Abstract
Purpose
This study aimed to investigate intra- and interfraction motion during liver stereotactic body radiation therapy for the purpose of planning target volume (PTV) margin estimation, comparing deep inspiration breath hold (DIBH) and deep expiration breath hold (DEBH).
Methods and materials
Pre- and posttreatment kV cone beam computed tomography (CT) images were acquired for patients with liver cancer who were treated using stereotactic body radiation therapy with DIBH or DEBH. A total of 188 images were analyzed from 18 patients. Positioning errors were determined based on a comparison with planning CT images and matching to the liver. Treatment did not proceed until errors were ≤3 mm. Standard deviations of random and systematic errors resulting from this image matching process were used to calculate PTV margin estimates.
Results
DIBH errors are generally larger than DEBH errors, especially in the anterior–posterior and superior–inferior directions. Posttreatment errors tend to be larger than pretreatment errors, especially for DIBH. Standard deviations of random errors are larger than those of systematic errors. Considering both pre- and posttreatment cone beam CT images, PTV margins for DIBH and DEBH are estimated as anterior–posterior, superior–inferior, right–left = (5.7, 6.3, 3.0) mm and (3.1, 3.4, 2.8) mm, respectively.
Conclusions
This study suggests that DEBH results in more reproducible target positioning, which could in turn justify the use of smaller PTV margins.
Introduction
Liver radiation therapy is a noninvasive treatment option for patients who are not surgical candidates or who have contraindications to chemotherapy.1 Stereotactic body radiation therapy (SBRT) potentially allows for improved normal tissue sparing and dose delivery accuracy. SBRT involves stringent patient positioning requirements, which allow for tighter planning target volume (PTV) margins compared with conventional radiation therapy where hypofractionated treatment regimens are used. Clinical trials for liver SBRT have shown encouraging results.2, 3, 4 Dawood et al1 presented a summary of SBRT for liver metastases, and most studies prescribed 30 Gy to 60 Gy in 3 fractions with approximately 10-mm margins in the superior–inferior (SI) direction and approximately 5-mm margins in the anterior–posterior (AP) and right–left (RL) directions.
Proximity of the liver to the diaphragm results in unwanted respiratory motion. Therefore, liver SBRT is typically delivered using either deep inspiration breath hold (DIBH) or deep expiration breath hold (DEBH). In addition to DIBH/DEBH, tumor motion can also be managed using gating, abdominal compression, or motion tracking.1 Capturing the motion using an internal target volume is also a possibility, but this results in more dose to normal tissues. Free breathing with gated treatment delivery prolongs treatment considerably. Abdominal compression reduces motion but is generally less effective than the breath hold technique. Motion tracking can be achieved with, for example, CyberKnife,4 but the current install base of this technology is small compared with conventional C-arm linacs. In contrast, breath hold techniques retain reasonably good treatment delivery efficiency while minimizing liver motion and allowing for treatment on a conventional C-arm linac. However, not all patients are capable of reproducible breath holds, and variations can lead to errors in treatment delivery,1 including target underdosage.5 Pretreatment imaging and subsequent repositioning shifts have been shown to be necessary to ensure accurate patient positioning.6,7
Dawson et al8 investigated a personalized approach to liver SBRT, considering PTV margins (minimum: 5 mm) based on breath hold reproducibility and liver motion, seeking to maintain the same risk of radiation-induced liver disease according to a radiobiologic model. Kitamura et al9 reported that the motion of the tumor during free breathing depends on various patient-specific factors, including tumor location, cirrhosis, and previous surgery. Yang et al10 compared positioning errors according to fiducial markers with those determined according to bony anatomy and found significant differences resulting from interfraction changes in patient anatomy. However, Balter et al11 showed that the diaphragm is a suitable surrogate for liver motion, resulting in positioning errors with a standard deviation (SD) of 1.04 mm in the SI direction compared with fiducial markers during free breathing.
This work investigated inter- and intrafraction motion during liver SBRT based on an analysis of pre- and posttreatment kV cone beam computed tomography (CT) images, considering both DIBH and DEBH. Random and systematic errors are computed based on a comparison of cone beam CT images with the planning CT images. The PTV margins are estimated.
Methods and Materials
A total of 18 patients with liver cancer were treated with SBRT for a total of 20 courses of treatment. Half of these treatments were delivered with DIBH, and DEBH was used for the other half. In the DIBH cohort, there were 6 women and 4 men, ranging in age from 46 to 74 years old (median age: 61 years). There was 1 case of fibroadenoma, 1 case of hepatocellular carcinoma, and the rest were metastases. Prescription doses ranged from 27.5 Gy to 50 Gy in 5 fractions, with an average of 40 Gy.
In the DEBH cohort, there were 5 women and 4 men. In 1 case, the patient received 2 separate treatments and appears in both cohorts. DEBH patients ranged in age from 54 to 81 years (median age: 72 years). There was 1 case of fibroadenoma, 2 cases of hepatocellular carcinoma, and the rest were metastases. Prescription doses ranged from 25 Gy to 50 Gy in 5 fractions, with an average of 44 Gy.
Treatment planning and delivery
A flowchart of the entire liver SBRT procedure is shown in Figure 1. Before acquisition of the planning CT, patient breath hold ability was assessed by the radiation oncologist using fluoroscopy. During breath hold, if the motion of the diaphragm (surrogate for the liver) is ≤3 mm and the patient is capable of holding their breath for at least ~20 seconds, then liver SBRT with breath hold may proceed.
Figure 1.
Flowchart illustrating the liver SBRT procedure.
The Real-time Position Management system (Varian Medical Systems, Palo Alto, CA) is used to monitor breath hold during both image acquisition and treatment delivery. An infrared camera system monitors height variation of a marker placed on the patient’s abdomen as a surrogate for internal motion. In the Real-time Position Management software, a 3-mm gating window is defined for both DIBH and DEBH, meaning that ±1.5 mm of amplitude variation is allowed around the breath hold position. The 3-mm window was considered an appropriate compromise that facilitated efficient treatment delivery with minimal allowable deviation of the respiratory trace from the planned position.
Liver SBRT was delivered using volumetric modulated arc therapy12 with 2 full coplanar arcs. Treatment was delivered using a 6 MV flattening filter-free photon beam with a maximum dose rate of 1400 MU per minute. Doses ranged from 25 Gy to 50 Gy prescribed to the 90% isodose surface, delivered in 5 fractions. PTV margins were between 5 mm and 8 mm depending on the variation in the diaphragm position observed during the pretreatment fluoroscopy session.
Treatments were delivered on a Varian TrueBeam linac. The kV on-board imaging system was used to acquire cone beam CT images before and after each treatment fraction. Patient positioning errors were determined based on a comparison of the cone beam CT with the planning CT, matching to the liver while also taking into account nearby organs at risk (eg, matching was determined by the location of the liver/bowel space interface in some cases). A total of 188 images were analyzed.
Before treatment delivery, if errors exceeded 3 mm in any direction, the couch was repositioned accordingly. Rotations were not considered. In most cases, multiple pretreatment cone beam CT images needed to be acquired, but only the final cone beam CT image was considered in this analysis. After treatment delivery, no additional couch shifts were applied, and another cone beam CT image was acquired. In 12 of the treatment fractions, posttreatment cone beam CT images were not acquired owing to patient fatigue. In all but 4 of the treatment courses, posttreatment cone beam CT images were acquired for at least 4 of 5 fractions. In all cases, posttreatment cone beam CT images were acquired for at least 3 of 5 fractions.
Estimation of planning target volume margins
Using the methodology introduced by van Herk et al,13,14 the SD of the systematic error (Σ) was calculated as follows: First, errors were averaged across all fractions for a given patient, then the SD was computed across all patients. The value of Σ quantifies treatment preparation errors that affected the entire course of treatment for a single patient. The SD of the random error (σ’) was found by first calculating the SD of errors across all fractions for a given patient, and next the root mean square was computed across all patients. The value of σ’ quantifies treatment execution errors that vary from 1 fraction to the next. The PTV margin (mPTV) was estimated as follows13:
| (1) |
where α = 2.5 and γ = 0.60 corresponds to 90% of patients having their clinical target volume enclosed by the 90% isodose surface. This equation assumes that the 50% to 90% penumbra is approximately 4 mm.
Van Herk et al13 pointed out that for a small number of fractions N (as in stereotactic radiation therapy), the average treatment execution (ie, random) error may be nonzero. In this case, the SD of the systematic error is redefined as as suggested by Leong et al15:
| (2) |
The aforementioned modification is adopted in this work. To quantify errors that systematically affect the treatment planning or delivery processes for all patients, we calculated the mean of means. First, errors were averaged across all fractions for a given patient, then the average was computed across all patients.
Results
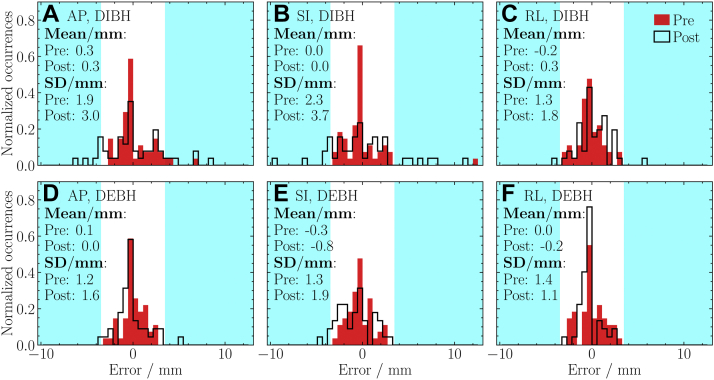
Histograms summarizing the entire data set are presented in Figure 2. In most cases, DIBH histogram SDs are larger than those of DEBH. For DIBH, posttreatment SDs are larger than pretreatment SDs, especially in the AP and SI directions. For DEBH, pre- and posttreatment SDs are similar. Also, DEBH SDs are similar in all directions (differing by <1 mm). Distribution means are equal to 0 within 1 mm, and there is no strong indication of an overall systematic error in any particular direction.
Figure 2.
Distributions of pre- and posttreatment errors for (A, D) anterior–posterior, (B, E) superior–inferior, and (C, F) right–left directions. The results are presented for (A, B, C) deep inspiration breath hold and (D, E, F) deep expiration breath hold. The mean and standard deviation are indicated. Errors outside of the 3-mm tolerance are in cyan. In 3 deep inspiration breath hold treatment fractions, pretreatment errors of >3 mm were accepted by the radiation oncologist for clinical reasons. (A color version of this figure is available at https://doi.org/10.1016/j.adro.2020.10.023.)
The SDs of errors across all patients are plotted as a function of fraction number in Figure 3. The SDs are generally smallest in the RL direction. The SDs of DIBH posttreatment errors in the SI direction tend to increase as the course of treatment progresses.
Figure 3.
Standard deviations of errors across all patients as a function of fraction number for (A) anterior–posterior, (B) superior–inferior, and (C) right–left directions.
SDs of random and systematic errors are presented in Figure 4. In general, values for DIBH are larger than those of DEBH, especially in the AP and SI directions. Posttreatment values tend to be larger than pretreatment values, especially for DIBH. SDs of random errors are larger than SDs of systematic errors.
Figure 4.
Standard deviations of (A) random and (B) systematic errors computed according to van Herk, using the modification introduced by Leong et al13,15 Results are presented for the anterior–posterior, superior–inferior, and right–left directions.
SDs of random and systematic errors, values of the mean of means, and PTV margin estimates are presented in Table 1. In Table 2, similar results are presented, but with pre- and posttreatment errors combined into a single data set. In all cases, the mean of means is equal to 0 within 1 mm, which suggests that there are no pronounced systematic errors in the overall treatment planning or delivery processes. In Table 1, the posttreatment mean of means in the SI direction is larger in magnitude for DEBH than for DIBH (−0.7 mm vs −0.1 mm), which suggests that there is a systematic change in posttreatment positioning for DEBH. Nonetheless, the magnitude of this systematic shift is sub-mm, and posttreatment positional variability in DIBH leads to considerably larger margin requirements.
Table 1.
Standard deviations of random and systematic errors, values of the mean of means, and PTV margin estimates for DIBH and DEBH
| Pretreatment DIBH |
Posttreatment DIBH |
||||||
|---|---|---|---|---|---|---|---|
| AP | SI | RL | AP | SI | RL | ||
| Random | 1.7 | 2.2 | 1.4 | 2.6 | 3.6 | 1.8 | mm |
| Systematic | 1.3 | 1.5 | 0.8 | 2.5 | 2.7 | 1.1 | mm |
| Mean of means | 0.3 | 0.0 | -0.2 | 0.2 | –0.1 | 0.3 | mm |
| PTV margin | 4.3 | 5.1 | 2.7 | 7.7 | 9.0 | 3.8 | mm |
| Pretreatment DEBH |
Posttreatment DEBH |
||||||
|---|---|---|---|---|---|---|---|
| AP | SI | RL | AP | SI | RL | ||
| Random | 1.1 | 1.4 | 1.3 | 1.5 | 2.0 | 1.2 | mm |
| Systematic | 0.8 | 0.8 | 1.0 | 1.2 | 1.2 | 0.7 | mm |
| Mean of means | 0.1 | –0.3 | 0.0 | 0.0 | –0.7 | –0.2 | mm |
| PTV margin | 2.6 | 2.9 | 3.3 | 3.8 | 4.3 | 2.6 | mm |
Abbreviations: AP = anterior–posterior; DEBH = deep expiration breath hold; DIBH = deep inspiration breath hold; PTV = planning target volume; RL = right–left; SI = superior–inferior.
Table 2.
Standard deviations of random and systematic errors, values of the mean of means, and PTV margin estimates for DIBH and DEBH with pre- and posttreatment images analyzed together
| DIBH |
DEBH |
||||||
|---|---|---|---|---|---|---|---|
| AP | SI | RL | AP | SI | RL | ||
| Random | 2.2 | 3.1 | 1.6 | 1.3 | 1.6 | 1.2 | mm |
| Systematic | 1.7 | 1.8 | 0.8 | 0.9 | 1.0 | 0.8 | mm |
| Mean of means | 0.3 | 0.0 | 0.0 | 0.1 | –0.5 | 0.0 | mm |
| PTV margin | 5.7 | 6.3 | 3.0 | 3.1 | 3.4 | 2.8 | mm |
Abbreviations: AP = anterior–posterior; DEBH = deep expiration breath hold; DIBH = deep inspiration breath hold; PTV = planning target volume; RL = right–left; SI = superior–inferior.
Discussion
Our results (Table 1) are comparable to values reported in the literature: Zhong et al7 report PTV margin estimates for AP, SI, RL of (5.2, 4.7, 3.7) mm and (6.9, 7.8, 5.7) mm according to the analysis of pre- and posttreatment cone beam CT images, respectively. The researchers used DIBH with the active breathing control (ABC) technique. With DEBH using ABC, Dawson et al6 reported pretreatment SDs of random and systematic errors for AP, SI, RL of (2.9, 2.5, 2.8) mm and (1.9, 1.4, 2.0) mm, respectively. These values are based on an analysis of MV orthogonal image pairs. In contrast, the kV cone beam CT images used herein likely resulted in improved image quality and soft tissue contrast, potentially allowing for more accurate patient positioning. Lu et al5 investigated the reproducibility of DIBH using ABC, considering liver centroid displacement across 3 CT images acquired using 3 separate breath holds. The researchers reported SDs for AP, SI, and RL of 2.51, 2.61, and 0.71 mm. In this work, the SD of errors across all patients and fractions for AP, SI, and RL was 2.5, 3.0, and 1.6 mm for DIBH and 1.4, 1.6, and 1.3 mm for DEBH, considering all pre- and posttreatment data.
We found that errors tended to be largest in the SI direction, which is in agreement with the results of previous research (eg, Yang et al10 and Zhong et al7). This SI error is likely due to variations from one breath hold to the next. In contrast, errors tended to be the smallest in the RL direction (Fig 4), which makes sense given that breath hold variations are not expected to have an effect on lateral positioning. In agreement with the results by Dawson et al,6 SDs of random errors were generally larger than SDs of systematic errors.
The observed increase in the SD of DIBH posttreatment errors in the SI direction (Fig 3B) could be due to increased patient fatigue over the course of treatment or the onset of treatment-related side effects,2,4 making DIBH more difficult. This trend is reflected in the relatively large DIBH posttreatment PTV margin estimates (Table 1). Such a trend is not apparent in the DEBH data, which suggests that DEBH is a more reproducible technique. In support of this claim, radiation therapists at our institution anecdotally report that with DIBH, patients may inhale slightly different amounts of air into their lungs from one attempt to the next. As a result, patients sometimes attempt DIBH several times before achieving a breath hold that corresponds to a position within the gating window. However, radiation therapists also report that patients find DEBH less comfortable than DIBH, resulting in breath hold durations of approximately 15 seconds and 20 seconds for DEBH and DIBH, respectively.
In addition, the time elapsed between the final pretreatment and posttreatment cone beam CT images is 1.65 times longer for DEBH than DIBH. This result could be due to the DEBH group having a higher median age or due to shorter DEBH durations resulting in a need for more breath holds per treatment. Despite all of this, DEBH results in smaller PTV margin estimates. Furthermore, the time elapsed between the beginning of the appointment (when the patient is brought into the treatment room) and the posttreatment cone beam CT images is 1.14 times longer for DEBH than DIBH.
Pre- and posttreatment PTV margins for DIBH differ considerably, but pre- and posttreatment PTV margin estimates for DEBH differ by <1.5 mm (Table 1). PTV margins range from 2.6 mm to 4.3 mm for DEBH. An analysis of DIBH posttreatment errors suggests that PTV margins of up to 9.0 mm would be required to obtain an acceptable level of clinical target volume coverage. In contrast, an analysis of DIBH pretreatment errors resulted in PTV margin estimates of at most 5.1 mm. This result emphasizes the importance of considering both pre- and posttreatment errors to obtain a more accurate estimation of intrafraction motion. Because treatments are delivered over the course of several breath holds (~3-8 per fraction), analysis of posttreatment cone beam CT data identifies a lower bound for the positioning error.
In most cases, multiple pretreatment cone beam CT images were needed to achieve patient positioning errors within the 3-mm tolerance. On average, 2.3 pretreatment images were required per fraction for DIBH, and 1.8 images were required for DEBH. PTV margin estimates can be recalculated, including these additional images; however, if multiple pretreatment images are acquired, then the first image is not used (because patient positioning is not based on image guidance in this case). This corresponds to 15 and 8 extra images for DIBH and DEBH, respectively. Redoing the analysis in this way, considering all pre- and posttreatment data, resulted in PTV margin estimates for AP, SI, and RL of (6.8, 6.7, 3.0) mm and (3.8, 4.2, 2.9) mm for DIBH and DEBH, respectively. These values are similar to those in Table 2, differing by at most 1.1 mm.
As mentioned, some patients were missing posttreatment cone beam CT image sets. To investigate the effect of these patients, the analysis was redone with the exclusion of the 4 courses of treatment where >1 posttreatment cone beam CT image was missing. In this case, PTV margins estimates for AP, SI, and RL became (5.6, 5.6, 2.9) mm and (3.3, 3.5, 2.3) mm for DIBH and DEBH, respectively, considering all pre- and posttreatment data. This corresponds to a difference of at most 0.7 mm compared to Table 2. Therefore, the missing posttreatment cone beam CT image sets do not have a drastic effect on results.
In the context of image guided radiation therapy (IGRT), examples of systematic errors include target delineation errors, lack of coincidence of the imaging and treatment isocenters, anatomic changes that IGRT cannot correct for, and appropriateness of the image matching surrogate for the true lesion. Random errors include patient motion during treatment delivery, random rotational errors that cannot be corrected without a 6 degrees-of-freedom couch, breath hold variations, and random physiological processes. Using Equation 2, this study estimated the PTV margins for liver SBRT with breath hold, taking into account intrafraction, interfraction, and interpatient errors that can be detected with IGRT. However, many of the errors listed earlier cannot be detected or corrected using IGRT. In particular, any error related to the assumed spatial relationships between the anatomy used for image registration and the target is not included in this analysis. In fact, any margin evaluation study considering targets that are not visible on cone beam CT images will encounter this limitation. Also, considering that the approach by van Herk et al13 involves various assumptions, the PTV margin estimates provided herein can be considered a lower limit.
Conclusions
Compared with DIBH, DEBH for liver SBRT generally resulted in smaller SDs of random and systematic errors and smaller PTV margin estimates according to analysis of pre- and posttreatment cone beam CT images. PTV margin estimates for AP, SI, and RL were (5.7, 6.3, 3.0) mm and (3.1, 3.4, 2.8) mm for DIBH and DEBH, respectively. DEBH is apparently more reproducible and therefore recommended over DIBH for liver SBRT. The use of DEBH could justify the use of smaller PTV margins compared with DIBH, leading to improved normal tissue sparing without compromising tumor control probability.
Footnotes
Sources of support: No funding was received for this project.
Disclosures: The authors have no conflicts of interest to declare.
Research data are stored in an institutional repository and can be shared upon request to the corresponding author.
Contributor Information
Patricia A.K. Oliver, Email: Patricia.Oliver2@albertahealthservices.ca.
Alasdair Syme, Email: Alasdair.Syme@nshealth.ca.
References
- 1.Dawood O., Mahadevan A., Goodman K.A. Stereotactic body radiation therapy for liver metastases. Eur J Cancer. 2009;45:2947–2959. doi: 10.1016/j.ejca.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Katz W., Carey-Sampson M., Muhs A.G., Milano M.T., Schell M.C., Okunieff P. Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Radiat Oncol Biol Phys. 2007;67:793–798. doi: 10.1016/j.ijrobp.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 3.Rusthoven K.E., Kavanagh B.D., Cardenes H. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 4.Mahadevan A., Dagoglu N., Mancias J. Stereotactic body radiotherapy (SBRT) for intrahepatic and hilar cholangiocarcinoma. J Cancer. 2015;6:1099. doi: 10.7150/jca.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu L., Ouyang Z., Lin S., Mastroianni A., Stephans K.I., Xia P. Dosimetric assessment of patient-specific breath-hold reproducibility on liver motion for SBRT planning. J Appl Clin Med Phys. 2020;21:77–83. doi: 10.1002/acm2.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson L.A., Eccles, Bissonnette J.P., Brock K.K. Accuracy of daily image guidance for hypofractionated liver radiotherapy with active breathing control. Int J Radiat Oncol Biol Phys. 2005;62:1247–1252. doi: 10.1016/j.ijrobp.2005.03.072. [DOI] [PubMed] [Google Scholar]
- 7.Zhong R., Wang J., Jiang X. Hypofraction radiotherapy of liver tumor using cone beam computed tomography guidance combined with active breath control by long breath-holding. Radiother Oncol. 2012;104:379–385. doi: 10.1016/j.radonc.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Dawson L.A., Eccles C., Craig T. Individualized image guided iso-NTCP based liver cancer SBRT. Acta Oncol. 2006;45:856–864. doi: 10.1080/02841860600936369. [DOI] [PubMed] [Google Scholar]
- 9.Kitamura K., Shirato H., Seppenwoolde Y. Tumor location, cirrhosis, and surgical history contribute to tumor movement in the liver, as measured during stereotactic irradiation using a real-time tumor-tracking radiotherapy system. Int J Radiat Oncol Biol Phys. 2003;56:221–228. doi: 10.1016/s0360-3016(03)00082-8. [DOI] [PubMed] [Google Scholar]
- 10.Yang W., Fraass B.A., Reznik R. Adequacy of inhale/exhale breathhold CT based ITV margins and image-guided registration for free-breathing pancreas and liver SBRT. Radiat Oncol. 2014;9:11. doi: 10.1186/1748-717X-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balter J.M., Dawson L.A., Kazanjian S. Determination of ventilatory liver movement via radiographic evaluation of diaphragm position. Int J Radiat Oncol Biol Phys. 2001;51:267–270. doi: 10.1016/s0360-3016(01)01649-2. [DOI] [PubMed] [Google Scholar]
- 12.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35:310–317. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 13.van Herk M., Remeijer P., Rasch C., Lebesque J.V. The probability of correct target dosage: Dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1121–1135. doi: 10.1016/s0360-3016(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 14.van Herk M. Errors and margins in radiotherapy. Semin Radiat Oncol. 2004;14:52–64. doi: 10.1053/j.semradonc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Leong J. Implementation of random positioning error in computerised radiation treatment planning systems as a result of fractionation. Phys Med Biol. 1987;32:327–334. doi: 10.1088/0031-9155/32/3/002. [DOI] [PubMed] [Google Scholar]