Abstract
On 2 June 2020, a marketing authorisation valid through the European Union (EU) was issued for encorafenib in combination with cetuximab in adult patients with metastatic colorectal carcinoma (mCRC) with the BRAFV600E mutation who had received prior systemic therapy. Encorafenib plus cetuximab was evaluated in a randomised phase III trial of encorafenib plus binimetinib plus cetuximab versus encorafenib plus cetuximab versus cetuximab plus irinotecan or FOLFIRI (control arm) to adult patients with BRAFV600E mCRC who had received prior therapy for metastatic disease. The median overall survival was 9.3 months [95% confidence interval (CI): 8.05-11.30] versus 5.88 months (95% CI: 5.09-7.10) for encorafenib plus cetuximab (doublet) versus the control arm, respectively [hazard ratio (HR) 0.61, 95% CI: 0.48-0.77]. Progression-free survival (PFS) was 4.27 months (95% CI: 4.07-5.45) versus 1.54 months (95% CI: 1.48-1.91) (HR 0.44; 95% CI: 0.35-0.55). The most frequent adverse events in patients receiving encorafenib plus cetuximab were fatigue, nausea, diarrhoea, acneiform dermatitis, abdominal pain, arthralgia, decreased appetite, vomiting and rash. The aim of this manuscript is to summarise the scientific review of the application leading to regulatory approval in the EU.
Key words: EMA, BRAF, V600E, encorafenib, colorectal
Highlights
-
•
Encorafenib was approved in combination with cetuximab for patients with previously treated BRAF plus colorectal carcinoma.
-
•
The original submission also included binimetinib, which was withdrawn during the procedure.
-
•
The benefit–risk balance was considered positive due to a large benefit on PFS and strong biologic rationale.
Introduction
Colorectal cancer (CRC) is the fourth most common cancer worldwide, with about 1.3 million new cases and over 550 000 deaths in 2018 according to the GLOBOCAN database.1 In Europe, CRC represents the second cancer in terms of prevalence and mortality, with 500 000 estimated new cases and 242 000 estimated deaths in 2018.2 Approximately 25% of newly diagnosed patients present with metastases and 50% of patients eventually develop metastatic disease.3
The median survival for patients who are originally diagnosed with metastatic CRC (mCRC) is about 2 years.4,5 The standard first-line therapy for patients with mCRC consists of a combination of chemotherapy (fluoropyrimidines/leucovorin with or without oxaliplatin or irinotecan) and monoclonal antibodies (mAbs) targeting the vascular endothelial growth factor (VEGF) or the epidermal growth factor receptor (EGFR).6 Recommended second-line options depend on initial therapy, but generally include fluoropyrimidine/leucovorin plus irinotecan (FOLFIRI) or irinotecan with or without the anti-EGFR mAbs.7, 8, 9, 10 Current European Society for Medical Oncology (ESMO) and National Comprehensive Cancer Network (NCCN) guidelines also recommend regorafenib and trifluridine plus tipiracil as additional options for patients with mCRC with progressive disease after standard therapy.7,8
Since 2008, there has been extensive research into biomarkers to guide treatment, namely RAS (KRAS and NRAS) mutations, BRAF mutations, microsatellite instability (MSI), and human EGFR2 (HER2) amplification. At diagnosis, 5%-21% of patients with mCRC harbour BRAF mutations,11 which are usually (>95%) located at the V600 codon and mutually exclusive with RAS mutations.12, 13, 14, 15 BRAF mutations lead to constitutive activation of BRAF kinase and sustained RAS/RAF/MEK/ERK pathway signalling, resulting in increased cell proliferation and survival.16 BRAF-mutated (BRAFmut) CRC is associated with unique clinical characteristics and reduced survival.17, 18, 19, 20 Standard therapy produces substantially poorer outcomes in patients with BRAFmut mCRC than in those with BRAF wild type (BRAFwt) disease both in the first-line17, 18, 19,21 and the relapse settings.14,22, 23, 24, 25 BRAF inhibitors in monotherapy or in combination with MEK inhibitors have shown minimal clinical activity in BRAFmut mCRC in the absence of EGFR inhibitors,26,27 potentially due to feedback reactivation of EGFR. This was the rationale behind the combination of MEK/BRAF inhibitors with an EGFR inhibitor.16,28
At the time of the marketing authorisation for encorafenib, there were no drugs specifically approved for BRAFmut mCRC. On 20 September 2018, encorafenib was approved, in combination with the MEK inhibitor binimetinib, for the treatment of unresectable or metastatic BRAFmut melanoma. On 14 October 2019, Pierre Fabre Medicament (marketing authorisation holder [MAH]) applied for an extension of indication for encorafenib to the European Medicines Agency (EMA). The review was conducted by the Committee for Medicinal Products for Human Use (CHMP) and a positive opinion was issued on 30 April 2020.
Nonclinical aspects and clinical pharmacology
Encorafenib is a selective ATP-competitive RAF kinase inhibitor that suppresses the RAF/MEK/ERK pathway in tumour cells expressing several mutated forms of BRAF kinase (V600E, D and K). Specifically, encorafenib inhibited in vitro and in vivo BRAFV600E, D and K mutant melanoma cell growth, but did not inhibit RAF/MEK/ERK signalling in cells expressing wild-type BRAF.29,30
Cetuximab is a chimeric immunoglobulin G1 mAb directed against EGFR. EGFR signalling pathways are involved in the control of cell survival, cell cycle progression, angiogenesis, cell migration and cellular invasion/metastasis.31 Cetuximab blocks binding of endogenous EGFR ligands resulting in functional inhibition of the receptor and its internalisation. Cetuximab also targets cytotoxic immune effector cells towards EGFR-expressing tumour cells.32
In order to support the indication, previous data from in vitro and in vivo studies with encorafenib in combination with cetuximab and encorafenib as single agent have been reanalysed with the focus of the doublet combination. These studies were carried out using a panel of BRAF-mutated cell lines and tumour xenografts considered representative of the main gene mutations and expression profiles reported for this patient population.12 Specifically, in the HT-29 CRC xenograft model the triple combination of encorafenib plus binimetinib plus cetuximab led to tumour growth inhibition, which was not statistically superior to the combinations encorafenib plus cetuximab or binimetinib plus cetuximab, suggesting little added value of the triplet versus doublet combination. Moreover, the tumour growth inhibition of the triple combination was significantly stronger when compared with encorafenib or binimetinib monotherapy, all in support of an additive/synergistic effect of cetuximab to BRAF/MEK inhibition that had been previously hypothesised.28
Trial design
The marketing authorisation application for the extension of indication was based on the ARRAY-818-302 (BEACON CRC) study: a multicentre, randomised, open-label, phase III clinical trial.33 Patients with BRAFmut mCRC who had disease progression after one or two previous treatment regimens were randomly assigned in a 1 : 1 : 1 ratio to 28-day courses of (i) ‘triplet’ arm: encorafenib (300 mg daily), binimetinib (45 mg twice daily) and cetuximab (400 mg/m2 and then 250 mg/m2 weekly); (ii) ‘doublet’ arm: encorafenib plus cetuximab (same doses as before); or (iii) control arm. Patients assigned to the control arm received cetuximab (same dose as before) plus irinotecan (180 mg/m2 on days 1 and 15) or cetuximab plus FOLFIRI. Doses for FOLFIRI were 5-FU: 400 mg/m2 followed by 1200 mg/m2/day for 2 days on days 1 and 15; leucovorin: 180 mg/m2 on days 1 and 15; and irinotecan (180 mg/m2 on days 1 and 15). This randomised trial was preceded by a phase Ib trial that determined the maximum tolerated dose of the encorafenib plus cetuximab doublet combination34 and a combined safety lead-in (CSLI) study evaluating the safety and tolerability of the triplet combination.35
The primary endpoints of the BEACON trial were overall survival (OS) and objective response rate (ORR) in the triplet arm as compared with the control arm (with a one-sided alpha of 0.025 and 0.005, respectively, using a ‘fallback procedure’36). The ORR and progression-free survival (PFS) were determined by a blinded independent review committee (BIRC) as per RECIST, version 1.1. A key secondary endpoint was OS in the doublet arm as compared with the control arm. Other secondary endpoints were PFS, duration of response and safety in all groups.
Clinical efficacy
A total of 1677 patients were screened for eligibility. From May 2017 through January 2019, a total of 665 patients underwent randomisation: 224 were assigned to the triplet arm, 220 to the doublet arm, and 221 patients to the control arm. Patients' baseline characteristics were comparable across the three groups, including age, Eastern Cooperative Oncology Group (ECOG) performance status (PS), proportion of patients with a completely resected primary tumour, primary tumour location and number and location of metastases.
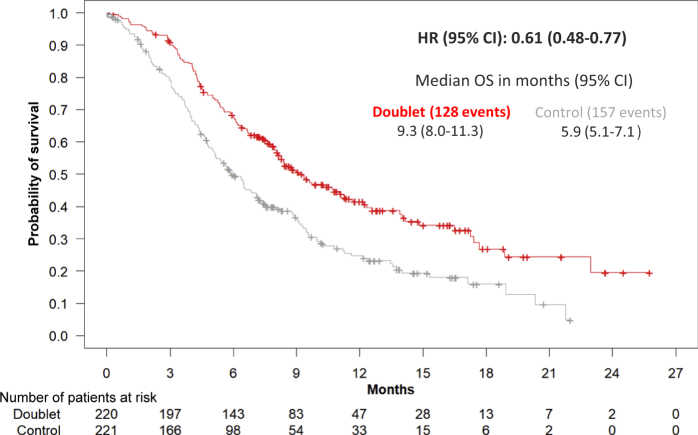
This submission was initially intended to support a new indication for encorafenib and binimetinib in combination with cetuximab but, during the procedure, the binimetinib application was withdrawn by the MAH and the assessment focused on the doublet versus control arm comparisons (secondary endpoints). At the cut-off date (COD) of August 2019, the median OS was 9.3 versus 5.88 months for the doublet versus the control group, respectively [hazard ration (HR) 0.61, 95% confidence interval (CI): 0.48-0.77; P < 0.0001]; median PFS was 4.27 versus 1.54 months, respectively (HR 0.44; 95% CI: 0.35-0.55); and ORR was 19.5% versus 1.8%, respectively (Table 1 and Figure 1).
Table 1.
Favourable and unfavourable effects for cetuximab plus encorafenib versus cetuximab plus irinotecan or FOLFIRI (BEACON trial; cut-off date August 2019)
| Effect | Unit | Cetuximab + encorafenib | Cetuximab + irinotecan or FOLFIRI | Uncertainties/strength of evidence |
|---|---|---|---|---|
| Favourable effects (updated results, August 2019) | ||||
| OS | Months (95% CI) | 9.3 (8.0-11.3) | 5.9 (5.1-7.1) | Secondary endpoint |
| HR (95% CI) | 0.61 (0.48-0.77) | |||
| PFS | Months (95% CI) | 4.3 (4.1-5.5) | 1.5 (1.5-1.9) | Secondary endpoint |
| HR (95% CI) | 0.44 (0.35-0.55) | |||
| Unfavourable effects | ||||
| Grade 3-4 AEs (related) | % | 21.3 | 42.5 | |
| Fatigue | % (grade 3-4) | 33.3 (4.2) | 28.0 (4.7) | |
| Diarrhoea | % (grade 3-4) | 38.4 (2.8) | 48.7 (10.4) | |
| Nausea | % (grade 3-4) | 38.0 (0.5) | 43.5 (1.6) | |
| Vomiting | % (grade 3-4) | 27.3- (1.4) | 31.6 (3.1) | |
| Dermatitis acneiform | % (grade 3-4) | 29.2 (0.5) | 39.4 (2.6) | |
| Headache | % (grade 3-4) | 19.9 (0.0) | 2.6 (0.0) | |
| Abdominal pain | % (grade 3-4) | 27.8 (3.2) | 28.0 (5.2) | |
| Decreased appetite | % (grade 3-4) | 31.0 (1.4) | 29.0 (3.2) | |
| Arthralgia | % (grade 3-4) | 22.7 (1.4) | 1.6 (0.0) | |
| Anaemia | % (grade 3-4) | 16.2 (5.6) | 19.2 (6.7) | |
| Haemorrhage | % (grade 3-4) | 19.0 (1.9) | 8.8 (0.0) | |
AEs, adverse events; CI, confidence interval; HR, hazard ration; OS, overall survival; PFS, progression-free survival.
Figure 1.
Updated overall survival analysis of the doublet (cetuximab plus encorafenib) versus control arm (cetuximab plus irinotecan or FOLFIRI) (BEACON trial, cut-off date August 2019).
CI, confidence interval; HR, hazard ratio; OS, overall survival.
An exploratory multivariate Cox regression model, stratified by ECOG PS, prior irinotecan use and cetuximab source revealed that the difference in OS for the doublet versus the control arm was consistent with the primary analysis (HR 0.49; 95% CI: 0.36-0.67).
Clinical safety
At the August 2019 COD, the median duration of exposure was 19.3 weeks in the doublet arm compared to 7.0 in the control arm. Over 40% of patients in the doublet arm received a minimum of 24 weeks of therapy, compared to only 12.4% of patients in the control arm. The median relative dose intensities were 98% (encorafenib) and 93.5% (cetuximab) in the doublet arm and 85.4% (cetuximab) and 75.7% (irinotecan) in the control arm.
Adverse events (AEs) occurred in 98.1% versus 97.4% of patients, and were grade (National Cancer Institute, Common Toxicity Criteria for Adverse Events, version 4.03)37 ≥3 in 57.4% versus 64.2%, respectively. The most frequent AEs were fatigue (33.3% versus 28.0%), nausea (38.0% versus 43.5%), diarrhoea (38.4% versus 48.7%), stomatitis (6.0% versus 23.3%), neutropenia (1.4% versus 18.7%), acneiform dermatitis (30.1% versus 39.9%), abdominal pain (27.8% versus 28.0%), arthralgia (22.7% versus 1.6%), decreased appetite (31.0% versus 29.0%), vomiting (27.3% versus 31.6%), myalgia (15.3% versus 2.1%), musculoskeletal pain (13.4% versus 2.6%), melanocytic nevus (15.7% versus none), headache (19.9% versus 2.6%) and pain in extremity (11.6% versus 1.0%) (Table 1). The doublet combination did not result in an increased frequency of secondary skin neoplasms. The percentage of patients who died on treatment was 17.6% in the doublet arm versus 15% in the control arm. The adjusted rate of on-treatment AEs resulting in death was 0.74 per 100 patient-months of exposure in the doublet arm and 2.06 in the control arm. No unexpected AEs of special interest were identified in the doublet combination.
Benefit–risk assessment
The pivotal trial supporting this application was a well-designed randomised controlled international clinical study. It had two primary endpoints (ORR and OS) of which OS was the most relevant from a regulatory point of view. Importantly, both primary endpoints referred to the comparison of triplet versus control arm, whereas the difference in OS of the doublet versus control arm was a secondary endpoint, without prespecified multiplicity adjustment for this comparison. This initial submission, incorporating data from the February 2019 COD, revealed a trend towards a longer OS for patients receiving the triplet versus doublet combination (HR 0.79; 95% CI: 0.59-1.06; P = 0.0582) but with a virtually identical PFS. This surprising result (different OS with a similar PFS) was raised as a major objection by the CHMP. Updated results from the August 2019 COD revealed an even smaller difference in OS between the triplet and doublet combination (HR 0.95; 95% CI: 0.74-1.21) which, coupled with a more favourable toxicity profile for the doublet combination, led to the withdrawal of binimetinib from this marketing authorisation.
When focusing on the doublet versus control arm comparison, the survival gain observed in the doublet arm was considered robust and clinically relevant. At the August COD, the median OS for the doublet arm was 3.42 months longer than for the control arm (P < 0.0001, stratified log-rank test), which was consistent with other efficacy endpoints such as PFS and ORR. This large and consistent effect, coupled with a strong biologic rationale (the combination of BRAF plus EGFR inhibitors may optimise MAPK suppression leading to improved efficacy in BRAF-mutated mCRC compared with BRAF inhibitor monotherapy), was deemed sufficient for approval despite being a secondary endpoint of the pivotal trial. The tolerability and safety profile of the doublet combination was acceptable and favourable compared with either the control arm (Table 1) or the triplet combination (data not shown).
In conclusion, the benefit–risk balance was considered positive. The CHMP recommended an approval for encorafenib in combination with cetuximab, for the treatment of adult patients with mCRC with a BRAFV600E mutation, who have received prior systemic therapy. The recommended dose of encorafenib is 300 mg (four 75-mg capsules) once daily, when used in combination with cetuximab. This was added to the prior indication in combination with binimetinib for the treatment of adult patients with unresectable or metastatic melanoma with a BRAFV600 mutation.
Acknowledgements
The scientific assessment as summarised in this report is based on the marketing authorisation application submitted by the applicant company and on important contributions from, among others, the rapporteur assessment team, CHMP members and additional experts.
Funding
None declared.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J., Colombet M., Soerjomataram I. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E., Cervantes A., Nordlinger B., Arnold D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii1–iii9. doi: 10.1093/annonc/mdu260. [DOI] [PubMed] [Google Scholar]
- 4.Bradley C.J., Lansdorp-Vogelaar I., Yabroff K.R. Productivity savings from colorectal cancer prevention and control strategies. Am J Prev Med. 2011;41:e5–e14. doi: 10.1016/j.amepre.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franchi M., Barni S., Tagliabue G. Effectiveness of first-line bevacizumab in metastatic colorectal cancer: the observational cohort study GRETA. Oncologist. 2019;24:358–365. doi: 10.1634/theoncologist.2017-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogel A., Kirstein M.M. First-line molecular therapies in the treatment of metastatic colorectal cancer – a literature-based review of phases II and III trials. Innov Surg Sci. 2018;3:85–86. doi: 10.1515/iss-2018-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Cutsem E., Cervantes A., Adam R. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 8.Messersmith W.A. NCCN guidelines updates: management of metastatic colorectal cancer. J Natl Compr Canc Netw. 2019;17:599–601. doi: 10.6004/jnccn.2019.5014. [DOI] [PubMed] [Google Scholar]
- 9.Peeters M., Price T.J., Cervantes A. Final results from a randomized phase 3 study of FOLFIRI ± panitumumab for second-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:107–116. doi: 10.1093/annonc/mdt523. [DOI] [PubMed] [Google Scholar]
- 10.Tabernero J., Yoshino T., Cohn A.L. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16:499–508. doi: 10.1016/S1470-2045(15)70127-0. [DOI] [PubMed] [Google Scholar]
- 11.Sforza V., Martinelli E., Ciardiello F. Mechanisms of resistance to anti-epidermal growth factor receptor inhibitors in metastatic colorectal cancer. World J Gastroenterol. 2016;22:6345–6361. doi: 10.3748/wjg.v22.i28.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barras D., Missiaglia E., Wirapati P. BRAF V600E mutant colorectal cancer subtypes based on gene expression. Clin Cancer Res. 2017;23:104–115. doi: 10.1158/1078-0432.CCR-16-0140. [DOI] [PubMed] [Google Scholar]
- 13.Clarke C.N., Kopetz E.S. BRAF mutant colorectal cancer as a distinct subset of colorectal cancer: clinical characteristics, clinical behavior, and response to targeted therapies. J Gastrointest Oncol. 2015;6:660–667. doi: 10.3978/j.issn.2078-6891.2015.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Roock W., Claes B., Bernasconi D. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 15.Sorbye H., Dragomir A., Sundstrom M. High BRAF mutation frequency and marked survival differences in subgroups according to KRAS/BRAF mutation status and tumor tissue availability in a prospective population-based metastatic colorectal cancer cohort. PLoS One. 2015;10:e0131046. doi: 10.1371/journal.pone.0131046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corcoran R.B., Ebi H., Turke A.B. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2:227–235. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cremolini C., Di Maio M., Petrelli F., Berenato R., Loupakis F., Pietrantonio F. BRAF-mutated metastatic colorectal cancer between past and future. Br J Cancer. 2015;113:1634–1635. doi: 10.1038/bjc.2015.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loupakis F., Cremolini C., Fioravanti A. EGFR ligands as pharmacodynamic biomarkers in metastatic colorectal cancer patients treated with cetuximab and irinotecan. Target Oncol. 2014;9:205–214. doi: 10.1007/s11523-013-0284-7. [DOI] [PubMed] [Google Scholar]
- 19.Ursem C., Atreya C.E., Van Loon K. Emerging treatment options for BRAF-mutant colorectal cancer. Gastrointest Cancer. 2018;8:13–23. doi: 10.2147/GICTT.S125940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran B., Kopetz S., Tie J. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623–4632. doi: 10.1002/cncr.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venderbosch S., Nagtegaal I.D., Maughan T.S. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res. 2014;20:5322–5330. doi: 10.1158/1078-0432.CCR-14-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundar R., Hong D.S., Kopetz S., Yap T.A. Targeting BRAF-mutant colorectal cancer: progress in combination strategies. Cancer Discov. 2017;7:558–560. doi: 10.1158/2159-8290.CD-17-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loupakis F., Di Maio M., Falcone A. Chemotherapy and immunotherapy in metastatic colorectal cancer. N Engl J Med. 2009;360:2135. author reply 2135-2136. [PubMed] [Google Scholar]
- 24.Morris V., Overman M.J., Jiang Z.Q. Progression-free survival remains poor over sequential lines of systemic therapy in patients with BRAF-mutated colorectal cancer. Clin Colorectal Cancer. 2014;13:164–171. doi: 10.1016/j.clcc.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seymour M.T., Brown S.R., Middleton G. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. Lancet Oncol. 2013;14:749–759. doi: 10.1016/S1470-2045(13)70163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyman D.M., Puzanov I., Subbiah V. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373:726–736. doi: 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopetz S., Tabernero J., Rosenberg R. Genomic classifier ColoPrint predicts recurrence in stage II colorectal cancer patients more accurately than clinical factors. Oncologist. 2015;20:127–133. doi: 10.1634/theoncologist.2014-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prahallad A., Sun C., Huang S. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–103. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 29.Adelmann C.H., Ching G., Du L. Comparative profiles of BRAF inhibitors: the paradox index as a predictor of clinical toxicity. Oncotarget. 2016;7:30453–30460. doi: 10.18632/oncotarget.8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delord J.P., Robert C., Nyakas M. Phase I dose-escalation and -expansion study of the BRAF inhibitor encorafenib (LGX818) in metastatic BRAF-mutant melanoma. Clin Cancer Res. 2017;23:5339–5348. doi: 10.1158/1078-0432.CCR-16-2923. [DOI] [PubMed] [Google Scholar]
- 31.Galizia G., Lieto E., De Vita F. Cetuximab, a chimeric human mouse anti-epidermal growth factor receptor monoclonal antibody, in the treatment of human colorectal cancer. Oncogene. 2007;26:3654–3660. doi: 10.1038/sj.onc.1210381. [DOI] [PubMed] [Google Scholar]
- 32.Roda J.M., Joshi T., Butchar J.P. The activation of natural killer cell effector functions by cetuximab-coated, epidermal growth factor receptor positive tumor cells is enhanced by cytokines. Clin Cancer Res. 2007;13:6419–6428. doi: 10.1158/1078-0432.CCR-07-0865. [DOI] [PubMed] [Google Scholar]
- 33.Kopetz S., Grothey A., Yaeger R. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med. 2019;381:1632–1643. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 34.van Geel R., Tabernero J., Elez E. A Phase Ib dose-escalation study of encorafenib and cetuximab with or without alpelisib in metastatic BRAF-mutant colorectal cancer. Cancer Discov. 2017;7:610–619. doi: 10.1158/2159-8290.CD-16-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Cutsem E., Huijberts S., Grothey A. Binimetinib, encorafenib, and cetuximab triplet therapy for patients with BRAF V600E-mutant metastatic colorectal cancer: safety lead-in results from the Phase III BEACON colorectal cancer study. J Clin Oncol. 2019;37:1460–1469. doi: 10.1200/JCO.18.02459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiens B.L., Dmitrienko A. The fallback procedure for evaluating a single family of hypotheses. J Biopharm Stat. 2005;15:929–942. doi: 10.1080/10543400500265660. [DOI] [PubMed] [Google Scholar]
- 37.National Cancer Institute. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events, version 4.03. Published on June 14, 2010. Available at: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.