Abstract
Objective.
To evaluate inheritance patterns and define the familial clustering rate of idiopathic subglottic stenosis (iSGS).
Study Design.
Retrospective observational study.
Setting.
International multicenter collaborative of >30 tertiary care centers.
Methods.
Patients with a clinically confirmed iSGS diagnosis within the North American Airway Collaborative’s iSGS1000 cohort consented between 2014 and 2018 were eligible for enrollment. Patient demographics and disease severity were abstracted from the collaborative’s iSGS longitudinal registry. Pedigrees of affected families were created.
Results.
A total of 810 patients with iSGS were identified. Positive family history for iSGS was reported in 44 patients in 20 families. The rate of familial clustering in iSGS is 2.5%. Mean age of disease onset is 42.6 years. Of the 44 patients with familial aggregation of iSGS, 42 were female and 2 were male; 13 were mother-daughter pairs and 2 were father-daughter pairs. There were 3 sister-sister pairs. There was 1 niece-aunt pair and 2 groups of 3 family members. One pedigree demonstrated 2 affected mother-daughter pairs, with the mothers being first-degree paternal cousins. Inheritance is non-Mendelian, and anticipation is present in 11 of 13 (84%) parent-offspring pairs. The mean age of onset between parents (48.4 years) and offspring (36.1 years) was significantly different (P = .016).
Conclusion.
This study quantifies the rate of familial clustering of iSGS at 2.5%. Inheritance is non-Mendelian, and disease demonstrates anticipation. These data suggest that there may be a genetic contribution in iSGS.
Keywords: idiopathic subglottic stenosis, laryngotracheal stenosis
Idiopathic subglottic stenosis (iSGS) is a rare (1:400,000 people per year)1 but devastating fibroinflammatory airway disease that occurs almost exclusively in adult Caucasian women.2 The disease is characterized by mucosal inflammation and localized fibrosis resulting in luminal obstruction of the upper airway.3 Because of high recurrence rates, many patients with iSGS will require multiple surgical procedures following their initial diagnosis.4 Given the significant emotional, physical, and financial costs associated with recurrent airway obstruction,5 most research efforts have focused on surgical techniques to improve patency rates. Highly focused scientific approaches to identify key elements of iSGS disease pathophysiology are critical to developing improved therapies.
Given the homogenous demographic and clinical characteristics of the iSGS patient population, several authors have postulated a genetic basis for disease.3 While clinical experience does not demonstrate a Mendelian inheritance pattern (diseases transmitted through generations in predictable ratios that are consistent with single-gene inheritance), a genetic contribution to disease susceptibility or severity cannot be excluded and warrants further investigation.
The first step in studying the genetic contribution to disease is through examining familial aggregation. Clustering of disease within a family may be due to genetic or environmental factors, but by studying affected individuals and their affected family members, attention may be placed on detecting shared exposures or potential inheritance patterns of the disease.6 Although there are limited data regarding familial clustering of iSGS, some familial aggregation has been reported. In 2013, 2 pairs of sisters and a mother and daughter with iSGS were described.7 However, the study reported only 6 patients with no iSGS prevalence data. To examine the prevalence of familial aggregation in iSGS, we utilized the North American Airway Collaborative (NoAAC) iSGS1000 cohort.2 We identified patients with iSGS with affected family members and analyzed their pedigrees to define the rate of familial clustering in iSGS.
Methods
This multicenter observational study was performed in accordance with the Declaration of Helsinki, good clinical practice, and applicable regulatory requirements. The study and clinical review of patient data were approved by the Institutional Review Board at all sites.
Participants
Patients with iSGS who met established diagnostic criteria were candidates in this study.2,8 Included were adult patients (> 18 years of age) with pathology and narrowing of the subglottis without vasculitis or positive autoimmune titers. Excluded were patients with known airway injury (intubation, caustic, or thermal), patients with a tracheostomy within the past 2 years, and patients without a confirmable index surgical procedure date.9 A set of monozygotic twins was excluded as they are considered genetically identical.
Setting
Patients were recruited by clinicians in the NoAAC network, which consists of 30 participating tertiary care centers throughout the United States and internationally that are referral centers for iSGS.2,4,10–13 Patients were also identified via online communities and patient advocacy groups, who directly contacted the study coordination team and subsequently consented to participate in the trial.
Study Protocol
Patient clinical characteristics and demographic information were extracted from the medical record. Demographic information included age, race, sex, body mass index (BMI), birth year, and medical comorbidities.
Statistical Analysis
Data management and analysis were performed with Prism 8 (GraphPad) and Microsoft Excel. Univariate analyses were performed with the Student’s t test, Fisher’s exact tests, and chi-square tests to compare 2 populations when examining difference in demographic factors, including age, ethnicity, BMI, as well as disease severity (defined as the interval between operative procedures to restore luminal caliber) and age of disease onset. Statistical significance was considered when the 2-sided P value was <.05.
The rate of familial clustering was defined as the number of representative familial groups divided by the total number of patients with iSGS. In parental pairs, disease onset intervals were calculated with age and year of symptom onset. The difference in year of onset is defined by subtracting the year in which a patient developed symptoms of iSGS from the year of onset of the affected family member. The relative difference in age of onset is calculated by subtracting the parent’s age of onset from the offspring’s age of onset; a negative number indicates that offspring presented at an earlier age than the parent. Disease severity was measured by the interval between operative procedures to restore luminal caliber. This interval was calculated by dividing the period (in months) from the date of disease onset to the date of last follow-up by the number of operative interventions performed.2 Patients with only 1 intervention recorded were excluded from this calculation.
Results
Demographic Data
Of 1056 patients in the NoAAC iSGS1000 cohort, 810 with iSGS met inclusion criteria. Table 1 demonstrates the baseline clinical characteristics of patients with iSGS and familial aggregation as compared with those with iSGS and no affected family members. The rate of familial clustering in our study was 2.5%. We identified a total of 44 patients in 20 groups with a positive family history of iSGS. All but 2 patients in our cohort are female, and all patients report Northern European ancestry. Of note, 2 sets of affected identical twins were found in the NoAAC but were excluded from this study, since they are considered genetically indistinguishable. There were no significant differences in the percentage of female patients, mean age at onset, ethnicity, BMI, or disease severity.
Table 1.
Clinical Characteristics of Patients With iSGS: Comparison Between Those With and Without Affected Relatives.a
| Familial iSGS | iSGS without affected relatives | P value | |
|---|---|---|---|
| Patients | 44 (5.4) | 766 (94.6) | |
| Familial groups | 20 (2.5) | ||
| Female sex | 42 (95.5) | 756 (98.7) | .08 |
| Age at onset, y | 42.6 ± 11.8 | 43.7 ± 12.6 | .36 |
| Ethnicity | |||
| Caucasian | 44 (100) | 743 (97.0) | .24 |
| Hispanic | 0 (0) | 17 (2.2) | |
| Black | 0 (0) | 1 (0.1) | |
| Other | 0 (0) | 5 (0.7) | |
| Body mass index, kg/m2 | 26.9 ± 5.2 | 29.3 ± 9.9 | .17 |
| Dilation interval, mo | 18.4 ± 20.0 | 19.4 ± 16.8 | .81 |
| Pairs | |||
| Mother-daughter | 13 | ||
| Sister-sister | 3 | ||
| Father-daughter | 2 | ||
| Niece-aunt | 1 | ||
| Group of 3 | |||
| Niece, aunt, cousin | 1 | ||
| Mother, 2 daughters | 1 | ||
Abbreviation: iSGS, idiopathic subglottic stenosis.
Values are presented as No. (%) or mean ± SD.
Table 2 demonstrates the clinical characteristics of pairings and groups of patients with iSGS and familial aggregation. For each parent-offspring pair, we evaluated whether disease presented at a similar time by calculating the difference in year of onset; the mean ± SD was 8.9 ± 10.9 years. Difference in age of onset was also calculated for each pair. The relative difference in age of onset was −12.3 ± 15.8 years. Dilation intervals ranged from 2.6 to 72 months. Anticipation, defined as whether a child presented at a younger age than the affected parent, is indicated by a negative value for relative difference in age of onset. This occurred in 11 out of 13 parental pairs (84.6%; Table 3). The mean age of onset between parents (48.4 years) and offspring (36.1 years) was significantly different (P = .016).
Table 2.
Clinical Characteristics of Patients With Idiopathic Subglottic Stenosis and Paired Affected Family Members.
| Pair/group: relationship | Sex | Birth year | Onset year | Onset age, y | Anticipation present? | Difference in year of onset, y | Relative difference in age of onset, y | Dilation interval, mo |
|---|---|---|---|---|---|---|---|---|
| 1 | ||||||||
| Daughter | F | 1971 | 2008 | 37 | Y | 4 | −18 | 38 |
| Mother | F | 1949 | 2004 | 55 | 7.5 | |||
| 2 | ||||||||
| Daughter | F | 1970 | 2011 | 41 | Y | 9 | −1 | |
| Mother | F | 1950 | 1992 | 42 | 6 | |||
| Paternal cousin | F | |||||||
| Daughter of paternal cousin | F | |||||||
| 3 | ||||||||
| Daughter | F | 1974 | 2015 | 41 | Y | 3 | −15 | |
| Mother | F | 1956 | 2012 | 56 | 6.3 | |||
| 4 | ||||||||
| Sister | F | 1980 | 2016 | 36 | NA | 3 | NA | |
| Sister | F | 1981 | 2013 | 32 | 4.7 | |||
| 5 | ||||||||
| Daughter | F | 1978 | 2008 | 30 | Y | 4 | −22 | |
| Father | M | 1952 | 2004 | 52 | 6 | |||
| 6 | ||||||||
| Daughter | F | 1969 | 2014 | 45 | N | +2 | ||
| Mother | F | 43 | ||||||
| 7 | ||||||||
| Daughter | F | |||||||
| Mother | F | |||||||
| 8 | ||||||||
| Daughter | F | 1979 | 2005 | 26 | Y | 5 | −22 | |
| Father | M | 1952 | 2000 | 48 | ||||
| 9 | ||||||||
| Daughter | F | 1956 | 2012 | 56 | Y | 22 | −2 | |
| Mother | F | 1932 | 1990 | 58 | 51 | |||
| 10 | ||||||||
| Daughter | F | 1950 | 2005 | 55 | N | 35 | +12 | |
| Mother | F | 1927 | 1970 | 43 | ||||
| 11 | ||||||||
| Daughter | F | 1973 | 2016 | 43 | ||||
| Mother | F | |||||||
| 12 | ||||||||
| Niece | F | 1973 | 2013 | 40 | NA | 3.5 | ||
| Maternal aunt | F | |||||||
| Cousin | F | |||||||
| 13 | ||||||||
| Paternal aunt | F | 1979 | 2013 | 34 | NA | 9 | ||
| Niece | F | |||||||
| 14 | ||||||||
| Sister | F | 1963 | 2005 | 42 | NA | 2 | ||
| Sister | F | 1965 | 2007 | 42 | 16.8 | |||
| 15 | ||||||||
| Daughter | F | 1998 | 2012 | 14 | Y | 2 | −40 | 2.6 |
| Mother | F | 1960 | 2014 | 54 | 6.5 | |||
| 16 | ||||||||
| Daughter | F | 1977 | 2005 | 33 | Y | 13 | −4 | |
| Mother | F | 1955 | 1992 | 37 | 72 | |||
| 17 | ||||||||
| Daughter | F | 1952 | 1994 | 42 | 39.4 | |||
| Mother | F | |||||||
| 18 | ||||||||
| Daughter | F | 1985 | 2017 | 32 | Y | 26 | −1 | 5.3 |
| Daughter | F | 1987 | 2011 | 24 | Y | 20 | −9 | 15.8 |
| Mother | F | 1957 | 1991 | 33 | 22.4 | |||
| 19 | ||||||||
| Daughter | F | 1965 | 2000 | 35 | Y | 11 | −40 | |
| Mother | F | 1936 | 2011 | 75 | ||||
| 20 | ||||||||
| Sister | F | |||||||
| Sister | F | |||||||
| Mean ± SD | 42.6 ± 11.8 | 84.6% | 8.9 ± 10.9 | −12.3 ± 15.8 | 18.4 ± 20.0 | |||
Abbreviation: NA, not applicable.
Table 3.
Parental Pairs: Age of Onset Comparison.
| Age, y |
||
|---|---|---|
| Pair | Parent | Offspring |
| 1 | 55 | 37 |
| 2 | 42 | 41 |
| 3 | 56 | 41 |
| 5 | 52 | 30 |
| 6 | 43 | 45 |
| 8 | 48 | 26 |
| 9 | 58 | 56 |
| 10 | 43 | 55 |
| 15 | 54 | 14 |
| 16 | 37 | 33 |
| 18 | 33 | 32 |
| 18 | 33 | 24 |
| 19 | 75 | 35 |
| Mean ± SDa | 48.4 ± 11.3 | 36.1 ± 11.4 |
P = .016.
The most frequent medical comorbidities were gastroesophageal reflux disease (6 of 31, 19.4%), asthma (5 of 31, 16.1%), and hypothyroidism (5 of 31, 16.1%).
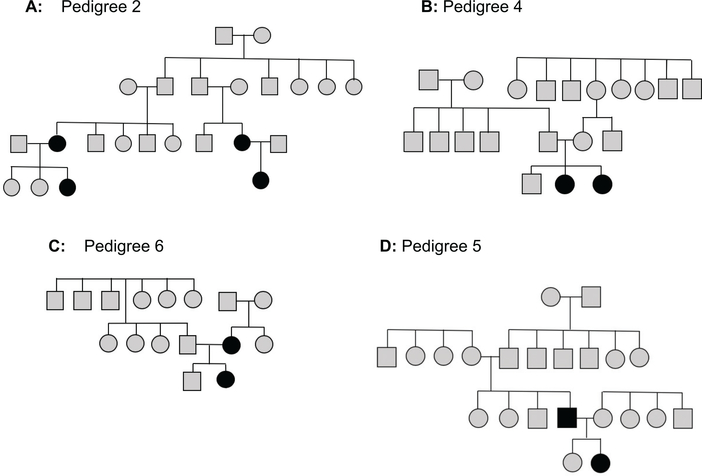
Figure 1 displays representative pedigrees of affected relatives. Figure 1A displays a lineage with 2 motherdaughter affected pairs who are related paternally. Figure 1B is an example of sisters with iSGS. Figure 1C shows a pedigree with a mother and daughter who are affected. Figure 1D shows a pedigree with an affected father-daughter pair. A comprehensive compilation of pedigrees for all affected groups is displayed in Supplemental Figure S1 (available online).
Figure 1.
Sample pedigrees. A filled-in marker denotes idiopathic subglottic stenosis. (A) Paternally related mother-daughter pairs. (B) Affected sisters. (C) A mother-daughter pair. (D) A father-daughter pair.
Discussion
Our study represents an initial investigation into familial iSGS by studying pedigrees, patterns of disease onset, and disease severity in 20 familial groups. We define the rate of familial aggregation in iSGS as 2.5%, and pedigree analysis shows a non-Mendelian pattern of inheritance. Disease is not present in every generation in an autosomal dominant fashion, nor does it necessitate 2 alleles for disease expression as an autosomal recessive disease. The cohort had a range of disease severity, as represented by the mean dilation interval, which suggests variable expression of disease. Furthermore, disease inheritance patterns exhibit anticipation, as offspring in parent-daughter pairs developed disease earlier than their parents. These findings suggest a hereditary basis for familial iSGS, although other determinants may modulate disease development.
Epidemiologic studies have characterized the patient population of iSGS as being demographically homogenous. The disease tends to present in perimenopausal Caucasian females without comorbid disease. In addition to demographic uniformity, iSGS is geographically restricted and rarely seen in areas such as Mediterranean Europe, Asia, and Africa.3 The new finding of familial clustering in iSGS is evidence of a genetic component of disease. We subsequently explored other clinical characteristics in our cohort to elucidate a potential genetic pathogenesis of iSGS.
While the demographics of our familial iSGS cohort are consistent with previously described homogeneity,3 disease severity, as measured by mean surgical interval for each individual, varied widely. The mean interval between surgical interventions ranged from 2.6 to 72 months (Table 2). This variation of disease severity among individuals with a potentially genetic disease underscores the complexity of disease inheritance. Expressivity is the result of variation in allelic composition. In many genetic diseases, an allele may not be fully penetrant, and this leads to inconsistency and diversity in phenotype.14 There are several hypotheses for differential expression of disease, including microRNA that regulates genes at a posttranscriptional level and affect phenotypic outcomes.15 The finding of variable expression in familial iSGS indicates that there are likely additional determinants that regulate phenotypic manifestation of disease.
A second clinical finding of anticipation suggests that familial iSGS may be genetic. In anticipation, the disease becomes more severe and assumes earlier expression in each generation. This phenomenon is due to expanded triplets: repetitive DNA sequences that show a high degree of mutation.16 These unstable DNA sequences, which comprise 30% of eukaryotic DNA,17 increase in number from one generation to the next and result in variable expression of disease. Anticipation has been well characterized in neurodegenerative disorders, most notably Huntington’s disease,18,19 as well as genetically complex disorders, including autoinflammatory conditions such as Crohn’s disease20 and psoriasis.21 In cases of genetic anticipation, parental sex can influence disease severity. While men rarely present with iSGS,2 our study included 2 men with daughters who developed stenosis at an age 22 years younger than their fathers’ presentation age. This pattern in the 2 father-daughter pairs with iSGS parallels Huntington’s disease, in which descendants of affected males have an earlier onset of the disease when compared with descendants of affected women.22
A third clinical characteristic studied in each familial pair was year of disease onset to consider the possibility of an external environmental exposure as a cause of iSGS. In the absence of gene-environmental studies, such as family-based monozygotic and dizygotic twin studies, estimates of genetic and environmental contributions to disease phenotypes and the interactions between them are difficult to partition and quantify. It is suggestive of a simultaneous exposure if disease onsets are temporally congregated, similar to acquisition of an infectious disease. The interval between successive cases of a disease is a measure used to interpret epidemiologic spread of disease.23 To investigate potential temporal association, we examined year of onset of disease. There was no consistent interval identified; years ranged from as few as 2 years between diagnosis to 35 years, suggesting that iSGS is not solely caused by an environmental exposure.
We hypothesize that familial patients with iSGS may possess a genotype rendering them more susceptible to disease initiation, which may explain the lack of full penetrance throughout generations. Depending on an individual’s inherited genotype, perhaps an external exposure may provide the “second hit” and create the iSGS phenotype in these familial groups. Both the underlying genotype and the identity and/or role of a “second hit” remain undefined. In other more prevalent fibrotic airway diseases that have been studied in greater depth, such as idiopathic pulmonary fibrosis, most patients do not have an affected family member. For these sporadic patients, weaker genetic risk alleles may contribute to the pathogenesis of fibrosis. Less commonly, patients with idiopathic pulmonary fibrosis have similarly affected family members and share more highly penetrant genetic risk alleles.24 These hypotheses may be generalized to patients with iSGS. We anticipate future studies identifying genes of interest in familial iSGS that may then be validated in a sporadic iSGS cohort.
Our study has limitations. Our cohort is small and reflects the rare prevalence of familial iSGS. Even so, these data are strengthened by our large multi-institutional collective of patients with iSGS. Broadly speaking, diseases characterized by progressive fibrosis, especially those of late onset, are complex and difficult to study. Environmental triggers are challenging to define. While we did study the year of disease onset in familial pairs as a proxy for an environmental cause, we were not able to evaluate patients’ and family members’ living locations at the time of disease onset, limiting our evaluation of geographically shared exposures. It will be beneficial to consider these factors as patient data are collected prospectively. Even with this constraint, the identification of these familial pairs facilitates future genetic studies to identify alleles that portend greater risk of disease.
In conclusion, this study reports novel clinical data about familial aggregation of iSGS and informs our understanding of iSGS. We define the rate of familial aggregation as 2.5%. We also provide data supporting a non-Mendelian pattern of disease inheritance and demonstrate anticipation in 11 of 13 parental pairs. The presence of anticipation is suggestive of a genetic contribution to familial iSGS. Further studies are necessary investigating the genomic composition of these familial pairs. Future directions include whole exome sequencing for comparison of affected family members to nonaffected relatives to identify candidate genes. Candidate genes uncovered in the familial pairs described in this work can then be validated in the larger iSGS1000 cohort to confirm their impact on iSGS disease pathogenesis.
Supplementary Material
Acknowledgments
Funding source: The National Institute on Deafness and Other Communication Disorders, National Institutes of Health (1K23D C014082 and 1R21DC017225 to A.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study was also financially supported by the Triological Society and American College of Surgeons (A.H.).
Footnotes
Disclosures
Competing interests: Alexander Hillel, Ambu, consultant fees.
Sponsorships: None.
Supplemental Material
Additional supporting information is available in the online version of the article.
References
- 1.Maldonado F, Loiselle A, DePew ZS, et al. Idiopathic subglottic stenosis: an evolving therapeutic algorithm. Laryngoscope. 2014; 124(2):498–503. doi: 10.1002/lary.24287 [DOI] [PubMed] [Google Scholar]
- 2.Gelbard A, Anderson C, Berry LD, et al. Comparative treatment outcomes for patients with idiopathic subglottic stenosis. JAMA Otolaryngol Neck Surg. 2019; 146(1): 1–10. doi: 10.1001/jamaoto.2019.3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gelbard A, Donovan DT, Ongkasuwan J, et al. Disease homogeneity and treatment heterogeneity in idiopathic subglottic stenosis. Laryngoscope. 2016; 126(6): 1390–1396. doi: 10.1002/lary.25708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hseu AF, Benninger MS, Haffey TM, Lorenz R. Subglottic stenosis: a ten-year review of treatment outcomes. Laryngoscope. 2014; 124(3):736–741. doi: 10.1002/lary.24410 [DOI] [PubMed] [Google Scholar]
- 5.Gnagi SH, Howard BE, Anderson C, Lott DG. Idiopathic subglottic and tracheal stenosis: a survey of the patient experience. Ann Otol Rhinol Laryngol. 2015;124(9):734–739. doi: 10.1177/0003489415582255 [DOI] [PubMed] [Google Scholar]
- 6.Matthews A, Finkelstein D, Betensky R. Analysis of familial aggregation studies with complex ascertainment schemes. Stat Med. 2008;27:5076–5092. doi: 10.1002/sim.3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dmnoulin E, Stather DR, Gelfand G, Maranda B, MacEachem P, Tremblay A. Idiopathic subglottic stenosis: a familial predisposition. Ann Thorac Surg. 2013;95(3):1084–1086. doi: 10.1016/j.athoracsur.2012.07.076 [DOI] [PubMed] [Google Scholar]
- 8.Gelbard A, Shyr Y, Berry L, et al. Treatment options in idiopathic subglottic stenosis: protocol for a prospective international multicentre pragmatic trial. BMJ Open. 2018;8(4): e022243. doi: 10.1136/bmjopen-2018-022243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nouraei S a. R, Sandliu GS. Outcome of a multimodality approach to the management of idiopathic subglottic stenosis. Laryngoscope. 2013;123(10):2474–2484. doi: 10.1002/lary.23949 [DOI] [PubMed] [Google Scholar]
- 10.Gelbard A, Francis DO, Sandulache VC, Simmons JC, Donovan DT, Ongkasuwan J. Causes and consequences of adult laryngotracheal stenosis. Laryngoscope. 2015;125(5):1137–1143. doi: 10.1002/lary.24956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dedo HH, Catten MD. Idiopathic progressive subglottic stenosis: findings and treatment in 52 patients. Ann Otol Rhinol Latyngol. 2001; 110(4):305–311. doi: 10.1177/000348940111000403 [DOI] [PubMed] [Google Scholar]
- 12.Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope. 2009; 119(2):272–283. doi: 10.1002/lary.20056 [DOI] [PubMed] [Google Scholar]
- 13.Taylor SC, Clayburgli DR, Rosenbaum JT, Schindler JS. Clinical manifestations and treatment of idiopathic and Wegener granulomatosis-associated subglottic stenosis. JAMA Otolaryngol Head Neck Surg. 2013;139(1):76–81. doi: 10.1001/jamaoto.2013.1135 [DOI] [PubMed] [Google Scholar]
- 14.Griffiths AJ, Miller JH, Suzuki DT, Lewontin RC, Gelbart WM. Penetrance and expressivity In: An Introduction to Genetic Analysis. 7th ed. W. H. Freeman; 2000. https://www.ncbi.nlm.nih.gov/books/NBK22090/ [Google Scholar]
- 15.Ahluwalia JK, Hariharan M, Bargaje R, Pillai B, Brahmachari V. Incomplete penetrance and variable expressivity: is there a microRNA connection? BioEssays. 2009;31(9):981–992. doi: 10.1002/bies.200900066 [DOI] [PubMed] [Google Scholar]
- 16.Sobczak K, Michlewski G, de Mezer M, et al. Structural diversity of triplet repeat RNAs. J Biol Chem. 2010;285(17):12755–12764.. doi: 10.1074/jbc.M109.078790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMurray CT. Mechanisms of trinucleotide repeat instability during human development. Nat Rev Genet. 2010;11(11 ):786–799.. doi: 10.1038/nrg2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross CA, McInnis MG, Margolis RL, Li SH. Genes with triplet repeats: candidate mediators of neuropsychiatric disorders. Trends Neurosci. 1993;16(7):254–260. doi: 10.1016/0166-2236(93)90175-1 [DOI] [PubMed] [Google Scholar]
- 19.Teisberg P. The genetic background of anticipation. J R Soc Med. 1995;88(4): 185–187. [PMC free article] [PubMed] [Google Scholar]
- 20.Heresbach D, Gulwani-Akolkar B, Lesser M, et al. Anticipation in Crohn’s disease may be influenced by gender and ethnicity of the transmitting parent. Am J Gastroenterol. 1998;93(12):2368–2372.. doi: 10.1111/j.1572-0241.1998.00689.x [DOI] [PubMed] [Google Scholar]
- 21.Zheng CJ, Thomson G, Pen YN. Allelic instability in mitosis can explain “genome imprinting” and other genetic phenomena in psoriasis. Am J Med Genet. 1994;51 (2): 163–164. doi: 10.1002/ajmg.1320510218 [DOI] [PubMed] [Google Scholar]
- 22.Ridley RM, Frith CD, Crow TJ, Comnneally PM. Anticipation in Huntington’s disease is inherited through the male line but may originate in the female. J Med Genet. 1988;25(9):589–595. doi: 10.1136/jmg.25.9.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine PEM. The interval between successive cases of an infectious disease. Am J Epidemiol. 2003;158(11):1039–1047. doi: 10.1093/aje/kwg251 [DOI] [PubMed] [Google Scholar]
- 24.Garcia CK. Insights from human genetic studies of lung and organ fibrosis. J Clin Invest. 2018; 128(1):36–44. doi: 10.1172/JCI93556 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.