Abstract
Background
The effect of palliative chemotherapy for non-small cell lung cancer (NSCLC) is well established. Recently, immune checkpoint inhibitors have shown promising efficacy in NSCLC patients. However, little is known about the efficacy of cytotoxic chemotherapy in patients whose tumors are refractory to first-line chemotherapy. We investigated the outcome of all consecutive and unselected patients receiving palliative chemotherapy in a single institution to assess the efficacy of second-line chemotherapy in primary refractory NSCLC.
Patients and methods
Patients with metastatic NSCLC diagnosed between 1990 and 2016 were assessed. Outcome parameters were collected and patients were characterized as either having primary progressive disease or clinical benefit [CB; defined as complete/partial remission (CR, PR) or stable disease (SD)]. Probabilities of survival were calculated using the Kaplan–Meier estimator. The log-rank test was used for comparing groups. Cox models were used to explore the prognostic value of covariables.
Results
The analysis included 576 patients. Median overall survival (OS) was 9.5 months [95% confidence interval (CI) 8.47-10.47]; 62.7% of patients were treated with a platinum-based first-line therapy. Two hundred twenty-two patients (38.5%) were primary refractory to first-line therapy. Median OS was significantly shorter for those patients [7.4 versus 11.5 months, hazard ratio (HR) 1.61 (95% CI 1.34-1.93), P < 0.0001]. Poorer initial performance status was significantly associated with primary refractory disease (P = 0.015). Eighty-one (36.5%) primary refractory patients received a second-line therapy. Median OS was significantly longer for refractory patients receiving second-line therapy versus best supportive care [10.1 versus 5.0 months, HR 0.53 (95% CI 0.40-0.72), P < 0.0001].
Conclusions
Nearly 40% of patients are primary refractory to palliative first-line therapy and have a poor prognosis. Active second-line therapy can significantly improve the outcome. Therefore, patients with primary refractory NSCLC should be offered further active therapy. These real-life data for primary refractory patients form the basis for further research in sequencing of current palliative treatment options.
Key words: non-small cell lung cancer, second-line therapy, refractory patients, chemotherapy
Highlights
-
•
Unselected patients with metastatic NSCLC have a poor outcome despite recent therapeutic advances.
-
•
A substantial number of NSCLC patients in a real-world setting is refractory to primary treatment.
-
•
Primary refractory patients have a poor prognosis.
-
•
It is unclear whether patients with primary refractory NSCLC should receive further active systemic treatment.
-
•
Our work shows that further treatment of primary refractory patients can lead to an improvement of the prognosis.
Introduction
For many years the prognosis for patients with metastatic non-small cell lung cancer (NSCLC) has improved at a painfully slow but nevertheless meaningful rate, even in unselected patients in the average oncology clinic.1 In recent years progress has accelerated dramatically, in part through the discovery of oncogenic driver mutations, e.g. the epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK), which can be targeted by tyrosine kinase inhibitors (TKIs),2, 3, 4, 5, 6 and currently through the introduction of immune checkpoint inhibitors (ICIs), which are now considered the new standard in first-line treatment, either in combination with chemotherapy, or even as monotherapy for tumors with high PD-L1 expression.7, 8, 9, 10, 11, 12, 13, 14
Today most guidelines propose a first-line combination chemotherapy with a platinum compound and pemetrexed as well as an ICI, plus a subsequent maintenance therapy with pemetrexed and ICI for adenocarcinoma.15 All other NSCLC histologic subtypes can be treated with platinum-containing combination treatment with paclitaxel in combination with an ICI. Upon progression patients are usually offered a second-line chemotherapy treatment, in general with docetaxel.16
One controversial topic is whether patients whose tumor is refractory to palliative first-line chemotherapy benefit from receiving further cytotoxic treatment. This is a common situation, in the pre-ICI era 23%-26% of all patients had progressive disease (PD) as best response and 6%-8% even died during first-line chemotherapy.17, 18, 19 Primary refractory tumors can exhibit resistance to other drugs, in addition the performance status of the patients generally declines due to PD during first-line therapy. So far, only one randomized trial investigated treatment options in NSCLC patients with primary refractory disease. In the TITAN study, patients with disease progression during or immediately after first-line platinum doublet chemotherapy for NSCLC were randomized to receive either erlotinib or chemotherapy (docetaxel or pemetrexed).20 Of 2590 chemotherapy-naïve patients treated with first-line platinum doublet chemotherapy, 424 (16.4%) were evaluable for randomization. There was no difference between the two arms and the median survival was about 5 months. Treatment with anti-PD-(L)1 drugs currently represents the mainstay of NSCLC immunotherapy and can result in impressive response rates and durable disease remission, but only in a subset of patients. With the positive results from randomized studies with ICI versus chemotherapy in the second- and third-line setting, ICIs have become the new standard for patients after progression on first-line platinum doublet chemotherapy.21, 22, 23, 24, 25 ICIs also showed activity in chemotherapy refractory patients after several lines of therapy.26 Due to the positive results of the randomized first-line studies, ICIs are now an integral part of first-line therapy either in combination with platinum-based chemotherapy or as monotherapy for selected patients.7, 8, 9, 10, 11, 12, 13, 14 Therefore, the question of the most effective second-line therapy has again become more important. Despite substantial improvement in prognosis through the use of ICIs in first-line therapy, a substantial proportion of patients are still refractory to first-line therapy. In the randomized studies, this rate was 3%-13.3% under combined chemo-immunotherapy.8,10,12,13,27 To our knowledge no study has yet assessed whether patients with primary refractory NSCLC should be offered best supportive care (BSC) alone or further chemotherapy.
We undertook a systematic analysis of all consecutive patients with palliative NSCLC and primary refractory disease at our center between 1991 and 2016, examining the efficacy of second-line chemotherapy in this population.
Patients and methods
Patients
All consecutive patients with NSCLC treated at the University Hospital of Basel between 1990 and 2016 were included, provided they had histologically verified stage IV NSCLC, or any earlier stage and receiving palliative chemotherapy because they were not fit enough to undergo curative treatment. Patients receiving curative treatment were excluded, unless they relapsed within 6 months after adjuvant or neoadjuvant chemotherapy, or relapsed or progressed within 6 months of definitive chemo-radiotherapy. This study was carried out in accordance with the institutional review board and was approved by the local ethical committee (ethical committee of northwestern and central part of Switzerland, EKNZ).
Data acquisition
Patient- and tumor-specific data were obtained from the patients' medical records. The staging of all patients included CT scans of the chest and upper abdomen (including the liver and adrenal glands), and since 2000, PET-CT (unless an initial examination demonstrated evident metastatic disease). Thus there was a change in staging procedures during the time of this study.
MRI or CT scans of the brain and bone scans were carried out only if the patients were symptomatic. Monitoring in the course of the disease was done with CT scans.
Analysis and definitions
Patients were divided into two groups according to their response to first-line palliative chemotherapy: (i) chemotherapy-sensitive group: patients with complete response (CR), partial remission (PR), or at least stable disease (SD), versus (ii) chemotherapy refractory group: PD at the first evaluation or patients who had to stop chemotherapy due to toxicity or other reasons. For this analysis the following parameters were recorded: age at diagnosis, sex, Eastern Cooperative Oncology Group performance status (ECOG PS) at diagnosis, histology, stage according to TNM sixth edition, primary metastatic disease versus relapse (as explained above), smoking history (smoker, former smoker, never smoker), laboratory values [hemoglobin, white blood cell count (WBC), serum albumin, corrected serum calcium, lactate dehydrogenase (LDH)], duration and type of chemotherapy (started cycles were counted as full cycles), objective response rate (ORR) and overall survival (OS).
ORR was defined according to the Response Evaluation Criteria in Solid Tumors (RECIST criteria 1.0 and 1.1).28 OS was measured from the time of diagnosis (stage IIIB, IV or inoperable situation) to date of death or date of last patient contact if lost to follow-up.
At each visit, a medical history was taken and a physical examination was carried out. The Swiss Group for Clinical Cancer Research (SAKK) has a standardized assessment form. This form was completed at every visit documenting the following parameters: pain, fever, night sweats, bleeding, cough, shortness of breath, nausea, vomiting, neurologic symptoms, infections, digestion, urinary tract problems, appetite, sleep and all medication. In addition, a physical examination was carried out with the following minimal examinations: ECOG PS, weight, blood pressure, lung and heart auscultation, palpation of the abdomen and lymph nodes and short neurologic examination. All findings were graded according to the National Cancer Institute Common Toxicity scale29; PS was graded according to the criteria of ECOG. At the first visit, blood samples were obtained from all patients for hematology and chemistry analyses. Patients receiving chemotherapy were seen every 1-3 weeks, during follow-up, while receiving BSC patients were seen every 6-12 weeks.
Statistical analysis
Qualitative variables were summarized by count and percentage, and quantitative variables by mean, median and range. Probabilities of survival were calculated using the Kaplan–Meier estimator. The log-rank test was used for comparing groups. Multivariable Cox regression was used to investigate potential prognostic factors with respect to OS. A two-sided P < 0.05 was considered statistically significant.
Fisher's exact and chi-square tests were used to assess correlations of nominal covariate distributions and response groups. The t-test was applied to compare metric variables among different subgroups.
Results
Between 1990 and 2016 a total of 576 consecutive patients with NSCLC were treated at the University Hospital Basel, receiving either palliative chemotherapy or BSC and meeting the inclusion criteria.
Patients
There were 183 female patients (31.8%) and 393 male patients (68.2%). Mean age was 62.9 years (range, 30.9-88.7) and 467 patients (81.0%) had a history of smoking. At diagnosis, 412 patients (71.5%) had stage IV disease, 101 (17.5%) had stage III disease, 39 (6.8%) had stage II disease, and 22 (3.8%) had stage I disease. All patients with stage I or II disease were medically inoperable. There were 317 adenocarcinomas (55.0%), 130 squamous cell carcinomas (SCC) (22.6%) and 65 large cell carcinomas (11.3%). In 61 cases (10.6%), histology was NSCLC not otherwise specified (NSCLC NOS) and three patients (0.5%) had another histological subtype.
There were 86 patients (14.9%) with ECOG PS 0, 210 patients (36.5%) had PS 1, 54 patients (9.4%) had PS 2, 10 patients (1.7%) had PS 3 and for 216 patients (37.5%), the PS at diagnosis was unknown.
The patients' characteristics are shown in Table 1.
Table 1.
Patient characteristics
| Characteristic | Mean (range) or number of patients (%) |
|---|---|
| Age, years | 62.9 (27.4-88.7) |
| Sex | |
| Male | 393 (68.2%) |
| Female | 183 (31.8%) |
| Histological subtype | |
| Adenocarcinoma | 317 (55.0%) |
| Squamous cell carcinoma | 130 (22.6%) |
| Large cell carcinoma | 65 (11.3%) |
| NOS | 61 (10.6%) |
| Other | 3 (0.5%) |
| Smoking history | |
| Current/former | 467 (81.0%) |
| Never | 60 (10.4%) |
| Unknown | 49 (8.5%) |
| Initial stage | |
| I | 22 (3.8%) |
| II | 39 (6.8%) |
| IIIA | 15 (2.6%) |
| IIIB | 86 (14.9%) |
| IV | 412 (71.5%) |
| Unknown | 2 (0.4%) |
| ECOG performance status | |
| 0 | 86 (14.9%) |
| 1 | 210 (36.5%) |
| 2 | 54 (9.4%) |
| 3 | 10 (1.7%) |
| Unknown | 216 (7.3%) |
ECOG, Eastern Cooperative Oncology Group; NOS, not otherwise specified.
Palliative first-line therapy
In our cohort 533 patients (92.5%) received at least one dose of palliative systemic first-line therapy with a median number of four cycles (range, 1-21); 361 patients (62.7%) were treated with a platinum-based combination therapy, and 248 patients (43.1%) received cisplatin-based therapy.
Median progression-free survival (PFS) for first-line therapy was 3.2 months [95% confidence interval (CI) 2.90-3.50], median OS for the whole cohort was 9.5 months (95% CI 8.47-10.47).
Response to first-line therapy
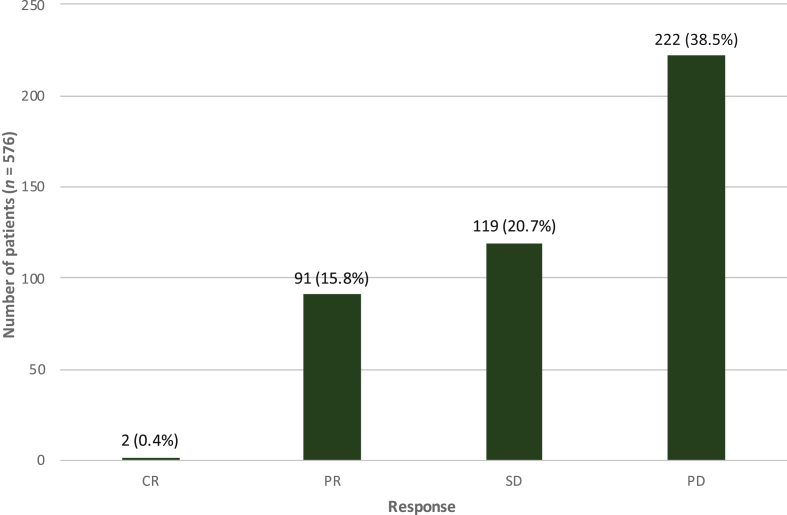
Four hundred thirty-five patients were accessible for response assessment. Of those, 222 patients (38.5%) had PD as best response, 119 patients (20.8%) had SD and 91 patients (10.9%) had PR. Only two patients (0.4%) had CR (Figure 1).
Figure 1.
Response to first-line therapy.
Of 576 patients, 141 patients (24.5%) were not evaluable for response. CR, complete remission; PD, progressive disease; PR, partial remission; SD, stable disease.
Primary treatment-refractory patients
Two hundred twenty-two patients were primary refractory to palliative first-line therapy. As shown in Table 2 there were no significant differences in baseline patient characteristics between primary refractory patients and those with at least SD to first-line chemotherapy except for ECOG PS (P = 0.015). Poorer initial ECOG PS was significantly associated with primary refractory disease.
Table 2.
Characteristics of patients with primary progressive disease compared with patients with a benefit from first-line therapy
| Variable | Benefit from first-line therapy (n = 354) | Primary progressive (n = 222) | P value |
|---|---|---|---|
| Sex (male/female) | 65.5%/34.5% | 72.5%/27.5% | 0.082a |
| Histology (SCC/non-SCC) | 22.9%/77.1% | 22.1%/77.9% | 0.563a |
| ECOG performance status | 0.015b | ||
| 0 | 16.1% | 13.1% | |
| 1 | 32.8% | 42.3% | |
| 2 | 8.5% | 10.8% | |
| 3 | 0.3% | 4.1% | |
| Unknown | 42.4% | 29.7% | |
| Smoking history | |||
| Current/former | 83.1% | 77.9% | 0.051b |
| Never | 9.6% | 11.7% | |
| Unknown | 7.3% | 10.4% | |
| First-line therapy | |||
| Platinum-based | 62.1% | 63.5% | 0.791a |
| Cisplatin-based | 40.4% | 47.3% | 0.120a |
ECOG, Eastern Cooperative Oncology Group; SCC, squamous cell carcinoma.
Fisher's exact test.
Chi-square test.
A combination therapy (P < 0.0001), treatment with a platinum compound (P < 0.0001) and treatment with cisplatin (P = 0.042) were significantly associated with response to first-line therapy.
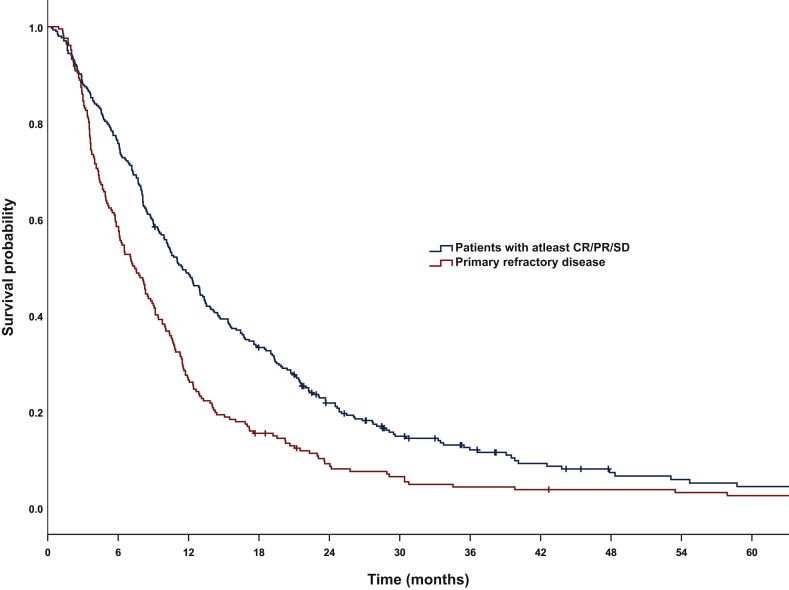
The median OS for primary refractory patients was 7.4 months (95% CI 6.08-8.73) and 11.5 months (95% CI 9.96-12.98) for patients with at least SD (Figure 2). One-year OS was 42.2%. Hazard ratio (HR) for OS was 1.61 (95% CI 1.34-1.93) with a statistically significant P value of <0.0001.
Figure 2.
Overall survival in patients with primary refractory disease versus patients with at least stable disease.
Median: 11.5 months [95% confidence interval (CI) 9.95-12.97] versus 7.4 months (95% CI 6.07-8.72), log-rank P < 0.0001. CR, complete remission; PR, partial remission; SD, stable disease.
Response to second-line therapy
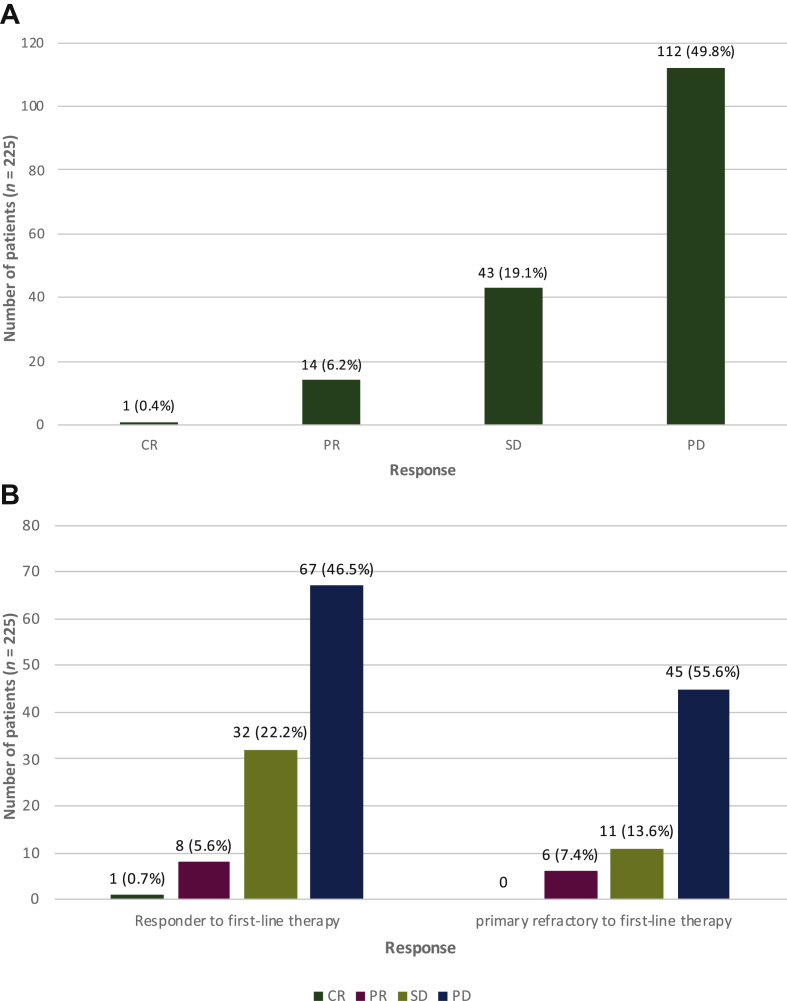
Of the total cohort, 225 patients received an active second-line therapy, and 170 patients were evaluable for response. Of those, 112 patients (49.8%) had PD as best response, 43 patients (19.1%) had SD and 14 patients (6.2%) had PR. Only one patient (0.4%) had CR (Figure 3A).
Figure 3.
(A) Response to second-line therapy. Of 225 patients, 55 patients (24.4%) were not evaluable for response. (B) Response to second-line chemotherapy in association with response to first-line therapy.
Of 144 responders, 36 patients (25%) were not evaluable for response and of 81 primary refractory patients, 19 patients (23.5%) were not evaluable for response. CR, complete remission; PD, progressive disease; PR, partial remission; SD, stable disease.
Primary treatment-refractory patients and response to second-line therapy
To evaluate the benefit of a second-line therapy in patients with primary refractory disease we analyzed the OS and PFS outcome of these patients. Eighty-one (36%) primary refractory patients received a second- or further-line therapy. The clinical benefit (CB) rate (CR + PR + SD) from second-line therapy was lower in primary refractory patients (21.0% versus 28.5%, P = 0.026) (Figure 3A and B).
The median PFS for second-line therapy was significantly shorter for primary refractory patients with 2.5 versus 4.7 months compared with the group of patients with good response to first-line therapy. HR for PFS was 1.63 (95% CI 1.11-2.39), P = 0.013.
As shown in Figure 3A and B, the CB to second-line therapy was significantly associated with previous response to first-line therapy (P = 0.026). There was one complete remission (CR) to second-line therapy in a patient with a PR under first-line therapy.
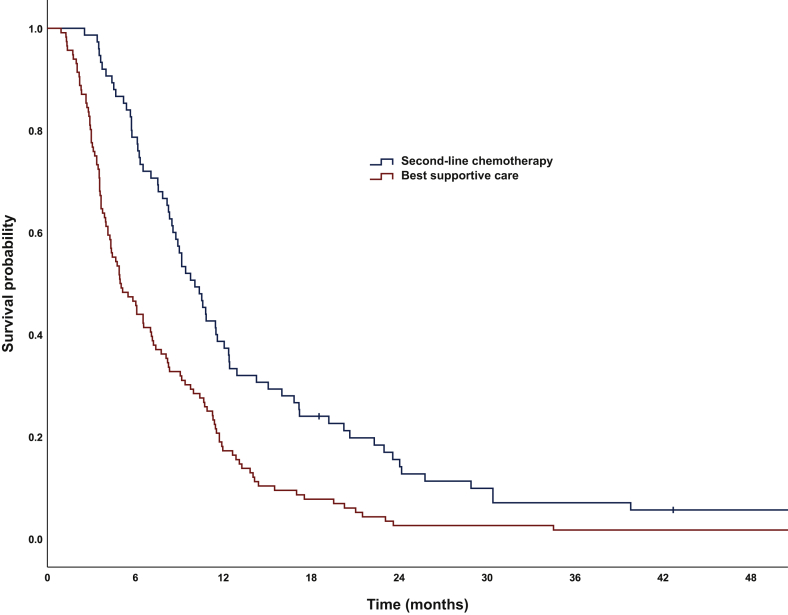
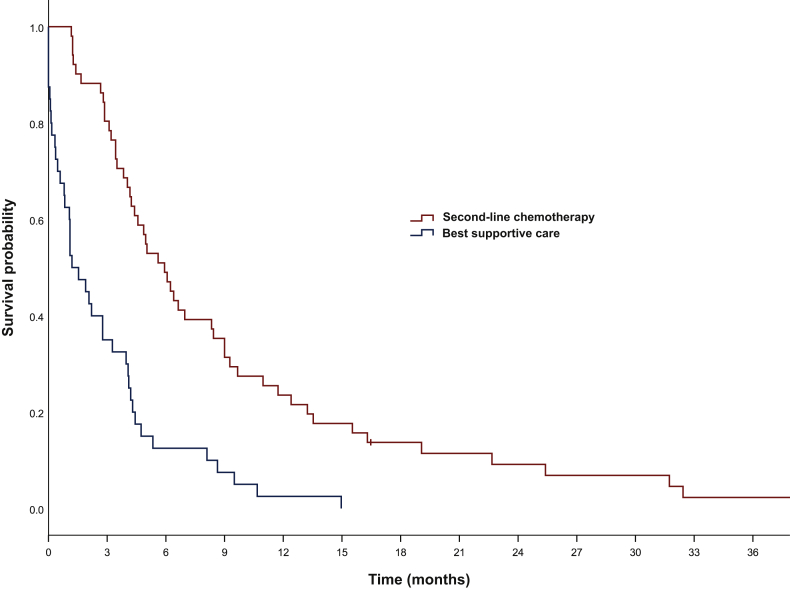
Median OS was significantly longer for refractory patients receiving second-line chemotherapy versus BSC (10.1 versus 5.0 months) (Figure 4). After 12 months OS was 39.7% versus 17.2% in patients receiving and not receiving second-line chemotherapy, respectively. HR for OS was 0.53 (95% CI 0.40-0.72) with a significant P value of <0.0001. More remarkably, median OS from the time of progression was 5.9 months (95% CI 4.40-7.47) in the group receiving further treatment, but only 1.2 months (95% CI 0.34-2.06) in the BSC group (Figure 5). One year after progression, OS was 24.5% and 2.5% in patients treated and not treated with second-line therapy, respectively. HR was 0.33 (95% CI 0.21-0.51), P < 0.0001.
Figure 4.
Overall survival for patients with refractory tumors receiving best supportive care only versus second-line chemotherapy.
Median OS: 5.0 months [95% confidence interval (CI) 3.6-6.2] versus 10.1 months (95% CI 8.4-11.8), log-rank P < 0.0001.
Figure 5.
Overall survival from time point of progression in primary progressive patients comparing patients with second-line therapy versus no second-line chemotherapy (n = 222).
Median OS: 1.2 months [95% confidence interval (CI) 0.34-2.06] versus 5.9 months (95% CI 4.4-7.47), log-rank P < 0.0001.
Discussion
In our retrospective analysis, we assessed the outcome of all consecutive patients with palliative treatment of NSCLC from 1990 to 2016 at the University Hospital of Basel.
Median OS of the cohort was about 9 months, somewhat shorter than published data before the use of immunotherapy, but a recent large international trial investigating the effect of denosumab in NSCLC treated with chemotherapy alone in first-line therapy also showed an OS of less than 9 months.30 Our cohort was unselected and included many patients with PS2 and even brain metastases.31 This fact could also explain the relatively low response rate of 18%, which is below the expected 20%-30% reported in many trials with platinum-based first-line chemotherapy.32,33 It is noteworthy that we saw an improvement of OS over time, with 8.1 months for patients treated before 1995 (before the availability of second-generation drugs such as taxanes and gemcitabine), and 12.4 months for patients treated since 2015, when immunotherapy was being first used. These findings are reported elsewhere.1,34 The distribution of histological subtypes in our cohort correlates well with the expected distribution, comprising 52% adenocarcinomas and 23% SCC.
There was a surprisingly high proportion of primary refractory patients at 42% (or 64%, depending on which denominator we take). This high percentage is in line with the SATURN trial, in which 54% of patients who received chemotherapy experienced primary progression and were subsequently offered participation in the TITAN study. The TITAN study is, to our knowledge, the only prospective randomized study in the population of primary refractory NSCLC.17,20
Our main question was to assess whether patients with a primary refractory NSCLC would benefit from a second or further line of chemotherapy in the real-world setting.
As expected, these patients as a whole have a significantly worse outcome compared with patients who respond to first-line treatment. However, we found a significant survival benefit for those patients who received a second-line therapy, compared with patients with only BSC (9.4 versus 5.5 months). The median OS for refractory patients receiving second-line chemotherapy was in fact similar to patients with initial response or SD (9.4 versus 11 months).
To our knowledge there is only one prospective randomized study investigating this issue. This study assessed the efficacy and tolerability of second-line therapy with a TKI against EGFR (erlotinib) versus chemotherapy (docetaxel) in patients with primary refractory NSCLC.20 In our study the partial response rate of 6.2% was similar to the TITAN study (6%-8%). Median survival of patients receiving second-line chemotherapy in our study was even better with 9.4 versus 5.3-5.5 months in the TITAN study.
Primary refractory patients without second-line therapy had a short therapy-free episode and short time of survival. Median OS in this group was 5.0 months which is slightly better compared with the median survival of the BSC group in a prospective randomized trial conducted by Shepherd et al., which compared the outcome of second-line therapy with docetaxel versus BSC in previously treated patients.16
In our study performance status was significantly associated with benefit from first-line therapy. CB was also associated with a combination therapy and treatment with a platinum compound especially with cisplatin. This corresponds to today's standards in which a combination chemotherapy is recommended as first-line therapy, preferably with cisplatin, if the patients' performance status allows it.15 Carboplatin is a valid alternative with comparable effectiveness but a different toxicity profile.35,36
In patients with PS 2 a combined chemotherapy significantly improved survival compared with monotherapy.37 In addition, in a sub-group analysis within a large phase III trial the superiority of carboplatin-based combinations over monotherapy in PS 2 patients have been identified.38
Finally, our results show that even though patients with primary refractory NSCLC have a poorer prognosis than those with good response to first-line chemotherapy, they should be offered further active therapy as they showed a similar median survival compared with patients with initial response or SD.
Limitations
Our study is a retrospective analysis, which creates the risk of selection bias. We cannot rule out or have to assume that the decision/indication for a second-line chemotherapy was due to performance status and other prognostic factors (e.g. age). In our retrospective analysis ECOG PS was only documented at initial presentation. ECOG PS at time point of initiation of second-line therapy is not available and might have influenced the decision on second-line therapy. However, we did not see a correlation between initial ECOG PS and the use of second-line therapy.
Furthermore, another bias of all retrospective analysis is caused by incompleteness of medical records. In particular, the ORR is missing in many patients in this analysis. However, we have comprehensive data on overall outcome with only very few patients lost to follow-up. Interestingly, a recent study suggests a good correlation between real-world progression data and OS.39 Another factor decreasing the risk of selection bias in our cohort is the fact that we included all consecutive patients with palliative treatment of NSCLC without any further selection.
Our findings are comparable with other randomized studies that analyzed the outcome of second-line therapy in previously treated patients,20 even including immunotherapies, the current standard for second-line treatment.21, 22, 23, 24 Thus we are confident that a second-line chemotherapy for primary refractory patients with good performance status has a positive impact.
Conclusion
The results of our study show the overall poor outcome of patients with metastatic NSCLC in an unselected patient population can considerably differ from a patient population represented in therapeutic clinical studies. However, our results may be closer to the real-life situation than the selected patient population in trials mentioned above. This is supported by the large international phase III SPLENDOUR trial, which recruited in many and also smaller centers and showed remarkably similar results to our retrospective trial.30 The rate of patients with primary refractory disease when undergoing standard of care first-line chemotherapy is substantial. This is a neglected patient population in the study setting with a poor prognosis. We were able to show that it is worthwhile to treat these patients with an active second-line therapy, if their general condition allows it. In light of the new data on immunotherapy, it will be interesting to see how patients with a lack of response to immunotherapy or immunochemotherapy in the first-line will respond to further treatment options. Our study can serve as an important reference value for such studies.
Acknowledgments
Disclosure
The authors report no conflicts of interest.
References
- 1.Waechter F., Passweg J., Tamm M. Significant progress in palliative treatment of non-small cell lung cancer in the past decade. Chest. 2005;127:738–747. doi: 10.1378/chest.127.3.738. [DOI] [PubMed] [Google Scholar]
- 2.Soria J.-C., Ohe Y., Vansteenkiste J. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 3.Yang J.C.-H., Wu Y.-L., Schuler M. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–151. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y.-L., Cheng Y., Zhou X. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:1454–1466. doi: 10.1016/S1470-2045(17)30608-3. [DOI] [PubMed] [Google Scholar]
- 5.Peters S., Camidge D.R., Shaw A.T. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 6.Camidge D.R., Kim H.R., Ahn M.-J. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med. 2018;379:2027–2039. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- 7.Reck M., Rodríguez-Abreu D., Robinson A.G. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37:537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 8.Gandhi L., Rodríguez-Abreu D., Gadgeel S. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 9.Mok T.S.K., Wu Y.-L., Kudaba I. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 10.Paz-Ares L., Luft A., Vicente D. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 11.Jotte R., Cappuzzo F., Vynnychenko I. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol. 2020;15(8):1351–1360. doi: 10.1016/j.jtho.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 12.Socinski M.A., Jotte R.M., Cappuzzo F. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 13.West H., McCleod M., Hussein M. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 14.Hellmann M.D., Paz-Ares L., Bernabe Caro R. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381:2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 15.Planchard D., Popat S., Kerr K. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(suppl 4):iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 16.Shepherd F.A., Dancey J., Ramlau R. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 17.Cappuzzo F., Ciuleanu T., Stelmakh L. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 18.Paz-Ares L., de Marinis F., Dediu M. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13:247–255. doi: 10.1016/S1470-2045(12)70063-3. [DOI] [PubMed] [Google Scholar]
- 19.Fidias P.M., Dakhil S.R., Lyss A.P. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:591–598. doi: 10.1200/JCO.2008.17.1405. [DOI] [PubMed] [Google Scholar]
- 20.Ciuleanu T., Stelmakh L., Cicenas S. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol. 2012;13:300–308. doi: 10.1016/S1470-2045(11)70385-0. [DOI] [PubMed] [Google Scholar]
- 21.Herbst R.S., Baas P., Kim D.-W. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 22.Borghaei H., Paz-Ares L., Horn L. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brahmer J., Reckamp K.L., Baas P. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rittmeyer A., Barlesi F., Waterkamp D. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garon E.B., Hellmann M.D., Rizvi N.A. Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol. 2019;37:2518–2527. doi: 10.1200/JCO.19.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizvi N.A., Mazières J., Planchard D. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langer C.J., Gadgeel S.M., Borghaei H. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17:1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 29.Oken M.M., Creech R.H., Tormey D.C. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 30.Peters S., Danson S., Hasan B. A randomized open-label phase III trial evaluating the addition of denosumab to standard first-line treatment in advanced NSCLC: the European Thoracic Oncology Platform (ETOP) and European Organisation for Research and Treatment of Cancer (EORTC) SPLENDOUR trial. J Thorac Oncol. 2020;15(10):1647–1656. doi: 10.1016/j.jtho.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Schiller J.H., Harrington D., Belani C.P. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 32.Scagliotti G.V., Parikh P., von Pawel J. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 33.Socinski M.A., Bondarenko I., Karaseva N.A. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30:2055–2062. doi: 10.1200/JCO.2011.39.5848. [DOI] [PubMed] [Google Scholar]
- 34.Rothschild S.I., Nachbur R., Herzog N. Significant progress in palliative treatment of NSCLC over the last decades – correlation of treatment milestones with survival in unselected patients. J Thorac Oncol. 2018;13:S1–S139. [Google Scholar]
- 35.Rossi A., Di Maio M., Chiodini P. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol. 2012;30:1692–1698. doi: 10.1200/JCO.2011.40.4905. [DOI] [PubMed] [Google Scholar]
- 36.Vasconcellos V.F., Marta G.N., da Silva E.M., Gois A.F., de Castria T.B., Riera R. Cisplatin versus carboplatin in combination with third-generation drugs for advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2020;1:CD009256. doi: 10.1002/14651858.CD009256.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lilenbaum R., Villaflor V.M., Langer C. Single-agent versus combination chemotherapy in patients with advanced non-small cell lung cancer and a performance status of 2: prognostic factors and treatment selection based on two large randomized clinical trials. J Thorac Oncol. 2009;4:869–874. doi: 10.1097/JTO.0b013e3181a9a020. [DOI] [PubMed] [Google Scholar]
- 38.Quoix E., Zalcman G., Oster J.-P. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet. 2011;378:1079–1088. doi: 10.1016/S0140-6736(11)60780-0. [DOI] [PubMed] [Google Scholar]
- 39.Griffith S.D., Miksad R.A., Calkins G. Characterizing the feasibility and performance of real-world tumor progression end points and their association with overall survival in a large advanced non-small-cell lung cancer data set. JCO Clin Cancer Inform. 2019;3:1–13. doi: 10.1200/CCI.19.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]