Abstract
Introduction
long-term environmental and occupational exposure to lead, which is a ubiquitous industrial pollutant, causes significant damage to tissues of kidney. This report aims to address this debilitating issue. A natural polyphenolic compound, Ellagic acid (EA) is having numerous potential medicinal properties. In this present study nephroprotective effects of EA has been evaluated in a rodent model with lead-induced toxicity.
Methods
Rats were treated with EA doses of 50 mg/kg and 25 mg/kg and simultaneously co-administered with lead acetate (60 mg/kg) for 2 months through oral route. The extent to which EA treatment provides nephroprotective effect was estimated by measurement of serum biomarkers, tissue antioxidants, inflammatory mediators, apoptosis, autophagy pathway and histological examination.
Results
EA treatment caused significant restoration in the level of serum biomarkers, tissue antioxidants and histological architecture of renal tissue. Treatment with either of the doses of EA causes restoration of pro-inflammatory mediators to approximately pre-exposure concentration. This phenomena is caused by suppression of expression levels of inflammatory molecules like tumour necrosis factor-α (TNF-α), nuclear factor kappa B (NF-κB), interleukin-6 (IL-6), and interleukin-1β (IL-1β), as well as functional expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). Moreover, it was also observed that EA suppressed apoptotic and autophagic pathway by reduction of expression of light chain 3B (LC3B) level which are the oxidative DNA damage markers of renal tissue.
Conclusion
It can be safely concluded that EA provides protection against lead-induced nephrotoxicity to a significant degree.
Keywords: Apoptosis, Autophagy, Lead, Inflammation, Nephrotoxicity, Ellagic acid
Apoptosis, Autophagy, Lead, Inflammation, Nephrotoxicity, Ellagic acid.
1. Introduction
Acute and chronic exposure to lead is often associated with a deficit of tubular transport mechanisms and corresponding appearance of degenerative perturbation in tubular epithelium responsible for debilitating pathological changes. Persistent inflammation has been reported to be an important characteristic of chronic renal disease and it plays an important role in pathophysiology of renal disorders. This toxic pathway is responsible for a significant percentage of all-cause mortality [1, 2]. Lead exposure causes initiation and development of inflammatory pathways in the body, acting as modulator on multiple levels of expression from genomic to proteomic profile. It affects, among others, expression and activity of enzymes involved in inflammation (such as COX-2) and expression of cytokines (IL8, TNF, IFNγ). Lead induced toxicity is associated with impairment of cellular constituents of the human immune system. Altered cellular and humoral immune response as a consequence of lead exposure is mediated by reduction of immunoglobulin production, which predisposes affected population to enhanced inflammatory disorders [3].
Phytochemicals isolated from different natural sources have been observed to be equally potent as compared to different synthetic active pharmaceutical ingredients with superior safety profile and efficacy when evaluated in different disease models. Polyphenols are a class of compounds that have multiple phenolic groups. Polyphenols have been isolated from numerous natural sources like different plants, fruits and vegetables. Their beneficial effects in ameliorating heavy metal toxicity have been demonstrated in many disease models. The polyphenols have been reported to detoxify different organs including kidney via removal of toxic heavy metals like lead [4, 5]. Possible mechanism of action of polyphenolic compounds in scavenging of free radicals. Reactive oxygen species (ROS) and similar free radicals are primarily responsible for development of toxicity mediated by lead and other heavy metals in different organs [6, 7].
Polyphenols have been reported to demonstrate ameliorative effect against lead-induced inflammatory reactions by attenuating ROS induced secretion of inflammatory cytokine through ERK/JNK/p38 pathways [8].
EA is a common polyphenolic phytoconstituent with well documented medicinal properties [9, 10, 11]. Among its many properties with potential therapeutic applications, most widely reported and significant are antioxidant, hepatoprotective, neuroprotective, anti-ulcer, anti-depressant, anti-bacterial, anti-inflammatory, cardioprotective, anti-cancer, Antiviral, and anti-cataract activities [12, 13, 14]. Different studies reported that medicinal properties attributed to EA is possibly due to reduced expression of IL-1β, IL-6, TNF-α, and MCP-1, and down-regulation of MCP-1 mRNA and TNF-α expression [15].
As of writing this report, no study has been performed to assess protective effect of EA against lead-induced nephrotoxicity. The current study has been framed to evaluate ameliorative effects of EA against lead-induced nephrotoxicity in a rodent model and to identify biochemical pathways responsible for observed nephroprotective action. .
2. Material and methods
2.1. Chemicals and phyto-chemicals
All chemicals purchased were of analytical grade. Lead acetate and biochemical kits were purchased from Loba Chemicals, Mumbai, India and Crest Biosystems, Goa, India respectively. EA samples were obtained from Sigma Aldrich, India. ELISA kits were procured from Thermo fisher scientific, USA.
2.2. Experimental animals
Adult male Wistar albino rats of 170–200 g were used for the study and housed under standardized condition (12 h L:D cycles, 25° ± 5 °C) with paddy husk bedding in polypropylene cages, at the Central Animal House, Soniya Education Trust's College of Pharmacy, Dharwad. Rats were fed with standard pellet food and provided with purified drinking water. The animals were maintained as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India. The study was conducted after the prior permission from the Institutional Animal Ethics Committee.
2.3. Dose selection of EA
High and low dose of EA were selected following extensive literature review. A high dose of 50 mg/kg and low dose of 25 mg/kg were selected to be administered through oral route [16].
2.4. Experimental design
After one week of acclimatization the rats were randomly divided into 4 groups of 8 animals in each group. Group I and Group II were selected to be negative and positive control respectively and received normal saline of 2 ml/kg body weight through oral route. Group III & IV animals administered with EA 50 & 25 mg/kg p.o. respectively. Experimental rats received both the treatment doses for 60 days. One hour following each treatment all groups were subjected for lead acetate (60 mg/kg) p.o. treatment daily for 60 months with exception of the negative control [17].
Twenty four hours following terminal treatment, animals were anaesthetized with light ether anesthesia following which blood was withdrawn by retro-orbital puncture. Blood serum was separated from collected blood by centrifugation. The serum was used for estimation of urea, uric acid, creatinine, and albumin using enzymatic assays (Agappe Diagnostics Limited) by a semi-autoanalyser instrument (Robonik, Mumbai). Thereafter the animals were sacrificed; kidneys extracted from euthanized animals were weighed and used for preparation of homogenate. The homogenate preparation was utilized for estimation of antioxidants like catalase, superoxide dismutase (SOD), reduced glutathione (GSH), malondialdehyde (MDA), pro-inflammatory cytokines, COX-2, iNOS and performance of assay of LC3B expression level. Remainder of renal tissues were embedded in saline solution (10%) of formalin for histological examination [18, 19].
2.5. Assay of pro-inflammatory cytokines
Pro-inflammatory cytokines in renal tissues were measured by Enzyme-linked immunosorbent assay (ELISA) using commercially purchased kits. ELISA kit (Thermo Fisher Scientific, USA) was used to determine expression levels of tumour necrosis factor-α (TNF-α), Nuclear factor-kappa B (NF-κB), interleukin-6 (IL-6), and interleukin-1β (IL-1β). Analysis was performed by Elisa Plate Reader (Varioskan LUX Multimode Microplate Reader) according to instruction of manufacturer [20].
2.6. Assay of COX-2 and iNOS activities
Inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) activities in renal tissues were assessed using rat ELISA kit (Thermo Fisher Scientific, USA) [20].
2.7. Assay of LC3B level
Light chain 3B (LC3B) level in tissues was determined using rat ELISA kit (Thermo Fisher Scientific, USA). The plates were read at 450 nm using ELISA microplate reader [21].
2.8. Preparation of kidney tissue homogenate
Kidneys were removed gently, rinsed with physiological saline solution (0.9% NaCl) to remove other debris. Following removal of debris each kidney was weighed and immediately homogenized in 50 mM potassium phosphate (pH 7.4) to get 10% homogenate. The homogenate was then subjected for centrifugation at 4000 rpm for 15 min at 4 °C. The supernatants were used for estimation of SOD, Catalase and GSH [22].
2.9. Histological analysis
For Remainder of the kidney samples, kidney sections were subjected to 10% formalin solution which were then embedded in paraffin and serial sections were incised for histopathological analysis. Hematoxylin and Eosin (H&E) dye was used to stain the sections and morphological changes in histology were observed [18]. The severity of damage in kidney tissue was determined by a scoring method determined by the following scoring method: no change - 0 scores, mild - 1 score (small multifocal degeneration with a slight degree of inflammation or focal tissue damage), moderate - 2 score (extensive degeneration of kidney tissue damage) and marked - 3 score (diffuse inflammation with necrosis).
2.10. Statistical analysis
Results were expressed as mean +/- SEM. Statistical test One-way Analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparison tests was applied for the analysis of documented results. P < 0.05 was considered significant.
3. Results
3.1. Effect on serum creatinine, urea, uric acid and albumin
Positive control (only lead acetate treated) group showed highly significant (P < 0.001) increase in serum creatinine, urea, uric acid and extremely significant (P < 0.001) reduction in albumin levels compared to negative control. Treatment groups such as EA 50, EA 25 witnessed highly significant (P < 0.001) restoration of serum biomarkers levels (Table 1).
Table 1.
Effect of Ellagic acid treatment on serum creatinine, urea, uric acid and albumin on lead acetate induced nephrototoxicity.
| Treatment | Blood serum level (mg/dl) |
|||
|---|---|---|---|---|
| Urea | Uric acid | Creatinine | Albumin | |
| Negative control | 31.87 ± 0.89 | 2.56 ± 0.09 | 0.65 ± 0.02 | 3.86 ± 0.90 |
| Positive control | 68.08 ± 1.93∗∗∗ | 7.30 ± 0.10∗∗∗ | 2.90 ± 0.08∗∗∗ | 2.80 ± 0.01∗∗∗ |
| EA 50 | 42.72 ± 1.17### | 3.58 ± 0.14### | 1.09 ± 0.03### | 3.60 ± 0.09### |
| EA 25 | 49.86 ± 1.29### | 4.44 ± 0.11### | 1.40 ± 0.05### | 3.30 ± 0.04### |
All values are mean ± SEM, n = 8, ∗∗∗P < 0.001 when compared to Negative control; ###P < 0.001 compared to Positive control group. EA 50: Ellagic acid (50 mg/kg) EA 25: Ellagic acid (25 mg/kg).
3.2. Effect on antioxidants in kidney tissue homogenate
3.2.1. Effect on SOD, catalase and GSH expression
The positive control group reported highly significant (P < 0.001) decrease in SOD, Catalase and GSH activity compared to negative control. Experimental groups EA 50 and EA 25 demonstrated significant increase (P < 0.001) in SOD, Catalase and GSH values compared to positive control group (Table 2).
Table 2.
Effect of Ellagic acid treatment on kidney weight, antioxidants and MDA in HTH against lead acetate induced nephrotoxicity.
| Treatment | Kidney Tissue Homogenate (Units/mg of protein) |
Kidney weight (gm) | Histological Score | |||
|---|---|---|---|---|---|---|
| SOD | CATALASE | GSH | MDA (nmol/g tissue) | |||
| Negative control | 32.41 ± 1.22 | 46.78 ± 0.57 | 8.10 ± 0.29 | 132.54 ± 2.18 | 0.87 ± 0.01 | 0.5 ± 0.12 |
| Positive control | 13.89 ± 0.82∗∗∗ | 21.61 ± 1.29∗∗∗ | 1.94 ± 0.12∗∗∗ | 207.12 ± 2.63∗∗∗ | 1.08 ± 0.3∗∗∗ | 2.71 ± 0.17 |
| EA 50 | 30.31 ± 0.59### | 39.74 ± 0.76### | 6.83 ± 0.06### | 148.90 ± 3.45### | 0.91 ± 0.01### | 0.89 ± 0.21 |
| EA 25 | 23.74 ± 1.26### | 32.54 ± 1.25### | 5.83 ± 0.05### | 157.46 ± 2.35### | 0.93 ± 0.02### | 1.35 ± 0.23 |
All values are mean ± SEM, n = 8, ∗∗∗P < 0.001 when compared to Negative control; ###P < 0.001 compared to Positive control group. EA 50: Ellagic acid (50 mg/kg) EA 25: Ellagic acid (25 mg/kg).
3.2.2. Effect on MDA expression
The positive control group demonstrated extremely significant (P < 0.001) elevation in MDA activity compared to negative control which is an indication of a significant rise in the lipid peroxidation.
Treatment groups such as EA 50, EA 25 reported highly significant (P < 0.001) reduction in MDA activity compared to the positive control group (Table 2).
3.3. Effect on lead-induced kidney inflammation in rats
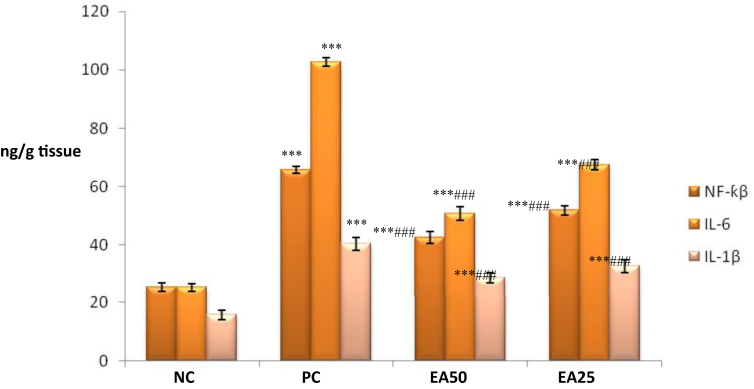
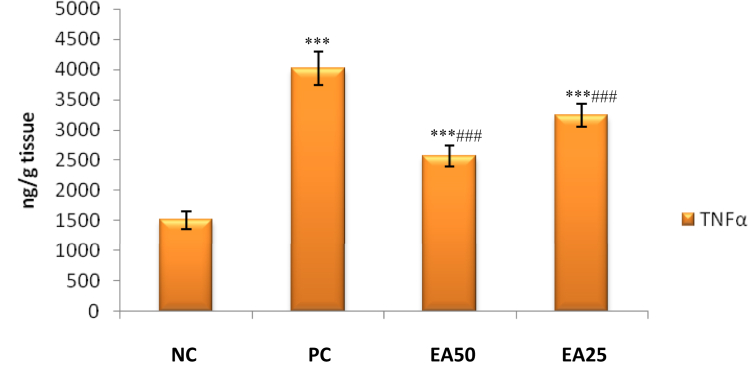
It was observed that only positive control treated group associated with significant (p < 0.001) increase in renal TNF-α, IL-1β, NF-κB, and IL-6 levels compared with negative control group. Experimental groups EA 50 and EA 25 exhibited a significant reduction (p < 0.001) in renal NF-κB, TNF-α, IL-1β, and IL-6 levels compared to the toxic control group (Figures 1 and 2).
Figure 1.
Effect of Ellagic acid treatment on kidney NF-κβ, IL-6 and IL-1β against lead acetate induced Nephrotoxicity. All values are mean ± SEM, n = 8, ∗∗∗P < 0.001 when compared to Negative control; ###P < 0.001 compared to Positive control group. EA 50: Ellagic acid (50 mg/kg) EA 25: Ellagic acid (25 mg/kg). NC: Negative Control, PC: Positive Control.
Figure 2.
Effect of Ellagic acid treatment on kidney TNF α against lead acetate induced Nephrotoxicity. All values are mean ± SEM, n = 8, ∗∗∗P < 0.001 when compared to Negative control; ###P < 0.001 compared to Positive control group. EA 50: Ellagic acid (50 mg/kg) EA 25: Ellagic acid (25 mg/kg). NC: Negative Control, PC: Positive Control.
3.4. Effect on kidney iNOS and COX-2 activities
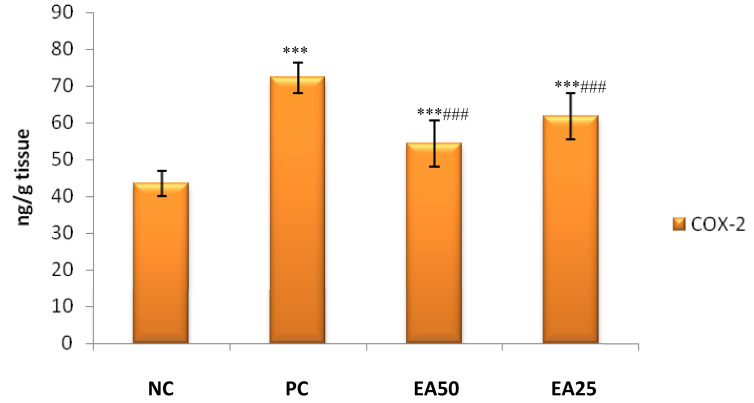
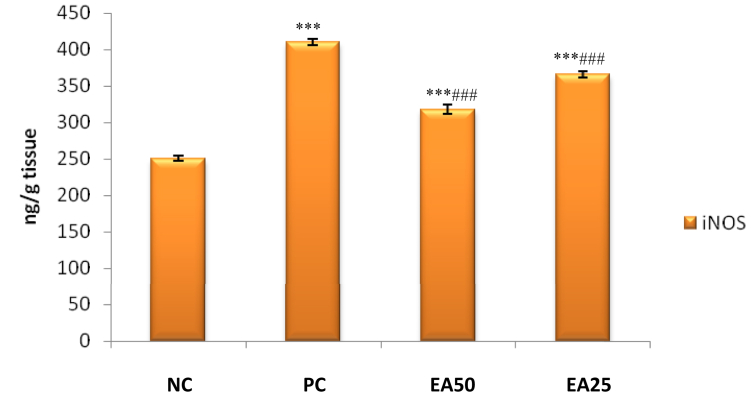
A significant increase (p < 0.001) in renal iNOS and COX-2 activities were observed for positive control group treated with only lead acetate in comparison with the negative control group. Both treatment groups EA 50 and EA 25 reported significant decrease (p < 0.001) in activities of renal iNOS and COX-2 as compared to positive control group (Figures 3 and 4).
Figure 3.
Effect of Ellagic acid treatment on kidney COX-2 against lead acetate induced Nephrotoxicity. All values are mean ± SEM, n = 8, ∗∗∗P < 0.001 when compared to Negative control; ###P < 0.001 compared to Positive control group. EA 50: Ellagic acid (50 mg/kg) EA 25: Ellagic acid (25 mg/kg). NC: Negative Control, PC: Positive Control.
Figure 4.
Effect of Ellagic acid treatment on kidney iNOS against lead acetate induced Nephrotoxicity. All values are mean ± SEM, n = 8, ∗∗∗P < 0.001 when compared to Negative control; ###P < 0.001 compared to Positive control group. EA 50: Ellagic acid (50 mg/kg) EA 25: Ellagic acid (25 mg/kg). NC: Negative Control, PC: Positive Control.
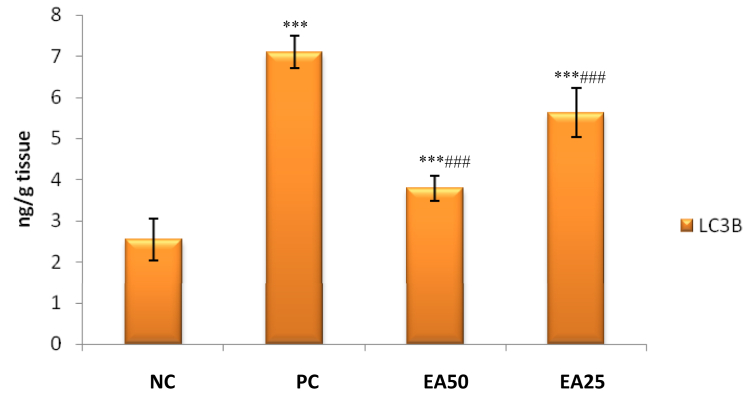
3.5. Effect on lead-induced kidney autophagy in rats
It has been observed that a significant increase (p < 0.001) in renal LC3B expression in the positive control group compared to the negative control group. However, EA 50 and EA 25 treatment (p < 0.001) significantly reduced levels of renal LC3B as compared to the positive control group treated with only lead acetate (Figure 5).
Figure 5.
Effect of Ellagic acid treatment on kidney LC3B against lead acetate induced Nephrotoxicity. All values are mean ± SEM, n = 8, ∗∗∗P < 0.001 when compared to Negative control; ###P < 0.001 compared to Positive control group. EA 50: Ellagic acid (50 mg/kg) EA 25: Ellagic acid (25 mg/kg). NC: Negative Control, PC: Positive Control.
3.6. Effect on histopathological study
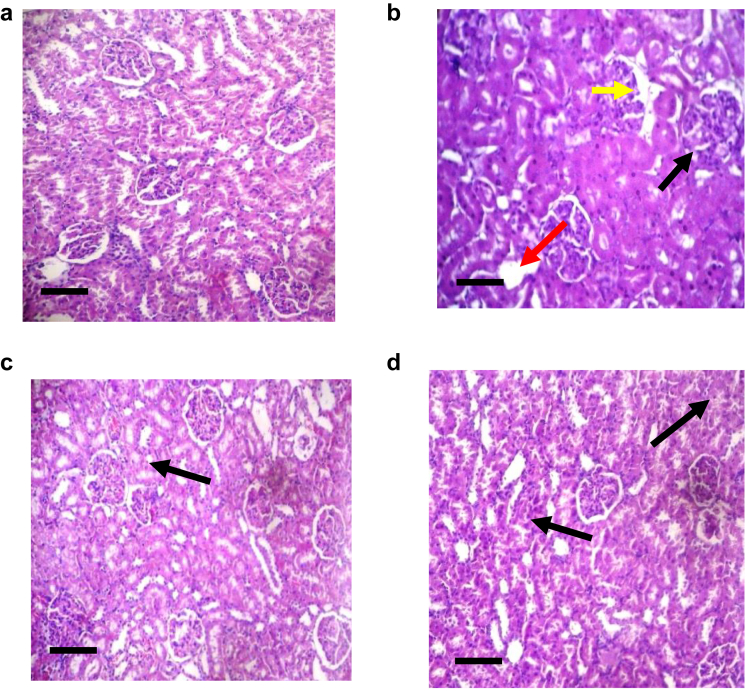
The negative control group exhibited typical renal structure, composed of an inner medulla with thin fibrous capsule, outer cortex and glomeruli (Figure 6a). The positive control group demonstrated structural distortion due to severe necrosis of tubules, separation of tubules, chronic inflammatory cell infiltration, distinct areas of hemorrhage (Figure 6b). Stroma exhibited a mild to moderate level of oedema in EA50. Glomerular congestion and mild peritubular were observed. Pyramids composed of straight tubules were present in medulla. There was mild to moderate congestion with no evidence of oedema, haemorrhage, inflammation or tubular necrosis (Figure 6c). EA25 treatment was responsible for inflammation of cells composed of small lymphocytes. Histological features suggested mild to moderate tubular damage (Figure 6d). The EA treatment was responsible for a significant reduction in histological score.
Figure 6.
Haematoxylin and eosin (H&E) stained section of kidney in lead acetate induced kidney damage. Necrosis (red arrow), inflammatory infiltration (black arrow) and increased thickness of basement membrane (yellow arrow) Photographed at magnification 100X; a: (H&E) stained microscopic kidney section (100X) of Negative control rats, Scale bar, (150 μm); b: (H&E) stained kidney section (100X) of Positive control rats showing areas of severe necrosis of proximal tubules, Scale bar, (150 μm); c: (H&E) stained kidney section (100X) of EA25 treated rats showing mild congestion. Scale bar, (150 μm); d: (H&E) stained kidney section (100X) of EA50 treated rats showing mild degree of peritubular and glomerular congestion. Scale bar, (150 μm).
4. Discussion
Lead is a highly toxic metal with no known physiological benefit. The present study was designed to find out protective effect of EA against lead acetate induced nephrotoxicity. Observed results suggest there is a definite dose-dependent ameliorative effect of EA against lead acetate induced nephrotoxicity.
The mechanism of lead-induced renal damage is due to interaction of lead with mitochondrial structures. It causes increased oxidative stress and production of reactive oxygen species and other free radicals. This in turn causes elevation of renal parameters like urea and creatinine. Lead exposure is associated with increase in production of superoxide anion and lipid peroxidation. Lead-induced toxicity may also be responsible for decreased tissue-specific functions of enzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT) and reduced glutathione (GSH). The expression level of these antioxidants may indicate extent of cytotoxic tissue damage to the kidney [22].
SOD, catalase and glutathione peroxidases (GPx) are important antioxidant enzymes. These enzymes provide protection against ROS. SOD decomposes superoxide radicals (O2-) to produce H2O. Therefore, these enzyme activities are used to determine extent of free radical generation and assess oxidative stress in cells. It has been observed that lead has a high affinity for SH groups in several enzymes such as SOD, responsible for inactivation of tissue antioxidants. In this present study CAT, SOD & GSH activities were significantly decreased in renal tissue of lead-treated group as compared to the negative control group. This suggested that lead acetate exposure induces oxidative stress by inhibiting activity of antioxidant enzymes. Interestingly, co-treatment of lead-acetate with EA leads to considerably improved function of CAT and SOD in kidneys to a level similar to that of negative control group. A significant and dose-dependent recovery of kidney GSH content was also noticed in EA50, EA25 treated groups in comparison with positive control group. These changes signify ability of EA to reduce accumulation of free radicals and protection it provides from cellular damage [22].
Lead toxicity causes free radical damage by formation of hydroperoxides, singlet oxygen and hydrogen peroxides. It can be evaluated by Malondialdehyde (MDA) levels as it is the final product of lipid peroxidation. In the present study, a significant increase of MDA level has been observed in kidney of rodents that were treated with lead acetate compared to negative control, which signifies increased oxidative stress in the lead acetate treated group. It was observed that in the tissues of rodents treated with EA50, EA25, MDA levels are significantly lower compared with rodents group which did not receive the EA doses but were dosed with lead acetate. This indicates attenuation of lipid peroxidation by EA. Intense lipid peroxidation caused by lead exposure may be responsible for damage to the mitochondrial and cytoplasmic membranes causing severe oxidative damage in tissues. As a consequence, tissues release lipid hydroperoxides into circulation which reflects induction of oxidative stress. It is known that lead acetate-induced oxidative stress tissue damage could be caused by two mechanisms: increased generation of ROS and by causing direct depletion of antioxidant reserves. As observed in this study, EA decreased MDA level in kidney tissue homogenate caused by lead acetate which signifies powerful antioxidant and free radical scavenging property [23].
The rise in creatinine and urea levels associated with exposure to lead acetate is an indicator of impaired renal function due to nephrotoxicity. Serum creatinine and urea are specific and sensitive indicators of kidney damage which are considered for assessment of renal injury. Kidney is a major organ in clearance and biotransformation of metals, so it is a primary target organ for metal-induced toxicity. The present study showed a significant increase in creatinine and urea levels in rats receiving lead acetate compared to negative controls. It may be because of degeneration of kidney tissues by necrosis. EA exhibited significant improvement in this aspect. It indicates that EA may possess nephroprotective activity against lead acetate-induced nephrotoxicity. This might be because of its action on generated free radicals, thus maintaining membrane integrity of kidney and protecting kidney from cellular damage [22, 23].
The hyperuricemia induced by lead acetate is possibly due to over-production and reduced renal excretion of uric acid, and also elevation of endogenous ROS levels. Treatment with EA demonstrated inhibition of lead-induced nephrotoxicity, by restoration of serum uric acid [24].
In the lead acetate treated group, the increase in kidney relative weight reflected renal hypertrophy following previous studies. Another possible explanation for relative increase in kidney weight in the lead acetate treated group may be initial DNA replication and proximal tubular proliferation induced by lead acetate. Treatment of rats with EA possibly prevented these events from taking place. These observations suggest that EA may protect kidney tissue from lead acetate induced hypertrophy [24, 25].
Exposure to lead is responsible for stimulation of circulating monocytes and tissue macrophages, which lead to synthesis and release of a variety of pro-inflammatory cytokines such as TNF-α, IL-1b and IL-6. Lead induced toxicity is associated with infiltration of inflammatory cells in interstitial space, which stimulate inflammation-mediated apoptosis via the extrinsic pathway [3].
In our present study positive control group of animals exhibited a significant increase in expression NF-κB, TNF-α, IL-6, IL-1β, COX-2 and iNOS activities.
Activation of JNK-MAPK pathway is responsible for ROS-mediated inflammation in the lead intoxicated rat. Generation of ROS act as a signalling molecule to activate NF-kB, which translocate to the nucleus to regulate the gene encoding inflammation by the release of mediators such as TNF-α, COX-2, and iNOS [26].
It was also observed that EA treatment with both high and low doses causes a significant reduction in NF-κB, IL-6, IL-1β, COX-2, TNF-α, and iNOS activities in kidney tissues this observation is in agreement with results reported in literature. The exact mechanism by which EA causes lower expression level of IL-1β and TNF-α has not been elucidated and needs further investigation. However it has been reported that EA suppress synthesis of IL-1β and TNF-α by inhibition of NF-kB pathway. Furthermore, it has been observed that pomegranate extract containing EA regulates transcriptional activation of many inflammatory biomarkers by activating mitogen-activated protein kinases and NK-kB. EA also downregulates inflammatory genes of molecules like IL-1β, MCP1, iNOS, TNF-α, COX-2, PGE2, and matrix metalloproteinases (MMPs). All these observations suggest that EA ameliorates lead-induced pathogenesis in renal cells by inhibition of pro-inflammatory cytokines [27].
Autophagy is a process responsible for degradation of lysosomes by cytosolic components of cytoplasmic organelles. Cellular stress causing events like nutrient deprivation, hypoxia, ROS, protein aggregates, DNA damage, intracellular pathogens, or damaged organelles may stimulate this pathway. Microtubule-associated protein light chains 3 (LC3) which is a mammalian autophagy protein acts as a marker of autophagosomes. Among the four LC3 isoforms, LC3B is the most widely used marker. In this current study, lead exposure was responsible for a significant increase in expression level of LC3B in renal tissues when compared to control group. EA treatment caused reduction in LC3B level and demonstrated an anti-autophagic effect [28].
The results obtained from histopathological study supported other findings where only lead acetate treated group was responsible for structural changes in renal tissues such as necrosis of tubules, separation of tubules, chronic inflammatory cell infiltration and areas of haemorrhage compared to negative control group. In EA50 treated animals, there was a mild to moderate congestion with no occurrence of oedema, haemorrhage, tubular necrosis or inflammation. In EA25, sparsely inflammatory cells along with mild to moderate tubular damage were observed.
The results of this study indicates lead acetate induced nephrotoxicity might be due to oxidative damage. EA administration along with lead acetate reduced effect of lead acetate induced nephrotoxicity, probably due to the defence mechanism against ROS.
5. Conclusion
From the current study, it may be concluded that EA may confer significant protection against lead-induced nephrotoxicity through their antioxidant mechanisms from oxygen-derived free radicals. This study will be potentially beneficial for segments of population who are chronically exposed to high-level lead pollution. Presence of EA in diet or its consumption as a supplement may keep kidney of affected people healthy and safe. Future studies need to be performed to establish the hypothesis with clinical studies.
Declarations
Author contribution statement
A. Bhattacharjee; A. Ray: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
V. H KulkarniI: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
M. Chakraborty; P. V Habbu: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Ritz E., Mann J., Stoeppler M. Lead and the kidney. Adv. Nephrol. Necker. Hosp. 1988;17:241–274. [PubMed] [Google Scholar]
- 2.Goyer R.A. Mechanisms of lead and cadmium nephrotoxicity. Toxicol. Lett. 1989 Mar;46(1-3):153–162. doi: 10.1016/0378-4274(89)90124-0. [DOI] [PubMed] [Google Scholar]
- 3.Metryka E., Chibowska K., Gutowska I., Falkowska A., Kupnicka P., Barczak K., Chlubek D., Baranowska-Bosiacka I. Lead (Pb) exposure enhances expression of factors associated with inflammation. Int. J. Mol. Sci. 2018 Jun;19(6):1813. doi: 10.3390/ijms19061813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H., Li D., Hu Z., Zhao S., Zheng Z., Li Wei. Protective effects of green tea polyphenol against renal injury through ROS-mediated JNK-MAPK pathway in lead exposed rats. Mol. Cell. 2016 Jun 30;39(6):508–513. doi: 10.14348/molcells.2016.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perron N.R., Brumaghim J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009;53(2):75–100. doi: 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- 6.Mezynska M., Brzoska M.M. Review of polyphenol-rich products as potential protective and therapeutic factors against cadmium hepatotoxicity. J. Appl. Toxicol. 2019;39:117–145. doi: 10.1002/jat.3709. [DOI] [PubMed] [Google Scholar]
- 7.Copello G.J., Pesenti M.P., Raineri M., Mebert A.M., Piehl L.L., de Celis E.R., Diaz L.E. Polyphenol-SiO2 hybrid biosorbent for heavy metal removal. Yerba mate waste (Ilex paraguariensis) as polyphenol source: kinetics and isotherm studies. Colloids Surf. B Biointerfaces. 2013;102:218–226. doi: 10.1016/j.colsurfb.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Lu A.Z., Hong W.Y., Mu T.X., Na W., Xin L.J. Study on effects of apple polyphenols on lead discharging in mice. Food Sci. (N. Y.) 2007;28(8):468–470. [Google Scholar]
- 9.Soh P.N., Witkowski B., Olagnier D. In vitro and in vivo properties of ellagic acid in malaria treatment. Antimicrob. Agents Chemother. 2009 Mar;53(3):1100–1106. doi: 10.1128/AAC.01175-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.https://pubchem.ncbi.nlm.nih.gov/compound/ellagic_acid#section=Top
- 11.Bae J.Y., Choi J.S., Kang S.W., Lee Y.J., Park J., Kang Y.H. Dietary compound ellagic acid alleviates skin wrinkle and inflammation induced by UV-B irradiation. Exp. Dermatol. 2010 Aug;19:e182–e190. doi: 10.1111/j.1600-0625.2009.01044.x. [DOI] [PubMed] [Google Scholar]
- 12.Singh K., Khanna A.K., Chander R. Hepatoprotective activity of ellagic acid against carbon tetrachloride induced hepatotoxicity in rats. Indian J. Exp. Biol. 1999 Oct;37(10):1025–1026. [PubMed] [Google Scholar]
- 13.Sarkaki A., Farbood Y., Dolatshahi M. Neuroprotective effects of ellagic acid in a rat model of Parkinson's disease. Acta Med. Iran. 2016 Aug;54(8):494–502. [PubMed] [Google Scholar]
- 14.Farbood Y., Sarkaki A., Dolatshahi M. Ellagic acid protects the brain against 6-hydroxydopamine induced neuroinflammation in a rat model of Parkinson’s disease. BCN. 2015 Apr;6(2):83–89. [PMC free article] [PubMed] [Google Scholar]
- 15.Chao C.Y., Mong M.C., Chan K.C., Yin M.C. Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol. Nutr. Food Res. 2010 Mar;54(3):388–395. doi: 10.1002/mnfr.200900087. [DOI] [PubMed] [Google Scholar]
- 16.Ambrose S.S., Solairaj P., Subramoniam A. Effectiveness of ellagic acid on isoniazid-rifampicin induced liver damage in rats. JPP. 2013 Jan-Mar;4(1):60–62. doi: 10.4103/0976-500X.107685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghoniem M.H., El-Sharkawy N.I., Hussein M.M.A., Moustafa G.G. Efficacy of curcumin on lead induced- nephrotoxicity in female albino rats. J. Am. Sci. 2012;8(6):502–510. [Google Scholar]
- 18.Chakraborty M., Kamath J.V., Bhattacharjee A. Pharmacodynamic interaction of green tea extract with hydrochlorothiazide against cyclophosphamide-induced myocardial damage. Toxicol. Int. 2014 May;21(2):196–202. doi: 10.4103/0971-6580.139810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sudjarwo S.A., Eraiko K., Wardani Sudjarwo G.W., Koerniasari Protective effects of piperine on lead acetate induced-nephrotoxicity in rats. Iran. J. Basic Med. Sci. 2017;20:1227–1231. doi: 10.22038/IJBMS.2017.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Somade O.T., Ajayi B.O., Safiriyu O.A., Oyabunmi O.S., Akamo A.J. Renal and testicular up-regulation of pro-inflammatory chemokines (RANTES and CCL2) and cytokines (TNF-α, IL-1β, IL-6) following acute edible camphor administration is through activation of NF-kB in rats. Toxicol Rep. 2019;1(6):759–767. doi: 10.1016/j.toxrep.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kandemir F.M., Kucukler S., Eldutar E., Caglayan C., Gülçin İ. Chrysin protects rat kidney from paracetamol-induced oxidative stress, inflammation, apoptosis, and autophagy: a multi-biomarker approach. Sci. Pharm. 2017 Mar;85(1):4. doi: 10.3390/scipharm85010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sudjarwo S.A., Koerniasari Protective effects of ethanol extract of mangosteen (Garcinia mangostana L) pericarp against lead acetate-induced nephrotoxicity in mice. GJP. 2015;9(4):385–391. [Google Scholar]
- 23.Laamech J., El-Hilaly J., Fetoui H., Chtourou Y., Tahraoui A., Lyoussi B. Nephroprotective effects of berberis vulgaris L. Total extract on lead acetate-induced toxicity in mice. Indian J. Pharmaceut. Sci. 2016;2016:326–333. [Google Scholar]
- 24.Amjad Z., Iqbal M.Z., Shoro A.A. Lead-induced reduction in body and kidney weight of wistar albino rats ameliorated by ginkgo biloba extract (EGb 761) Biochem. Physiol. 2013;2:113. [Google Scholar]
- 25.Dkhil M.A., Al-Khalifa M.S., Al-Quraishy S., Zrieq R., Moneim A.E.A. Indigofera oblongifolia mitigates lead-acetateinduced kidney damage and apoptosis in a rat model. Drug Des. Dev. Ther. 2016;10:1847–1856. doi: 10.2147/DDDT.S105511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu C.M., Sun Y.Z., Sun J.M., Ma J.Q., Cheng C. Protective role of quercetin against lead-induced inflammatory response in rat kidney through the ROS-mediated MAPKs and NF-κB pathway. Biochim. Biophys. Acta Gen. Subj. 2012 Oct 1;1820(10):1693–1703. doi: 10.1016/j.bbagen.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Rana M.N., Tangpong J., Rahman M.A. Xanthones protects lead-induced chronic kidney disease (CKD) via activating Nrf-2 and modulating NF-kB, MAPK pathway. Biochem. Biophys. Rep. 2020 Mar 1;21:100718. doi: 10.1016/j.bbrep.2019.100718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sui L., Zhang R.H., Zhang P., Yun K.L., Zhang H.C., Liu L., Hu M.X. Lead toxicity induces autophagy to protect against cell death through mTORC1 pathway in cardiofibroblasts. Biosci. Rep. 2015 Apr 1;35(2) doi: 10.1042/BSR20140164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.