Abstract
The endocannabinoid system is involved in the regulation of the stress response, but the relative contribution of N-arachidonoylethanolamine (AEA) and 2-arachidonoylglycerol (2-AG) and their mechanisms have to be elucidated. In this study, we compared the effects of the pharmacological inhibition of the two major endocannabinoid-degrading enzymes [fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) for AEA and 2-AG, respectively] on stress-coping [forced swim test (FST) and tail suspension test (TST)] and anxiety-like [elevated-plus maze (EPM) and light-dark test (LDT)] behaviors in wild-type and FAAH knockout mice. In vivo microdialysis estimated the effects of FAAH and MAGL inhibition on dopamine (DA) and serotonin (5-HT) levels in the medial prefrontal cortex (mPFC) during an FST. Mice were treated with PF-3845 (FAAH inhibitor), JZL184 (MAGL inhibitor), JZL195 (dual FAAH/MAGL inhibitor) or vehicle. Our data showed that PF-3845 increased latency to immobility and decreased total immobility time in FST, but no effects were observed in TST compared with vehicle-treated wild-type mice. By contrast, JZL184 decreased latency and increased immobility in TST and FST. JZL195 in wild-type mice and JZL184 in FAAH knockout mice reproduced the same passive coping behaviors as JZL184 in wild-type mice in TST and FST. In the microdialysis experiment, FST was associated with increased DA and 5-HT levels in the mPFC. However, JZL184-treated wild-type mice displayed a significant attenuation of forced swim stress-induced DA release compared with vehicle-treated wild-type mice and PF-3845-treated wild-type mice. Finally, FAAH and/or MAGL inhibitors induced robust and consistent anxiolytic-like effects in EPM and LDT. These results suggested differences between FAAH and MAGL inhibition in stress-coping behaviors. Notably, MAGL inhibition induced a consistent avoidant coping behavior and attenuated the stress-induced mPFC DA response in FST. However, more investigation is needed to elucidate the functional association between DA and 2-AG signaling pathways, and the molecular mechanism in the regulation of passive coping strategies during inescapable stress.
Keywords: 2-Arachidonoylglycerol, Stress-coping behavior, Dopamine, Mouse, Microdialysis
Highlights
-
•
FAAH and/or MAGL inhibition induce opposite changes in stress-coping behaviors.
-
•
MAGL inhibition increases passive stress-coping behaviors in mice.
-
•
Passive stress-coping behaviors are regulated by 2-AG rather than AEA signaling.
-
•
MAGL inhibition attenuates mPFC dopamine increase in the forced swim test.
-
•
FAAH and/or MAGL inhibitors are associated with anxiolytic-like effects.
Abbreviations
- AEA
N-arachidonoylethanolamine
- ANOVA
analysis of variance
- AUC
area under the curve
- CB
cannabinoid receptor
- DA
dopamine
- EPM
elevated-plus maze
- FAAH
fatty acid amide hydrolase
- FAAH KO
FAAH knockout
- FST
forced swim test
- LDT
light-dark test
- MAGL
monoacylglycerol lipase
- mPFC
medial prefrontal cortex
- NAc
nucleus accumbens
- OEA
N-oleoylethanolamine
- OTR
open-arm time ratio
- PPAR-α
Peroxisome proliferator-activated receptor alpha
- SEM
standard error of the mean
- TRPV1
transient receptor potential vanilloid type-1 channel
- TST
tail suspension test
- VTA
ventral tegmental area
- WT
wild-type
- 2-AG
2-arachidonoylglycerol
- 5-HT
serotonin
1. Introduction
The endogenous cannabinoid system is a neuromodulatory lipid system that is involved in the regulation of numerous physiological functions. N-arachidonoylethanolamine (anandamide, AEA) and 2-arachidonoylglycerol (2-AG) are the best characterized endogenous ligands that act on the two major cannabinoid receptors (CB1 and CB2) (Devane et al., 1992; Sugiura et al., 1995). Unlike classical neurotransmitters, the endocannabinoids are primarily synthesized on demand in the postsynaptic neuron and exert their signaling effects via activation of CB1 expressed on presynaptic terminals to suppress neurotransmitters release. Inactivation of endocannabinoid signaling occurs by enzymatic hydrolysis mediated by multiple enzymes, being the serine hydrolases fatty acid amide hydrolase (FAAH) and the monoacylglycerol lipase (MAGL) the major enzymes responsible for the degradation of AEA and 2-AG, respectively (Blankman and Cravatt, 2013).
A growing body of evidence implicates the endocannabinoid system as an integral regulator of stress responses (for review see (Morena et al., 2016)). In fact, CB1 receptors and the enzymes contributing to endocannabinoid metabolism are prominently expressed in the mesocorticolimbic dopamine (DA) system, which regulates emotion, reward and stress (Covey et al., 2017; Sullivan et al., 2014), involving brain regions such as the ventral tegmental area (VTA), nucleus accumbens (NAc), amygdala and prefrontal cortex (PFC). Stress exposure alters endocannabinoid levels in both human and rodents, although bidirectional effects on brain levels of AEA and 2-AG have been described (Dlugos et al., 2012; Hill et al., 2009; Patel and Hillard, 2008). Thus, stress has been reported to induce rapid and transient decrements in brain AEA levels, and somewhat delayed but more sustained elevations in brain 2-AG levels (Morena et al., 2016). Moreover, chronic stress is generally associated with a down-regulation of CB1 receptors in most brain regions except for the PFC (Lee and Hill, 2013; McLaughlin et al., 2013). In addition, pharmacological or genetic inhibition of the endocannabinoid signaling consistently produces a phenotype similar to classical stress responses, including increased anxiety-related behaviors and activation of hypothalamic-pituitary-adrenal axis (Bellocchio et al., 2013; Haller et al., 2004; Patel et al., 2004).
Given that the development of emotional disorders is associated with stress and the endocannabinoid influence in the regulation of stress responses, it is conceivable that a dysregulation of endocannabinoid function may contribute to abnormal anxiety- and depression-related behaviors. In this regard, a substantial preclinical literature indicates that elevation of interstitial AEA levels through inhibition of FAAH activity produces anxiolytic-like effects, mainly under stressful or aversive conditions (Bedse et al., 2018; Marco et al., 2015; Moreira et al., 2008; Natividad et al., 2017), and decreases passive coping in classical models of depression or behavioral despair such as the forced swim test (FST) and the tail suspension test (TST) (Gobbi et al., 2005; Hill and Gorzalka, 2005; Naidu et al., 2007; Wang and Zhang, 2017). Similar to the results from pharmacological studies, mice lacking FAAH also display reduced anxiety-like behavior under anxiogenic testing conditions and reduced immobility time in both the TST and FST (Bambico et al., 2007, 2010; Moreira et al., 2008; Naidu et al., 2007). While all these previous studies in rodents have shown the role that AEA/FAAH system plays on the regulation of emotional behaviors, the involvement of 2-AG/MAGL system is only beginning to be elucidated. Similar to FAAH inhibition, MAGL inhibition has been reported to produce anxiolytic-like effects in heightened stress conditions (Aliczki et al., 2012, 2013; Bedse et al., 2018; Busquets-Garcia et al., 2011; Serrano et al., 2018). Regarding coping behaviors for stress, it has been reported a dose-dependent biphasic effect of the selective MAGL inhibitor JZL184 (Long et al., 2009a) on immobility of acutely stressed mice (Wang et al., 2017). Other studies have shown that repeated treatment with JZL184 decreases passive coping behavior in chronically stressed mice (Zhang et al., 2015; Zhong et al., 2014). In addition to selective inhibitors of either FAAH or MAGL, dual inhibitors of both endocannabinoid-degrading enzymes have been also developed (e.g., JZL195) (Long et al., 2009c). Unlike FAAH or MAGL inhibition, dual blockade of FAAH and MAGL is not able to prevent stress-induced anxiety and even has been reported to induce anxiogenic-like effects (Bedse et al., 2018; Manduca et al., 2015). In contrast to anxiety-like behaviors, there is little information on stress-induced coping behaviors.
Evidence shows that the DA and serotonin (5-HT) systems play a key role in the response to stress, and cortical and limbic areas contribute to appraisal and development of coping strategies (Bai et al., 2017; Bland et al., 2003). Thus, stress induces an increase in DA release from the VTA mediated by the amygdala that has been associated with active coping behaviors. However, DA transmission shows a biphasic response in uncontrolled or inescapable stressful conditions because the initial increase of DA levels is followed by a DA decrease (Cabib and Puglisi-Allegra, 2012). Interestingly, this inhibition of DA response is mainly mediated by the medial PFC (mPFC) and supports avoidance coping (Arnsten, 2009; Cabib and Puglisi-Allegra, 2012). In addition to the potential control of DA in the NAc and its potential role in stress-coping by the PFC, microdialysis studies have demonstrated increased extracellular DA levels in the mPFC during exposure to diverse stressors, although higher DA levels were observed in animals exposed to inescapable stress (Bland et al., 2003; Chen et al., 2016).
In the present study, we explored the effects of different doses of selective and dual inhibitors of FAAH and MAGL on anxiety-like and stress-coping behaviors in mice. Because the mPFC is a key region in the regulation of stress response and emotion, and the dopaminergic transmission is modulated by the cannabinoid signaling (Covey et al., 2017), we examined whether the effects of FAAH and/or MAGL inhibition on coping behaviors were associated with stress-induced changes in extracellular DA and 5-HT in the mPFC.
2. Material and methods
2.1. Animals
All studies were performed on adult male C57BL/6J (i.e. wild-type, WT) and null FAAH allele (i.e. FAAH knockout, KO) mice. FAAH KO mice were created by using homologous recombination as previously described (Cravatt et al., 2001) and were maintained on the original 129/SvJ x C57BL/6J genetic background for at least 13 generations. All mice weighed 20–30g and were group housed (4 mice/cage) in a humidity- and temperature-controlled (22 °C) room on a reverse 12-h/12-h light/dark cycle (lights off at 09:00AM) with food and water ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). All efforts were made to reduce the number of animals used and to minimize unnecessary pain and/or distress.
2.2. Drugs
PF-3845 (selective FAAH inhibitor), JZL184 (selective MAGL inhibitor) and JZL195 (dual FAAH/MAGL inhibitor) were synthesized and provided by the Cravatt Laboratory. Each compound was dissolved in a vehicle of ethanol:emulphor:saline (1:1:18) and injected intraperitoneally (i.p.) in a volume of 10 mL/kg body weight. The following doses were used for behavioral tests (PF-3845: 1, 3 and 10 mg/kg; JZL184: 2, 10 and 40 mg/kg; and JZL195: 3, 8, 20 mg/kg) and in vivo microdialysis (PF-3845: 3 mg/kg; and JZL184: 10 mg/kg). Pretreatment times were 180min for PF-3845 and JZL195, and 60min for JZL184 based on the temporal profiles for efficacious enzyme inhibition (Ahn et al., 2009; Long et al., 2009a, 2009c). The number of animals per group size is indicated for each experiment in the figure legends.
2.3. Behavioral procedures
The tail suspension test (TST), forced swim test (FST), elevated-plus maze (EPM) and light-dark test (LDT) were conducted at room temperature (21–23 °C) between 10:00h and 15:00h during the dark phase of the diurnal cycle. WT and FAAH KO mice were treated and tested with at least 7 days separating each test. All tests were conducted in the presence of 70-dB white noise to minimize auditory distraction and 60-W white light (illuminance between 100 and 120lx, except for the light compartment in the LDT). To minimize behavioral disruption animals were transported to an anteroom beside the testing environment at least 2h prior to testing. Each mouse was placed in the testing apparatus and behavior was recorded on digital video. Behavioral tests were conducted by trained observers who were unaware of the experimental conditions.
2.3.1. Tail suspension test
Each mouse was suspended from its tail using adhesive tape on a metal bar located 30 cm above a flat surface for 6 min. Immobility was defined as the absence of any limb or body movements, except those caused by respiration. The apparatus was wiped with a cleaning solution and dried with paper towels between each test. The following variables were scored in the TST: the latency to the first episode of immobility (duration of vigorous movements at the beginning) and the total immobility time (sec).
2.3.2. Forced swim test
The FST was based on the original version the Porsolt swim test for mouse with certain procedural recommendations (Can et al., 2012). Mice were placed in an inescapable 5 L cylindrical tank (40 cm high and 25 cm diameter) that was filled with 3.5 L of water. Water temperature was set at room temperature. Immobility was determined when the mouse was only making small movements necessary to balance the body and keep the head above the water. Mice were placed and recorded in the tank containing water for 6 min. At the end of testing period, the animals were dried using paper towels and a heat lamp to prevent hypothermia, and subsequently placed back into their homecages. Water in the tank was changed between each test. The following variables were scored in the FST: the latency to the first episode of immobility (duration of active swimming at the beginning) and the total time of immobility (sec).
2.3.3. Elevated-plus maze
The EPM apparatus consisted of a cross-shaped apparatus consisting of four arms of equal dimension (5 cm width × 35 cm length) with each arm positioned 90° relative to the adjacent arms. Two arms on opposite sides were enclosed by 15 cm-high walls made of black plexiglass, and the remaining two arms were open without walls. The four arms were connected by a central area (5 cm width × 5 cm length). The entire maze was on supports that lifted the arm surfaces to 30 cm above the floor. At the start of the test, each mouse was placed in the center of the maze facing an open arm, and was allowed to freely explore the maze for 5 min. The EPM was wiped with a cleaning solution and dried with paper towels between each test. The following variables were scored in the EPM: the percent time spent in the open arm (time spent on open arms/time spent in open and closed arms × 100) (OTR) and the total number closed arm entries.
2.3.4. Light-dark test
The light-dark box consisted of a dark compartment (15 cm width × 30 cm length × 30 cm height) and a light compartment (30 cm width × 30 cm length × 30 cm height) that were connected through an opening (8 cm width × 8 cm height). Testing was performed in a dark experimental room. A 60-W light bulb was positioned above the light compartment, such that the illuminance in the middle of the light compartment was approximately 450 lux. At the start of the test, each mouse was placed in the dark compartment with its head facing away from the opening, and was allowed to freely explore the light-dark box for 5 min. After testing, the box was wiped with a cleaning solution and dried with paper towels. The following variables were scored in the LDT for the present study: the time spent in the light compartment and the number of transitions between compartments.
2.4. In vivo microdialysis procedures
Additional naïve WT mice were used to study the effects of PF-3845 (3 mg/kg, i.p.) and JZL184 (10 mg/kg, i.p.) treatments on DA and 5-HT levels in the mPFC during an FST session. These mice were different from those used previously in the behavioral assessments.
2.4.1. Surgery and probe implantation
All in vivo microdialysis sessions were performed using probes of cellulose membrane with 2 mm active membrane length constructed as previously described (Pavon et al., 2018) and aimed at mPFC. The stereotaxic coordinates relative to Bregma were as follows: +2.0 mm AP, ±0.5 mm ML and −4.0 mm DV from the skull surface (Paxinos and Franklin, 2001). During the implantation procedure the probes were continuously perfused with artificial cerebrospinal fluid [aCSF; 149 mM NaCl, 2.8 mM KCl, 1.2 mM CaCl2, 1.2 mM MgCl2, 0.25 mM ascorbic acid and 5.4 mM d-glucose (pH 7.2–7.4)] and secured to the skull as previously described (Pavon et al., 2018).
2.4.2. Microdialysis and FST
Probes were perfused with aCSF at 0.10 μL/min during a 12h postsurgical recovery period. Then, the perfusion flow rate was increased to 0.60 μL/min for 1h. Dialysate fractions of aCSF were collected in 2 stages (26 samples): 15-min intervals during 240min [60-min baseline period (samples 1–4), drug injection and 180-min post-injection period (samples 5–16)]; and 9-min intervals during 90min [8-min FST and return to original cages (sample 17) and 81-min post-FST period (samples 18–26)]. Samples were stored at −80 °C. Prior to monoamine determinations, histological verifications were conducted to include all cases that the active dialysis membrane was properly located within the mPFC (n = 22) using a mouse brain atlas (Paxinos and Franklin, 2001) (Fig. S1).
2.5. High-performance liquid chromatography coupled with electrochemical detection
Dialysate samples were analyzed for DA and 5-HT using HPLC-ECD (model HTEC-500, from EiCOM Co., Kyoto, Japan). Five μL aliquots of dialysate were injected onto an analytical column (particle size 2 mm, PP-ODS 4.6 × 30 mm maintained at 25 °C) and eluted using an isocratic mobile phase consisting of a 100 mM NaH2PO4 buffer containing 134 μM 2Na-EDTA, 3.27 mM SDS and 0.75% (v/v) methanol (pH 6.0) delivered at 400 μL/min. Both monoamines were detected electrochemically using a graphite working electrode set at +450 mV against an Ag/AgCl reference electrode. Quantification was performed using external calibration curves that were constructed daily using freshly prepared standards. All chemicals for the mobile phase and chromatographic standards were of the highest obtainable grade from Sigma-Aldrich Co. (St. Louis, MO, USA).
2.6. Statistical analysis
The behavioral and biochemical data are expressed as mean ± standard error of the mean (SEM). Behavioral variables in the TST, FST, EPM and LDT were analyzed using one-way analysis of variance (ANOVA) to explore the dose-response of drugs in mice. Sidak's test was used for post hoc multiple comparisons. Between-group differences in basal dialysate DA and 5-HT levels (nM) were first compared by repeated-measures ANOVA in the mPFC. For each treatment group, the mean baseline level was calculated as the average of all dialysate samples that were collected before the drug administration (4 samples/animal). Subsequent analyses were conducted on dialysate data (baseline %) using a two-way repeated-measures ANOVA, with treatment as the between-subjects factor and sampling time as the within-subjects factor to evaluate the impact of the FST on mPFC dialysate monoamine levels. Sidak's test was used for post hoc multiple comparisons (simple effects). Area under the curve (AUC) calculations were used for comparison of overall FST-induced alterations in drug-treated mice. The AUC was calculated for each animal by subtracting the basal average (100) from the percent value for each data point following the FST, and subsequently summing all these data points. AUC values were analyzed by one-way ANOVA. Test statistic values and degrees of freedom are indicated in the results where appropriate. Differences were considered statistically significant at p-value of 0.05. All the statistical analyses were performed using GraphPad Prism (GraphPad Software, La Jolla, CA, USA).
3. Results
3.1. Effects of FAAH and MAGL inhibition on stress-coping behavior
3.1.1. Tail suspension test
3.1.1.1. Selective FAAH inhibition
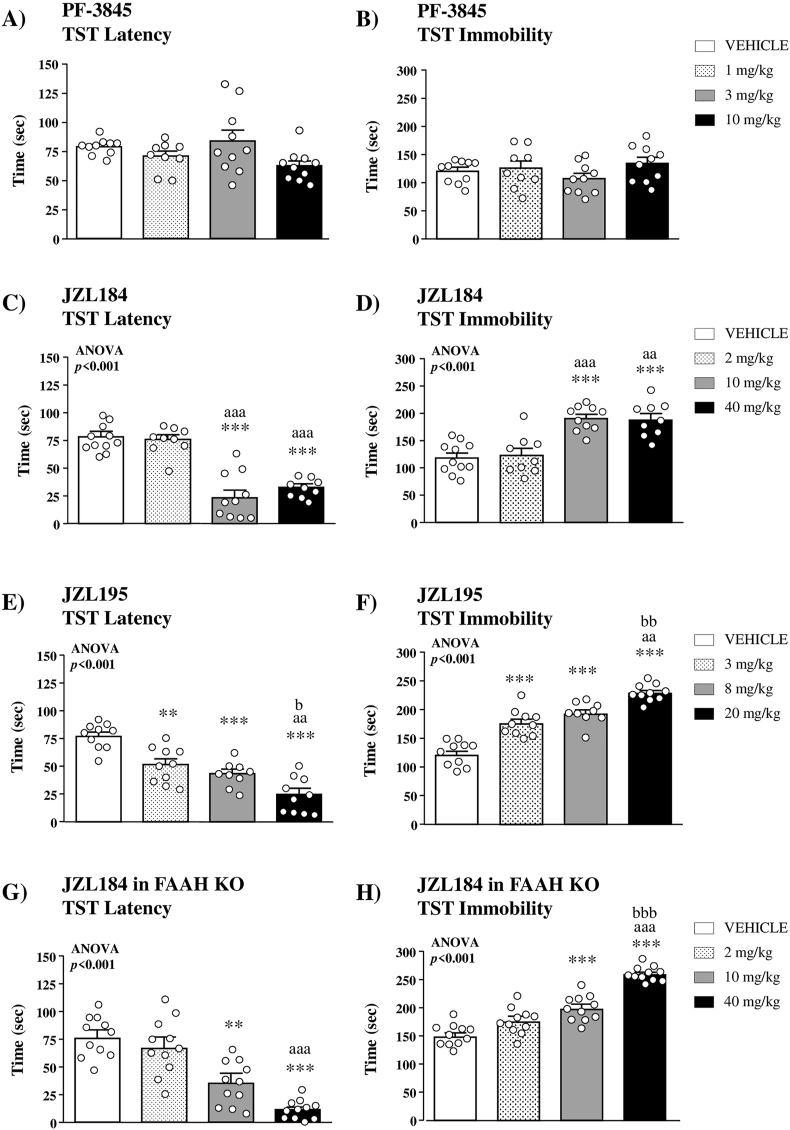
Treatment with PF-3845 failed to elicit significant effects on the latency to the first episode of immobility (Fig. 1A) and the total immobility time (Fig. 1B) in the TST.
Fig. 1.
Effects of selective and dual inhibitors of FAAH and MAGL on stress-coping behavior in the TST. Effects of the selective FAAH inhibitor PF-3845 (1, 3 or 10 mg/kg, i.p.) on the latency to the first episode of immobility (A) and the total immobility time (B) in WT mice (n = 9–10 mice per group). Effects of the selective MAGL inhibitor JZL184 (2, 10 or 40 mg/kg, i.p.) on the latency to the first episode of immobility (C) and the total immobility time (D) in WT mice (n = 9–11 per group). Effects of the dual FAAH/MAGL inhibitor JZL195 (3, 8 or 20 mg/kg, i.p.) on the latency to the first episode of immobility (E) and the total immobility time (F) in WT mice (n = 9–10 per group). Effects of the selective MAGL inhibitor JZL184 on the latency to the first episode of immobility (G) and the total immobility time (H) in FAAH KO mice (n = 11 per group). Bars are mean ± SEM. Symbols in the bars denote significant differences in the post hoc test after one-way ANOVA: (**)p < 0.01 and (***)p < 0.001 denote significant differences vs. the vehicle group; (aa)p < 0.01 and (aaa)p < 0.001 denote significant differences vs. the low-dose group; (b)p < 0.05, (bb)p < 0.01 and (bbb)p < 0.001 denote significant differences vs. the intermediate-dose group.
3.1.1.2. Selective MAGL inhibition
There were significant differences in the latency and the total immobility time among different doses of JZL184 with respect to the vehicle group in the TST. The analysis revealed a significant main effect of treatment (F(3,39) = 27.22;p < 0.001) on the latency, and the post hoc test indicated that 10 and 40 mg/kg of JZL184 significantly decreased the latency compared with the vehicle group (p < 0.001) (Fig. 1C). Accordingly, there was a significant main effect of treatment on the total immobility time (F(3,39) = 15.60;p < 0.001), and mice treated with 10 and 40 mg/kg of JZL184 showed higher immobility than the vehicle group (p < 0.001) (Fig. 1D).
3.1.1.3. Dual FAAH/MAGL inhibition
One-way ANOVA of the latency revealed a significant main effect of JZL195 treatment (F(3,35) = 21.35;p < 0.001), and the post hoc comparisons indicated a dose-dependent decrease in the latency of JZL195 relative to the vehicle group (3 mg/kg, p < 0.01; 8 mg/kg, p < 0.001; and 20 mg/kg, p < 0.001) (Fig. 1E). In agreement to the latency, there was a significant main effect of treatment on the total immobility time (F(3,35) = 48.96;p < 0.001) and JZL195 was associated with a dose-dependent increase in the total immobility relative to the vehicle group (p < 0.001) (Fig. 1F).
3.1.1.4. Selective MAGL inhibition in FAAH KO mice
There was a significant main effect of JZL184 treatment on the latency to immobility (F(3,41) = 11.96;p < 0.001) in FAAH KO mice, and a dose-dependent effect of JZL184 was observed with a significant decrease in the latency at 10 and 40 mg/kg compared with the vehicle group (p < 0.01 and p < 0.001, respectively) (Fig. 1G). Regarding the total immobility time, there was a significant main effect of treatment (F(3,41) = 37.78;p < 0.001), and higher doses of JZL184 (10 and 40 mg/kg) produced a significant increase in the immobility relative to the vehicle group (p < 0.001) (Fig. 1H).
3.1.2. Forced swim test
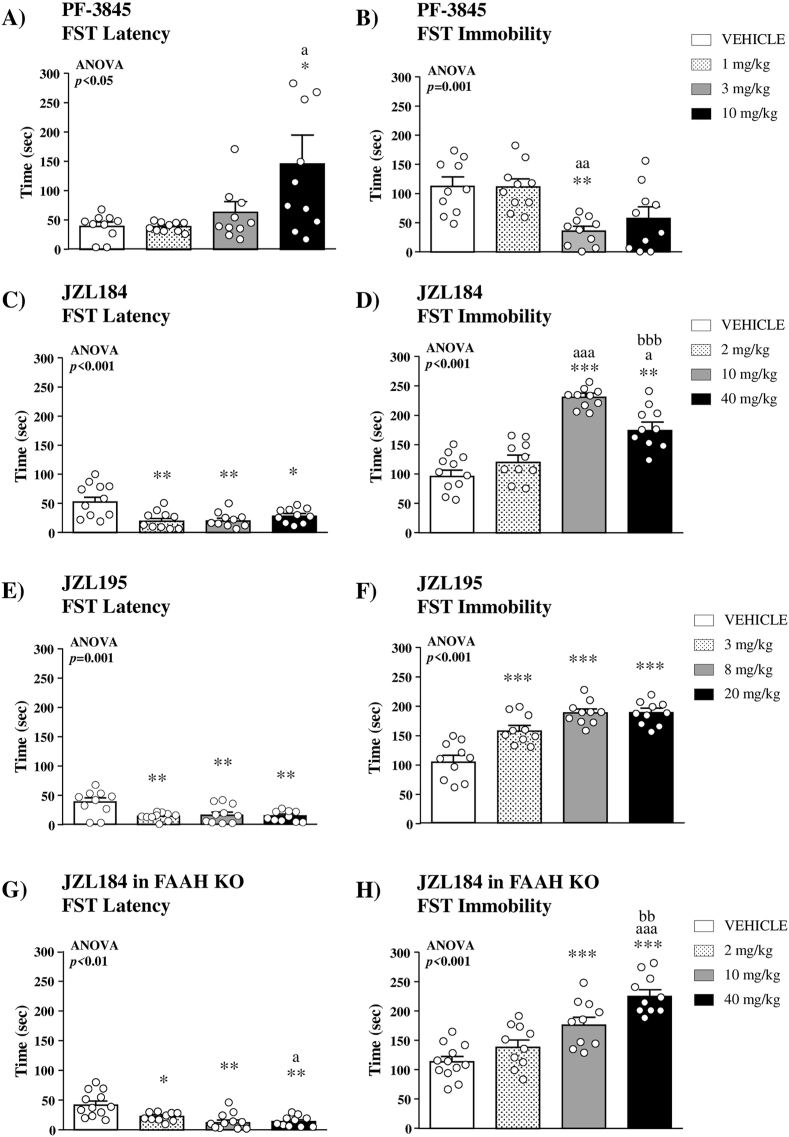
3.1.2.1. Selective FAAH inhibition
There was a significant main effect of PF-3845 treatment on the latency (F(3,36) = 3.600;p = 0.023) in the FST, and the post hoc comparison showed that 10 mg/kg of PF-3845 significantly increased the latency to the first immobility compared with the vehicle group (p < 0.05) (Fig. 2A). Moreover, there was a significant main effect of treatment on the total immobility time (F(3,36) = 7.493;p = 0.001), and mice treated with 3 mg/kg of PF-3845 showed significantly lower immobility than the vehicle group (p < 0.01) (Fig. 2B).
Fig. 2.
Effects of selective and dual inhibitors of FAAH and MAGL on stress-coping behavior in the FST. Effects of the selective FAAH inhibitor PF-3845 on the latency to the first episode of immobility (A) and the total immobility time (B) in WT mice (n = 10 per group). Effects of the selective MAGL inhibitor JZL184 on the latency to the first episode of immobility (C) and the total immobility time (D) in WT mice (n = 10–11 per group). Effects of the dual FAAH/MAGL inhibitor JZL195 on the latency to the first episode of immobility (E) and the total immobility time (F) in WT mice (n = 10 per group). Effects of the selective MAGL inhibitor JZL184 on the latency to the first episode of immobility (G) and the total immobility time (H) in FAAH KO mice (n = 10–12 per group). Bars are mean ± SEM. Symbols in the bars denote significant differences in the post hoc test after one-way ANOVA: (*)p < 0.05, (**)p < 0.01 and (***)p < 0.001 denote significant differences vs. the vehicle group; (a)p < 0.05, (aa)p < 0.01 and (aaa)p < 0.001 denote significant differences vs. the low-dose group; (bb)p < 0.01 and (bbb)p < 0.001 denote significant differences vs. the intermediate-dose group.
3.1.2.2. Selective MAGL inhibition
There was a main effect of JZL184 treatment on the latency to the first immobility (F(3,39) = 8.235;p < 0.001), and the post hoc test indicated that all doses of JZL184 significantly decreased the latency relative to the vehicle group (2 mg/kg, p < 0.01; 10 mg/kg, p < 0.01; and 40 mg/kg, p < 0.05) (Fig. 2C). Accordingly, there was a significant main effect of treatment (F(3,39) = 47.59, p < 0.001) on the total immobility time, and mice treated with 10 and 40 mg/kg of JZL184 showed significantly higher immobility than the vehicle group (p < 0.01) (Fig. 2D).
3.1.2.3. Dual FAAH/MAGL inhibition
The effects of JZL195 on the latency and the total immobility time were found to be similar to the effects of JZL184 in the FST. One-way ANOVA revealed a significant main effect of treatment on the latency (F(3,36) = 36.622;p = 0.001), and mice treated with JZL195 showed significantly lower latency than the vehicle group (p < 0.01) (Fig. 2E). In addition, there was a significant main effect of treatment on the total immobility time (F(3,36) = 21.32;p < 0.001), and the post hoc test revealed significant increases in the immobility time of mice treated with JZL195 as compared with the vehicle group (p < 0.001) (Fig. 2F).
3.1.2.4. Selective MAGL inhibition in FAAH KO mice
There was a main effect of JZL184 treatment on the latency to the first immobility (F(3,42) = 4.531;p = 0.008) in FAAH KO mice, and a significant decrease was found for all doses of JZL184 compared with the vehicle group (2 mg/kg, p < 0.05; 10 mg/kg, p < 0.01; and 40 mg/kg, p < 0.01) (Fig. 2G). Additionally, there was a main effect of treatment on the total immobility time (F(3,42) = 46.98;p < 0.001). Thus, post hoc comparisons revealed a dose-dependent effect of JZL184, and significant increases in the total immobility time were observed in mice treated with 10 and 40 mg/kg compared with the vehicle group (p < 0.001) (Fig. 2H).
3.2. Effects of FAAH and MAGL inhibition on stress-induced DA and 5-HT levels in the mPFC
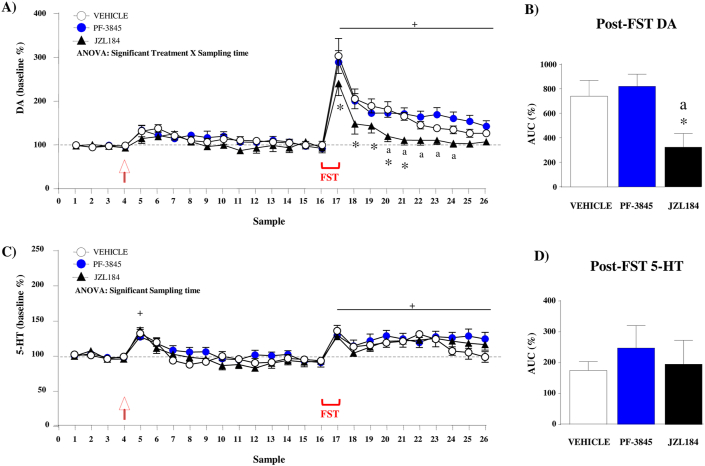
3.2.1. Extracellular DA levels
The analysis of baseline DA levels revealed no differences among groups of mice in the first 4 samples [vehicle: 0.255 ± 0.012 nM; PF-3845 (3 mg/kg): 0.273 ± 0.012 nM; and JZL184 (10 mg/kg): 0.280 ± 0.012 nM]. Overall, a two-way repeated-measures ANOVA during the microdialysis experiment revealed main effects of treatment (F(2,19) = 4.173;p = 0.031) and sampling time (F(25,475) = 43.32;p < 0.001) on relative DA levels, but also a significant interaction between the two factors (F(50,475) = 1.845;p < 0.001) (Fig. 3A). Post hoc comparisons for simple effects were performed to explore the interaction among treatment and sampling time. A slight increase in extracellular DA levels was observed immediately after drug injection in all treatment groups but there were no differences relative to their basal levels. In contrast, the FST session induced a rapid and robust increase in DA levels (vehicle: samples 17–23, PF-3845: samples 17–26; and JZL184: samples 17–19) relative to their basal levels and significant differences among the treatment groups. Specifically, mice treated with JZL184 showed lower DA levels than the vehicle group at samples 17–21 (p < 0.05). The FST-induced increases in DA levels and differences among treatments were confirmed in the AUC analysis using a one-way ANOVA (F(2,19) = 5.538;p = 0.013) (Fig. 3B). Thus, mice treated with JZL184 showed significantly lower post-FST AUC values than the vehicle group (p < 0.05). However, no differences were observed between mice treated with PF-3845 and the vehicle group.
Fig. 3.
Effects of the FST on mPFC dialysate DA and 5-HT levels in WT mice treated with selective inhibitors of FAAH and MAGL. Microdialysis experiment expressing DA levels as the percentage change from baseline levels (mean ± SEM) (A). The AUC for DA summarizes the effect of the FST on dialysate DA (mean ± SEM) (B). Microdialysis experiment expressing 5-HT levels as the percentage change from baseline levels (mean ± SEM) (C). The AUC for 5-HT summarizes the effect of the FST on dialysate DA (mean ± SEM) (D). The drug injection is indicated by the arrow. FAAH inhibitor [PF-3845, 3 mg/kg (i.p.)], MAGL inhibitor [(JZL184, 10 mg/kg (i.p.)] or vehicle were administered in groups of 7–8 mice. (*)p < 0.05 denotes significant differences vs. the vehicle group. (a)p < 0.05 denotes significant differences vs. the PF-3845 group. (+)p < 0.05 denotes significant differences vs. baseline levels (100%).
3.2.2. Extracellular 5-HT levels
There were no significant differences among groups of mice in baseline 5-HT levels (vehicle: 0.377 ± 0.010 nM; PF-3845: 0.370 ± 0.010 nM; and JZL184: 0.347 ± 0.020 nM). The analysis of 5-HT levels during the microdialysis experiment revealed only a main effect of sampling time (F(25,475) = 18.29;p < 0.001) (Fig. 3C). Post hoc comparisons showed a significant increase in 5-HT levels immediately after drug injections [sample 5 (p < 0.001)] and the FST session [samples 17–26 (p < 0.05)] relative to their baselines. The AUC analysis for dialysate 5-HT using one-way ANOVA revealed no differences among treatments (Fig. 3D).
3.3. Effects of FAAH and MAGL inhibition on anxiety-like behavior and locomotor activity
We examined anxiety-like behavior and locomotor activity in mice treated with FAAH and/or MAGL inhibitors in the EPM and LDT.
3.3.1. Elevated plus maze
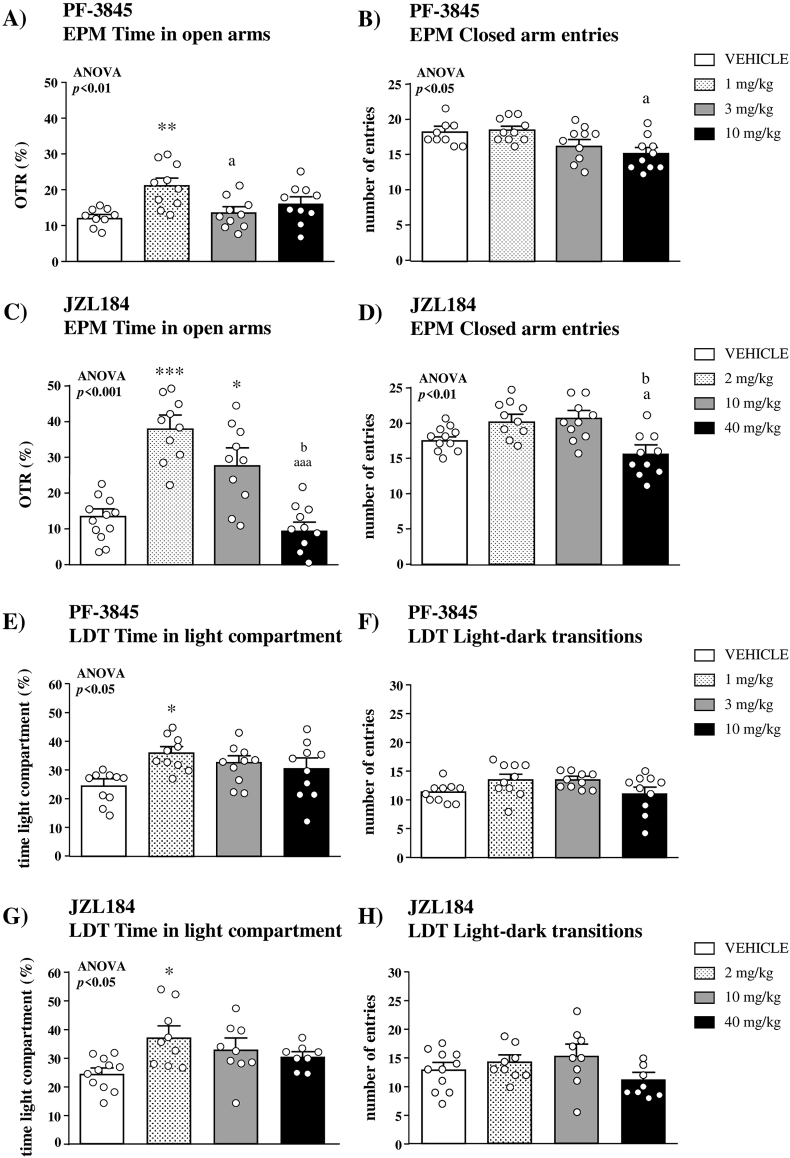
3.3.1.1. Selective FAAH inhibition
In the EPM, one-way ANOVA of the OTR revealed a significant main effect of PF-3845 treatment (F(3,35) = 4.678;p = 0.008), and the post hoc test indicated that only 1 mg/kg of PF-3845 significantly increased the OTR compared with the vehicle group (p < 0.01) (Fig. 4A). In addition to the OTR, there was a significant main effect of treatment (F(3,35) = 3.697;p = 0.021) on the closed arm entries but the post hoc test found no significant differences when mice treated with PF-3845 were compared with the vehicle group (Fig. 4B).
Fig. 4.
Effects of selective inhibitors of FAAH and MAGL on anxiety-like behavior and locomotor activity. Effects of the selective FAAH inhibitor PF-3845 on the time spent in open arms (A) and the number of entries in the closed arms (B) in the EPM with WT mice (n = 9–10 per group). Effects of the selective MAGL inhibitor JZL184 on the time spent in open arms (C) and the number of entries in the closed arms (D) in the EPM with WT mice (n = 10–11 per group). Effects of the selective FAAH inhibitor PF-3845 on the time spent in the light compartment (E) and the number of transitions between light and dark compartments (F) in the LDT with WT mice (n = 10 per group). Effects of the selective MAGL inhibitor JZL184 on the time spent in the light compartment (G) and the number of transitions between light and dark compartments (H) in the LDT with WT mice (n = 8–11 per group). Bars are mean and SEM. Symbols in the bars denote significant differences in the post hoc test after one-way ANOVA: (*) p < 0.05, (**) p < 0.01 and (***) p < 0.001 denote significant differences vs. the vehicle group; (a) p < 0.05 and (aaa) p < 0.001 denote significant differences vs. the low-dose group; (b) p < 0.05 denotes significant differences vs. the intermediate-dose group.
3.3.1.2. Selective MAGL inhibition
There was a significant main effect of JZL184 treatment on the OTR (F(3,38) = 13.19;p < 0.001). The post hoc test showed that 2 and 10 mg/kg of JZL184 significantly increased the OTR compared with the vehicle group (p < 0.001 and p < 0.05, respectively), and a dose-dependent effect of JZL184 was observed (Fig. 4C). Regarding the closed arm entries, the analysis indicated a significant main effect of treatment (F(3,38) = 5.110;p = 0.005), but we observed no significant differences when mice treated with JZL184 were compared with the vehicle group (Fig. 4D). Unlike WT mice, JZL184 treatment in FAAH KO mice showed no significant effects on the OTR or the closed arm entries in the EPM (Fig. S2A and S2B).
3.3.2. Light-dark test
3.3.2.1. Selective FAAH inhibition
Similarly to the results in the EPM, the statistical analysis of the time spent in the light compartment of the light-dark box revealed a significant main effect of PF-3845 treatment (F(3,36) = 3.202;p = 0.035), and 1 mg/kg of this FAAH inhibitor significantly increased the time in the light compartment relative to the vehicle group (p < 0.05) (Fig. 4E). In contrast, there was no significant effects of treatment on the light-dark transitions in the LDT (Fig. 4F).
3.3.2.2. Selective MAGL inhibition
There was a significant main effect of JZL184treatment on the time spent in the light compartment of the LDT (F(3,33) = 3.005;p = 0.044), and the post hoc test showed a significant increase with 2 mg/kg of JZL184 compared with the vehicle group (p < 0.05) (Fig. 4G). Regarding the locomotor activity, we found no statistical differences among doses of treatment in the light-dark transitions (Fig. 4H). In FAAH KO mice, there was a significant increase in the time spent in the light compartment of the light-dark box compared with the vehicle group (Fig. S2C and S2D) with the highest dose of JZL184 (40 mg/kg).
In addition to the selective inhibition of FAAH (PF-3845) and MAGL (JZL184), the dual inhibition with JZL195 was also examined in both the EPM and LDT. Thus, while we observed a significant increase in the OTR at higher doses (8 and 20 mg/kg) in the EPM (Fig. S3A and S3B), this anxiolytic-like effect was not statistically significant in the LDT (Fig. S3C and S3D).
4. Discussion
In the last decade, the development of new FAAH and/or MAGL inhibitors has allowed the characterization of the physiological implications of FAAH-AEA and MAGL-2-AG pathways in specific behavioral processes. Because substantial evidence indicates a main role of the endocannabinoid system in the modulation of emotional behaviors, the present study was mainly designed to provide a better understanding of the specific involvement of these two main endocannabinoid pathways in the modulation of stress-coping behaviors.
4.1. Stress-coping behavior and endocannabinoid signaling pathways
Numerous studies have reported the ability of CB1 receptor agonists to increase proactive stress coping strategies (Bambico et al., 2007, 2012; El-Alfy et al., 2010). Accordingly, it has been described that the enhancement of AEA signaling through the pharmacological or genetic inhibition of FAAH produces a significant decrease in immobility in the TST and FST, which is appraised as avoidance coping (Bambico et al., 2010; Gobbi et al., 2005). In line with these studies, swim stress has been reported to produce a significant decrease in AEA content in the mPFC, and local administration of a FAAH inhibitor into this brain region decreases passive coping behaviors that are interpreted as antidepressant-like effects (McLaughlin et al., 2012). In agreement, we also observed a significant increase in the latency to immobility and a decrease in the total immobility time in mice treated with PF-3845 in the FST, but no effects in the TST. The inconsistency between both paradigms may be related to the differential sensitivity of each test to detect the influence of AEA in passive coping behaviors, but also to the testing conditions. In this regard, previous studies have reported that the effects on emotional behaviors using FAAH inhibitors (URB597) or FAAH KO mice are detected in the TST only altering the ambient lighting conditions and increasing sample sizes (Naidu et al., 2007). In addition to increased AEA levels, FAAH inhibition increases other N-acylethanolamines [e.g., N-palmitoylethanolamine (PEA) and N-oleoylethanolamine (OEA)] that could contribute to stress-coping behaviors through other targets (non-CB1/non-CB2 receptors) such as peroxisome proliferator-activated receptors (PPAR-α) (Fu et al., 2003), whose activation has been shown as a natural response to stress (Hillard, 2018).
In contrast to FAAH, the relative influence of MAGL inhibition on stress-coping responses has not been deeply explored and apparently contradictory findings have been reported. Despite one study described an increase in the immobility time in the FST after chronic treatment with JZL184 (Lomazzo et al., 2015), other studies in mice found no effects after acute treatment or even a decrease of passive stress-coping behavior after chronic treatment with JZL184 in stressed mice, but not in control mice (Aliczki et al., 2013; Zhang et al., 2015; Zhong et al., 2014). Recently, it has been reported a dose-dependent biphasic effect of the selective MAGL inhibitor JZL184 on immobility in acutely stressed mice (Wang et al., 2017). Furthermore, Wang and colleagues demonstrate that these effects of JZL184 in the FST depending on the duration of stress exposure; whereas 20 mg/kg increases immobility in acutely stressed mice, immobility is decreased in chronic corticosterone-treated mice. In the present study, mice acutely treated with JZL184 displayed a significant decrease in the latency to the first immobility and a significant increase in the total immobility time in both the TST and FST at doses of 10 and 40 mg/kg. The reasons for the discrepancy between studies are not clear, but differences in variables related to the pharmacological and environmental conditions or the use of different mouse strains among others may explain this disparity of effects (Table S1).
Because the selective inhibition of FAAH or MAGL was associated with opposite effects on immobility, we examined whether there was an interactive or antagonistic effect of AEA and 2-AG signaling in the regulation of stress-coping behaviors. Overall, the combined FAAH/MAGL inhibition in WT mice and the MAGL inhibition in FAAH KO mice produced a dose-dependent increase in the total immobility time in both the TST and FST, which suggests that the 2-AG signaling prevails over the AEA signaling on regulating coping behavior. This discrepancy in coping behavior is not unprecedented and multiple studies have found opposite effects of FAAH and MAGL inhibition on many emotional behaviors linked to stress such as fear expression (Llorente-Berzal et al., 2015), fear extinction (Gunduz-Cinar et al., 2013; Hartley et al., 2016) and predator-induced flight behavior (Heinz et al., 2017).
Immobility expressed by rodents in the FST and TST has been classically used as measure of depression-like behavior, and we could differentiate the antidepressant-like effects of PF-3845 from the pro-depressant-like (or depressive-like) effects of JZL184. However, we appraise this behavioral response as an adaptive strategy to cope with a stressful situation that cannot be escaped (i.e., strategy for survival and energy conservation) (Campus et al., 2015; Commons et al., 2017; Molendijk and de Kloet, 2019). Therefore, both FAAH and MAGL inhibitors in mice show different adaptative strategies to inescapable stress (swim stress in the FST) and more investigation is needed to determine their pharmacological characteristics as drugs for depression or related conditions characterized by altered stress response such as autism spectrum disorders (Commons et al., 2017).
4.2. FAAH and MAGL inhibition and monoamines in the mouse mPFC
For a better understanding of the neurochemical substrate underlying the behavioral effects observed herein, we evaluated the effects of swim stress on extracellular DA and 5-HT levels in the mouse mPFC after blocking FAAH or MAGL. Growing evidence indicates that exposure to an acute stress activates dopaminergic neurons, increasing DA levels in the mesocorticolimbic pathway (Butts and Phillips, 2013). Namely, microdialysis studies have reported increased DA levels in the mPFC during exposure to diverse stressors and this dopaminergic response is enhanced in animals exposed to inescapable stress (Bland et al., 2003; Chen et al., 2016). Accordingly, our experiment revealed rapid and significant elevations of extracellular DA levels (over a 300% of basal DA levels in the vehicle group) in the mPFC during the swim stress that were gradually decreasing. Previous studies have showed a biphasic response of DA transmission in uncontrolled or inescapable stressful conditions because the initial increase of DA levels is followed by a DA decrease from the VTA(Cabib and Puglisi-Allegra, 2012). However, there were differences among treatments and JZL184-treated mice displayed a significant attenuation of the DA increase and a faster return to baseline compared with mice treated with PF-3845 or vehicle. MAGL inhibition produces a significant elevation in brain 2-AG levels (Long et al., 2009b) that could reduce synaptic transmission at both excitatory glutamatergic and inhibitory GABAergic synapses onto DA neurons via CB1 activation, as observed in mouse mPFC(Domoto et al., 2018). Therefore, the enhanced 2-AG tone induced by the acute administration of JZL184 could be associated with the attenuation of the swim stress-induced DA release in the mPFC.
The doses of JZL184 (10 mg/kg) and PF-3845 (3 mg/kg) for the microdialysis experiment induced significant effects on the total immobility time in the FST. Previous studies have suggested that stress-coping behaviors associated with alterations in the endocannabinoid signaling may be mediated through modulating monoaminergic neurotransmission (Gobbi et al., 2005; Haring et al., 2013; McLaughlin et al., 2012). Furthermore, a recent study in rats reported that a reduction in the PFC dopaminergic inputs from the VTA is associated with an increase in passive coping responses in the FST (Bai et al., 2017). However, while increased immobility was paralleled to changes in the DA response to stress with a selective MAGL inhibitor, decreased immobility was uncorrelated with changes in the DA response with a selective FAAH inhibitor.
4.3. FAAH and MAGL inhibition and anxiety-like behavior
Because the mesocortical dopaminergic system is also involved in the modulation of the anxiety-like behavior, and anxiety is considered an adaptive component of the acute stress response (Zarrindast and Khakpai, 2015), we examined the effects of FAAH and MAGL inhibition on anxiety. Overall, we found a robust anxiolytic-like effect of all FAAH and/or MAGL inhibitors in the EPM and LDT.
In agreement with previous reports, we observed that the indirect stimulation of AEA signaling influences anxiety (for review see (Gaetani et al., 2009)) and PF-3845 induced a significant anxiolytic-like effect at 1 mg/kg. However, the effects of PF-3845 on anxiety-like behavior depends on the in vivo efficacy of AEA levels and the influence of other molecules and targets that are affected by the FAAH inhibition. Thus, it has been reported that mice treated with 10 mg/kg of PF-3845 show a dramatic increase (>10-fold) in AEA levels and in other non-cannabinoid congeners (i.e., N-acylethanolamines), such as OEA that acts as agonist for PPAR-α, but also for the transient receptor potential vanilloid type-1 (TRPV1) channel (Ahn et al., 2009). Interestingly, CB1 and TRPV1 receptors are co-expressed in several brain regions and exert opposite roles on different brain functions, including the control of affective and anxiety-like behaviors (Cristino et al., 2006; Micale et al., 2009).
Similar to FAAH inhibition, we also observed that mice treated with the MAGL inhibitor displayed a significant anxiolytic-like response, although PF-3845 and JZL184 show different molecular mechanisms of action. In this regard, previous studies have reported that while the anxiolytic-like effects produced by the FAAH inhibitor URB597 are primarily CB1-dependent (Moreira et al., 2008), the anxiolytic-like effects of JZL184 are mediated through a CB2-dependent mechanism (Busquets-Garcia et al., 2011). However, other studies have found that the anxiolytic effects of MAGL inhibition are also mediated by CB1 receptors (Bedse et al., 2017; Morena et al., 2016).
In addition to the selective FAAH and MAGL inhibition, we found that the dual increase of AEA and 2-AG signaling pathways using JZL195 produced anxiolytic-like responses in the EPM. However, these findings appear to stand in contrast to previous studies that show that dual FAAH/MAGL inhibition has no effect or induces an increased anxiety-like behavior (Bedse et al., 2018; Manduca et al., 2015). Because it has been reported that the effects of JZL195 are dependent on the experimental context (Bedse et al., 2018), this discrepancy may be related to experimental conditions.
4.4. Summary and conclusions
Our results demonstrated that FAAH and MAGL inhibition induced different effects on stress coping behavior. The FAAH inhibitor PF-3845 increased latency (10 mg/kg) to immobility and decreased total immobility time (3 mg/kg) in the FST, whereas the MAGL inhibitor JZL184 significantly decreased latency and increased immobility in both the TST and FST at 10 and 40 mg/kg. The strong and consistent effects of JZL184 on latency and immobility across tests were also observed in FAAH KO mice, and the dual FAAH/MAGL inhibitor JZL195 decreased latency and increased immobility in both TST and FST at 3, 8 and 20 mg/kg in a way similar to JZL184. Therefore, passive stress-coping behavior was primarily associated with MAGL inhibition. The in vivo microdialysis experiment revealed that the forced swim induced a rapid and significant increase in extracellular DA and 5-HT levels in the mPFC. While 5-HT levels were not affected by treatments, mice treated with the MAGL inhibitor JZL184 (10 mg/kg) showed a significant attenuation of stress-induced DA release relative to mice treated with vehicle. By contrast, PF-3845 (3 mg/kg) treatment produced no effects on increased DA levels during the FST. Finally, despite these differences between FAAH and MAGL inhibition on coping behaviors and stress-induced DA response in the mPFC, the FAAH and/or MAGL inhibitors showed robust and consistent anxiolytic-like effects under our experimental conditions.
In conclusion, we show a different profile for FAAH and MAGL inhibitors in the effects on stress coping behavior, although their association with depression-like effects need more investigation. Collectively, our data suggest an association between the 2-AG and DA signaling pathways in the mPFC during an acute inescapable or uncontrolled stress. However, we are aware that additional experiments are needed to elucidate the mechanism of this functional association between both pathways regarding avoidance coping strategies, and whether alternate mechanisms are involved in the regulation of these behavioral and biochemical responses related to stress.
Role of the funding sources
None of the funding sources had a role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
CRediT authorship contribution statement
Francisco Javier Pavón: Formal analysis, Writing - original draft, performed the experiments, analyzed the data and wrote the manuscript, contributed to and have approved the final manuscript. Ilham Y. Polis: performed the experiments, contributed to and have approved the final manuscript. David G. Stouffer: performed the experiments, contributed to and have approved the final manuscript. Benjamin F. Cravatt: contributed new reagents tools, contributed to and have approved the final manuscript. Marisa Roberto: provided critical revision of the manuscript for important intellectual content, contributed to and have approved the final manuscript. Rémi Martin-Fardon: provided critical revision of the manuscript for important intellectual content, contributed to and have approved the final manuscript. Fernando Rodríguez de Fonseca: Formal analysis, Writing - original draft, analyzed the data and wrote the manuscript, contributed to and have approved the final manuscript. Loren H. Parsons: conceived and designed the study, contributed to and have approved the final manuscript. Antonia Serrano: Formal analysis, Writing - original draft, conceived and designed the study, analyzed the data and wrote the manuscript, contributed to and have approved the final manuscript.
Declaration of competing interest
All authors declare that they have no conflicts of interest.
Acknowledgements
This study was conceived and led by Dr. Loren (Larry) Parsons at The Scripps Research Institute during the postdoctoral training period of Antonia Serrano and Francisco Javier Pavón. We believe this study serves as suitable recognition of the notable research carried out by Dr. Parsons and his team in the fields of neurochemistry and neuropharmacology over the past many years.
This work was supported by the following funding sources and grants: the National Institute on Alcohol Abuse and Alcoholism (AA020404, AA022249, AA024146 and AA026999 to RMF, AA006420 to RMF and MR, AA017447 and AA015566 to MR); Pearson Center for Alcoholism and Addiction Research; Instituto de Salud Carlos III (ISCIII) and European Regional Development Funds-European Union (ERDF-EU) [Subprograma RETICS Red de Trastornos Adictivos (RD16/0017/0001), Ministerio de Economía y Competitividad (PI16/01953, PI16/01698, PI17/02026, PI19/01577 and PI19/00886)]; Ministerio de Sanidad and Delegación del Gobierno para el Plan Nacional sobre Drogas (PND2017/043, PND2018/044 and PND2018/033); and Junta de Andalucía and ERDF-EU [Consejería de Economía, Innovación y Ciencia (CTS-433 and CTS-1052) and Servicio Andaluz de Salud (C1-0049-2019)]. AS and FJP hold a “Miguel Servet” research contract funded by ISCIII and ERDF-EU (CPII19/00031 and CPII19/00022, respectively). This is article number 29846 from The Scripps Research Institute.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100293.
Contributor Information
Francisco Javier Pavón, Email: javier.pavon@ibima.eu.
Fernando Rodríguez de Fonseca, Email: fernando.rodriguez@ibima.eu.
Antonia Serrano, Email: antonia.serrano@ibima.eu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ahn K., Johnson D.S., Mileni M., Beidler D., Long J.Z., McKinney M.K., Weerapana E., Sadagopan N., Liimatta M., Smith S.E., Lazerwith S., Stiff C., Kamtekar S., Bhattacharya K., Zhang Y., Swaney S., Van Becelaere K., Stevens R.C., Cravatt B.F. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem. Biol. 2009;16:411–420. doi: 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliczki M., Balogh Z., Tulogdi A., Haller J. The temporal dynamics of the effects of monoacylglycerol lipase blockade on locomotion, anxiety, and body temperature. Behav. Pharmacol. 2012;23:348–357. doi: 10.1097/FBP.0b013e3283564dfa. [DOI] [PubMed] [Google Scholar]
- Aliczki M., Zelena D., Mikics E., Varga Z.K., Pinter O., Bakos N.V., Varga J., Haller J. Monoacylglycerol lipase inhibition-induced changes in plasma corticosterone levels, anxiety and locomotor activity in male CD1 mice. Horm. Behav. 2013;63:752–758. doi: 10.1016/j.yhbeh.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai M., Zhu X., Zhang L., Zhang Y., Xue L., Wang Y., Zhong M., Zhang X. Divergent anomaly in mesocorticolimbic dopaminergic circuits might be associated with different depressive behaviors, an animal study. Brain Behav. 2017;7 doi: 10.1002/brb3.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambico F.R., Cassano T., Dominguez-Lopez S., Katz N., Walker C.D., Piomelli D., Gobbi G. Genetic deletion of fatty acid amide hydrolase alters emotional behavior and serotonergic transmission in the dorsal raphe, prefrontal cortex, and hippocampus. Neuropsychopharmacology. 2010;35:2083–2100. doi: 10.1038/npp.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambico F.R., Hattan P.R., Garant J.P., Gobbi G. Effect of delta-9-tetrahydrocannabinol on behavioral despair and on pre- and postsynaptic serotonergic transmission. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2012;38:88–96. doi: 10.1016/j.pnpbp.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Bambico F.R., Katz N., Debonnel G., Gobbi G. Cannabinoids elicit antidepressant-like behavior and activate serotonergic neurons through the medial prefrontal cortex. J. Neurosci. 2007;27:11700–11711. doi: 10.1523/JNEUROSCI.1636-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedse G., Bluett R.J., Patrick T.A., Romness N.K., Gaulden A.D., Kingsley P.J., Plath N., Marnett L.J., Patel S. Therapeutic endocannabinoid augmentation for mood and anxiety disorders: comparative profiling of FAAH, MAGL and dual inhibitors. Transl. Psychiatry. 2018;8:92. doi: 10.1038/s41398-018-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedse G., Hartley N.D., Neale E., Gaulden A.D., Patrick T.A., Kingsley P.J., Uddin M.J., Plath N., Marnett L.J., Patel S. Functional redundancy between canonical endocannabinoid signaling systems in the modulation of anxiety. Biol. Psychiatr. 2017;82:488–499. doi: 10.1016/j.biopsych.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio L., Soria-Gomez E., Quarta C., Metna-Laurent M., Cardinal P., Binder E., Cannich A., Delamarre A., Haring M., Martin-Fontecha M., Vega D., Leste-Lasserre T., Bartsch D., Monory K., Lutz B., Chaouloff F., Pagotto U., Guzman M., Cota D., Marsicano G. Activation of the sympathetic nervous system mediates hypophagic and anxiety-like effects of CB(1) receptor blockade. Proc. Natl. Acad. Sci. U. S. A. 2013;110:4786–4791. doi: 10.1073/pnas.1218573110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland S.T., Hargrave D., Pepin J.L., Amat J., Watkins L.R., Maier S.F. Stressor controllability modulates stress-induced dopamine and serotonin efflux and morphine-induced serotonin efflux in the medial prefrontal cortex. Neuropsychopharmacology. 2003;28:1589–1596. doi: 10.1038/sj.npp.1300206. [DOI] [PubMed] [Google Scholar]
- Blankman J.L., Cravatt B.F. Chemical probes of endocannabinoid metabolism. Pharmacol. Rev. 2013;65:849–871. doi: 10.1124/pr.112.006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets-Garcia A., Puighermanal E., Pastor A., de la Torre R., Maldonado R., Ozaita A. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol. Psychiatr. 2011;70:479–486. doi: 10.1016/j.biopsych.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Butts K.A., Phillips A.G. Glucocorticoid receptors in the prefrontal cortex regulate dopamine efflux to stress via descending glutamatergic feedback to the ventral tegmental area. Int. J. Neuropsychopharmacol. 2013;16:1799–1807. doi: 10.1017/S1461145713000187. [DOI] [PubMed] [Google Scholar]
- Cabib S., Puglisi-Allegra S. The mesoaccumbens dopamine in coping with stress. Neurosci. Biobehav. Rev. 2012;36:79–89. doi: 10.1016/j.neubiorev.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Campus P., Colelli V., Orsini C., Sarra D., Cabib S. Evidence for the involvement of extinction-associated inhibitory learning in the forced swimming test. Behav. Brain Res. 2015;278:348–355. doi: 10.1016/j.bbr.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Can A., Dao D.T., Arad M., Terrillion C.E., Piantadosi S.C., Gould T.D. The mouse forced swim test. JoVE. 2012 doi: 10.3791/3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Nakagawa S., Kitaichi Y., An Y., Omiya Y., Song N., Koga M., Kato A., Inoue T., Kusumi I. The role of medial prefrontal corticosterone and dopamine in the antidepressant-like effect of exercise. Psychoneuroendocrinology. 2016;69:1–9. doi: 10.1016/j.psyneuen.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Commons K.G., Cholanians A.B., Babb J.A., Ehlinger D.G. The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chem. Neurosci. 2017;8:955–960. doi: 10.1021/acschemneuro.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey D.P., Mateo Y., Sulzer D., Cheer J.F., Lovinger D.M. Endocannabinoid modulation of dopamine neurotransmission. Neuropharmacology. 2017;124:52–61. doi: 10.1016/j.neuropharm.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt B.F., Demarest K., Patricelli M.P., Bracey M.H., Giang D.K., Martin B.R., Lichtman A.H. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl. Acad. Sci. U. S. A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristino L., de Petrocellis L., Pryce G., Baker D., Guglielmotti V., Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- Devane W.A., Hanus L., Breuer A., Pertwee R.G., Stevenson L.A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dlugos A., Childs E., Stuhr K.L., Hillard C.J., de Wit H. Acute stress increases circulating anandamide and other N-acylethanolamines in healthy humans. Neuropsychopharmacology. 2012;37:2416–2427. doi: 10.1038/npp.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domoto M., Sasase H., Wada S., Ito S., Deyama S., Hinoi E., Kaneko S., Kaneda K. The synthetic cannabinoid 5F-AMB changes the balance between excitation and inhibition of layer V pyramidal neurons in the mouse medial prefrontal cortex. Psychopharmacology. 2018;235:2367–2376. doi: 10.1007/s00213-018-4933-5. [DOI] [PubMed] [Google Scholar]
- El-Alfy A.T., Ivey K., Robinson K., Ahmed S., Radwan M., Slade D., Khan I., ElSohly M., Ross S. Antidepressant-like effect of delta9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol. Biochem. Behav. 2010;95:434–442. doi: 10.1016/j.pbb.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Gaetani S., Oveisi F., Lo Verme J., Serrano A., Rodriguez De Fonseca F., Rosengarth A., Luecke H., Di Giacomo B., Tarzia G., Piomelli D. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- Gaetani S., Dipasquale P., Romano A., Righetti L., Cassano T., Piomelli D., Cuomo V. The endocannabinoid system as a target for novel anxiolytic and antidepressant drugs. Int. Rev. Neurobiol. 2009;85:57–72. doi: 10.1016/S0074-7742(09)85005-8. [DOI] [PubMed] [Google Scholar]
- Gobbi G., Bambico F.R., Mangieri R., Bortolato M., Campolongo P., Solinas M., Cassano T., Morgese M.G., Debonnel G., Duranti A., Tontini A., Tarzia G., Mor M., Trezza V., Goldberg S.R., Cuomo V., Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O., MacPherson K.P., Cinar R., Gamble-George J., Sugden K., Williams B., Godlewski G., Ramikie T.S., Gorka A.X., Alapafuja S.O., Nikas S.P., Makriyannis A., Poulton R., Patel S., Hariri A.R., Caspi A., Moffitt T.E., Kunos G., Holmes A. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol. Psychiatr. 2013;18:813–823. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J., Varga B., Ledent C., Freund T.F. CB1 cannabinoid receptors mediate anxiolytic effects: convergent genetic and pharmacological evidence with CB1-specific agents. Behav. Pharmacol. 2004;15:299–304. doi: 10.1097/01.fbp.0000135704.56422.40. [DOI] [PubMed] [Google Scholar]
- Haring M., Grieb M., Monory K., Lutz B., Moreira F.A. Cannabinoid CB(1) receptor in the modulation of stress coping behavior in mice: the role of serotonin and different forebrain neuronal subpopulations. Neuropharmacology. 2013;65:83–89. doi: 10.1016/j.neuropharm.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Hartley N.D., Gunduz-Cinar O., Halladay L., Bukalo O., Holmes A., Patel S. 2-arachidonoylglycerol signaling impairs short-term fear extinction. Transl. Psychiatry. 2016;6:e749. doi: 10.1038/tp.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz D.E., Genewsky A., Wotjak C.T. Enhanced anandamide signaling reduces flight behavior elicited by an approaching robo-beetle. Neuropharmacology. 2017;126:233–241. doi: 10.1016/j.neuropharm.2017.09.010. [DOI] [PubMed] [Google Scholar]
- Hill M.N., Gorzalka B.B. Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test. Eur. Neuropsychopharmacol. 2005;15:593–599. doi: 10.1016/j.euroneuro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Hill M.N., Miller G.E., Carrier E.J., Gorzalka B.B., Hillard C.J. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology. 2009;34:1257–1262. doi: 10.1016/j.psyneuen.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard C.J. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology. 2018;43:155–172. doi: 10.1038/npp.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.T., Hill M.N. Age of stress exposure modulates the immediate and sustained effects of repeated stress on corticolimbic cannabinoid CB(1) receptor binding in male rats. Neuroscience. 2013;249:106–114. doi: 10.1016/j.neuroscience.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Llorente-Berzal A., Terzian A.L., di Marzo V., Micale V., Viveros M.P., Wotjak C.T. 2-AG promotes the expression of conditioned fear via cannabinoid receptor type 1 on GABAergic neurons. Psychopharmacology. 2015;232:2811–2825. doi: 10.1007/s00213-015-3917-y. [DOI] [PubMed] [Google Scholar]
- Lomazzo E., Bindila L., Remmers F., Lerner R., Schwitter C., Hoheisel U., Lutz B. Therapeutic potential of inhibitors of endocannabinoid degradation for the treatment of stress-related hyperalgesia in an animal model of chronic pain. Neuropsychopharmacology. 2015;40:488–501. doi: 10.1038/npp.2014.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J.Z., Li W., Booker L., Burston J.J., Kinsey S.G., Schlosburg J.E., Pavon F.J., Serrano A.M., Selley D.E., Parsons L.H., Lichtman A.H., Cravatt B.F. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat. Chem. Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J.Z., Nomura D.K., Cravatt B.F. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem. Biol. 2009;16:744–753. doi: 10.1016/j.chembiol.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J.Z., Nomura D.K., Vann R.E., Walentiny D.M., Booker L., Jin X., Burston J.J., Sim-Selley L.J., Lichtman A.H., Wiley J.L., Cravatt B.F. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc. Natl. Acad. Sci. U. S. A. 2009;106:20270–20275. doi: 10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manduca A., Morena M., Campolongo P., Servadio M., Palmery M., Trabace L., Hill M.N., Vanderschuren L.J., Cuomo V., Trezza V. Distinct roles of the endocannabinoids anandamide and 2-arachidonoylglycerol in social behavior and emotionality at different developmental ages in rats. Eur. Neuropsychopharmacol. 2015;25:1362–1374. doi: 10.1016/j.euroneuro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Marco E.M., Rapino C., Caprioli A., Borsini F., Laviola G., Maccarrone M. Potential therapeutic value of a novel FAAH inhibitor for the treatment of anxiety. PloS One. 2015;10 doi: 10.1371/journal.pone.0137034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin R.J., Hill M.N., Bambico F.R., Stuhr K.L., Gobbi G., Hillard C.J., Gorzalka B.B. Prefrontal cortical anandamide signaling coordinates coping responses to stress through a serotonergic pathway. Eur. Neuropsychopharmacol. 2012;22:664–671. doi: 10.1016/j.euroneuro.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin R.J., Hill M.N., Dang S.S., Wainwright S.R., Galea L.A., Hillard C.J., Gorzalka B.B. Upregulation of CB(1) receptor binding in the ventromedial prefrontal cortex promotes proactive stress-coping strategies following chronic stress exposure. Behav. Brain Res. 2013;237:333–337. doi: 10.1016/j.bbr.2012.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micale V., Cristino L., Tamburella A., Petrosino S., Leggio G.M., Drago F., Di Marzo V. Anxiolytic effects in mice of a dual blocker of fatty acid amide hydrolase and transient receptor potential vanilloid type-1 channels. Neuropsychopharmacology. 2009;34:593–606. doi: 10.1038/npp.2008.98. [DOI] [PubMed] [Google Scholar]
- Molendijk M.L., de Kloet E.R. Coping with the forced swim stressor: current state-of-the-art. Behav. Brain Res. 2019;364:1–10. doi: 10.1016/j.bbr.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Moreira F.A., Kaiser N., Monory K., Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008;54:141–150. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Morena M., Patel S., Bains J.S., Hill M.N. Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology. 2016;41:80–102. doi: 10.1038/npp.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu P.S., Varvel S.A., Ahn K., Cravatt B.F., Martin B.R., Lichtman A.H. Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology. 2007;192:61–70. doi: 10.1007/s00213-006-0689-4. [DOI] [PubMed] [Google Scholar]
- Natividad L.A., Buczynski M.W., Herman M.A., Kirson D., Oleata C.S., Irimia C., Polis I., Ciccocioppo R., Roberto M., Parsons L.H. Constitutive increases in amygdalar corticotropin-releasing factor and fatty acid amide hydrolase drive an anxious phenotype. Biol. Psychiatr. 2017;82:500–510. doi: 10.1016/j.biopsych.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S., Hillard C.J. Adaptations in endocannabinoid signaling in response to repeated homotypic stress: a novel mechanism for stress habituation. Eur. J. Neurosci. 2008;27:2821–2829. doi: 10.1111/j.1460-9568.2008.06266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S., Roelke C.T., Rademacher D.J., Cullinan W.E., Hillard C.J. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- Pavon F.J., Serrano A., Sidhpura N., Polis I., Stouffer D., de Fonseca F.R., Cravatt B.F., Martin-Fardon R., Parsons L.H. Fatty acid amide hydrolase (FAAH) inactivation confers enhanced sensitivity to nicotine-induced dopamine release in the mouse nucleus accumbens. Addiction Biol. 2018;23:723–734. doi: 10.1111/adb.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Franklin K.B.J. Academic Press; San Diego: 2001. The Mouse Brain in Stereotaxic Coordinates. [Google Scholar]
- Serrano A., Pavon F.J., Buczynski M.W., Schlosburg J., Natividad L.A., Polis I.Y., Stouffer D.G., Zorrilla E.P., Roberto M., Cravatt B.F., Martin-Fardon R., Rodriguez de Fonseca F., Parsons L.H. Deficient endocannabinoid signaling in the central amygdala contributes to alcohol dependence-related anxiety-like behavior and excessive alcohol intake. Neuropsychopharmacology. 2018;43:1840–1850. doi: 10.1038/s41386-018-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T., Kondo S., Sukagawa A., Nakane S., Shinoda A., Itoh K., Yamashita A., Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Sullivan R.M., Dufresne M.M., Siontas D., Chehab S., Townsend J., Laplante F. Mesocortical dopamine depletion and anxiety-related behavior in the rat: sex and hemisphere differences. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;54:59–66. doi: 10.1016/j.pnpbp.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Wang Y., Gu N., Duan T., Kesner P., Blaskovits F., Liu J., Lu Y., Tong L., Gao F., Harris C., Mackie K., Li J., Tan Q., Hill M.N., Yuan Z., Zhang X. Monoacylglycerol lipase inhibitors produce pro- or antidepressant responses via hippocampal CA1 GABAergic synapses. Mol. Psychiatr. 2017;22:215–226. doi: 10.1038/mp.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang X. FAAH inhibition produces antidepressant-like efforts of mice to acute stress via synaptic long-term depression. Behav. Brain Res. 2017;324:138–145. doi: 10.1016/j.bbr.2017.01.054. [DOI] [PubMed] [Google Scholar]
- Zarrindast M.R., Khakpai F. The modulatory role of dopamine in anxiety-like behavior. Arch. Iran. Med. 2015;18:591–603. [PubMed] [Google Scholar]
- Zhang Z., Wang W., Zhong P., Liu S.J., Long J.Z., Zhao L., Gao H.Q., Cravatt B.F., Liu Q.S. Blockade of 2-arachidonoylglycerol hydrolysis produces antidepressant-like effects and enhances adult hippocampal neurogenesis and synaptic plasticity. Hippocampus. 2015;25:16–26. doi: 10.1002/hipo.22344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P., Wang W., Pan B., Liu X., Zhang Z., Long J.Z., Zhang H.T., Cravatt B.F., Liu Q.S. Monoacylglycerol lipase inhibition blocks chronic stress-induced depressive-like behaviors via activation of mTOR signaling. Neuropsychopharmacology. 2014;39:1763–1776. doi: 10.1038/npp.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.