Abstract
Many arboviral diseases are uncontrolled, and the viruses that cause them are globally emerging or reemerging pathogens that produce significant disease throughout the world. The increased spread and prevalence of disease are occurring during a period of substantial scientific growth in the vectorborne disease research community. This growth has been supported by advances in genomics and proteomics, and by the ability to genetically alter disease vectors. For the first time, researchers are elucidating the molecular details of vector host-seeking behavior, the susceptibility of disease vectors to arboviruses, the immunological control of infection in disease vectors, and the determinants that facilitate transmission of arboviruses from a vector to a host. These discoveries are facilitating the development of novel strategies to combat arboviral disease, including the release of transgenic mosquitoes harboring dominant lethal genes, the introduction of arbovirus-blocking microbes into mosquito populations, and the development of acquisition- and transmission-blocking therapeutics. Understanding the role of the vector in arbovirus transmission has provided critical practical and theoretical tools to control arboviral disease.
Keywords: mosquito, host, immune response, saliva protein
INTRODUCTION
Arboviruses are defined as viruses that are transmitted to a mammalian host by an arthropod vector. In humans, relevant disease-spreading arthropods include mosquitoes and ticks, among others. This enzootic transmission cycle requires that virus, vector, and host spatially and temporally interact in a way that facilitates acquisition of a virus from an infected host into a susceptible vector, dissemination of the virus throughout the vector to the salivary glands, and transmission of the virus to a host. Numerous complex factors play a role in this dynamic relationship, including vector capacity and host susceptibility. Vector capacity describes all aspects of a vector’s ability to acquire, maintain, and transmit a pathogen, including the feeding habits and life span of the vector species (1). Host susceptibility includes a complex interplay of genetic and immunological determinants.
Currently, the Centers for Disease Control and Prevention’s list of arboviruses and related zoonotic viruses encompasses more than 600 known arboviruses. Over 80 of these are known human pathogens. Our global survey of arboviruses is likely incomplete, and ongoing surveillance is necessary to understand how viruses spill over into the human population. Many arboviruses have evolved the ability to infect both arthropod and mammalian hosts, leading to widespread human infection and disease. Understanding the role of the vector in arbovirus transmission is critical to the development of novel strategies to control the spread of disease. Here we review features of vector mosquitoes that influence arbovirus transmission. We compile work from diverse research fields and identify novel targets for rational therapeutic and vaccine design based on vector rather than viral or host components.
DENGUE AND WEST NILE VIRUSES
Currently, dengue virus (DENV) is the most problematic arbovirus to the human population; globally, it infects 100–390 million individuals, causing up to 96 million symptomatic infections and leading to 12,500 deaths per year—mostly among young children (2, 3). DENV is considered a reemerging pathogen largely because of the increasing range of Aedes aegypti and Aedes albopictus vectors. These mosquito species are spreading around the world due to globalization, a warming climate, and other factors (4). Epidemics of DENV have primarily been restricted to resource-limited areas of the world, regions that include 2.5 billion individuals or 40% of the world population, making DENV a significant burden on these struggling economies (3).
Unpredictable outbreaks of arboviral disease, such as the introduction of West Nile virus (WNV) into the United States in 1999, highlight the need to control the spread of arboviruses and their disease vectors. WNV has become endemic to the Unites States and is responsible for unpredictable epidemics that result in hundreds to thousands of reported cases of neuroinvasive diseases each year (5). Dozens to a few hundred deaths occur each year from disease caused by WNV infection (5). Since the introduction of WNV in the United States, over 3 million people have been infected (6). The reason for the variability in epidemic severity is unclear, but it may be in part due to interactions between the vector mosquito and its environment, including temperature changes and availability of preferred hosts (7). There are no specific treatments or prophylactics available for either DENV or WNV, and treatments are limited for many other human disease-causing arboviruses.
MOSQUITO VECTORS
A number of mosquito species serve as vectors of arboviral disease in nature, and many more are competent vectors in a laboratory setting. We can expect fluctuations in time of the predominant species involved in arbovirus transmission. At present, Aedes and Culex spp. are the major vectors of medically important arboviral diseases in humans (Table 1).
Table 1.
Mosquito-borne arboviruses that cause disease in humans
| Arbovirus | Main vector(s)a | Main reservoir(s) | Endemic region(s) |
|---|---|---|---|
| Dengue virus | Aedes | Primates, humans | Africa, Asia, South America, Pacific |
| West Nile virus | Culex | Birds | Europe, North America, Africa, Asia |
| Yellow fever virus | Aedes | Primates, humans | Africa, South America |
| Japanese encephalitis virus | Culex | Birds, pigs | Asia |
| St. Louis encephalitis virus | Culex | Birds | Americas |
| Chikungunya virus | Aedes | Primates, bats, rodents | Africa, Asia |
| Venezuelan equine encephalitis virus | Culex, Aedes | Rodents | Americas |
| Ross River virus | Culex, Aedes | Mosquitoes | Australia, New Zealand |
| Eastern equine encephalitis virus | Culex, Aedes | Birds, rodents | Americas |
| Western equine encephalitis virus | Culex | Birds | Americas |
| O’nyong-nyong virus | Anopheles | Mosquitoesb | East Africa |
| Rift Valley fever virus | Culex, Aedes | Sheep, cattle | Africa, Asia |
| Murray Valley encephalitis virus | Culex | Birds | Australia, New Guinea |
| Orungo virus | Anopheles, Aedes | Mosquitoesb | Africa |
| La Crosse encephalitis virus | Aedes | Squirrels, chipmunks | North America |
| Sindbis virus | Culex | Birds | Europe, Africa, Asia |
| Vesicular stomatitis virus | Widespreadc | Widespreadc | Americas |
Only the genus of each main vector is listed.
Other reservoirs may exist.
More than three vectors or reservoirs have been identified.
Aedes aegypti and Aedes albopictus
Aedes aegypti is a tropical mosquito that has recently become endemic to many novel geographic locations due to globalization, a warming climate, and the disuse of DDT (dichlorodiphenyltrichloroethane) as an insecticide. Aedes aegypti is the primary vector of DENV, chikungunya virus (CHIKV), and yellow fever virus (YFV) and has colonized southern regions of the United States including parts of Florida, California, and Texas (Table 1) (8, 9). Aedes albopictus is also a competent vector for many arboviruses including DENV and CHIKV, although it typically leads to milder epidemics than Aedes aegypti does (10). Aedes albopictus has also been found in nature infected with WNV, eastern equine encephalitis virus (EEEV), and Japanese encephalitis virus (JEV)(11).
Aedes albopictus is a temperate mosquito that has colonized a major portion of the United States. In the north, Aedes albopictus has become endemic from New Jersey to the Midwest. In the south, it has become endemic from Florida to Texas (10). It does not appear that Aedes albopictus can overwinter north of Chicago; however, genetic and climatic variation can lead to enhanced survival of mosquito eggs during winter conditions (12–14).
At this point, it is unclear whether DENV can become established in the United States Aedes albopictus population (15). However, studies suggest that Aedes albopictus can maintain a pool of DENV that is utilized by Aedes aegypti (10). This possibility suggests that DENV can move between these two species. Currently, they overlap in multiple regions throughout the southern United States. Interestingly, when inhabiting the same geographical region, Aedes albopictus tends to displace Aedes aegypti from competing environments due to the one-directional insemination of Aedes aegypti females by Aedes albopictus males (16, 17). It should be determined whether the competitive advantage of Aedes albopictus over Aedes aegypti can lead to the selection of DENV variants that are more effectively transmitted by Aedes albopictus. This scenario would facilitate the dissemination of DENV into the United States and other more temperate regions that are refractory to colonization by Aedes aegypti.
Culex Species
Several species of Culex have the ability to serve as vectors of arboviruses such as WNV, JEV, and St. Louis encephalitis virus (SLEV) (Table 1). Though Culex spp. typically obtain their blood meals from birds instead of mammals, their ability to harbor and transmit human pathogens during the occasional human blood meal can lead to severe and potentially fatal disease (18). WNV is maintained in an enzootic transmission cycle between Culex spp. and avian hosts. Humans, horses, and other animals are considered dead-end hosts and are usually targeted by Culex spp. when the preferred avian host is not available (18, 19). Avian hosts may not be available due to changes in climate or migration patterns, leading to increased human exposure to infected mosquitoes (18). The most important Culex spp. in terms of arbovirus exposure and human infection vary depending on the geographical region and may be subject to change depending on climate and host availability. Generally, Culex pipiens, Culex tarsalis, and Culex quinquefasciatus are responsible for transmission of WNV in the United States. Different Culex spp. have been implicated in disease transmission in other countries.
VIRUS-VECTOR INTERACTIONS
Virus Acquisition
Mosquitoes become infected with arboviruses during feeding on an infected host. Virus travels into the mosquito midgut along with the blood meal. In order for the mosquito to acquire an arboviral infection, it is essential that the arbovirus has evolved a mechanism to breach the midgut barrier. This consists of both immunological and physical barriers including proteolytic enzyme upregulation, the RNA interference (RNAi) pathway, peritrophic matrix formation, antimicrobial molecule influx, and the physical barrier of midgut epithelial cells (20–22). The presence of normal bacterial flora in the insect midgut also negatively influences arbovirus acquisition. This antiviral state may be due to the production of reactive oxygen species by the vector to control bacterial growth, or perhaps to competition for metabolic resources (23).
It is largely unknown how arboviruses evade the midgut barrier, although incoming arboviral populations do undergo a genetic bottleneck (24). Presumably, certain genotypes are maladapted to the midgut environment, and a strong selective pressure is exerted (25). If this is the case, it is not clear what phenotype the selected population has that makes it well adapted to the mosquito midgut environment. It is also possible that a nonselective reduction in the viral population leads to the genetic bottleneck and that infection of midgut epithelial cells simply requires a high titer of virus in the blood meal.
Vector Response to Infection
The acquisition of an arbovirus leads to transcriptomic and proteomic alterations in mosquito vectors. Many studies have been performed to evaluate the effect of arbovirus infection on mosquito gene expression, protein levels, and immune system responses, as well as the impacts these changes have on the vector’s life cycle (26–28). For many genes, it is unclear whether altered gene and protein expression is directed by the virus or the vector. Generally, microarray analysis has shown that flavivirus infection leads to the up regulation of many genes in Aedes aegypti, including transcription factors, ion-binding proteins, and many metabolic proteins, and leads to the downregulation of protease and pupal cuticle protein genes, among others (26). Transcriptomic analysis of Culex spp. during infection has revealed that many genes related to transport and metabolism are upregulated upon infection with WNV (29). The immune response of both Culex and Aedes spp. to arbovirus infection includes the RNAi pathway, the JAK-STAT pathway, and Toll signaling (30–32). Immune responses may also be triggered by factors in the blood such as insulin, which may stimulate the ERK pathway and lead to a broad antiviral response (33). It is likely that viral infection directs some alteration in gene expression and immune function in the mosquito, and these genes and proteins may represent ideal targets for blocking arbovirus infections in the vector.
Dissemination to the Salivary Glands
For those arbovirus genotypes that do survive the midgut barrier, it takes several days to disseminate to distal tissues including the salivary gland (34, 35). It remains to be seen whether arboviruses undergo further selective pressure in the hemolymph or other distal tissues. However, after the midgut, a second bottleneck has been observed in the salivary glands, suggesting either that certain viral genotypes are maladapted to infect this organ or that a strong nonselective reduction in the viral population occurs at that site (24). While mutations can become fixed in viral populations after passage in insect cell culture or live mosquitoes, it is clear that alternating between vector and host constrains the rate of evolutionary change (36–38). These constraints may limit the interaction of viral and cellular components to evolutionarily conserved molecules that are present in both vector and host. However, selection and accumulation of vector-specific mutations may occur in a single round of infection. These mutations may impact transmission or pathogenesis in the host. For example, research on DENV replication kinetics in Aedes aegypti has shown that virus isolates that are more commonly associated with dengue hemorrhagic fever epidemics can outcompete virus isolates associated with dengue fever (39).
VIRUS-VECTOR-HOST INTERACTIONS
Establishment of an Enzootic Transmission Cycle
Mosquitoes as we know them have been on this planet for at least 50 million years (40). An increasing number of arboviruses have been classified as infecting only arthropods, raising the questions of where these viruses came from and which genetic changes were required for adaptation to their new arthropod hosts (41, 42). These viruses may have been introduced into arthropods from the environment or during a sugar or blood feeding event. Other arboviruses can infect both arthropods and mammals. Unknown genetic changes are required for the adaptation of arboviruses to mammalian hosts. At a minimum, the establishment of an enzootic transmission cycle would require the selection of viral proteins that can interact with diverse molecules including cell surface receptors and entry factors, immune components, protein translation machinery, and protein export machinery in both arthropod and mammalian cells. It is reasonable to hypothesize that many individual mutations are required for an arthropod-only virus to transition to an enzootic transmission cycle that includes a mammalian host. It is unclear how the selective environment in disease vectors influences this process, and it is possible that certain arthropods or environments more effectively select for viruses that can be transmitted to a mammalian host. Understanding how this selective process leads to the evolution of pathogenic arboviruses is critical to control emerging arboviral diseases.
Factors Influencing Host Seeking
Mosquito species feed on either plant nectar, vertebrate blood, or both plant nectar and vertebrate blood (43–46). Mosquitoes use different visual, chemical, and sensory cues to seek out nectar and blood meals (47,48). Disease vectors sense various attractive cues to host seek including movement, body heat, CO2, and volatile compounds released from host skin and normal bacterial flora (48–52). Discrimination of hosts can be further stratified at the genus level. For instance, Culex spp. prefer to feed on American robins in some locations and will feed on humans only if their preferred avian host is not available (18, 19). The genetic alterations required for host discrimination are largely unknown, although it is clear that insects detect attractive cues through several molecules including odorant receptors and an obligate coreceptor called orco (48). Importantly, genetic disruption of Aedes aegypti orco protein led to reduced discrimination between animal and human scent in the presence of CO2 and to reduced attraction to honey and human scent in the absence of CO2 (48). Detection of skin odor has been mapped to CO2-sensitive olfactory neurons, which suggests that orco is expressed in this cell type (53). Detection of CO2 appears to amplify scent signals in the mosquito. These data confirm that molecular evolution is key to host detection and discrimination.
Influence of Saliva on Transmission
When infected mosquitoes probe host skin for a source of blood, they inoculate virus-infected saliva mostly into extravascular spaces in the dermis (54–56). The majority of in vivo arbovirus research uses laboratory techniques such as needle inoculation of virus that may alter or miss elements of the natural infectious process, which is typically modified by vector saliva (57). Accordingly, multiple reports have identified a role for mosquito saliva in the modulation of arbovirus infectivity and transmission both in vitro and in vivo (Table 2) (58–67).
Table 2.
Effects of mosquito saliva, salivary gland extracts, and salivary gland proteins on arbovirus infectivity
| Arbovirus | Vector | Model | Infection route | Infectivity | Candidate | Immunomodulation | Reference |
|---|---|---|---|---|---|---|---|
| Dengue virus | Aedes | C6/36 cells | In vitro | Inhibited | AAEL000598 | NA | 28 |
| Keratinocytes | In vitro | Enhanced | NA | Suppressed IFN, AMP | 74 | ||
| Keratinocytes | In vitro | Enhanced | 34-kDa protein | Suppressed IFN, IRF, AMP | 62 | ||
| Mouse | Mosquito bite | Enhanced | NA | Long-term effects on IFN and others | 69 | ||
| Mouse | Intradermal | Enhanced | NA | Suppressed TLR7, RelA, IFN, IL-10 | 71 | ||
| Mouse | Intradermal | Enhanced | NA | Suppressed IFN | 70 | ||
| Dengue virus, West Nile virus | Aedes | Fibroblast, mouse | In vitro, intradermal | Enhanced | AAEL005718 | Suppressed TFN-α | 63 |
| West Nile virus | Aedes | Mouse | Intraperitoneal | Inhibited | AAEL011045 | NA | 26 |
| Mouse | Mosquito bite | Enhanced | NA | NA | 60 | ||
| Mouse | Mosquito bite | Enhanced | NA | Reduced T cells, suppressed IFN | 72 | ||
| Culex | Mouse | Mosquito bite | NA | D7 | NA | 78 | |
| Mouse | Mosquito bite | Enhanced | NA | NA | 61 | ||
| Rift Valley fever virus | Aedes | Mouse | Intradermal, mosquito bite | Enhanced | NA | NA | 64 |
| Chikungunya virus | Aedes | Mouse | Mosquito bite | Enhanced | NA | Induced IL-4, suppressed TLR3 | 57 |
| Cache Valley virus | Aedes, Culex | Mouse | Mosquito bite | Enhanced | NA | NA | 75 |
| La Crosse encephalitis virus | Aedes | Deer, chipmunk | Mosquito bite | Enhanced | NA | NA | 66 |
| Western equine encephalitis virus, St. Louis encephalitis virus | Culex | Bird | Mosquito bite | No change | NA | NA | 67 |
| Vesicular stomatitis virus | Aedes | Mouse | Mosquito bite | Enhanced | NA | Enhanced seroconversion | 65 |
| Fibroblast | In vitro | Enhanced | NA | Reduced IFN-α | 73 | ||
| Sindbis virus | Aedes | Mouse | Intradermal | NA | NA | increased IL-10 and IL-4 | 59 |
Abbreviation: NA, not applicable.
Although a correlation between saliva-mediated infectivity enhancement and the modulation of interferon (IFN), tumor necrosis factor (TNF), and T helper 1/2 (Th1/Th2) immune responses has been shown, no study has directly tested whether modulation of the immune response is required for saliva-mediated infectivity enhancement or is just a consequence of exposure to saliva allergens (57, 59, 61, 62, 68). That said, most studies do show that saliva can suppress IFN expression in both in vitro and in vivo model systems (62, 69–74). Suppression of the host innate immune response would be expected to have an impact on virus transmission; however, there are no conclusive experimental data that implicate a specific saliva factor in immunomodulation. Further, saliva-mediated enhancement of DENV infectivity occurs in cells and mice lacking the type I IFN response, suggesting that enhancement in this context is not the result of modulation of the innate immune system (63). The role of the immune response in saliva-mediated infectivity enhancement is not clear. It is clear, however, that both mosquito saliva and virus must be inoculated at the same cutaneous site for infectivity enhancement to occur (61, 75), suggesting that a mosquito saliva component alters the local inoculation site in favor of virus transmission.
Complex Nature of Mosquito Saliva
Salivary gland transcriptomes (sialotranscriptomes) have been generated for multiple mosquito species, and these data suggest that over 100 proteins are expressed and secreted into saliva (76, 77). All hematophagous arthropods appear to express saliva proteins with antiplatelet, anticoagulation, and vasodilation activities, and many of these proteins have been characterized genetically and biochemically (76). There are many other proteins whose role in blood feeding and virus transmission remains largely uncharacterized, including D7 proteins, odorant-binding proteins, antimicrobial proteins, serpins, nucleotidases, serine proteases, lectins, mucins, and various other antigens of unknown homology and function (76). It will be important to determine whether any of these proteins play a role in arbovirus transmission.
Due to the complex nature of mosquito saliva, certain saliva factors may inhibit and others may enhance virus infectivity (63). In fact, a cecropin-like peptide that is expressed in the salivary glands of Aedes aegypti has been shown to lower DENV infectivity (28). Additionally, immunization with recombinant D7 protein, one of the most immunogenic proteins in saliva, led to enhanced WNV infection in mice, suggesting that the protein itself is directly or indirectly inhibitory to infection (78). A recombinant pupal cuticle protein that is expressed in Aedes aegypti salivary glands was also able to inhibit WNV infection in an encephalitic mouse model of infection (26). Interestingly, we found that certain fractions of high-performance liquid chromatography (HPLC)-fractionated Aedes aegypti salivary gland extract (SGE) increased and others decreased DENV infectivity in vitro, whereas transmission enhancement was observed using nonfractionated SGE in vivo (63). This suggested that enhancing factors may be dominant over inhibiting factors in vivo. The vast majority of studies using coinoculation of virus with SGE or live mosquitoes to deliver a virus inoculum suggest that whole saliva enhances rather than inhibits infectivity (Table 2).
Saliva-Induced Allergic Response and Transmission
Mosquito saliva contains potent allergens. The bite of a mosquito and subsequent injection of saliva into human skin almost always trigger an allergic reaction. Treatment of skin with irritants or allergens modulates the dermal environment and induces the migration of Langerhans cells to draining lymph nodes (79). Although the mechanistic details of how irritants and allergens induce Langerhans cell migration are not fully defined, it is correlated with the breakdown of integrinmediated interactions with the extracellular matrix and a fibroblast and interleukin 10 (IL-10)-dependent switch of Langerhans cells to a macrophage-like phenotype (80, 81). Many allergens such as dust mite Der p proteins, Aspergillus spp. Asp proteins, certain pollens, and cockroach proteins are proteases that cleave tight-junction molecules and activate PAR2 (82). This results in increased epithelial permeability and production of chemokines. These proteases also cleave components of complement, CD40, CD25, and CD23, leading to various cellular effects ranging from recruiting innate immune cells to stimulating immunoglobulin E (IgE) production by B cells (82). Protease allergens also elicit a Th2 response, which has been suggested as the cause of saliva-mediated infectivity enhancement (59, 68, 83).
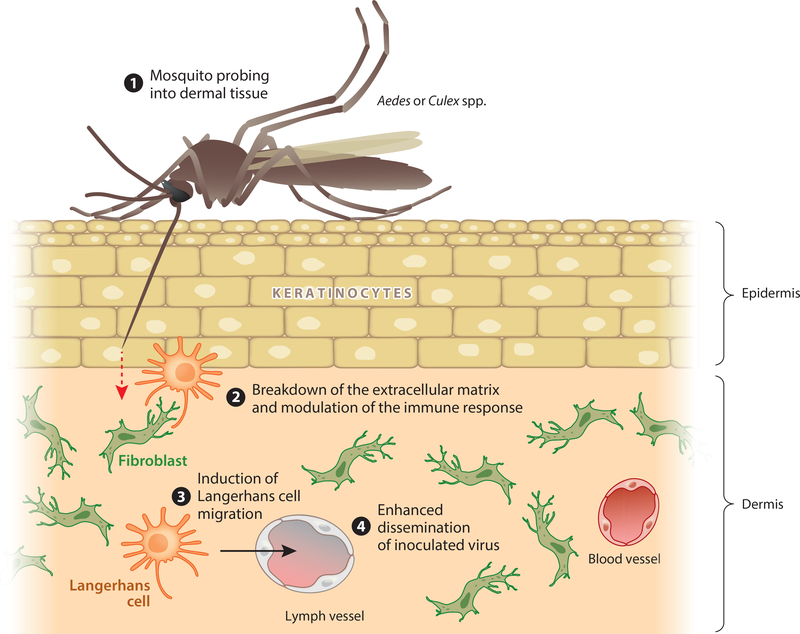
We identified that serine protease activity in mosquito saliva is responsible for transmission enhancement in vivo (63). Our mechanistic studies suggested that the salivary serine protease breaks down the extracellular matrix laid down by interstitial fibroblasts, which may lead to increased cell migration at the inoculation site. Blocking extracellular matrix breakdown with a chemical inhibitor completely inhibited saliva-mediated enhancement of viral RNA in draining lymph nodes (Figure 1) (63). Langerhans cells are targets of DENV infection in vivo (84), whereas macrophages may serve to control infection at this early time point (85). Given this context, we hypothesize that a mosquito saliva serine protease, like known protease allergens, disrupts the barrier function of skin and induces Langerhans cell migration to draining lymph nodes. Induction of Langerhans cell migration would increase the probability of interaction with immobilized virions and dissemination to distal sites in the host (Figure 1).
Figure 1.
Model of saliva-mediated infectivity enhancement. ❶ Infected mosquitoes inoculate virus-laden saliva mostly into host dermal tissue during probing for a blood meal. ❷ Saliva serine proteases break down dermal extracellular matrix, which modulates the immune response. ❸ Langerhans cell migration is induced, which increases the probability of interaction with immobilized virions. ❹ Infected Langerhans cells migrate to draining lymph nodes, thereby enhancing the dissemination of virus into the host.
TARGETING THE VECTOR: THE FUTURE OF ACQUISITION-AND TRANSMISSION-BLOCKING TECHNOLOGIES
The development of therapeutics that target either a pathogen or vector protein to prevent transmission to human hosts is essential to the eradication of many vector-borne diseases. Acquisitionblocking vaccines (ABVs) are currently being developed and have been successful at preventing malaria infection of Anopheles mosquitoes (86–88). One of these, an ABV developed against the Plasmodium protein Pfs25, was able to prevent the acquisition of malaria from infected mice by naive mosquitoes (86). Another group found that vaccinating mice with the mosquito protein serpin-2 prevented the acquisition of Plasmodium berghei by a naive group of mosquitoes (89). In addition, an arthropod-specific transmission-blocking vaccine (TBV) based on the outer surface protein A (OspA) of Borrelia burgdorferi, the causative agent of Lyme disease, has been shown to protect mice from spirochete infection. Proteins from sand fly saliva have also been used successfully as TBVs to prevent the transmission of Leishmania (90–92). The studies above suggest that it is theoretically possible to use mosquito proteins as TBVs to prevent the transmission of DENV and other arboviruses.
Advances in techniques to biochemically, genetically, and physically manipulate mosquito vectors have resulted in an explosion of novel vector control strategies to combat arboviral disease. For instance, the adaptation of RNAi and transgenic techniques used in Drosophila and other species has facilitated experimentation with gene-altered mosquitoes. Genes can now be upregulated, downregulated, knocked in, and knocked out of disease-causing mosquitoes. Further, the annotation of disease vector genomes has facilitated the use of high-throughput technologies such as RNA Seq and proteomics. These technologies will become more refined in the near future, leading to tissue-targeted gene modulation and a global collection of transgenic mosquito colonies with various genetic alterations, as well as a detailed understanding of the molecular determinants that govern virus transmission. Novel strategies are already being developed and tested that could provide valuable tools to reduce arboviral disease (Figure 2). The following sections detail exciting progress in the development of various vector-based control measures and therapeutics.
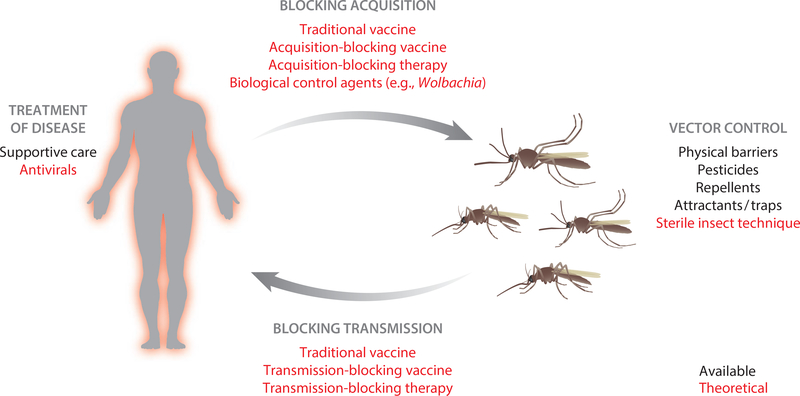
Figure 2.
Available (black) and theoretical (red) interventions to combat arboviral disease. Physical barriers, pesticides, repellants, and attractants/traps are the only methods available to prevent arboviral diseases. Supportive care is the only treatment option available in most cases. The development of a traditional vaccine for dengue virus has been problematic due to antibody-dependent enhancement. New research has paved the way for vector-based interventions including acquisition- and transmission-blocking vaccines and therapeutics, biological control agents, and the release of genetically altered, sterile mosquitoes.
Repellents and Attractants
Many stimuli attract mosquitoes, including movement, body heat, CO2, and skin volatiles. Recently, targeted mutations in an obligate coreceptor of the odorant-binding protein receptors, orco, led to the generation of orco knockouts (48). These mosquitoes lost their host-seeking preference for humans and were refractory to the effects of DEET (N,N-diethyl-meta-toluamide). Importantly, high-throughput screening identified orco agonists that interfere with its function and that may interfere with host seeking thousands of times more effectively than DEET (93, 94). Another high-throughput screen discovered both agonists and antagonists of cpA, a mosquito neuron that is critical for detection of human skin odor (53). Antagonists blocked mosquito attraction to human stimuli. Agonists increased attraction, suggesting that they could be used as bait to trap and control local mosquito populations. Field studies of the above chemicals in addition to structural analysis of orco and other olfactory proteins from vector mosquitoes will help in the design of more effective, targeted repellents that are less toxic to the human population and are environmentally friendly.
The identification of powerful attractants and the development of effective traps also provide opportunities for control of vector-borne disease. Mosquito traps have been developed that utilize CO2, octenol, and human skin volatiles to attract and remove disease vectors from the environment. Detailed molecular and structural analysis of odorant-binding proteins and their preferred ligands will provide important information for the design of powerful attractants. High-throughput assays will need to be developed using recombinant odorant-binding proteins and insect cell culture to probe interactions between vector sensory proteins and candidate attractant molecules.
Wolbachia
The use of biological control as a method to reduce mosquito numbers as well as to reduce the transmission of arboviruses by mosquito vectors is a relatively novel and creative intervention technique. Mosquitoes infected with the obligate intracellular bacteria Wolbachia are unable to successfully reproduce due to the phenomenon known as cytoplasmic incompatibility. Wolbachia is naturally present in several mosquito species, including Aedes albopictus and Culex pipiens, though it is not present in Aedes aegypti. The release of Wolbachia-infected males can reduce Culex spp. mosquito populations in nature, and new models predict that population replacement strategies could successfully establish dominant Wolbachia-infected mosquitoes (95, 96).
Naturally occurring strains of Wolbachia can also restrict salivary gland infection of Aedes albopictus with DENV and limit transmission, because the number of infectious particles is greatly reduced in the saliva of mosquitoes infected with Wolbachia (97). In addition, Culex quinquefasciatus mosquitoes are less susceptible to WNV when they are infected with Wolbachia and are less able to transmit the virus (98). Anon-native Wolbachia infection of Aedes albopictus has also been shown to inhibit CHIKV infection (99). Although Aedes aegypti is not naturally infected with Wolbachia, several groups have successfully generated mosquitoes with stable, inheritable infections (100, 101) and have shown that these infected mosquitoes are resistant to DENV and CHIKV infection (102–104). Field trials have already been initiated to test whether Wolbachia-infected Aedes aegypti will be less prone to DENV infection in nature; these trials are based on the release and establishment of infected mosquitoes in Australian populations and follow-up to assess whether the endosymbiont remains in the local mosquito population over time. Trials with Wolbachia suggest that biological control of arboviral disease may play a key role in disease mitigation (102, 105). Time will tell whether the Wolbachia technique will continue to suppress arboviral pathogens. It is possible that the targeted viruses will evolve resistance or that the effect of Wolbachia on the vector will wane.
Other Viruses
Attempts have also been made to utilize arboviruses as a biological control measure, either to inhibit the replication of pathogenic arboviruses or to limit the life span of vector mosquitoes (106, 107). A genetically altered mosquito virus could also be used as a means to deliver a lethal or inhibitory gene (108). The focus has been on densoviruses, but a rapid increase in the discovery of insect-only flaviviruses may lead to the identification of viruses that effectively compete with the DENV life cycle yet do not cause disease in humans (42, 106). Use of viruses as a biological control agent may benefit from their inherent high mutation rate, which may compete with adaptation of pathogenic arboviruses, and their ability to rapidly spread through a local mosquito population. Mutations may also be selected or engineered that facilitate effective competition with pathogenic arboviruses. However, recombination events between insect-only and pathogenic arboviruses must be taken into account, which could theoretically lead to the development of novel human pathogens. Comprehensive studies should first be performed to elucidate the genetic changes that are required for insect-only arboviruses to adapt to a mammalian host.
Transgenic Mosquitoes
Another biological intervention to control mosquito populations and reduce their capacity for arbovirus infection is genetic modification. One method, called release of insects with dominant lethality (RIDL), has successfully created insects that carry a dominant-lethal gene; this technique involves males delivering female-acting transgenes to the mosquito population. Transgenes currently in use include those to reduce flight (109) and to induce mortality with age (110, 111), and the RIDL method is now being implemented in Brazil and Malaysia (112). Another use of genetic control is to create mosquitoes with enhanced viral resistance. An example is the recent creation of mosquitoes expressing RNAi against DENV RNA, which reduced infection in expressing mosquitoes (113). Finally, transgenic mosquitoes containing bacterial homing endonuclease gene (HEG) elements have been created by using simulation modeling; these have lowered vector competence and have been predicted to eliminate mosquito populations (114, 115).
Transmission-Blocking Therapeutics
Arthropod saliva can modulate the infectivity of a number of arboviruses in vitro and in vivo (60, 63). Currently, little is known about the molecular components in mosquito saliva that are responsible for infectivity enhancement, and it is unclear whether this phenomenon occurs in the human host. Our data suggest that a saliva serine protease is responsible for enhancing dissemination of DENV to draining lymph nodes in a mouse model of infection. A serine protease inhibitor was able to completely block saliva-mediated enhanced dissemination, suggesting that prophylactic intervention with a chemical inhibitor or vaccine could significantly reduce the probability that DENV would disseminate beyond the inoculation site (63). Further research is required to conclusively identify the molecular components in vector saliva responsible for infectivity enhancement before vector saliva-based TBVs can be developed.
Importantly, no saliva-mediated transmission enhancement experiments have been performed using human subjects or tissues. Therefore, it is unknown whether saliva-mediated infectivity or transmission enhancement is relevant to human disease. These experiments would be difficult to perform considering the danger of human infection with most arboviruses and would be further complicated by preexisting memory responses against multiple arthropod saliva proteins. Further, clinical trials testing the efficacy of saliva proteins as TBVs should take into account experimental data suggesting that immunization with certain saliva proteins can enhance virus transmission and disease (63, 78). Establishment of human explant models of saliva-mediated infectivity enhancement will be critical for understanding how mosquito saliva proteins alter the epithelium and induce migration of target immune cells, and for developing novel therapeutics and vaccines to block transmission enhancement.
Acquisition-Blocking Therapeutics
Multiple components are likely required for successful acquisition of an arboviral infection in the vector midgut. As an example, recent research identified both a soluble C-type lectin coreceptor and a human CD45 homolog receptor that facilitate WNV acquisition into midgut cells (116). It is theoretically possible to develop chemical inhibitors or vaccines that would prevent the acquisition of arboviruses by uninfected vector populations. Although this strategy would not help the infected host, it would reduce the number of infected vectors within a population, mitigating the total number of human infections. This would be of huge importance from a public health perspective. Future research is required to identify target molecules that are required for acquisition of arboviruses in the mosquito midgut, and to develop chemical or biological interventions.
CONCLUSIONS
The impact of arboviral diseases is global in scope and has become a great concern due to the expanding geographical range of many mosquito species. Many arboviral diseases occur during unpredictable epidemics that increase the difficulty of controlling these infections. Further, after decades of research, a traditional vaccine is not available for DENV, which is arguably the most important arbovirus causing disease in the world today. Novel targets are desperately needed to combat arboviral diseases. In recent years, there has been a shift in the fight against arboviral disease toward a focus on vector-based instead of host- or virus-based therapies. These therapies span a wide range of technologies, some of which are being tested in the field and some of which are still in their infancy in the molecular biology lab. In the near future, we can expect to see a new arsenal of technologies available to combat arboviral disease, including transgenics, biological control agents, second-generation repellents, and transmission- and acquisition-blocking vaccines, among other possibilities.
ACKNOWLEDGMENTS
We thank the Fikrig lab and Ruth Montgomery at Yale University and Linlin Zhao at Central Michigan University for informative discussions and editing of this manuscript.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Anderson JR, Rico-Hesse R. 2006. Aedes aegypti vectorial capacity is determined by the infecting genotype of dengue virus. Am. J. Trop. Med. Hyg. 75:886–92 [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, et al. 2013. The global distribution and burden of dengue. Nature 496:504–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, et al. 2010. Dengue: a continuing global threat. Nat. Rev. Microbiol. 8:S7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kilpatrick AM, Randolph SE. 2012. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet 380:1946–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindsey NP, Lehman JA, Staples JE, Fischer M. 2013. West Nile virus and other arboviral diseases—United States, 2012. CDC Morb. Mortal. Wkly. Rep. 62:513–17. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6225a1.htm [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen LR, Carson PJ, Biggerstaff BJ, Custer B, Borchardt SM, Busch MP. 2012. Estimated cumulative incidence of West Nile virus infection in US adults, 1999–2010. Epidemiol. Infect. 141:591–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray KO, Ruktanonchai D, Hesalroad D, Fonken E, Nolan MS. 2013. West Nile virus, Texas, USA, 2012. Emerg. Infect. Dis. 19. doi: 10.3201/eid1911.130768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray KO, Rodriguez LF, Herrington E, Kharat V, Vasilakis N, et al. 2013. Identification of dengue fever cases in Houston, Texas, with evidence of autochthonous transmission between 2003 and 2005. Vector Borne Zoonotic Dis. 13:835–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisen L, Moore CG. 2013. Aedes (Stegomyia) aegypti in the continental United States: a vector at the cool margin of its geographic range. J. Med. Entomol. 50:467–78 [DOI] [PubMed] [Google Scholar]
- 10.Rezza G. 2012. Aedes albopictus and the reemergence of dengue. BMC Public Health 12:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell CJ, Niebylski ML, Smith GC, Karabatsos N, Martin D, et al. 1992. Isolation of eastern equine encephalitis virus from Aedes albopictus in Florida. Science 257:526–27 [DOI] [PubMed] [Google Scholar]
- 12.Hanson SM, Craig GB Jr. 1995. Aedes albopictus (Diptera: Culicidae) eggs: field survivorship during northern Indiana winters. J. Med. Entomol. 32:599–604 [DOI] [PubMed] [Google Scholar]
- 13.Swanson J, Lancaster M, Anderson J, Crandell M, Haramis L, et al. 2000. Overwintering and establishment of Aedes albopictus (Diptera: Culicidae) in an urban La Crosse virus enzootic site in Illinois. J. Med. Entomol. 37:454–60 [DOI] [PubMed] [Google Scholar]
- 14.Hawley WA, Pumpuni CB, Brady RH, Craig GB Jr. 1989. Overwintering survival of Aedes albopictus (Diptera: Culicidae) eggs in Indiana. J. Med. Entomol. 26:122–29 [DOI] [PubMed] [Google Scholar]
- 15.Lambrechts L, Scott TW, Gubler DJ. 2010. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl. Trop. Dis. 4:e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bargielowski IE, Lounibos LP, Carrasquilla MC. 2013. Evolution of resistance to satyrization through reproductive character displacement in populations of invasive dengue vectors. Proc. Natl. Acad. Sci. USA 110:2888–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tripet F, Lounibos LP, Robbins D, Moran J, Nishimura N, Blosser EM. 2011. Competitive reduction by satyrization? Evidence for interspecific mating in nature and asymmetric reproductive competition between invasive mosquito vectors. Am. J. Trop. Med. Hyg. 85:265–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. 2006. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 4:e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. 2006. Host heterogeneity dominates West Nile virus transmission. Proc. R. Soc. B 273:2327–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khoo CC, Piper J, Sanchez-Vargas I, Olson KE, Franz AW. 2010. The RNA interference pathway affects midgut infection- and escape barriers for Sindbis virus in Aedes aegypti. BMC Microbiol. 10:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato N, Mueller CR, Fuchs JF, McElroy K, Wessely V, et al. 2008. Evaluation of the function of a type I peritrophic matrix as a physical barrier for midgut epithelium invasion by mosquito-borne pathogens in Aedes aegypti. Vector Borne Zoonotic Dis. 8:701–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brackney DE, Foy BD, Olson KE. 2008. The effects of midgut serine proteases on dengue virus type 2 infectivity of Aedes aegypti. Am.J. Trop. Med. Hyg. 79:267–74 [PMC free article] [PubMed] [Google Scholar]
- 23.Oliveira JH, Goncalves RL, Lara FA, Dias FA, Gandara AC, et al. 2011. Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathog. 7:el001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forrester NL, Guerbois M, Seymour RL, Spratt H, Weaver SC. 2012. Vector-borne transmission imposes a severe bottleneck on an RNA virus population. PLoS Pathog. 8:e1002897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsetsarkin KA, Weaver SC. 2011. Sequential adaptive mutations enhance efficient vector switching by chikungunya virus and its epidemic emergence. PLoS Pathog. 7:e1002412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colpitts TM, Cox J, Vanlandingham DL, Feitosa FM, Cheng G, et al. 2011. Alterations in the Aedes aegypti transcriptome during infection with West Nile, dengue and yellow fever viruses. PLoS Pathog. 7:e1002189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonizzoni M, Dunn WA, Campbell CL, Olson KE, Marinotti O, James AA. 2012. Complex modulation of the Aedes aegypti transcriptome in response to dengue virus infection. PLoS ONE 7:e50512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luplertlop N, Surasombatpattana P, Patramool S, Dumas E, Wasinpiyamongkol L, et al. 2011. Induction of a peptide with activity against a broad spectrum of pathogens in the Aedes aegypti salivary gland, following infection with dengue virus. PLoS Pathog 7:e1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartholomay LC, Waterhouse RM, Mayhew GF, Campbell CL, Michel K, et al. 2010. Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science 330:88–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Souza-Neto JA, Sim S, Dimopoulos G. 2009. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc. Natl. Acad. Sci. USA 106:17841–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brackney DE, Beane JE, Ebel GD. 2009. RNAi targeting of West Nile virus in mosquito midguts promotes virus diversification. PLoS Pathog. 5:e1000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keene KM, Foy BD, Sanchez-Vargas I, Beaty BJ, Blair CD, Olson KE. 2004. RNA interference acts as a natural antiviral response to o’nyong-nyong virus (Alphavirus; Togaviridae) infection of Anopheles gamhiae. Proc. Natl. Acad. Sci. USA 101:17240–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu J, Hopkins K, Sabin L, Yasunaga A, Subramanian H, et al. 2013. ERK signaling couples nutrient status to antiviral defense in the insect gut. Proc. Natl. Acad. Sci. USA 110:15025–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Girard YA, Klingler KA, Higgs S. 2004. West Nile virus dissemination and tissue tropisms in orally infected Culex pipiens quinquefasciatus. Vector Borne Zoonotic Dis. 4:109–22 [DOI] [PubMed] [Google Scholar]
- 35.Salazar MI, Richardson JH, Sanchez-Vargas I, Olson KE, Beaty BJ. 2007. Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weaver SC, Brault AC, Kang W, Holland JJ. 1999. Genetic and fitness changes accompanying adaptation of an arbovirus to vertebrate and invertebrate cells. J. Virol. 73:4316–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasilakis N, Deardorff ER, Kenney JL, Rossi SL, Hanley KA, Weaver SC. 2009. Mosquitoes put the brake on arbovirus evolution: experimental evolution reveals slower mutation accumulation in mosquito than vertebrate cells. PLoS Pathog. 5:e1000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coffey LL, Vasilakis N, Brault AC, Powers AM, Tripet F, Weaver SC. 2008. Arbovirus evolution in vivo is constrained by host alternation. Proc. Natl. Acad. Sci. USA 105:6970–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cologna R, Armstrong PM, Rico-Hesse R. 2005. Selection for virulent dengue viruses occurs in humans and mosquitoes. J. Virol. 79:853–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenwalt DE, Goreva YS, Siljestrom SM, Rose T, Harbach RE. 2013. Hemoglobin-derived porphyrins preserved in a Middle Eocene blood-engorged mosquito. Proc. Natl. Acad. Sci. USA. In press, doi: 10.1073/pnas.1310885110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cook S, Moureau G, Harbach RE, Mukwaya L, Goodger K, et al. 2009. Isolation of a novel species of flavivirus and a new strain of Culex flavivirus (Flaviviridae) from a natural mosquito population in Uganda. J. Gen. Virol. 90:2669–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cook S, Moureau G, Kitchen A, Gould EA, de Lamballerie X, et al. 2012. Molecular evolution of the insect-specific flaviviruses. J. Gen. Virol. 93:223–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edman JD, Strickman D, Kittayapong P, Scott TW. 1992. Female Aedes aegypti (Diptera: Culicidae) in Thailand rarely feed on sugar. J. Med. Entomol. 29:1035–38 [DOI] [PubMed] [Google Scholar]
- 44.Foster WA. 1995. Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 40:443–74 [DOI] [PubMed] [Google Scholar]
- 45.Vinogradova EB, Karpova SG. 2006. Cultivation of the mosquito Culex pipiens pipiens f. molestus (Diptera, Culicidae) without blood feeding. Parazitologiia 40:306–11 [PubMed] [Google Scholar]
- 46.O'Meara GF, Edman JD. 1975. Autogenous egg production in the salt-marsh mosquito, Aedes taeniorhynchus. Biol. Bull. 149:384–96 [DOI] [PubMed] [Google Scholar]
- 47.Foster WA, Hancock RG. 1994. Nectar-related olfactory and visual attractants for mosquitoes. J. Am. Mosq. Control Assoc. 10:288–96 [PubMed] [Google Scholar]
- 48.DeGennaro M, McBride CS, Seeholzer L, Nakagawa T, Dennis EJ, et al. 2013. orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature 498:487–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dormont L, Bessiere JM, McKey D, Cohuet A. 2013. New methods for field collection of human skin volatiles and perspectives for their application in the chemical ecology of human-pathogen-vector interactions. J. Exp. Biol. 216:2783–88 [DOI] [PubMed] [Google Scholar]
- 50.Verhulst NO, Beijleveld H, Knols BG, Takken W, Schraa G, et al. 2009. Cultured skin microbiota attracts malaria mosquitoes. Malar. J. 8:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smallegange RC, Knols BG, Takken W. 2010. Effectiveness of synthetic versus natural human volatiles as attractants for Anopheles gambiae (Diptera: Culicidae) sensu stricto. J. Med. Entomol. 47:338–44 [DOI] [PubMed] [Google Scholar]
- 52.Bohbot JD, Durand NF, Vinyard BT, Dickens JC. 2013. Functional development of the octenol response in Aedes aegypti. Front. Physiol. 4:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tauxe GM, Mac William D, Boyle SM, Guda T, Ray A. 2013. Targeting a dual detector of skin and CO2 to modify mosquito host seeking. Cell 155:1365–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turell MJ, Tammariello RF, Spielman A. 1995. Nonvascular delivery of St. Louis encephalitis and Venezuelan equine encephalitis viruses by infected mosquitoes (Diptera: Culicidae) feeding on a vertebrate host. J. Med. Entomol. 32:563–68 [DOI] [PubMed] [Google Scholar]
- 55.Turell MJ, Spielman A. 1992. Nonvascular delivery of Rift Valley fever virus by infected mosquitoes. Am.J. Trop. Med. Hyg. 47:190–94 [DOI] [PubMed] [Google Scholar]
- 56.Styer LM, Kent KA, Albright RG, Bennett CJ, Kramer LD, Bernard KA. 2007. Mosquitoes inoculate high doses of West Nile virus as they probe and feed on live hosts. PLoS Pathog. 3:1262–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thangamani S, Higgs S, Ziegler S, Vanlandingham D, Tesh R, Wikel S. 2010. Host immune response to mosquito-transmitted chikungunya virus differs from that elicited by needle inoculated virus. PLoS ONE 5:el2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ader DB, Celluzzi C, Bisbing J, Gilmore L, Gunther V, et al. 2004. Modulation of dengue virus infection of dendritic cells by Aedes aegypti saliva. Viral Immunol. 17:252–65 [DOI] [PubMed] [Google Scholar]
- 59.Schneider BS, Soong L, Zeidner NS, Higgs S. 2004. Aedes aegypti salivary gland extracts modulate anti-viral and TH1/TH2 cytokine responses to Sindbis virus infection. Viral Immunol. 17:565–73 [DOI] [PubMed] [Google Scholar]
- 60.Schneider BS, Soong L, Girard YA, Campbell G, Mason P, Higgs S. 2006. Potentiation of West Nile encephalitis by mosquito feeding. Viral Immunol. 19:74–82 [DOI] [PubMed] [Google Scholar]
- 61.Styer LM, Lim PY, Louie KL, Albright RG, Kramer LD, Bernard KA. 2011. Mosquito saliva causes enhancement of West Nile virus infection in mice. J. Virol. 85:1517–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Surasombatpattana P, Ekchariyawat P, Hamel R, Patramool S, Thongrungkiat S,et al. 2013. Aedes aegypti saliva contains a prominent 34-kDa protein that strongly enhances dengue virus replication in human keratinocytes. J. Investig. Dermatol. 134:281–84 [DOI] [PubMed] [Google Scholar]
- 63.Conway MJ, Watson AM, Colpitts TM, Dragovic SM, Li Z, et al. 2014. Mosquito saliva serine protease enhances dissemination of dengue virus into the mammalian host. J. Virol. 88:164–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Le Coupanec A, Babin D, Fiette L, Jouvion G, Ave P, et al. 2013. Aedes mosquito saliva modulates Rift Valley fever virus pathogenicity. PLoS Negl. Trop. Dis. 7:e2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Limesand KH, Higgs S, Pearson LD, Beaty BJ. 2000. Potentiation of vesicular stomatitis New Jersey virus infection in mice by mosquito saliva. Parasite Immunol. 22:461–67 [DOI] [PubMed] [Google Scholar]
- 66.Osorio JE, Godsey MS, Defoliart GR, Yuill TM. 1996. La Crosse viremias in white-tailed deer and chipmunks exposed by injection or mosquito bite. Am. J. Trop. Med. Hyg. 54:338–42 [DOI] [PubMed] [Google Scholar]
- 67.Reisen WK, Chiles RE, Kramer LD, Martinez VM, Eldridge BF. 2000. Method of infection does not alter response of chicks and house finches to western equine encephalomyelitis and St. Louis encephalitis viruses. J. Med. Entomol. 37:250–58 [DOI] [PubMed] [Google Scholar]
- 68.Schneider BS, Higgs S. 2008. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans. R. Soc. Trop. Med. Hyg. 102:400–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cox J, Mota J, Sukupolvi-Petty S, Diamond MS, Rico-Hesse R. 2012. Mosquito bite delivery of dengue virus enhances immunogenicity and pathogenesis in humanized mice. J. Virol. 86:7637–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Christofferson RC, McCracken MK, Johnson AM, Chisenhall DM, Mores CN. 2013. Development of a transmission model for dengue virus. Virol. J. 10:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCracken MK, Christofferson RC, Chisenhall DM, Mores CN. 2014. Analysis of early dengue viral infection in mice as modulated by Aedes aegypti probing. J. Virol. 88:1881–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schneider BS, McGee CE, Jordan JM, Stevenson HL, Soong L, Higgs S. 2007. Prior exposure to uninfected mosquitoes enhances mortality in naturally-transmitted West Nile virus infection. PLoS ONE 2:e1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Limesand KH, Higgs S, Pearson LD, Beaty BJ. 2003. Effect of mosquito salivary gland treatment on vesicular stomatitis New Jersey virus replication and interferon α/β expression in vitro. J. Med. Entomol. 40:199–205 [DOI] [PubMed] [Google Scholar]
- 74.Surasombatpattana P, Patramool S, Luplertlop N, Yssel H, Misse D. 2012. Aedes aegypti saliva enhances dengue virus infection of human keratinocytes by suppressing innate immune responses. J. Investig. Dermatol. 132:2103–5 [DOI] [PubMed] [Google Scholar]
- 75.Edwards JF, Higgs S, Beaty BJ. 1998. Mosquito feeding-induced enhancement of Cache Valley virus (Bunyaviridae) infection in mice. J. Med. Entomol. 35:261–65 [DOI] [PubMed] [Google Scholar]
- 76.Ribeiro JM, Area B, Lombardo F, Calvo E, Phan VM, et al. 2007. An annotated catalogue of salivary gland transcripts in the adult female mosquito, Aedes aegypti. BMC Genomics 8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Calvo E, Sanchez-Vargas I, Favreau AJ, Barbian KD, Pham VM, et al. 2010. An insight into the sialotranscriptome of the West Nile mosquito vector, Culex tarsalis. BMC Genomics 11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reagan KL, Machain-Williams C, Wang T, Blair CD. 2012. Immunization of mice with recombinant mosquito salivary protein D7 enhances mortality from subsequent West Nile virus infection via mosquito bite. PLoS Negl. Trop. Dis 6:el935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jacobs JJ, Lehe CL, Hasegawa H, Elliott GR, Das PK. 2006. Skin irritants and contact sensitizers induce Langerhans cell migration and maturation at irritant concentration. Exp. Dermatol. 15:43 2–40 [DOI] [PubMed] [Google Scholar]
- 80.Ouwehand K, Oosterhoff D, Breetveld M, Scheper RJ, de Gruijl TD, Gibbs S. 2011. Irritant-induced migration of Langerhans cells coincides with an IL-10-dependent switch to a macrophage-like phenotype. J. Investig. Dermatol. 131:418–2 5 [DOI] [PubMed] [Google Scholar]
- 81.Price AA, Cumberbatch M, Kimber I, Ager A. 1997. α6 integrins are required for Langerhans cell migration from the epidermis. J. Exp. Med. 186:1725–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hammad H, Lambrecht BN. 2008. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat. Rev. Immunol. 8:193–204 [DOI] [PubMed] [Google Scholar]
- 83.Zeidner NS, Higgs S, Happ CM, Beaty BJ, Miller BR. 1999. Mosquito feeding modulates Th1 and Th2 cytokines in flavivirus susceptible mice: an effect mimicked by injection of sialokinins, but not demonstrated in flavivirus resistant mice. Parasite Immunol. 21:35–44 [DOI] [PubMed] [Google Scholar]
- 84.Wu SJ, Grouard-Vogel G, Sun W, Mascola JR, Brachtel E, et al. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 6:816–20 [DOI] [PubMed] [Google Scholar]
- 85.Fink K, Ng C, Nkenfou C, Vasudevan SG, van Rooijen N, Schul W. 2009. Depletion of macrophages in mice results in higher dengue virus titers and highlights the role of macrophages for virus control. Eur.J. Immunol. 39:2809–21 [DOI] [PubMed] [Google Scholar]
- 86.Farrance CE, Chichester JA, Musiychuk K, Shamloul M, Rhee A, et al. 2011. Antibodies to plantproduced Plasmodium falciparum sexual stage protein Pfs25 exhibit transmission blocking activity. Hum. Vaccines 7(Suppl.): 191–98 [DOI] [PubMed] [Google Scholar]
- 87.Mathias DK, Plieskatt JL, Armistead JS, Bethony JM, Abdul-Majid KB, et al. 2012. Expression, immunogenicity, histopathology, and potency of a mosquito-based malaria transmission-blocking recombinant vaccine. Infect. Immun. 80:1606–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miyata T, Harakuni T, Sugawa H, Sattabongkot J, Kato A, et al. 2011. Adenovirus-vectored Plasmodium vivax ookinete surface protein, Pvs25, as a potential transmission-blocking vaccine. Vaccine 29:2720–26 [DOI] [PubMed] [Google Scholar]
- 89.Williams AR, Zakutansky SE, Miura K, Dicks MD, Churcher TS, et al. 2013. Immunisation against a serine protease inhibitor reduces intensity of Plasmodium berghei infection in mosquitoes. Int. J. Parasitol. 43:869–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kamhawi S, Ramalho-Ortigao M, Pham VM, Kumar S, Lawyer PG, et al. 2004. A role for insect galectins in parasite survival. Cell 119:329–41 [DOI] [PubMed] [Google Scholar]
- 91.Gomes R, Oliveira F, Teixeira C, Meneses C, Gilmore DC, et al. 2012. Immunity to sand fly salivary protein LJM11 modulates host response to vector-transmitted leishmania conferring ulcer-free protection. J. Investig. Dermatol 132:2735–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gomes R, Teixeira C, Teixeira MJ, Oliveira F, Menezes MJ, et al. 2008. Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model. Proc. Natl. Acad. Sci. USA 105:7845–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jones PL, Pask GM, Rinker DC, Zwiebel LJ. 2011. Functional agonism of insect odorant receptor ion channels. Proc. Natl. Acad. Sci. USA 108:8821–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Taylor RW, Romaine IM, Liu C, Murthi P, Jones PL, et al. 2012. Structure-activity relationship of a broad-spectrum insect odorant receptor agonist. ACS Chem. Biol. 7:1647–52 [DOI] [PubMed] [Google Scholar]
- 95.Laven H. 1967. Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature 216:383–84 [DOI] [PubMed] [Google Scholar]
- 96.Dobson SL, Fox CW, Jiggins FM. 2002. The effect of Wolbachia-induced cytoplasmic incompatibility on host population size in natural and manipulated systems. Proc. R. Soc. B 269:437–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mousson L, Zouache K, Arias-Goeta C, Raquin V, Mavingui P, Failloux AB. 2012. The native Wolbachia symbionts limit transmission of dengue virus in Aedes albopictus. PLoS Negl. Trop. Dis 6:el989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Glaser RL, Meola A1A 2010. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS ONE 5:el1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rlagrove MS, Arias-Goeta C, Di Genua C, Failloux AB, Sinkins SP. 2013. A Wolbachia wMel transinfection in Aedes albopictus is not detrimental to host fitness and inhibits chikungunya virus. PLoS Negl. Trop. Dis 7:e2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xi Z, Khoo CC, Dobson SL. 2005. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 310:326–28 [DOI] [PubMed] [Google Scholar]
- 101.McMeniman CJ, Lane RV, Cass BN, Fong AW, Sidhu M, et al. 2009. Stable introduction of a lifeshortening Wolbachia infection into the mosquito Aedes aegypti. Science 323:141–44 [DOI] [PubMed] [Google Scholar]
- 102.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, et al. 2011. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476:450–53 [DOI] [PubMed] [Google Scholar]
- 103.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, et al. 2009. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell 139:1268–78 [DOI] [PubMed] [Google Scholar]
- 104.Bian G, Xu Y, Lu P, Xie Y, Xi Z. 2010. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 6:e 1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, et al. 2011. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476:454–57 [DOI] [PubMed] [Google Scholar]
- 106.Burivong P, Pattanakitsakul SN, Thongrungkiat S, Malasit P, Flegel TW. 2004. Markedly reduced severity of dengue virus infection in mosquito cell cultures persistently infected with Aedes albopictus densovirus (AalDNV). Virology 329:261–69 [DOI] [PubMed] [Google Scholar]
- 107.Hirunkanokpun S, Carlson J O, Kittayapong P. 2008. Evaluation of mosquito densoviruses for controlling Aedes aegypti (Diptera: Culicidae): variation in efficiency due to virus strain and geographic origin of mosquitoes. Am. J. Trop. Med. Hyg. 78:784–90 [PubMed] [Google Scholar]
- 108.Afanasiev BN, Kozlov YV, Carlson JO, Beaty BJ. 1994. Densovirus of Aedes aegypti as an expression vector in mosquito cells. Exp. Parasitol. 79:322–39 [DOI] [PubMed] [Google Scholar]
- 109.Fu G, Lees RS, Nimmo D, Aw D, Jin L, et al. 2010. Female-specific flightless phenotype for mosquito control. Proc. Natl. Acad. Sci. USA 107:4550–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Phuc HK, Andreasen MH, Burton RS, Vass C, Epton MJ, et al. 2007. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol. 5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bargielowski I, Nimmo D, Alphey L, Koella JC. 2011. Comparison of life history characteristics of the genetically modified OX513 A line and a wild type strain of Aedes aegypti. PLoS ONE 6:e20699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harris AF, McKemey AR, Nimmo D, Curtis Z, Black I, et al. 2012. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat. Biotechnol. 30:828–30 [DOI] [PubMed] [Google Scholar]
- 113.Franz AW, Sanchez-Vargas I, Adelman ZN, Blair CD, Beaty BJ, et al. 2006. Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc. Natl. Acad. Sci. USA 103:4198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Deredec A, Godfray HC, Burt A. 2011. Requirements for effective malaria control with homing endonuclease genes. Proc. Natl. Acad. Sci. USA 108:E874–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Traver BE, Anderson MA, Adelman ZN. 2009. Homing endonucleases catalyze double-stranded DNA breaks and somatic transgene excision in Aedes aegypti. Insect. Mol. Biol. 18:623–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cheng G, Cox J, Wang P, Krishnan AIN, Dai J, et al. 2010. A C-type lectin collaborates with a CD45 phosphatase homolog to facilitate West Nile virus infection of mosquitoes. Cell 142:714–25 [DOI] [PMC free article] [PubMed] [Google Scholar]