Abstract
Here we report 3 cases of neuromyelitis optica spectrum disorder (NMOSD), who were all treated with eculizumab and could be observed with monitoring serum C3, C4 and 50% hemolytic complement (CH50) before and after the treatment. Serum C3 and C4 were not dramatically changed during the treatment, in contrast serum CH50 level of each patient had diminished and kept under the detection limit after the treatment without clinical worsening, even in the situation of extending dosing. Serum CH50 level is useful to monitor the drug efficacy during eculizumab treatment.
Keywords: Neuromyelitis Optica, Neuromyelitis optica spectrum disorder, Eculizumab, Biomarker, CH50, 50% hemolytic complement, C3, C4, C5, Astrocytopathy
Neuromyelitis Optica; Neuromyelitis optica spectrum disorder; Eculizumab; Biomarker; CH50; 50% hemolytic complement; C3; C4; C5; Astrocytopathy
1. Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is an autoimmune inflammatory disease that is characterized by recurrent optic neuritis and myelitis. The pathogenesis of NMOSD is mainly characterized by aquaporin-4 antibody(AQP4-Ab)-mediated and complement-dependent cytotoxicity against astrocyte.
Eculizumab is a humanized monoclonal antibody against complement protein C5, which inhibits the formation of terminal complement activated membrane attacked complex C5b-9. So far, this compound was approved in paroxysmal nocturnal hemoglobinuria (PNH), atypical hemolytic uremic syndrome (aHUS), and myasthenia gravis (MG). After the double-blinded randomized placebo-controlled trial (RCT), eculizumab was recently approved by United states, Europe, and Japanese government for the prevention of relapse in NMOSD with AQP4-IgG (Pittock et al., 2019).
Serum 50% hemolytic complement (CH50) activity is a commonly used marker for the functional activity of classical pathway and is suggested to be useful for monitoring efficacy of eculizumab in PNH (Peffault de Latour et al., 2015), aHUS (Wehling et al., 2017), and myasthenia gravis (MG) (Yanagidaira et al., 2020), but there is no previous report in NMOSD patients using Eculizumab.
Here, we present three cases of NMOSD with AQP4-IgG, who were successfully treated with Eculizumab and monitored complement activity C3, C4, and CH50 before and after the treatment.
2. Materials and methods
2.1. Patients
3 NMOSD patients who presented to Tohoku University Hospital and participated in ECU-NMO-301 (PREVENT) study (Pittock et al., 2019) were included. Each patient received same eculizumab regimen as the trial; 900mg of eculizumab weekly for the first 4 doses, followed by 1200mg biweekly. During the trial, immunosuppressive therapies at baseline were basically continued according to the trial protocol. Clinical data was collected retrospectively from the medical records. This study was approved by ethical committee of Tohoku University Hospital and we got the informed consent of each patients.
2.2. Cell-based assay for AQP4 antibody
AQP4 antibody was detected by cell-based assay, described in previous report (Takahashi et al., 2007).
2.3. Complement assays
CH50 activity, C3 and C4 level of serum samples were measured using the following; liposome immunoassay kit (Complement activity-HA test Wako, Wako) for CH50 activity, C3 turbidimetric immunoassay (TIA) kit (N-Assay TIA C3-SH Nittobo, Nittobo Medical) for C3 level, C4 TIA kit (N-Assay TIA C4-SH Nittobo, Nittobo Medical) for C4 level. Each was measured using an automated analyzer (LABOSPECT 008, Hitachi).
3. Case presentation
3.1. Case 1
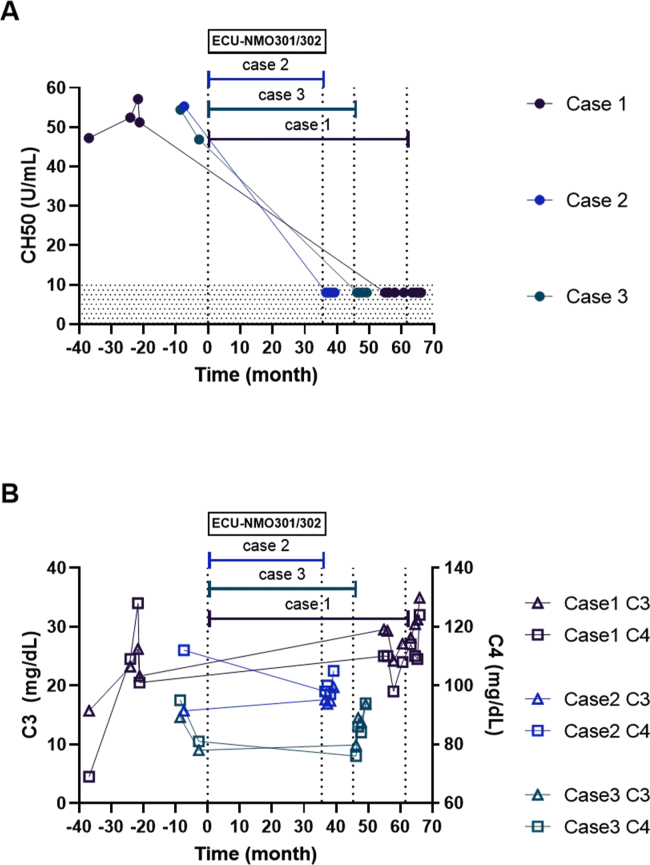
This female patient developed unilateral optic neuritis (ON) at the age of 53. Serum AQP4 antibody was positive (antibody titer was 1:524288), and she was diagnosed as NMOSD with AQP4 antibody. Oral prednisolone (PSL) and azathioprine (AZP) treatment were started, but she experienced 8 attacks of ON or myelitis during 4 years after onset. When she was 57-year-old she had entered to the RCT-based eculizumab trial. At the beginning of the trial, her EDSS score was 7.0. After the trial started, PSL and AZP were continued according to the trial protocol but AZP was tapered off because of recurrent hematuria. As a result, she never had relapse during the eculizumab treatment. 55 months later, she was admitted to our hospital because of bacterial pneumonia. Therefore, eculizumab was suspended once but was restarted 4 weeks after last administration. Serum CH50 level declined to the normal level below 10 U/mL through this trial even during the interruption of eculizumab (Figure 1A), though C3 and C4 level had no marked change (Figure 1B). EDSS score did not change during eculizumab treatment.
Figure 1.
A Time courses of serum CH50 level in each patient, showing complete decline under the detection limit after the treatment of eculizumab. B Serum C3 (trigone) and C4 (square) before and after the eculizumab administration in each patient.
3.2. Case 2
This female patient experienced pain in neck and arms at the age of 46. Brain MRI revealed brainstem lesion. 3 months later she experienced a relapse. Serum AQP4 antibody was revealed as positive (titer data was not available), and she was diagnosed as NMOSD with AQP4 antibody. Oral PSL treatment was started, but 5 months later she experienced another relapse. She entered to the RCT of eculizumab continuing PSL treatment. At this time her EDSS score was 2.0. She had no relapse throughout the trial. Serum CH50 level declined to the normal level below 10 U/mL (Figure 1A), though C3 and C4 level had little change (Figure 1B). EDSS score did not change during eculizumab treatment.
3.3. Case 3
This woman developed unilateral ON at the age of 28. AQP4-IgG was revealed as positive (titer data was not available) and she was diagnosed with NMOSD. Oral PSL treatment was started, but 7 months later she experienced relapse of thoracic myelitis. She entered to the RCT trial of eculizumab, continuing PSL treatment. At this time her EDSS score was 3.5. She had no relapse throughout the trial. Serum CH50 level decreased to the normal level below 10 U/mL after eculizumab started (Figure 1A), though C3 and C4 level had little change (Figure 1B). EDSS score did not change during eculizumab treatment.
4. Discussion
In all these 3 NMOSD patients, CH50 activity completely declined to the level under detection limit after starting eculizumab treatment. In contrast, the concentration of main complement C3 and C4 level did not change. C4 are mainly decreased with CH50 level in classical pathway, in contrast C3 are mainly consumed influencing on CH50 in alternative pathway. This indicates that eculizumab specifically inhibits complement C5, related to both pathway, and reduces CH50 level in NMOSD patients, same as in PNH, aHUS, and MG patients. We cannot show when CH50 levels reached to the lower detection limit in NMOSD patients, because our patients participated in the double-blind trial. But we consider that CH50 activity may decrease soon after starting eculizumab, as previous report showed in PNH patients CH50 activity significantly decreased 1 h after first eculizumab administration (Peffault de Latour et al., 2015). All our patients experienced no relapse during eculizumab treatment. In one patient (case 1), eculizumab administration was skipped once because of bacterial pneumonia, but her serum CH50 activity had kept under the detection limit and she had no relapse. These facts suggest that monitoring serum CH50 activity may be useful to assess the efficacy of eculizumab.
The relationship between CH50 level and NMOSD disease activity has not been established yet. A previous report showed the relatively elevated CH50 activity in remission phase compared to healthy control (Veszeli et al., 2014). In contrast, another report showed the lower CH50 activity in NMOSD patients, including acute stage diseases, compared to control (Chen et al., 2014). These phenomena may reflect the increase of complement consumption in acute phase of NMOSD, possibly relating to our Case 1 showing the low level of complement only in the initial acute stage but relatively high at the follow-up.
This report has some limitation. We could observe only a small number of NMOSD patients retrospectively. As our patients participated in the double-blind trial, we were not able to get some clinical data during the trial. In addition, our patients had no relapse thus we could not assess complement activity at relapsing phase under eculizumab treatment. Further research should be needed to know the significance of CH50 monitoring in disease activity of NMOSD. Furthermore, it is still unknown whether serum CH50, not C5 itself or its attacked complex C5b-9, is adequate to monitor the drug efficacy, but may be reasonable to avoid the possible masking of targeting C5 structure by eculizumab.
5. Conclusion
Serum CH50 activity measurement can be useful for assessing efficacy of eculizumab treatment in NMOSD patients. Further data collection is needed to check the reliability of CH50 as a drug monitoring marker.
Declarations
Author contribution statement
C. Namatame: Performed the experiments, Analyzed and interpreted the data, Wrote the paper.
T. Misu: Conceived and designed the experiments, Analyzed and interpreted the data, Wrote the paper.
S. Nishiyama and Y. Takai: Analyzed and interpreted the data, Contributed reagents, materials, analysis tools or data.
M. Aoki, K. Fujihara and I. Nakashima: Contributed reagents, materials, analysis tools or data.
Funding statement
This study was supported in part by JSPS KAKENHI Grant Number 19K07953.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
K. Fujihara serves on the advisory boards for Bayer Schering Pharma, Biogen Idec, Mitsubishi Tanabe Pharma Corporation, Novartis Pharma, Chugai Pharmaceutical, Ono Pharmaceutical, Nihon Pharmaceutical, Alexion Pharmaceuticals, and Medimmune; has received travel funding and speaker honoraria from Bayer Schering Pharma, Biogen Idec, Eisai Inc, Mitsubishi Tanabe Pharma Corporation, Novartis Pharma, Astellas Pharma Inc., Takeda Pharmaceutical Company Limited, Asahi Kasei Medical Co., Daiichi Sankyo, and Nihon Pharmaceutical; is on the editorial board for Clinical and Experimental Neuroimmunology; is an advisory board member for Sri Lanka Journal of Neurology; and received research support from Bayer Schering Pharma, Biogen Idec Japan, Asahi Kasei Medical, The Chemo-Sero-Therapeutic Research Institute, Teva Pharmaceutical, Mitsubishi Tanabe Pharma, Teijin Pharma, Chugai Pharmaceutical, Ono Pharmaceutical, Nihon Pharmaceutical, Genzyme Japan, Ministry of Education, Science and Technology of Japan, and Ministry of Health, Welfare and Labor of Japan.
M. Aoki received research support from Japanese Ministry of Health Labor and Welfare, Japanese Ministry of Education, Culture, Sports, Science and Technology.
Additional information
No additional information is available for this paper.
References
- Chen Y., Li R., Wu A.M. The complement and immunoglobulin levels in NMO patients. Neurol. Sci. 2014;35:215–220. doi: 10.1007/s10072-013-1481-y. [DOI] [PubMed] [Google Scholar]
- Peffault de Latour R., Fremeaux-Bacchi V., Porcher R. Assessing complement blockade in patients with paroxysmal nocturnal hemoglobinuria receiving eculizumab. Blood. 2015;125(5):775–783. doi: 10.1182/blood-2014-03-560540. [DOI] [PubMed] [Google Scholar]
- Pittock S.J., Berthele A., Fujihara K. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N. Engl. J. Med. 2019;381(7):614–625. doi: 10.1056/NEJMoa1900866. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Fujihara K., Nakashima I. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain. 2007;130(Pt 5):1235–1243. doi: 10.1093/brain/awm062. [DOI] [PubMed] [Google Scholar]
- Veszeli N., Fust G., Csuka D. A systematic analysis of the complement pathways in patients with neuromyelitis optica indicates alteration but no activation during remission. Mol. Immunol. 2014;57:200–209. doi: 10.1016/j.molimm.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Wehling C., Amon O., Bommer M. Monitoring of complement activation biomarkers and eculizumab in complement-mediated renal disorders. Clin. Exp. Immunol. 2017;187(2):304–315. doi: 10.1111/cei.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagidaira M., Nishida Y., Yokota T. Temporal correlation between serum CH50 level and symptom severity of myasthenia gravis during eculizumab therapy. Clin. Neurol. Neurosurg. 2020;189:105630. doi: 10.1016/j.clineuro.2019.105630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.