health, 2, e356–e366. doi: 10.1016/S2468-2667(17)30118-4 [DOI] [PubMed] [Google Scholar]
- Humphreys K. L., Zeanah C. H. (2015). Deviations from the expectable environment in early childhood and emerging psychopathology. Neuropsychopharmacology, 40, 154–170. doi: 10.1038/npp.2014.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter A. L., Minnis H., Wilson P. (2011). Altered stress responses in children exposed to early adversity: A systematic review of salivary cortisol studies. Stress, 14, 614–626. doi: 10.3109/10253890.2011.577848 [DOI] [PubMed] [Google Scholar]
- Ironside M., Kumar P., Kang M.-S., Pizzagalli D. A. (2018). Brain mechanisms mediating effects of stress on reward sensitivity. Current Opinion in Behavioral Sciences, 22, 106–113. doi: 10.1016/J.COBEHA.2018.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M. R., Cole S. W. (2011). Reciprocal regulation of the neural and innate immune systems. Nature Reviews Immunology, 11, 625–632. doi: 10.1111/j.1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy A. S., Rex C. S., Chen Y., Dubé C., Maras P. M., Grigoriadis D. E., . . . Baram T. Z. (2010). Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. The Journal of Neuroscience, 30, 13005–13015. doi: 10.1523/JNEUROSCI.1784-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. P., Hangen E. J., Lee H. Y., Yeager D. S. (2018). Capitalizing on appraisal processes to improve affective responses to social stress. Emotion Review, 10, 30–39. doi: 10.1177/1754073917693085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepma M., Koban L., van Doorn J., Jones M., Wager T. D. (2018). Behavioural and neural evidence for self-reinforcing expectancy effects on pain. Nature Human Behaviour, 2, 838–855. doi: 10.1038/s41562-018-0455-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentner A. C., Cryan J. F., Brummelte S. (2018). Resilience priming: Translational models for understanding resiliency and adaptation to early life adversity. Developmental Psychobiology, 61, 350–375. doi: 10.1002/dev.21775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L. S., Colich N. L., LeMoult J., Humphreys K. L., Ordaz S. J., Price A. N., Gotlib I. H. (2017). The impact of the severity of early life stress on diurnal cortisol: The role of puberty. Psychoneuroendocrinology, 77, 68–74. doi: 10.1016/J.PSYNEUEN.2016.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte S. M., Buwalda B., Bouws G. A. H., Koolhaas J. M., Maes F. W., Bohus B. (1992). Conditioned neuroendocrine and cardiovascular stress responsiveness accompanying behavioral passivity and activity in aged and in young rats. Physiology & Behavior, 51, 815–822. doi: 10.1016/0031-9384(92)90120-Q [DOI] [PubMed] [Google Scholar]
- Korte S. M., De Boer S. F., De Kloet E. R., Bohus B. (1995). Anxiolytic-like effects of selective mineralocorticoid and glucocorticoid antagonists on fear-enhanced behavior in the elevated plus-maze. Psychoneuroendocrinology, 20, 385–394. doi: 10.1016/0306-4530(94)00069-7 [DOI] [PubMed] [Google Scholar]
- Korte S. M., Koolhaas J. M., Wingfield J. C., McEwen B. S. (2005). The Darwinian concept of stress: Benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neuroscience and Biobehavioral Reviews, 29, 3–38. doi: 10.1016/j.neubiorev.2004.08.009 [DOI] [PubMed] [Google Scholar]
- Koslov K., Mendes W. B., Pajtas P. E., Pizzagalli D. A. (2011). Asymmetry in resting intracortical activity as a buffer to social threat. Psychological Science, 22, 641–649. doi: 10.1177/0956797611403156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss K. J., Gunnar M. R. (2017). Annual research review: Early adversity, the hypothalamic-pituitary-adrenocortical axis, and child psychopathology. Journal of Child Psychology and Psychiatry, 59, 327–346. doi: 10.1111/jcpp.12784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman K. R., Chiang J. J., Horn S., Bower J. E. (2017). Developmental psychoneuroendocrine and psychoneuroimmune pathways from childhood adversity to disease. Neuroscience and Biobehavioral Reviews, 80, 166–184. doi: 10.1016/j.neubiorev.2017.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman K. R., Geiss E. G., Vargas I., Lopez-Duran N. (2018). HPA-axis activation as a key moderator of childhood trauma exposure and adolescent mental health. Journal of Abnormal Child Psychology, 46, 149–157. doi: 10.1007/s10802-017-0282-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson G. M., Camins J. S., Wisse L., Wu J., Duda J. T., Cook P. A., . . . Farah M. J. (2017). Childhood socioeconomic status and childhood maltreatment: Distinct associations with brain structure. PLOS ONE, 12(4), Article e0175690. doi: 10.1371/journal.pone.0175690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus R. S. (1990). Theory-based stress measurement. Psychological Inquiry, 1, 3–13. [Google Scholar]
- LeDoux J. E. (2015). Anxious. New York, NY: Penguin Books. [Google Scholar]
- Lipina S. J., Evers K. (2017). Neuroscience of childhood poverty: Evidence of impacts and mechanisms as vehicles of dialog with ethics. Frontiers in Psychology, 8, Article 61. doi: 10.3389/fpsyg.2017.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Diorio J., Day J. C., Francis D. D., Meaney M. J. (2000). Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nature Neuroscience, 3, 799–806. doi: 10.1038/77702 [DOI] [PubMed] [Google Scholar]
- Loggia M. L., Juneau M., Bushnell M. C. (2011). Autonomic responses to heat pain: Heart rate, skin conductance, and their relation to verbal ratings and stimulus intensity. Pain, 152, 592–598. doi: 10.1016/J.PAIN.2010.11.032 [DOI] [PubMed] [Google Scholar]
- Lunkenheimer E., Busuito A., Brown K. M., Skowron E. A. (2018). Mother–child coregulation of parasympathetic processes differs by child maltreatment severity and subtype. Child Maltreatment, 23, 211–220. doi: 10.1177/1077559517751672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S. J., McEwen B. S., Gunnar M. R., Heim C. M. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10, 434–445. [DOI] [PubMed] [Google Scholar]
- Maier S. F., Watkins L. R. (1998). Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychological Review, 105, 83–107. doi: 10.1037/0033-295X.105.1.83 [DOI] [PubMed] [Google Scholar]
- Manly J. T., Kim J., Rogosch F. A., Ciccheti D. (2001). Dimensions of child maltreatment and children’s adjustment: Contributions of developmental timing and subtype. Development & Psychopathology, 13, 759–782. [PubMed] [Google Scholar]
- Manuck S. B., Cohen S., Rabin B. S., Muldoon M. F., Bachen E. A. (1991). Individual differences in cellular immune response to stress. Psychological Science, 2, 111–115. doi: 10.1111/j.1467-9280.1991.tb00110.x [DOI] [Google Scholar]
- Mason J. W. (1959). Psychological influences on the pituitary-adrenal cortical system. Recent Progress in Hormone Research, 15, 345–389. [Google Scholar]
- Mason J. W. (1975). A historical view of the stress field. Journal of Human Stress, 1(2), 22–36. doi: 10.1080/0097840X.1975.9940405 [DOI] [PubMed] [Google Scholar]
- Masten A. S. (2011). Resilience in children threatened by extreme adversity: Frameworks for research, practice, and translational synergy. Development and Psychopathology, 23, 493–506. doi: 10.1017/S0954579411000198 [DOI] [PubMed] [Google Scholar]
- McCrory E., De Brito S. A., Viding E. (2010). Research review: The neurobiology and genetics of maltreatment and adversity. Journal of Child Psychology and Psychiatry and Allied Disciplines, 51, 1079–1095. doi: 10.1111/j.1469-7610.2010.02271.x [DOI] [PubMed] [Google Scholar]
- McEwen B. S. (2012). Brain on stress: How the social environment gets under the skin. Proceedings of the National Academy of Sciences, USA, 109(Suppl. 2), 17180–17185. doi: 10.1073/pnas.95.9.5335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S. (2017). Neurobiological and systemic effects of chronic stress. Chronic Stress, 1, 1–11. doi: 10.1177/2470547017692328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S. (2019). Resilience of the brain and body. In Fink G. (Ed.), Stress: Physiology, biochemistry, and pathology (Vol. 3, pp. 19–33). doi: 10.1016/B978-0-12-813146-6.00002-3 [DOI] [Google Scholar]
- McEwen B. S., Gianaros P. J. (2010). Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences, 1186, 190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S., Magarinos A. M. (2001). Stress and hippocampal plasticity: Implications for the pathophysiology of affective disorders. Human Psychopharmacology, 16(Suppl. 1), S7–S19. doi: 10.1002/hup.266 [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Morrison J. H. (2013). The brain on stress: Vulnerability and plasticity of the prefrontal cortex over the life course. Neuron, 79, 16–29. doi: 10.1016/J.NEURON.2013.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S., Nasca C., Gray J. D. (2016). Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology, 41, 3–23. doi: 10.1038/npp.2015.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen C. A., McEwen B. S. (2017). Social structure, adversity, toxic stress, and intergenerational poverty: An early childhood model. Annual Review of Sociology, 43, 445–472. doi: 10.1146/annurev-soc-060116-053252 [DOI] [Google Scholar]
- McLafferty M., O’Neill S., Armour C., Murphy S., Bunting B. (2018). The mediating role of various types of social networks on psychopathology following adverse childhood experiences. Journal of Affective Disorders, 238, 547–553. doi: 10.1016/J.JAD.2018.06.020 [DOI] [PubMed] [Google Scholar]
- McLaughlin K. A., Lambert H. K. (2017). Child trauma exposure and psychopathology: Mechanisms of risk and resilience. Current Opinion in Psychology, 14, 29–34. doi: 10.1016/j.copsyc.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K. A., Sheridan M. A. (2016). Beyond cumulative risk: A dimensional approach to childhood adversity. Current Directions in Psychological Science, 25, 239–245. doi: 10.1177/0963721416655883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K. A., Sheridan M. A., Tibu F., Fox N. A., Zeanah C. H., Nelson C. A. (2015). Causal effects of the early caregiving environment on development of stress response systems in children. Proceedings of the National Academy of Sciences, USA, 112, 5637–5642. doi: 10.1073/pnas.1423363112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P. H., Jones A. C., Josephs R. A. (2008). The social endocrinology of dominance: Basal testosterone predicts cortisol changes and behavior following victory and defeat. Journal of Personality and Social Psychology, 94, 1078–1093. doi: 10.1037/0022-3514.94.6.1078 [DOI] [PubMed] [Google Scholar]
- Mendes W. B., Blascovich J., Major B., Seery M. (2001). Challenge and threat responses during downward and upward social comparisons. European Journal of Social Psychology, 31, 477–497. doi: 10.1002/ejsp.80 [DOI] [Google Scholar]
- Mendes W. B., Park J. (2014). Neurobiological concomitants of motivational states. In Elliot A. J. (Ed.), Advances in motivation science (Vol. 1, pp. 233–270). San Diego, CA: Academic Press. [Google Scholar]
- Mennen F. E., Kim K., Sang J., Trickett P. K. (2010). Child neglect: Definition and identification of youth’s experiences in official reports of maltreatment. Child Abuse & Neglect, 34, 647–658. doi: 10.1016/J.CHIABU.2010.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino J. J., Cordero M. I., Sandi C. (2000). Regulation of hippocampal cell adhesion molecules NCAM and L1 by contextual fear conditioning is dependent upon time and stressor intensity. European Journal of Neuroscience, 12, 3283–3290. doi: 10.1046/j.1460-9568.2000.00191.x [DOI] [PubMed] [Google Scholar]
- Messman-Moore T. L., Bhuptani P. H. (2017). A review of the long-term impact of child maltreatment on posttraumatic stress disorder and its comorbidities: An emotion dysregulation perspective. Clinical Psychology: Science and Practice, 24, 154–169. doi: 10.1111/cpsp.12193 [DOI] [Google Scholar]
- Meyer B. D., Sullivan J. X. (2009). Five decades of consumption and income poverty (NBER Working Paper No. 14827). Retrieved from the National Bureau of Economic Research website: https://www.nber.org/papers/w14827
- Michaelson L. E., Munakata Y. (2020). Same dataset, different conclusions: Preschool delay of gratification predicts later behavioral outcomes in a preregistered study. Psychological Science, 31, 193–201. doi: 10.1177/0956797619896270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M. R., Wright C. I., Orr S. P., Pitman R. K., Quirk G. J., Rauch S. L. (2007). Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry, 62, 446–454. doi: 10.1016/J.BIOPSYCH.2006.10.011 [DOI] [PubMed] [Google Scholar]
- Miller G.E., Chen E. (2010) Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science, 21, 848–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milojevich H. M., Norwalk K. E., Sheridan M. A. (2019). Deprivation and threat, emotion dysregulation, and psychopathology: Concurrent and longitudinal associations. Development and Psychopathology, 31, 847–857. doi: 10.1017/S0954579419000294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D., Petrovic P., Marchant J. L., Hassabis D., Weiskopf N., Seymour B., . . . Frith C. D. (2007). When fear is near: Threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science, 317, 1079–1083. doi: 10.1126/science.1144298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad G., Chowdhury I., Fujioka T., Nakamura S. (2000). Induction and adaptation of Fos expression in the rat brain by two types of acute restraint stress. Brain Research Bulletin, 52, 171–182. doi: 10.1016/S0361-9230(00)00231-8 [DOI] [PubMed] [Google Scholar]
- Molet J., Maras P. M., Kinney-Lang E., Harris N. G., Rashid F., Ivy A. S., . . . Baram T. Z. (2016). MRI uncovers disrupted hippocampal microstructure that underlies memory impairments after early-life adversity. Hippocampus, 26, 1618–1632. doi: 10.1002/hipo.22661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy E., Hernández-Torres E., Floréz G., Flores G. (2010). Maternal separation disrupts dendritic morphology of neurons in prefrontal cortex, hippocampus, and nucleus accumbens in male rat offspring. Journal of Chemical Neuroanatomy, 40, 93–101. doi: 10.1016/J.JCHEMNEU.2010.05.005 [DOI] [PubMed] [Google Scholar]
- Moriceau S., Shionoya K., Jakubs K., Sullivan R. (2009). Early-life stress disrupts attachment learning: The role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. The Journal of Neuroscience, 29, 15754–15755. doi: 10.1523/jneurosci.4106-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormede P., Dantzer R., Michaud B., Kelley K. W., Le Moal M. (1988). Influence of stressor predictability and behavioral control on lymphocyte reactivity, antibody responses and neuroendocrine activation in rats. Physiology and Behavior, 43, 577–583. [DOI] [PubMed] [Google Scholar]
- Muehlenhard C. L., Kimes L. A. (1999). The social construction of violence: The case of sexual and domestic violence. Personality and Social Psychology Review, 3, 234–245. doi: 10.1207/s15327957pspr0303_6 [DOI] [PubMed] [Google Scholar]
- Muller M. J. (2012). Will it hurt less if I believe I can control it? Influence of actual and perceived control on perceived pain intensity in healthy male individuals: A randomized controlled study. Journal of Behavioral Medicine, 35, 529–537. [DOI] [PubMed] [Google Scholar]
- Müller N., Krause D., Barth R., Myint A.-M., Weidinger E., Stettinger W., . . . Schwarz M. J. (2019). Childhood adversity and current stress are related to pro- and anti-inflammatory cytokines in major depression. Journal of Affective Disorders, 253, 270–276. doi: 10.1016/J.JAD.2019.04.088 [DOI] [PubMed] [Google Scholar]
- Mychasiuk R., Muhammad A., Kolb B. (2016). Chronic stress induces persistent changes in global DNA methylation and gene expression in the medial prefrontal cortex, orbitofrontal cortex, and hippocampus. Neuroscience, 322, 489–499. doi: 10.1016/J.NEUROSCIENCE.2016.02.053 [DOI] [PubMed] [Google Scholar]
- Nephew B. C., Huang W., Poirier G. L., Payne L., King J. A. (2017). Altered neural connectivity in adult female rats exposed to early life social stress. Behavioural Brain Research, 316, 225–233. doi: 10.1016/J.BBR.2016.08.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick A. M., Levandowski M. L., Laumann L. E., Philip N. S., Price L. H., Tyrka A. R. (2018). The effects of early life stress on reward processing. Journal of Psychiatric Research, 101, 80–103. doi: 10.1016/J.JPSYCHIRES.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani C., Thayer J. F., Verkuil B., Lonigro A., Medea B., Couyoumdjian A., Brosschot J. F. (2016). Physiological concomitants of perseverative cognition: A systematic review and meta-analysis. Psychological Bulletin, 142, 231–259. doi: 10.1037/bul0000036 [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I., Robitaille M.-P., Langevin S., Cantave C., Brendgen M., Lupien S. J. (2019). Enduring effect of childhood maltreatment on cortisol and heart rate responses to stress: The moderating role of severity of experiences. Development and Psychopathology, 31, 497–508. doi: 10.1017/S0954579418000123 [DOI] [PubMed] [Google Scholar]
- Pacák K., Palkovits M. (2001). Stressor specificity of central neuroendocrine responses: Implications for stress-related disorders. Endocrine Reviews, 22, 502–548. doi: 10.1210/edrv.22.4.0436 [DOI] [PubMed] [Google Scholar]
- Palacios-Barrios E. E., Hanson J. L. (2019). Poverty and self-regulation: Connecting psychosocial processes, neurobiology, and the risk for psychopathology. Comprehensive Psychiatry, 90, 52–64. doi: 10.1016/j.comppsych.2018.12.012 [DOI] [PubMed] [Google Scholar]
- Paulesu E., Sambugaro E., Torti T., Danelli L., Ferri F., Scialfa G., . . . Sassaroli S. (2010). Neural correlates of worry in generalized anxiety disorder and in normal controls: A functional MRI study. Psychological Medicine, 40, 117–124. doi: 10.1017/S0033291709005649 [DOI] [PubMed] [Google Scholar]
- Pechtel P., Pizzagalli D. A. (2011). Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology, 214, 55–70. doi: 10.1007/s00213-010-2009-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroud N., Paoloni-Giacobino A., Prada P., Olié E., Salzmann A., Nicastro R., . . . Malafosse A. (2011). Increased methylation of glucocorticoid receptor gene (NR3C1) in adults with a history of childhood maltreatment: A link with the severity and type of trauma. Translational Psychiatry, 1(12), Article e59. doi: 10.1038/tp.2011.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. E., Finegood E. D., Braren S. H., Dejoseph M. L., Putrino D. F., Wilson D. A., . . . Blair C. (2019). Developing a neurobehavioral animal model of poverty: Drawing cross-species connections between environments of scarcity-adversity, parenting quality, and infant outcome. Development and Psychopathology, 31, 399–418. doi: 10.1017/S095457941800007X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A., McEwen B. S., Friston K. J. (2017). Uncertainty and stress: Why it causes diseases and how it is mastered by the brain. Progress in Neurobiology, 156, 164–188. doi: 10.1016/j.pneurobio.2017.05.004 [DOI] [PubMed] [Google Scholar]
- Platt J. M., McLaughlin K. A., Luedtke A. R., Ahern J., Kaufman A. S., Keyes K. M. (2018). Targeted estimation of the relationship between childhood adversity and fluid intelligence in a US population sample of adolescents. American Journal of Epidemiology, 187, 1456–1466. doi: 10.1093/aje/kwy006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletto R., Siegford J. M., Steibel J. P., Coussens P. M., Zanella A. J. (2006). Investigation of changes in global gene expression in the frontal cortex of early-weaned and socially isolated piglets using microarray and quantitative real-time RT-PCR. Brain Research, 1068, 7–15. doi: 10.1016/J.BRAINRES.2005.11.012 [DOI] [PubMed] [Google Scholar]
- Pollak S. D. (2005). Early adversity and mechanisms of plasticity: Integrating affective neuroscience with developmental approaches to psychopathology. Development and Psychopathology, 17, 735–752. doi: 10.1017/S0954579405050352 [DOI] [PubMed] [Google Scholar]
- Pollak S. D., Cicchetti D., Hornung K., Reed A. (2000). Recognizing emotion in faces: Developmental effects of child abuse and neglect. Developmental Psychology, 36, 679–688. doi: 10.1037/0012-1649.36.5.679 [DOI] [PubMed] [Google Scholar]
- Pollak S. D., Tolley-Schell S. A. (2004). Attention, emotion, and the development of psychopathology. In Posner M. I. (Ed.), Cognitive neuroscience of attention (pp. 357–368). New York, NY: Guilford Press. [Google Scholar]
- Pollak S. D., Vardi S., Bechner A. M. P., Curtin J. J. (2005). Physically abused children’s regulation of attention in response to hostility. Child Development, 76, 968–977. doi: 10.1111/j.1467-8624.2005.00890.x [DOI] [PubMed] [Google Scholar]
- Porges S. W. (2015). Making the world safe for our children: Down-regulating defence and up-regulating social engagement to “optimise” the human experience. Children Australia, 40, 114–123. doi: 10.1017/cha.2015.12 [DOI] [Google Scholar]
- Pruessner J. C., Baldwin M. W., Dedovic K., Renwick R., Mahani N. K., Lord C., . . . Lupien S. (2005). Self-esteem, locus of control, hippocampal volume, and cortisol regulation in young and old adulthood. NeuroImage, 28, 815–826. doi: 10.1016/J.NEUROIMAGE.2005.06.014 [DOI] [PubMed] [Google Scholar]
- Pruessner J. C., Dedovic K., Khalili-Mahani N., Engert V., Pruessner M., Buss C., . . . Lupien S. (2008). Deactivation of the limbic system during acute psychosocial stress: Evidence from positron emission tomography and functional magnetic resonance imaging studies. Biological Psychiatry, 63, 234–240. doi: 10.1016/J.BIOPSYCH.2007.04.041 [DOI] [PubMed] [Google Scholar]
- Pryce C. R., Dettling A. C., Spengler M., Schnell C. R., Feldon J. (2004). Deprivation of parenting disrupts development of homeostatic and reward systems in marmoset monkey offspring. Biological Psychiatry, 56, 72–79. doi: 10.1016/J.BIOPSYCH.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Quigley K. S., Barrett L. F., Weinstein S. (2002). Cardiovascular patterns associated with threat and challenge appraisals: A within-subjects analysis. Psycho-physiology, 39, 292–302. doi: 10.1017/S0048577201393046 [DOI] [PubMed] [Google Scholar]
- Raineki C., Cortés M. R., Belnoue L., Sullivan R. M. (2012). Effects of early-life abuse differ across development: Infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. The Journal of Neuroscience, 32, 7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben A., Moffitt T. E., Caspi A., Belsky D. W., Harrington H., Schroeder F., . . . Danese A. (2016). Lest we forget: Comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. Journal of Child Psychology and Psychiatry, 57, 1103–1112. doi: 10.1111/jcpp.12621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón-Cortés M., Sullivan R. M. (2016). Emergence of social behavior deficit, blunted corticolimbic activity and adult depression-like behavior in a rodent model of maternal maltreatment. Translational Psychiatry, 6(10), Article e930. doi: 10.1038/tp.2016.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough V. B., Glynn L. M., Davis E. P., Snadman C. A., Obenause A., Stern H. S., . . . Baker D. G. (2018). Does anhedonia presage increased risk of posttraumatic stress disorder. In Vermetten E., Baker D. G., Risbrough V. B. (Eds.), Behavioral neurobiology of PTSD (pp. 249–265). Cham, Switzerland: Springer. doi: 10.1007/7854_2018_51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues G. M., Toffoli L. V., Manfredo M. H., Francis-Oliveira J., Silva A. S., Raquel H. A., . . . Gomes M. V. (2015). Acute stress affects the global DNA methylation profile in rat brain: Modulation by physical exercise. Behavioural Brain Research, 279, 123–128. doi: 10.1016/J.BBR.2014.11.023 [DOI] [PubMed] [Google Scholar]
- Rodrigues S. M., LeDoux J. E., Sapolsky R. M. (2009). The influence of stress hormones on fear circuitry. Annual Review of Neuroscience, 32, 289–313. doi: 10.1146/annurev.neuro.051508.135620 [DOI] [PubMed] [Google Scholar]
- Romens S. E., McDonald J., Svaren J., Pollak S. D. (2015). Associations between early life stress and gene methylation in children. Child Development, 86, 303–309. doi: 10.1111/cdev.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B., McEwen B. S., Chattarji S. (2009). Stress, memory and the amygdala. Nature Reviews Neuroscience, 10, 423–433. doi: 10.1038/nrn2651 [DOI] [PubMed] [Google Scholar]
- Roy M. P., Kirschbaum C., Steptoe A. (2001). Psychological, cardiovascular, and metabolic correlates of individual differences in cortisol stress recovery in young men. Psychoneuroendocrinology, 26, 375–391. doi: 10.1016/S0306-4530(00)00061-5 [DOI] [PubMed] [Google Scholar]
- Runyan D. K., Cox C. E., Dubowitz H., Newton R. R., Upadhyaya M., Kotch J. B., . . . Knight E. D. (2005). Describing maltreatment: Do child protective service reports and research definitions agree? Child Abuse and Neglect, 29, 461–477. doi: 10.1016/j.chiabu.2004.06.015 [DOI] [PubMed] [Google Scholar]
- Rutter M. (2012). Resilience as a dynamic concept. Development and Psychopathology, 24, 335–344. doi: 10.1017/S0954579412000028 [DOI] [PubMed] [Google Scholar]
- Sammy N., Anstiss P. A., Moore L. J., Freeman P., Wilson M. R., Vine S. J. (2017). The effects of arousal reappraisal on stress responses, performance and attention. Anxiety, Stress, & Coping, 30, 619–629. doi: 10.1080/10615806.2017.1330952 [DOI] [PubMed] [Google Scholar]
- Sanchez M. M., McCormack K. M., Howell B. R. (2015). Social buffering of stress responses in nonhuman primates: Maternal regulation of the development of emotional regulatory brain circuits. Social Neuroscience, 10, 512–526. doi: 10.1080/17470919.2015.1087426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M. M., Pollak S. D. (2009). Socio-emotional development following early abuse and neglect: Challenges and insights from translational research. In De Haan M., Gunnar M. R. (Eds.), Handbook of Developmental Social Neuroscience (pp. 497–520). New York, NY: Guilford Press. [Google Scholar]
- Sapolsky R. M. (2015). Stress and the brain: Individual variability and the inverted-U. Nature Neuroscience, 18, 1344–1346. doi: 10.1038/nn.4109 [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M. (2017). Behave: The biology of humans at our best and worst. New York, NY: Penguin Press. [Google Scholar]
- Schiller D., Levy I., Niv Y., LeDoux J. E., Phelps E. A. (2008). From fear to safety and back: Reversal of fear in the human brain. The Journal of Neuroscience, 28, 11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seery M. D. (2011). Challenge or threat? Cardiovascular indexes of resilience and vulnerability to potential stress in humans. Neuroscience and Biobehavioral Reviews, 35, 1603–1610. doi: 10.1016/j.neubiorev.2011.03.003 [DOI] [PubMed] [Google Scholar]
- Seltzer L. J., Ziegler T. E., Pollak S. D. (2010). Social vocalizations can release oxytocin in humans. Proceedings of the Royal Society B: Biological Sciences, 277, 2661–2666. doi: 10.1098/rspb.2010.0567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman J. E., Shackman A. J., Pollak S. D. (2007). Physical abuse amplifies attention to threat and increases anxiety in children. Emotion, 7, 838–852. doi: 10.1037/1528-3542.7.4.838 [DOI] [PubMed] [Google Scholar]
- Sheridan M. A., McLaughlin K. A. (2014). Dimensions of early experience and neural development: Deprivation and threat. Trends in Cognitive Sciences, 18, 580–585. doi: 10.1016/j.neubiorev.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan M. A., Peverill M., Finn A. S., McLaughlin K. A. (2017). Dimensions of childhood adversity have distinct associations with neural systems underlying executive functioning. Development and Psychopathology, 29, 1777–1794. doi: 10.1017/S0954579417001390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson G. G. (1945). The principles of classification and a classification of mammals [Monograph]. Bulletin of the American Museum of Natural History, 85, 1–350. Retrieved from http://hdl.handle.net/2246/1104 [Google Scholar]
- Sirevaag A. M., Greenough W. T. (1987). Differential rearing effects on rat visual cortex synapses: III. Neuronal and glial nuclei, boutons, dendrites, and capillaries. Brain Research, 424, 320–332. doi: 10.1016/0006-8993(87)91477-6 [DOI] [PubMed] [Google Scholar]
- Skoluda N., Strahler J., Schlotz W., Niederberger L., Marques S., Fischer S., . . . Natar U. M. (2015). Intra-individual psychological and physiological responses to acute laboratory stressors of different intensity. Psychoneuroendocrinology, 51, 227–236. doi: 10.1016/J.PSYNEUEN.2014.10.002 [DOI] [PubMed] [Google Scholar]
- Slopen N., Tang A., Nelson C. A., Zeanah C. H., McDade T. W., McLaughlin K. A., Fox N. (2019). The consequences of foster care versus institutional care in early childhood on adolescent cardiometabolic and immune markers. Psychosomatic Medicine, 81, 449–457. doi: 10.1097/PSY.0000000000000696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani A., Izquierdo A. (2019). Adaptive learning under expected and unexpected uncertainty. Nature Reviews Neuroscience, 20, 635–644. doi: 10.1038/s41583-019-0180-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli S., Chefer S., Suomi S. J., Higley J. D., Barr C. S., Stein E. (2009). Early-life stress induces long-term morphologic changes in primate brain. Archives of General Psychiatry, 66, 658–665. doi: 10.1001/archgenpsychiatry.2009.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair M. C., Croudace T., Dunn V. J., Jones P. B., Herbert J., Goodyer I. M. (2015). Childhood adversity subtypes and depressive symptoms in early and late adolescence. Development and Psychopathology, 27, 885–899. doi: 10.1017/S0954579414000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele H., Bate J., Steele M., Dube S. R., Danskin K., Knafo H., . . . Murphy A. (2016). Adverse childhood experiences, poverty, and parenting stress. Canadian Journal of Behavioral Science, 48, 32–38. [Google Scholar]
- Steine I. M., Winje D., Krystal J. H., Bjorvatn B., Milde A. M., Grønli J., . . . Pallesen S. (2017). Cumulative childhood maltreatment and its dose-response relation with adult symptomatology: Findings in a sample of adult survivors of sexual abuse. Child Abuse & Neglect, 65, 99–111. doi: 10.1016/J.CHIABU.2017.01.008 [DOI] [PubMed] [Google Scholar]
- Stroud C. B., Chen F. R., Doane L. D., Granger D. A. (2019). Early adversity and internalizing symptoms in adolescence: Mediation by individual differences in latent trait cortisol. Development and Psychopathology, 31, 509–524. doi: 10.1017/S0954579418000044 [DOI] [PubMed] [Google Scholar]
- Stroufe L. A. (1988). The role of infant-caregiver attachment in development. In Belsky J., Nezworski T. (Eds.), Clinical implications of attachment (pp. 18–41). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Strüber N., Strüber D., Roth G. (2014). Impact of early adversity on glucocorticoid regulation and later mental disorders. Neuroscience and Biobehavioral Reviews, 38, 17–37. doi: 10.1016/j.neubiorev.2013.10.015 [DOI] [PubMed] [Google Scholar]
- Sullivan R. M., Opendak M. (2018). Developmental and neurobehavioral transitions in survival circuits. Current Opinion in Behavioral Sciences, 24, 50–55. doi: 10.1016/j.cobeha.2018.03.005 [DOI] [Google Scholar]
- Sumner J. A., Colich N. L., Uddin M., Armstrong D., McLaughlin K. A. (2019). Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biological Psychiatry, 85, 268–278. doi: 10.1016/j.biopsych.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. E., Way B. M., Seeman T. E. (2011). Early adversity and adult health outcomes. Development and Psychopathology, 23, 939–954. doi: 10.1017/S0954579411000411 [DOI] [PubMed] [Google Scholar]
- Teicher M. H., Anderson C. M., Ohashi K., Khan A., McGreenery C. E., Bolger E. A., . . . Vitaliano G. D. (2018). Differential effects of childhood neglect and abuse during sensitive exposure periods on male and female hippocampus. NeuroImage, 169, 443–452. doi: 10.1016/j.neuroimage.2017.12.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M. H., Samson J. A. (2016). Annual research review: Enduring neurobiological effects of childhood abuse and neglect. Journal of Child Psychology and Psychiatry and Allied Disciplines, 57, 241–266. doi: 10.1111/jcpp.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M. E., Marusak H. A. (2017). Toward understanding the impact of trauma on the early developing human brain. Neuroscience, 342, 55–67. doi: 10.1016/j.neuroscience.2016.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N. (2015). Social scaffolding of human amygdala-mPFCcircuit development. Social Neuroscience, 10, 489–499. doi: 10.1080/17470919.2015.1087424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Sheridan M. A. (2009). A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Frontiers in Human Neuroscience, 3, Article 68. doi: 10.3389/neuro.09.068.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treister R., Kliger M., Zuckerman G., Aryeh I. G., Eisenberg E. (2012). Differentiating between heat pain intensities: The combined effect of multiple autonomic parameters. Pain, 153, 1807–1814. doi: 10.1016/J.PAIN.2012.04.008 [DOI] [PubMed] [Google Scholar]
- Tsoory M., Cohen H., Richter-Levin G. (2007). Juvenile stress induces a predisposition to either anxiety or depressive-like symptoms following stress in adulthood. European Neuropsychopharmacology, 17, 245–256. doi: 10.1016/J.EURONEURO.2006.06.007 [DOI] [PubMed] [Google Scholar]
- Turecki G., Meaney M. J. (2016). Effects of the social environment and stress on glucocorticoid receptor gene methylation: A systematic review. Biological Psychiatry, 79, 87–96. doi: 10.1016/J.BIOPSYCH.2014.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka A. R., Price L. H., Marsit C., Walters O. C., Carpenter L. L. (2012). Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: Preliminary findings in healthy adults. PLOS ONE, 7(1), Article 30148. doi: 10.1371/journal.pone.0030148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen A.-L., Gibson J. L., St Clair M. C., Owens M., Brodbeck J., Dunn V., . . . Goodyer I. M. (2016). Friendships and family support reduce subsequent depressive symptoms in at-risk adolescents. PLOS ONE, 11(5), Article 0153715. doi: 10.1371/journal.pone.0153715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen A.-L., Hauber K., Gunther Moor B., Spinhoven P., Boon A. E., Crone E. A., Elzinga B. M. (2014). Childhood emotional maltreatment severity is associated with dorsal medial prefrontal cortex responsivity to social exclusion in young adults. PLOS ONE, 9(1), Article 85107. doi: 10.1371/journal.pone.0085107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hasselt F. N., Cornelisse S., Yuan Zhang T., Meaney M. J., Velzing E. H., Krugers H. J., Joëls M. (2012). Adult hippocampal glucocorticoid receptor expression and dentate synaptic plasticity correlate with maternal care received by individuals early in life. Hippocampus, 22, 255–266. doi: 10.1002/hipo.20892 [DOI] [PubMed] [Google Scholar]
- VanTieghem M. R., Tottenham N. (2018). Neurobiological programming of early life stress: Functional development of amygdala prefrontal circuitry and vulnerability for stress related psychopathology. Current Topics in Behavioral Neuroscience, 38, 117–136. doi: 10.1007/7854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez D. M., Bailey C., Dent G. W., Okimoto D. K., Steffek A., López J. F., Levine S. (2006). Brain corticotropin-releasing hormone (CRH) circuits in the developing rat: Effect of maternal deprivation. Brain Research, 1121, 83–94. doi: 10.1016/J.BRAINRES.2006.08.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedhara K., Hyde J., Gilchrist I., Tytherleigh M., Plummer S. (2000). Acute stress, memory, attention and cortisol. Psychoneuroendocrinology, 25, 535–549. doi: 10.1016/S0306-4530(00)00008-1 [DOI] [PubMed] [Google Scholar]
- Veenema A. H., Meijer O. C., De Kloet E. R., Koolhaas J. M. (2003). Genetic selection for coping style predicts stressor susceptibility. Journal of Neuroendocrinology, 15, 256–267. doi: 10.1046/j.1365-2826.2003.00986.x [DOI] [PubMed] [Google Scholar]
- Veenema A. H., Meijer O. C., de Kloet E. R., Koolhaas J. M., Bohus B. G. (2003). Differences in basal and stress-induced HPA regulation of wild house mice selected for high and low aggression. Hormones and Behavior, 43, 197–204. doi: 10.1016/S0018-506X(02)00013-2 [DOI] [PubMed] [Google Scholar]
- Verbeek M. E., De Goede P., Drent P. J., Wiepkema P. R. (1999). Individual behavioral characteristics and dominance in aviary groups of great tits. Behaviour, 136, 23–48. Retrieved from https://www.jstor.org/stable/pdf/4535592.pdf [Google Scholar]
- Verbeek M. E. M., Drent P. J., Wiepkema P. R. (1994). Consistent individual differences in early exploratory behaviour of male great tits. Animal Behaviour, 48, 1113–1121. doi: 10.1006/ANBE.1994.1344 [DOI] [Google Scholar]
- Vyas A., Mitra R., Rao B. S. S., Chattarji S. (2002). Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. The Journal of Neuroscience, 22, 6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T. D., Waugh C. E., Lindquist M., Noll D. C., Fredrickson B. L., Taylor S. F. (2009). Brain mediators of cardiovascular responses to social threat: Part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. NeuroImage, 47, 821–835. doi: 10.1016/J.NEUROIMAGE.2009.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. L., Senn T. E., Carey M. P. (2012). Exposure to different types of violence and subsequent sexual risk behavior among female sexually transmitted disease clinic patients: A latent class analysis. Psychology of Violence, 2, 339–354. doi: 10.1037/a0027716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.-D., Chen Y., Wolf M., Wagner K. V., Liebl C., Scharf S. H., . . . Schmidt M. V. (2011). Forebrain CRHR1 deficiency attenuates chronic stress-induced cognitive deficits and dendritic remodeling. Neurobiology of Disease, 42, 300–310. doi: 10.1016/J.NBD.2011.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Gould E., McEwen B. S. (1992). Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Research, 588, 341–345. doi: 10.1016/0006-8993(92)91597-8 [DOI] [PubMed] [Google Scholar]
- Weaver I. C. G., Cervoni N., Champagne F. A., D’Alessio A. C., Sharma S., Seckl J. R., . . . Meaney M. J. (2004). Epigenetic programming by maternal behavior. Nature Neuroscience, 7, 847–854. doi: 10.1038/nn1276 [DOI] [PubMed] [Google Scholar]
- Wethington E., Brown G. W., Kessler R. C. (1997). Interview measurement of stressful life events. In Cohen S., Kessler R. C., Gordon L. U. (Eds.), Measuring stress (pp. 59–79). Oxford, England: Oxford University Press. [Google Scholar]
- Winiarski D. A., Engel M. L., Karnik N. S., Brennan P. A. (2018). Early life stress and childhood aggression: Mediating and moderating effects of child callousness and stress reactivity. Child Psychiatry and Human Development, 49, 730–739. doi: 10.1007/s10578-018-0785-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wismer Fries A. B., Pollak S. D. (2017). The role of learning in social development: Illustrations from neglected children. Developmental Science, 20, Article 12431. doi: 10.1111/desc.12431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt A., Münzer A., Ganser H. G., Fegert J. M., Goldbeck L., Plener P. L. (2016). Experience by children and adolescents of more than one type of maltreatment: Association of different classes of maltreatment profiles with clinical outcome variables. Child Abuse & Neglect, 57, 1–11. doi: 10.1016/J.CHIABU.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Wittbrodt M. T., Moazzami K., Lima B. B., Alam Z. S., Corry D., Hammadah M., . . . Bremner D. J. (2019). Early childhood trauma alters neurological responses to mental stress in patients with coronary artery disease. Journal of Affective Disorders, 254, 49–58. doi: 10.1016/J.JAD.2019.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard K., Pollak S. D. (2020). Is there evidence for sensitive periods in emotional development? Current Opinion in Behavioral Sciences, 36, 1–6. doi: 10.1016/j.cobeha.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon F. L., Hedges D. W. (2008). Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: A meta-analysis. Hippocampus, 18, 729–736. doi: 10.1002/hipo.20437 [DOI] [PubMed] [Google Scholar]
- Yao S., Qi S., Kendrick K. M., Mobbs D. (2018). Attentional set to safety recruits the ventral medial prefrontal cortex. Scientific Reports, 8(1), Article 15395. doi: 10.1038/s41598-018-33953-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. S., Farrell A. K., Carlson E. A., Englund M. M., Miller G. E., Gunnar M. R., . . . Simpson J. A. (2019). The dual impact of early and concurrent life stress on adults’ diurnal cortisol patterns: A prospective study. Psychological Science, 30, 739–747. doi: 10.1177/0956797619833664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac L., Raby K. L., Dozier M. (2019). Attachment state of mind and childhood experiences of maltreatment as predictors of sensitive care from infancy through middle childhood: Results from a longitudinal study of parents involved with Child Protective Services. Development and Psychopathology, 31, 113–125. doi: 10.1017/S0954579418001554 [DOI] [PubMed] [Google Scholar]
- Zhang T., Chrétien P., Meaney M. J., Gratton A. (2005). Influence of naturally occurring variations in maternal care on prepulse inhibition of acoustic startle and the medial prefrontal cortical dopamine response to stress in adult rats. The Journal of Neuroscience, 25, 1493–1502. doi: 10.1523/jneurosci.3293-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Smith_Supplemental_Material for Rethinking Concepts and Categories for Understanding the Neurodevelopmental Effects of Childhood Adversity by Karen E. Smith and Seth D. Pollak in Perspectives on Psychological Science