Abstract
Fishmeal substitutes (such as insect-based feeds) in pig diets can promote sustainable pork production. Insect powders contain chitin, a nitrogen-containing indigestible material, and pigs must have the capacity to secrete chitin-degrading enzymes to benefit from these diets. The chitin-degrading enzyme (acidic mammalian chitinase; AMCase) and its gene expression have been detected in the stomach tissue of approximately 6-month-old fattening pigs; however, it remains unclear from which stage chitin-degrading enzymes are secreted. In the present study, the stomach tissue of piglets was collected from the suckling stage (14 d old) to 56 d to evaluate chitin-degrading enzymes and associated gene expression. AMCase mRNA and protein expression was detected in the stomach tissue of all piglets from days 14 to 56. AMCase secretion might increase with the increase in stomach tissue weight as piglets grow. Insect powders can therefore be used in the diets of pre-weaning piglets. The gastric AMCase level was approximately 30% that of fattening pigs. The appropriate inclusion of insect meals in the diets of pigs at different growth stages still needs to be determined.
Subject terms: Animal physiology, Sustainability, Entomology
Introduction
Since the publication of "Edible insects" by the Food and Agriculture Organization in 20131, insects have garnered the attention of researchers as potential feed material globally. Food wastes, which are mostly incinerated or landfilled, can be converted to animal proteins by using them as a feed for insects2. In addition, the utilization of insect larvae for recycling resources is consistent with the Sustainable Development Goals, and many firms have been set up worldwide to produce insects as a feed source3,4.
Some insects that are being studied for their potential use as animal feeds are the mealworm (Tenebrio molitor) and black soldier fly (BSF, Hermetia illucens)5–9. The mealworms have been produced as feed for pets (e.g., birds and reptiles) for a long time10, and therefore, their husbandry and breeding methods have been well established11. Similarly, the BSF has attracted considerable attention as a potential animal feed and in waste management during recent years3,7,8,12–15, and its larvae can be cultivated using either food waste or livestock feces; however, the reproductive efficiency of adult BSFs is low16.
The amino acid contents of insect powders are similar to those of fishmeal. Thus, defatted powders of such insects could be used as alternatives to fishmeal, whose availability is influenced by natural resource status, as a protein source in pig and poultry diets13. In addition, the supplementation of pig diets with insect powder has been reported to enhance growth and nutrient digestibility with no detrimental effects on the immune system in piglets17,18.
The insect cuticle consists of chitin, proteins, phenolic compounds, and lipids19. Chitin is a nitrogen-containing fiber20 that can only be degraded by chitinase21. Numerous studies have reported the immunomodulatory effects of chitin22,23. According to these previous studies, the immunomodulatory effects of chitin implied that chitin is not fully digested and eventually reaches the cecum and colon. The substitution of an easily digestible protein source with insect powder, which is relatively difficult to digest, could decrease the growth rates of piglets that have an immature digestive system; therefore, caution should be exercised when adopting insect powder as a protein source.
Mammals produce two types of chitinase, chitotriosidase (Chit1) and acidic mammalian chitinase (AMCase)24. Tabata et al.24 reported AMCase expression in the stomach tissue of approximately 6-month–old pigs. This suggests that mature pigs have the ability to secrete chitinase. However, it remains unclear from which growth stage chitinase is secreted. Therefore, in the present study, we investigated whether piglets have the capacity to secrete chitinase, from the suckling to growing stages.
Results
Body and stomach tissue weights of piglets
The body weight of the piglets at birth was 1344.4 ± 312.3 g. The piglets weighed > 1 kg at birth, and they were not runts. The body and stomach tissue weights at different ages are listed in Table 1.
Table 1.
Body and stomach tissue weights of the piglets at different age.
| Age | Male (n = 3) | Female (n = 3) | All (n = 6) | ANOVA of body weight | ANOVA of stomach tissue weight | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Body weight (kg) | Stomach tissue weight (g) | Body weight (kg) | Stomach tissue weight (g) | Body weight (kg) | Stomach tissue weight (g) | Age | Sex | Age × sex | Age | Sex | Age × sex | |
| 14d | 5.0 ± 0.4 | 21.1 ± 2.3 | 4.1 ± 0.3 | 19.2 ± 1.1 | 4.5 ± 0.3a | 20.2 ± 1.2a | < 0.01 | 0.15 | 0.94 | < 0.01 | 0.15 | 0.68 |
| 21d | 6.6 ± 0.8 | 28.9 ± 3.4 | 6.0 ± 1.2 | 31.4 ± 6.8 | 6.3 ± 0.6ab | 30.2 ± 3.5a | ||||||
| 28d | 8.7 ± 0.1 | 37.2 ± 1.5 | 6.5 ± 1.2 | 35.3 ± 1.9 | 7.6 ± 0.7abc | 36.3 ± 1.1abc | ||||||
| 35d | 11.1 ± 1.3 | 68.3 ± 3.0 | 10.6 ± 1.0 | 67.3 ± 2.2 | 10.8 ± 0.7bc | 67.8 ± 1.7bc | ||||||
| 42d | 12.9 ± 1.2 | 90.0 ± 9.0 | 12.5 ± 2.3 | 77.0 ± 4.7 | 12.7 ± 1.2c | 83.5 ± 5.4c | ||||||
| 49d | 21.4 ± 3.8 | 149.2 ± 28.5 | 19.9 ± 0.9 | 137.0 ± 8.7 | 20.6 ± 1.8d | 143.1 ± 13.6d | ||||||
| 56d | 24.9 ± 1.7 | 185.2 ± 6.3 | 20.7 ± 4.1 | 150.4 ± 24.9 | 22.8 ± 2.2d | 167.8 ± 13.9d | ||||||
Data is mean ± SE. Different alphabet characters indicate significant differences at the 0.05 level.
Chitinase-related mRNA expression in the stomach of piglets
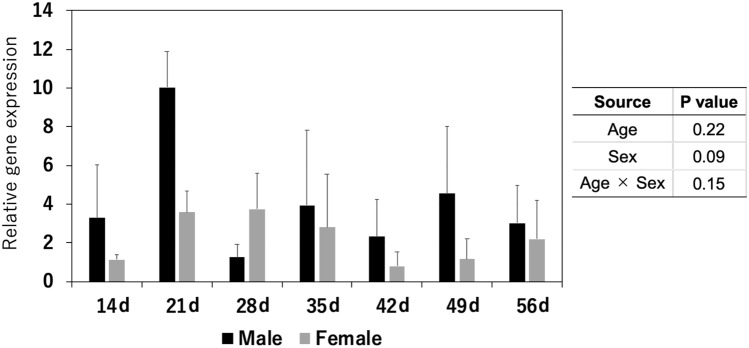
AMCase mRNA was expressed in all piglets. There were no significant differences in the mRNA expression of AMCase among piglets of different ages (P > 0.05, Fig. 1). Moreover, there was no significant difference in the relative expression of AMCase between the sexes of piglets (P > 0.05, Fig. 1). Chit1 mRNA expression was observed in only 4 (two 21-, one 35-, and one 56-d-old) of the 42 piglets, and the threshold cycle (Ct) of Chit1 was 15–20-fold that of AMCase.
Figure 1.
AMCase mRNA expression level in the stomach tissue of piglets. Data are presented as mean ± standard error. Values are expressed as relative gene expression.
AMCase level in the stomach of piglets
AMCase was secreted in the stomach tissue of all piglets. The AMCase levels per unit stomach weight did not differ significantly among piglets of different ages (P > 0.05, Fig. 2). However, the analysis of variance (ANOVA) showed a significant difference in AMCase level per unit stomach weight between piglet sexes (P < 0.05, Fig. 2).
Figure 2.
AMCase levels per unit stomach tissue weight in piglets.
The AMCase level per whole stomach weight tended to increase with an increase in age (Fig. 3). The AMCase level in the whole stomach of piglets was significantly higher at 56 d than at 14 d (P < 0.05). However, the ANOVA did not reveal significant differences in the AMCase level in the whole stomach between piglet sexes (P > 0.05, Fig. 3).
Figure 3.
AMCase levels in whole stomach weight of piglets. Different lowercase letters indicate a significant difference (Bonferroni post-hoc test, P < 0.01).
Discussion
In the present study, we aimed to clarify the stage from which chitin-degrading enzymes are secreted. To this end, the stomach tissue of piglets was collected from the suckling (14 d old) to the growing stages (56 d old) to investigate chitin-degrading enzyme and associated gene expression levels. Piglet AMCase gene expression did not vary among the growth stages, suggesting that the piglets had the ability to secrete AMCase from the suckling stage. Similar to the findings of Tabata et al.24, Chit1 mRNA expression in the stomach was low compared to AMCase mRNA expression. In addition, the relative AMCase mRNA expression did not significantly change when the piglets began to wean, and the stress of weaning did not seem to influence the gene expression levels considerably. Although the piglets were allowed to consume only maternal milk and water until weaning, the piglets exhibited interest in commercial feed from around 9 d of age when the feed was placed in the rearing enclosures; they have been reported to poke the feed with their noses or eat it25. In the wild, piglets probably begin to eat insects and mushrooms that contain chitin. Thus, pigs may have the ability to secrete AMCase in the pre-weaning stage.
In the present study, the AMCase level did not exhibit an increasing trend with age between the pre-weaning and post-weaning stages; however, it was approximately 28% that in the stomach tissue of fattened pigs24. The results suggest that AMCase secretion increases with the growth of pigs. As the weight and area of the gastric mucosa increase with pig growth, even if the AMCase level per unit area of gastric mucosa is the same, the degraded chitin amount would increase with age. This implies that insect powder concentrations in pig diets can be increased with the growth stage of pigs.
Several studies in which the protein source in piglet diets has been substituted with insect powder have been reported; however, in most of these studies, the protein has been substituted with soybean meal, and a few studies have used animal-based proteins18,26–28. In addition, it has been reported that soybean meal can be substituted with 10% insect powder26; however, the insect powder used to replace soybean meal was defatted under high pressure and may not contain a considerable amount of chitin. Another study that substituted non-defatted insect powders with animal protein in piglet diets concluded that 2% substitution was possible29.
Protein concentrations in pig diets decrease from the pre-weaning stage to the fattening stage, when their diets shift to contain more grains than animal proteins30. In addition, based on the composition of the diet, the suckling stage diets contain more animal proteins, but the fattening stage diets contain more grains than animal proteins; therefore, the proportion of animal proteins in the diets of fattening pigs is low30. Consequently, animal proteins in the diet of fattening pigs, which are thought to secrete a higher amount of chitin-degrading enzymes than piglets, can be entirely substituted with insect powder. However, as the proportion of animal proteins in the diet during the pre-weaning and early weaning stages are high30, it is necessary to consider the chitin level when replacing animal proteins in the diet with insect powder. Otherwise, the chitin nitrogen would be calculated as the amount of nitrogen in the diet, and the amount of piglet digestible nitrogen could be lower than that presumed. Furthermore, inappropriate concentrations of insect powder in diets could inhibit weight gain in piglets.
The piglets exhibited the ability to secrete AMCase in the stomach tissue from day 14. Therefore, insect powder could be adopted in the diets of pre-weaning piglets. However, the gastric AMCase level in piglets was approximately 28% that in fattening pigs; this necessitates further investigation to determine the appropriate insect powder concentration in the diets at different growth stages.
Methods
The protocol of this study was approved by the Animal Care and Use Committee of Kagawa University (Permit number: 2019–19,631) and carried out according to the Kagawa University Animal Experimentation Regulation.
Stomach tissue samples from piglets
Forty-two piglets (Landrace × Large white × Duroc: 21 males and 21 females) delivered by six sows (Landrace × Large white) were used. The sows and piglets were reared in farrowing stalls (1.8 m × 2.0 m) until weaning. Weaning was carried out at 28 d. After weaning, out of the 42 piglets, 24 piglets were reared in piglet cages (1.8 m × 1.8 m) in an open-type pig house with an average temperature of 21.8 °C ± 5.5 °C and average humidity of 70.3% ± 6.5%. The piglets were fed commercial feeds (Nosan, Yokohama, Japan) according to the feeding program of the university farm. The feeding program and the feed compositions are shown in Fig. S1 and Table S1, respectively. At 14 d, six piglets (3 males and 3 females) were euthanized by administering 90.0 mg/kg body weight of pentobarbital via the auricular vein and dissected. This process was repeated weekly for 7 weeks with the remaining piglets. Stomach tissue was collected from the gastric body to the pylorus, and then weighed. The collected samples were separated for gene expression analyses and chitinase measurements. The stomach tissue samples for gene expression analyses were stored at 4 °C overnight in RNAlater stabilizer solution (Thermo Fisher Scientific, Waltham, MA, USA), and then frozen at -80 °C until analysis. The samples for chitinase secretion assays were frozen at -80 °C until analysis.
Expression of piglet AMCase
Total RNA was isolated from piglet stomach tissue using TRIzol Reagent (Thermo Fisher Scientific), according to the manufacturer’s instructions. The RNA was reverse transcribed into cDNA using ReverTra Ace qPCR RT Master Mix with gDNA Remover (TOYOBO, Osaka, Japan), according to the manufacturer’s instructions. The sequences of primers used for real-time PCR were as follows: AMCase forward primer 5ʹ-TGACTTCACAGGCACTTTCT-3ʹ, AMCase reverse primer 5ʹ-CGGTGCAACTTGTGCTATTC-3ʹ; Chit1 forward primer 5ʹ-GTCAACTCAGCCATCAGGTT-3ʹ, Chit1 reverse primer 5ʹ-CAAGGTCAAGGCCATCAAA-3ʹ; and GAPDH forward primer 5ʹ-ACCTCCACTACATGGTCTACA-3ʹ, GAPDH reverse primer 5ʹ-ATGACAAGCTTCCCGTTCTC-3ʹ24. A StepOnePlus Real-Time PCR system (Applied Bioscience, Waltham, MA, USA) was used for real-time PCR, and each reaction mixture consisted of 0.4 µl of 50 × ROX reference dye (TOYOBO, Osaka, Japan), 10 µl of THUNDERBIRD SYBR qPCR Mix (TOYOBO, Osaka, Japan), 2 µl of DNA template, 0.5 µM of forward primer, 0.5 µM of reverse primer, and 5.6 µl of sterile water, with a total volume of 20 µl. Each reaction was performed in triplicate. The PCR cycling conditions were as follows: initial denaturation at 95 °C for 1 min; 40 cycles of denaturation at 95 °C for 15 s, and extension at 60 °C for 1 min.
Measurement of piglet AMCase
Twenty milligrams of gastric mucosa and 1 ml of RIPA Buffer (Nacalai Tesque, Kyoto, Japan) were added into a 1.5 ml tube. Thereafter, the samples were completely homogenized using a bead crusher (µT-12; TAITEC, Saitama, Japan) by shaking at 3,500 rpm for 20 s, and then cooling on ice for 30 s; this process was repeated three times. Subsequently, the samples were centrifuged at 10,000 × g for 20 min at 4 °C, and the cells were precipitated. The supernatant was transferred to another 1.5 ml tube without disturbing the pellet.
The AMCase level was measured using a fluorometer (Gemini EM; Molecular Devices, San Jose, CA, USA) and the CycLex Acidic Mammalian Chitinase Fluorometric Assay Kit (Medical & Biological Laboratories, Nagoya, Japan), according to the manufacturers’ instructions.
Statistical analysis
The relative AMCase mRNA and protein expression levels were analyzed using a two-way ANOVA and Bonferroni post-hoc test using IBM SPSS Statistics 25 (IBM Corp, Armonk, NY, USA).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 19K15962. The authors thank for technical assistance from Mr. Takumi Shiota, Mr. Toshiya Kawasaki, and Mr. Atsushi Nakazu (students of Kagawa University, Kagawa, Japan).
Author contributions
K.K. and T.O. conceived and designed the experiment; all authors performed the experiment; K.K. and T.O. performed RNA isolation, cDNA synthesis, and real-time quantitative P.C.R.; K.K. and T.O. analyzed the data; K.K. and T.O. wrote the first draft of the manuscript; all the authors read and approved publishing the final manuscript.
Competing interests
The authors declare no competing interests
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41598-020-80368-0.
References
- 1.Van Huis, A. et al. Edible insects Future prospects for food and feed security. Food and Agriculture Organization of the United Nations171, (2013).
- 2.Cortes Ortiz JA, et al. Insect mass production technologies. In: Dossey AT, Morales-Ramos JA, Guadalupe Rojas M, et al., editors. Insect as Sustainable Food Ingredients Production, Processing and Food Applications. Cambridge: Academic Press; 2016. pp. 153–201. [Google Scholar]
- 3.Kawasaki K, Kawasaki T, Hirayasu H, Matsumoto Y. Evaluation of fertilizer value of residues obtained after processing household organic waste with black soldier fly larvae (Hermetia illucens) Sustainability. 2020;12:4920. doi: 10.3390/su12124920. [DOI] [Google Scholar]
- 4.International Platform of Insects for Food and Feed (IPIFF). International Platform of Insects for Food and Feed. Available at: https://ipiff.org/. (Accessed: 23rd June 2020)
- 5.Thévenot A, et al. Mealworm meal for animal feed: Environmental assessment and sensitivity analysis to guide future prospects. J. Clean. Prod. 2018;170:1260–1267. doi: 10.1016/j.jclepro.2017.09.054. [DOI] [Google Scholar]
- 6.Van Zanten HHE, et al. From environmental nuisance to environmental opportunity: housefly larvae convert waste to livestock feed. J. Clean. Prod. 2015;102:362–369. doi: 10.1016/j.jclepro.2015.04.106. [DOI] [Google Scholar]
- 7.Smetana S, Palanisamy M, Mathys A, Heinz V. Sustainability of insect use for feed and food: life cycle assessment perspective. J. Clean. Prod. 2016;137:741–751. doi: 10.1016/j.jclepro.2016.07.148. [DOI] [Google Scholar]
- 8.Sánchez-Muros MJ, Barroso FG, Manzano-Agugliaro F. Insect meal as renewable source of food for animal feeding: a review. J. Clean. Prod. 2014;65:16–27. doi: 10.1016/j.jclepro.2013.11.068. [DOI] [Google Scholar]
- 9.Makkar HPS, Tran G, Heuzé V, Ankers P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014;197:1–33. doi: 10.1016/j.anifeedsci.2014.07.008. [DOI] [Google Scholar]
- 10.Grau T, Vilcinskas A, Joop G. Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed. Zeitschrift fur Naturforsch Sect. C J. Biosci. 2017;72:337–349. doi: 10.1515/znc-2017-0033. [DOI] [PubMed] [Google Scholar]
- 11.Dossey AT, Tatum JT, McGill WL. Modern insect-based food industry: current status, insect processing technology, and recommendations moving forward. In: Dossey AT, Morales-Ramos JA, Guadalupe Rojas M, editors. Insects as sustainable food ingredients. Cambridge: Academic Press; 2016. pp. 113–152. [Google Scholar]
- 12.Wang Y-S, Shelomi M. Review of black soldier fly (Hermetia illucens) as animal feed and human food. Foods. 2017;6:91. doi: 10.3390/foods6100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veldkamp T, Bosch G. Insects: A protein-rich feed ingredient in pig and poultry diets. Anim. Front. 2015;5:45–50. [Google Scholar]
- 14.Schiavone A, et al. Nutritional value of a partially defatted and a highly defatted black soldier fly larvae (Hermetia illucens L.) meal for broiler chickens: apparent nutrient digestibility, apparent metabolizable energy and apparent ileal amino acid digestibility. J. Anim. Sci. Biotechnol. 2017;8:1–9. doi: 10.1186/s40104-017-0181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawasaki K, et al. Evaluation of black soldier fly (Hermetia illucens) larvae and pre-pupae raised on household organic waste, as potential ingredients for poultry feed. Animals. 2019;9:98. doi: 10.3390/ani9030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura S, Ichiki RT, Shimoda M, Morioka S. Small-scale rearing of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae), in the laboratory: low-cost and year-round rearing. Appl. Entomol. Zool. 2016;51:161–166. doi: 10.1007/s13355-015-0376-1. [DOI] [Google Scholar]
- 17.Jin XH, Heo PS, Hong JS, Kim NJ, Kim YY. Supplementation of dried mealworm (Tenebrio molitor larva) on growth performance, nutrient digestibility and blood profiles in weaning pigs. Asian-Australas. J. Anim. Sci. 2016;29:979–986. doi: 10.5713/ajas.15.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu M, et al. Hermetia illucens larvae as a Fishmeal replacement alters intestinal specific bacterial populations and immune homeostasis in weanling piglets. J. Anim. Sci. 2020;98:skz395. doi: 10.1093/jas/skz395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hackman R. Chemistry of the insect cuticle. In: Rockstein M, editor. The Physiology of Insect. Amsterdam: Elsevier; 1974. pp. 216–260. [Google Scholar]
- 20.Khoushab F, Yamabhai M. Chitin research revisited. Mar. Drugs. 2010;8:1988–2012. doi: 10.3390/md8071988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamid R, et al. Chitinases: An update. J. Pharm. Bioallied Sci. 2013;5:21–29. doi: 10.4103/0975-7406.106559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koller B, Müller-Wiefel AS, Rupec R, Korting HC, Ruzicka T. Chitin modulates innate immune responses of keratinocytes. PLoS ONE. 2011;6:e16594. doi: 10.1371/journal.pone.0016594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CG, Da Silva CA, Lee J-Y, Hartl D, Elias JA. Chitin regulation of immune responses: an old molecule with new roles. Curr. Opin. Immunol. 2008;20:684–689. doi: 10.1016/j.coi.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabata E, et al. Protease resistance of porcine acidic mammalian chitinase under gastrointestinal conditions implies that chitin-containing organisms can be sustainable dietary resources. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Middelkoop A, Costermans N, Kemp B, Bolhuis JE. Feed intake of the sow and playful creep feeding of piglets influence piglet behaviour and performance before and after weaning. Sci. Rep. 2019;9:16140. doi: 10.1038/s41598-019-52530-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biasato I, et al. Partially defatted black soldier fly larva meal inclusion in piglet diets: Effects on the growth performance, nutrient digestibility, blood profile, gut morphology and histological features. J. Anim. Sci. Biotechnol. 2019;10:1–11. doi: 10.1186/s40104-019-0325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji YJ, et al. Use of insect powder as a source of dietary protein in early-weaned piglets1. J. Anim. Sci. 2016;94:111–116. doi: 10.2527/jas.2015-9555. [DOI] [Google Scholar]
- 28.Spranghers T, et al. Gut antimicrobial effects and nutritional value of black soldier fly (Hermetia illucens L.) prepupae for weaned piglets. Anim. Feed Sci. Technol. 2018;235:33–42. doi: 10.1016/j.anifeedsci.2017.08.012. [DOI] [Google Scholar]
- 29.Ao, X. & Kim, I. H. Effects of dietary dried mealworm (Ptecticus tenebrifer) larvae on growth performance and nutrient digestibility in weaning pigs. Livest. Sci.230, (2019).
- 30.National Agriculture and Food Research Organization (NARO). Japanese Feeding Standard for Swine. (Japan Livestock Industry Association, 2013).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.