Summary
Electroactive microbes is the driving force for the bioelectrochemical degradation of organic pollutants, but the underlying microbial interactions between electrogenesis and pollutant degradation have not been clearly identified. Here, we combined stable isotope-assisted metabolomics (SIAM) and 13C-DNA stable isotope probing (DNA-SIP) to investigate bisphenol S (BPS) enhanced degradation by electroactive mixed-culture biofilms (EABs). Using SIAM, six 13C fully labeled transformation products were detected originating via hydrolysis, oxidation, alkylation, or aromatic ring-cleavage reactions from 13C-BPS, suggesting hydrolysis and oxidation as the initial and key degradation pathways for the electrochemical degradation process. The DNA-SIP results further displayed high 13C-DNA accumulation in the genera Bacteroides and Cetobacterium from the EABs and indicated their ability in the assimilation of BPS or its metabolites. Collectively, network analysis showed that the collaboration between electroactive microbes and BPS assimilators played pivotal roles the improvement in bioelectrochemically enhanced BPS degradation.
Subject areas: Biochemistry applications, isotope, microbial metabolism, microbiofilms, pollution
Graphical abstract

Highlights
-
•
BPS can be effectively degraded by the electroactive biofilms in BES
-
•
DNA-SIP showed Bacteroides and Cetobacterium were the dominant BPS assimilators
-
•
SIAM highlighted hydrolysis and oxidation as initial degradation pathways for BPS
-
•
The enhanced degradation is attributed to interactions between EAM and degradators
Biochemistry applications; isotope; microbial metabolism; microbiofilms; pollution
Introduction
Bioelectrochemical system (BES) is a sustainable technology that can provide continuous electrons to enhance bioremediation efficiency through a combination of biological and electrochemical processes (Miran et al., 2018; Wang et al., 2016). BESs have been applied for in degradation of various recalcitrant pollutants, such as BTEX compounds (benzene, toluene, ethylbenzene, and xylenes) (Daghio et al., 2018), sulfa drugs (Miran et al., 2018; Wang et al., 2016), halogenated aromatic hydrocarbons (2-fluoroaniline, 2,4,6-trichlorophenol, and 2,4-dichlorophenol) (Cao et al., 2016; Xu et al., 2018; Zhang et al., 2014), and organophosphate esters (Hou et al., 2019). A mixed-culture BES exhibited the most promising stable electrochemical expression and degradation cooperation (Ng et al., 2017), and the electricity generated by electrochemically active biofilms (EABs) was deemed the central driving force for the biocatalysis of recalcitrant compounds in the BES (Hou et al., 2019). The degradation of organic pollutants is commonly accomplished by anodic oxidation by EABs, where the pollutant discharges electrons to oxidative metabolites (Hasany et al., 2016; Song et al., 2016). Therefore, comprehensive investigation of the microbial community and pollutant transformation in BES is crucial and will lead to a better understanding of bioelectrochemical reaction mechanisms and assessment of treatment efficiency (Yan et al., 2019).
Considering that most electroactive microbes (EAMs) in EABs can only use restricted substrates as electron donors, such as acetate and glucose (Chae et al., 2009; Fernando et al., 2019; Kiely et al., 2011; Logan et al., 2019), several studies recognized that unique non-EAMs in EABs were responsible for metabolizing organic pollutants (Capellades et al., 2016; Yan et al., 2019). The degradation process for organic pollutants can be divided into the decomposition of complex compounds by non-EAMs and the subsequent utilization of simple products by EAMs (Cao et al., 2020). To reveal the actual microbial characteristics during the electrochemical degradation of organic pollutants in BES, high-throughput sequencing and even metagenomic approaches have been used to scan the pollution-associated microbiome in EABs. The existence of target organic pollutants can alter the microbial community on the anode surface of the BES, where some microbial communities with unique pollutant degradation capacities were enriched on acclimatized EABs (Hou et al., 2019; Lu et al., 2014; Pham et al., 2009; Yan et al., 2017). It was suggested that the coexistence and the potential synergy between EMs and multiple organic-degrading microbes may be the underlying mechanisms for the enhanced degradation of biorefractory compounds in BES (Hou et al., 2019). However, the contribution of non-EAMs to pollutant degradation and the possible interaction mechanisms between EAMs and non-EAMs during pollutant degradation are still unknown.
The degradation pathways for several biorefractory compounds in mixed-culture EABs have been previously demonstrated via target screening methods using high-resolution mass spectrometry. For example, the degradation pathway of sulfamethoxazole in a BES was determined to include the cracking of S–N bonds, N–O bonds, and carbon–carbon double bonds (Wang et al., 2016); and oxidized furan derivatives, open-ring products, and dimers were generated from the bioelectrochemical transformation of furanic and phenolic compounds via exoelectrogenesis (Zeng et al., 2015). However, targeted screening methods for metabolite identification require predetermined or suspected metabolic pathways, which may overlook potential novel pathways, reaction priority, and sequences (Creek et al., 2012). In the targeted method, secondary analytical approaches are also needed for confident identification, where the purchase, synthesis, or isolation of pure authentic metabolite standards is required (Creek 2013). The degradation mechanisms for xenobiotic pollutants are quite complicated in BESs with different operation modes, and the detected products may not derive from classical pathways, even in well-defined bioelectrochemical processes (Yan et al., 2019). Therefore, there is still a limitation on the ability to determine pollutant degradation processes and mechanisms in BESs with traditional target screening methods.
In recent years, pathway identification and mechanism elucidation in bioremediation processes have progressed, aided by advancements in stable isotope probing (SIP) techniques, such as DNA-SIP and stable isotope-assisted metabolomics (SIAM). DNA-SIP allows the identities of microorganisms in environmental samples to be linked with their activities and functions using particular growth substrates that are highly enriched in a stable isotope (e.g., 13C or 15N) (Bernard et al., 2007; Dumont and Murrell 2005; Zhan et al., 2018). This technique has been used to identify the functional microbes in the biodegradation of a large number of pollutants, including pharmaceuticals and personal care products (Badia-Fabregat et al., 2014), phenanthrene (Jones et al., 2011; Li et al., 2017b), bisphenol A (Sathyamoorthy et al., 2018), triclosan (Lee et al., 2014), and nonylphenol (Zemb et al., 2012). For bioelectrochemical process, DNA-SIP has also been used to identify the predominant roles of Geobacter sulfurreducens and Hydrogenophaga in acetate consumption and current generation in microbial fuel cells (Kimura and Okabe 2013). However, few studies have applied DNA-SIP to investigate the microbial mechanisms of pollutant degradation by EABs. In addition, SIAM can provide the identification of stable isotopes labeled metabolites on a global scale through comparing to their nonlabeled pendants according to appropriate experimental protocols (e.g., X13CMS, geoRge, and MetExtract II) (Baran et al., 2010; Bueschl et al., 2017; Capellades et al., 2016; Creek et al., 2012). Recently, SIAM has been applied to quantitatively unveil a variety of biological processes, such as carbon and nitrogen fixation by Synechococcus sp. (Baran et al., 2010), the intracellular metabolic fluxes of G. sulfurreducens under different electron donors (Yang et al., 2010), and the biotransformation of polycyclic aromatic hydrocarbons in soil (Tian et al., 2018). Inspired by these studies, the combination of these stable isotopic tracing techniques is expected to offer an opportunity to elucidate not only the global pollutant transformation pathways but also key microbial interplay mechanisms between EAMs and non-EAMs for pollutant degradation in BESs.
Bisphenol S (4-hydroxyphenyl sulfone; BPS) is one of the major substitutes for the replacement of bisphenol A (BPA) in various applications with endocrine-disrupting effects of concern (Ruth and Louise 2015). BPS exhibits similar structures and environmental behaviors to other bisphenol analogues including BPA (Chen et al., 2016), and similar removal effect of BPS is expected for BES (Li et al., 2019). However, to the best of our knowledge, there is a limited report on the removal strategies of BPS in the bioelectrochemical approach and the unique microbial cooperation mechanisms in EAMs during degradation of this kind of pollutants. In this study, we expand the application of SIP to elucidate the biodegradation mechanisms of BPS by acclimated EABs in a three-electrode BES, combining the technologies of DNA-SIP and SIAM. Our objectives were (1) to investigate the relationship between electricity generation characteristics and the removal kinetics of BPS in acclimated EABs; (2) to apply SIAM to identify the global biodegradation pathways for BPS; and (3) to utilize DNA-SIP to characterize the contributions of EAMs and non-EAMs in biofilms and BES suspensions to BPS degradation. These results might provide novel information on microbial behavior in the bioelectrochemical remediation of biorefractory compounds and promote the better exploitation of appropriate BES strategies.
Results
Bioelectrochemical degradation of BPS in the BESs
Figure S1 shows the changes in the BPS biodegradation ratio in the BES reactors during 9 cycles of acclimation, at the initial administrated concentration of 2 mg/L. BPS was rather difficult to remove over the 48 hr after it was initially added to the reactors. After acclimation to BPS, it was found that the changes in BPS concentration in the BESs were rather obvious, where approximately 78.2% of the BPS was removed after 48 h of operation. On the other hand, the reactor current density severely declined and then gradually increased to 4.0 mA/cm2, which indicated that the metabolic activity of EAMs inhibited by BPS was recovered and that the reactor microbes became highly efficient at BPS removal after 9 cycles of operation.
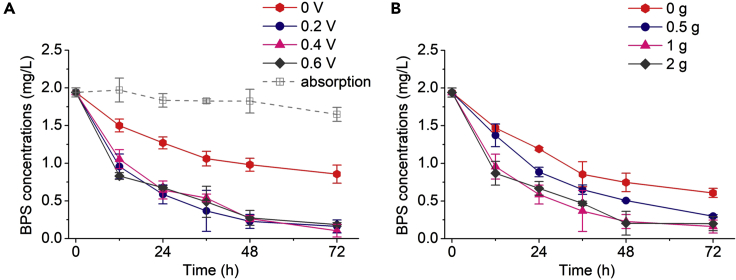
Three different modes of BES reactors were tested for comparison, including a closed-circuit mode, an open-circuit mode, and an abiotic control. Figure 1A shows the degradation kinetics of 2 mg/L BPS during 72 h of incubation for the BES groups. Significant changes in BPS concentration were observed in all closed-circuit groups, with the 72 h degradation ratios for BPS calculated as 55.9%, 91.7%, 94.6%, and 90.4% under anode potentials of 0, 0.2, 0.4, and 0.6 V, respectively. However, only approximately 15.1% and 24.8% of BPS was removed in the abiotic control and open-circuit groups after 72 h of operation, respectively. These results indicated that the physical adsorption of BPS by the BES anode was slight and that the existence of an electric field in the BES reactors could increase the removal of BPS. In addition, the calculated pseudo-first order rate constants (k) for BPS at an anode potential of 0 V were significantly lower than those at anode potentials of 0.2, 0.4 and 0.6 V (Table S1; p < 0.05), but no significance was found among the degradation rate constants of BES reactors at anode potentials of 0.2, 0.4 and 0.6 V (p > 0.05). This phenomenon is in agreement with previous studies in which EAMs at 0.2 V exhibited higher catalytic activity than those at other potentials ranging from −0.1 V to +0.6 V (Li et al., 2017a).
Figure 1.
BPS degradation by the acclimatized biofilm in BESs
(A) degradation curves under different external voltages.
(B) degradation curves under different sodium acetate concentrations. Other test conditions: BPS 2 mg/L, ambient temperature (28 ± 2°C), pH = 7.0. Data are represented as mean ± SEM.
To ascertain the role of the acclimatized EAMs in BPS removal in the BESs, the degradation kinetics of BPS were evaluated under various sodium acetate concentrations (Figure 1B). The BES without sodium acetate showed a BPS removal of only 68.7%, and this removal increased significantly to 84.6% when 0.5 g/L sodium acetate was added at the beginning of incubation into the BES as cosubstrate. BPS degradation in the BES with 2 g/L sodium acetate showed the highest rate constants (Table S1). The results were in accord with previous studies on the enhanced degradation of aromatic compounds in BESs obtained by supplementing carbon cosubstrates (Fan et al., 2017; Luo et al., 2009). We also found elevated electricity generation with increasing sodium acetate concentrations in the BESs (Figure S2). Therefore, the acceleration in the removal of BPS in the BESs can be linked to the presence of the anode in providing an electron transfer pathway to enhance the metabolic rate of bacteria using acetate as an electron acceptor (Miran et al., 2018). The permeability of the cell membrane, increasing the absorbance of extracellular substances, and altering the microbial metabolism of EAMs caused by an electric field might also be the possible reason for the enhancement of BPS degradation in BESs (Hasany et al., 2016; Saratale et al., 2017; Yan et al., 2019).
Metabolites and novel pathway of BPS biodegradation as revealed by SIAM
To unequivocally demonstrate the degradation mechanisms of BPS in BESs, we employed an untargeted SIAM study, wherein a 1:1 ratio of 12C- and 13C12-BPS was used as substrate with sodium acetate. A total of 13 feature groups were detected in full-scan chromatograms of samples using MetExtract (Figures 2 and S3–S5). The nonlabeled and the corresponding isotopically labeled metabolites were matched to the ion pairs, and the different feature pairs originating from the same transformed products (TPs) were convoluted into one feature group. All the corresponding feature groups for the detected TPs showed divisible mass differences from 2 to 12 times the mass of a neutron (1.0033 Da), indicating the presence of 2–12 isotopic carbon atoms in the features (Table 1). For all the detected TPs, their tentative structures were assigned based on the exact mass, MS/MS fragmentation patterns and deduced number of labeled carbons, which were processed with the Compound Discovery platform and searched in the Chemspider database (Table 1, Figure S3 and S4). The identity of BPS (Fg 7) was confirmed by measurements of the 12C-BPS standard (Level 1 certainty, Schymanski et al. (2014)). Among these detected TPs, 7 formulas (Fg 1, 2, 3, 7, and 9) were qualified with both the exact mass and MS/MS fragmentation patterns (Level 2 certainty with diagnostic evidence). However, the other 5 TPs (Fg 4, 5, 6, and 8) could only be tentatively identified with MS data, but the MS/MS data were insufficient to confirm their structures (Level 3 certainty) (Figure S6).
Figure 2.
Comparison visualization of the extracted native (12C; red line) and labeled (13C; blue line) feature groups
The metabolites shown in gray are unmatched ions.
Table 1.
Detected metabolites of BPS from the processed feature groups obtained by MetExtract
| Features | RT | Ionization | m/z12C | m/z13C | Xn | Formula | ΔMass (ppm) | Tentative idenetification | Confidence levela | CSID | Intensities (peak area) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fg1 | 2.00 | + | 143.06884 | 150.09600 | 7 | C7H10O3 | 17.5 | Triacetylmethane | 2 | 63143 | 14176.89 |
| Fg2 | 2.08 | + | 111.04402 | 117.04654 | 6 | C6H6O2 | 35.0 | Dihydroxybenzene | 2 | 13837760 | 25479.99 |
| Fg3 | 2.19 | – | 165.02900 | 173.05578 | 8 | C8H6O4 | 34.8 | Terephthalic acid | 2 | 7208 | 18748.60 |
| Fg4 | 2.21 | + | 123.07332 | 131.09021 | 8 | C8H10O | −26.0 | Phenylethyl alcohol | 3 | 5830 | 16239.42 |
| Fg5 | 2.28 | – | 188.99243 | 194.99495 | 6 | C6H6O5S | 11.6 | Dihydroxy benzenesulfonic acid | 3 | 164229 | 45024.94 |
| Fg6 | 2.95 | + | 137.05917 | 145.08602 | 8 | C8H8O2 | 24.7 | Methylbenzoate | 3 | 6883 | 13064.11 |
| Fg7 | 14.98 | – | 249.02292 | 261.06318 | 12 | C12H10O4S | 18.7 | BPS | 1 | 6374 | 935016.75 |
| Fg8 | 15.23 | + | 183.08249 | 195.12288 | 12 | C13H10O | 32.6 | 2-fluorene | 3 | 68072 | 10786.83 |
| Fg9 | 17.29 | + | 301.10167 | 313.14183 | 12 | C18H20S2 | −7.7 | 2,2-dibenzyl-1,3-dithiane | 2 | 530757 | 17060.69 |
Confidence levels for metabolite identification were assigned according to the criteria established by Schymanski et al. Level 2: tentative candidates (compounds identified by molecular formula and substructures), based on accurate mass, MS/MS pattern, and database search. Level 3: unequivocal molecular formula based on accurate mass, but no other supporting data.
Accordingly, except for BPS, three major groups of TPs were detected in the BES suspension, and the specific degradation pathway of BPS was tentatively proposed as shown in Figure 3. In the first group, we detected two formulas (Fg 2, dihydroxybenzene, C6H6O2; and Fg 5, dihydroxybenzene sulfonic acid, C6H6O5S) originating from the hydroxylated hydrolysis products, which all contained 6 labeled carboxyl groups. The two TPs from the first and second groups (i.e., Fg 2 and Fg 5) were also exhibited relatively higher peak intensities than those of other detected TPs. Previous studies on advanced oxidative degradation of BPS also reported the formation of these two TPs (Kovačič et al., 2019; Luo et al., 2019). This result suggested the hydrolysis and oxidation processes were the initial and key transformation pathways for bioelectrochemical degradation of BPS. The second groups of the features (Fg 1, 3, 4, and 6) were formed through the pathways of alkyl substitution, rearrangement and oxidation from the hydrolysis TPs. All of the carbon skeletons in each of these compounds were labeled with 13C. Fg 1 and Fg 3 were identified using their MS/MS fragmentation patterns as triacetyl methane and terephthalic acid, respectively, and the other accumulated TPs (Fg 4 and Fg 6) were hypothesized to be phenylethyl alcohol and methylbenzoate, respectively, according to their exact mass. Therefore, we propose that further oxidation, alkylation, and aromatic ring-cleavage reactions occurred as the next degradation pathways for BPS.
Figure 3.
Proposed degradation pathway for BPS based on detected metabolites
Note that substituent positions are either inferred from known mass spectra or hypothesized. Transformation products (TPs) that have been validated by fragment mass spectrometry are labeled in red. The number of 13C for each TP is indicated in brackets after its name. Pathways with solid arrows are predicted based on identified labeled metabolites in the current study and literature, and dotted arrows denote unknown pathways. Bold arrows represent higher flux and thin arrows represent stable isotope flux.
For the third group of TPs, the two features both had twelve labeled carbons in their 13C paired ions, which means that unlabeled carbons occurred in their structure. The two TPs (Fg 8 and Fg 9) were assigned to 2-fluorenol and 2,2-dibenzyl-1,3-dithiane, respectively. It was indicated that carbon-carbon (C-C), carbon-oxygen (C-O) or multivalent couplings occurred in the formation of these resultant dimers from hydrolysis products of BPS by constant transfer of electrons (Wang et al., 2017; Zeng et al., 2015). The results indicated that 13C-labeled byproducts (i.e., CH3OH, CH₃CHO, HCHO, C2H5OH, etc.) from the first and second degradation pathways can be reprocessed by EAMs to reconstitute low-isotopic-ratio products.
The formation of the low-isotopic-ratio products (i.e., the third group) indicated that monomeric product coupling and carboxyl coupling as the further BPS degradation pathways, which reflects the complexity of the degradation process for BPS in BES. For the TPs from the third degradation pathways, considering that no previous research has described these complex degradation processes in BES, the structural formula and possible compounds can only be roughly inferred according to the exact mass number of ions. The best explanation for most of these detected TPs is anabolic and catabolic processes in microbial metabolism. The oxidative products of BPS from the first and second groups had a variety of hydroxyl, aldehyde, ketone, sulfate or carboxyl groups, which might contribute to cross-linking reactions with various 12C organic substrates. A previous study observed that several hydroxylated PAH metabolites were able to undergo coupling reactions with compounds in natural organic matter through ether, C-C, or multivalent bonds during microbial degradation (Kästner et al., 1999). In addition, coupling reactions can be enhanced by chemoautotrophic EAMs when taking up electrons from the electrode to transform substrates into products containing more carbon (Pellis et al., 2018; Rabaey and Rozendal 2010; Srikanth et al., 2018). Accordingly, it is possible for EAMs to utilize directly degraded metabolites to form low-isotopic-ratio TPs; the environmental safety of these compounds is also currently unknown and needs to be further investigated.
Identification of microorganisms assimilating 13C-BPS in BES
After ultracentrifugation of the DNA extracted from the 13C-BPS assimilated biofilm and BES suspension, the “light” and “heavy” fractions were selected according to the total DNA concentrations across the isotopically fractionated gradients (Figure S7). The DNA concentration curves for gradient fractions from the 12C-BPS assimilated biofilm and BES suspension both indicated a single peak in the mass range of 1.72–1.74 g/mL. A similar trend was also found in the DNA curve for the 13C-BPS assimilated suspension, whereas in the 13C-BPS assimilated biofilm, a second higher peak for DNA concentration was present at a buoyant density of 1.75–1.77 g/mL. This result implies that some microbes that assimilated 13C atoms derived from 13C-BPS were successfully isolated by ultracentrifugation, which made their appearance in the “heavy” DNA fraction.
Accordingly, the “light” (12C-L and 13C-L) and “heavy” (12C-H and 13C-H) fractions of 16S rRNA were selected to be phylogenetically characterized by high-throughput Illumina sequencing, along with the noncentrifuged biofilm and suspension samples from the initial (Ini.) and acclimated (Accli.) BES (Figure 4A). Proteobacteria were the preponderant phylum in the noncentrifuged biofilm and suspension, accounting for greater than 47.3% of bacterial reads, and the genus Geobacter comprised between 12.7% and 60.4% of the bacterial reads in these biofilm samples (Figures 4A and S8). For the 13C-BPS SIP experiment, significant shifts in community composition were observed between the “heavy” and “light” DNA fractions of the biofilm (p < 0.05). The relative abundances of Bacteroides and Cetobacterium in the “13C-H” pooled samples were significantly higher than those measured in the “13C-L” and “12C-H” samples collected for SIP experiments or the noncentrifuged biofilm. However, there was no significant difference in the community composition of “13C-L” and “13C-H” from the BES suspension.
Figure 4.
Phylogenetic tree and network associations in the BES biofilm and suspension
(A) Phylogenetic tree of bacterial 16S rRNA genes showing detected genera (left plot) and their relative abundance (right bubble plot) in (i) biomass collected from the light (L) and heavy (H) fractions from the 12C and 13C experiment and (ii) the initial (Ini.) and the acclimatized (Accli.) anode biofilm and suspension.
(B) the visualization of the bacterial network associations in the biofilm and suspension based on RMT analysis of OTU profiles.
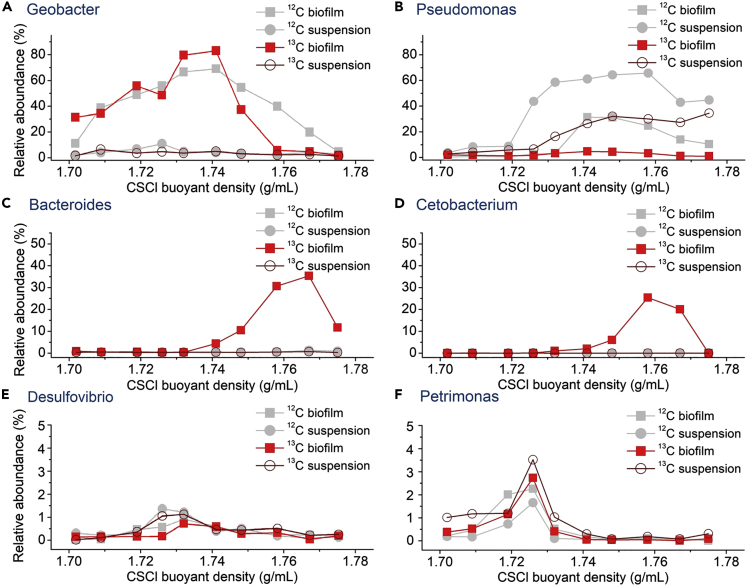
In addition, we assessed the relative abundances of the important microbes in each fraction from the biofilm and suspension of the SIP experiment (Figure 5). The results indicated that Geobacter, Pseudomonas, Desulfovibrio, and Petrimonas were not significantly enriched in the “heavy” BD fraction (>1.748 g/mL) in the 13C-BPS biofilm and suspension samples compared with those in the 12C-BPS samples. Geobacter can generate current using acetate as an electron donor (Yang et al., 2012); Petrimonas was also reported in previous studies to be a degradator of protein and carbohydrates in BESs (Kang et al., 2014); Desulfovibrio is associated with sulfate removal in BESs (Zheng et al., 2014); and Pseudomonas plays a key role in electron transfer media for nitrate and nitrite reduction (Zhang et al., 2014). Although these microbes are typical exoelectrogenic microbes in electrode biofilms (Lovley 2011), the present results suggested that these EAMs were not potential degradators of BPS in the BES.
Figure 5.
Relative abundance of representative bacterial genera across the entire buoyant density gradient of DNA fractions from the 12C- and 13C-BPS acclimatized biofilm and suspension
Compared to the relative abundances of Bacteroides and Cetobacterium in the same fractions of the 12C-BPS biofilm sample (0.82%, 0.45%, and 0.02%, respectively), the abundances in the heavy fractions from the 13C-BPS biofilm sample were higher (35.4%, 10.0%, and 25.4%, respectively). Bacteria belonging to the genus Bacteroides were frequently detected in anodic biofilm (Call et al., 2009; Rismani-Yazdi et al., 2013; Zakaria and Dhar 2020), which also been suggested to have an important role in electricity generation in BES (Ha et al., 2012; Shehab et al., 2017). Bacteroides have also been previously identified as degradators of BPA or other aromatic hydrocarbon degradation (Comte et al., 2006; Yang et al., 2019). Cetobacterium most frequently occurs as gut microbes (Sheng et al., 2010) and sludge microbes (Song et al., 2015a; Tian et al., 2020), which were previously found to be involved in the consumption of alcohols (Bhute et al., 2020), fatty acids, and protein (Hao et al., 2017). Recent studies also found the existence of Cetobacterium in sludge and landfill sites (Song et al., 2015a; Tian et al., 2020) and their abilities in degradation of surfactants (Motteran et al. 2016, 2020), dye (Ji et al., 2020), and several aromatic compounds (Tian et al., 2020). The enhanced contributions of the electrode biofilms to BPS degradation in the BESs were likely derived from these microbes. Similar to the total DNA concentrations, Bacteroides and Cetobacterium were not enriched in the “heavy” BD fraction of the 13C-BPS suspension, which confirmed the validity of our criteria for high-sensitivity SIP. Accordingly, these results suggested that the non-EAMs in EABs, rather than bacteria in the BES suspension, play key roles in the degradation of BPS, and Bacteroides and Cetobacterium may be preferentially involved in the degradation and assimilation of BPS and its metabolites.
Microbial interactions during bioelectrochemical degradation of BPS
After identification of the BPS-assimilating microbes, we constructed phylogenetic molecular ecological networks to determine the interspecies interaction between the typical EAMs and BPS-assimilating microbes in the BES biofilm and suspension (Figure 4B). As shown in Table S2, networks with 227 and 110 nodes were constructed from the biofilm and suspension samples, respectively, and the generated random networks showed significance of the network indices between the biofilm and suspension samples. Specifically, the values of density, average clustering coefficient, and transitivity in the suspension were statistically higher than those in the biofilm samples (p < 0.01), whereas the values of modularity, connectedness, and harmonic geodesic distance were significantly higher in the biofilm (p < 0.01). As expected, these phenomena suggest that the co-occurrence patterns of the bacterial communities in the biofilm and suspension were quite different, and the bacterial molecular ecological network in the biofilm was more complicated, intense, and centralized than that in the suspension. This community similarity was confirmed by the lower Shannon index for the biofilm than for the BES suspension (Table S3). The tighter microbial network interaction in the biofilms is also in accordance with the observation of higher 13C accumulation in BES biofilms. Therefore, electron transfer between EABs and the electrode not only simultaneously enriched electrochemical bacteria and specific degradation bacteria but also enhanced their interaction as consortia.
According to the topographical roles of each node in the network (Figure S9), two nodes in the biofilm (OTU_2955, Geobacter; OTU_426, Bacteroides) were identified as keystone species in the bacterial network. Principal coordinates analysis (PCoA) using the weighted UniFrac approach was conducted to illustrate the difference of bacterial communities from BPS-acclimated biofilm and suspension (Figure S10A). Results from partial least squares discriminant analysis based cluster analysis (Figures S10B and S10C) well agreed with the PCoA analysis, where the bacterial genus such as Geobacter was identified to be the most dominant contributors to the separation based on their variable importance in projection values. These results suggested that electron transfer processes made EAMs (i.e., Geobacter) pivotal in the BPS-acclimated biofilm consortium, which resulted in the efficient and robust degradation of BPS via specific microbial metabolic interactions between EAMs and the (i.e., Bacteroides and Cetobacterium).
Discussion
The present study first investigated the accelerated degradation of a novel bisphenol analog (i.e., BPS) in a BES using combined stable isotope-assisted approaches, which can provide a clear clue to the accelerated degradation mechanism of BPS. We identified that the genera Bacteroides and Cetobacterium accounted for the majority in assimilating BPS, and found that these assimilators were colonized in the biofilm but not suspended in the BES. Cetobacterium was not been reported as important components in the anodic biofilm from BES, but Bacteroides were identified as a fermentative bacterium which can produce the simple organic acids from a wide range of substrates (i.e., glucose, acetate, cellulose, lactate, etc) to be utilized by EAMs (Jia et al., 2013; Wei et al., 2019). In a recent investigation of microbial communities from anodic biofilm via DNA-SIP (Song et al., 2015b), Bacteroides were not found to be involved in uptake 13C-labeled sodium acetate, while Geobacter, Rhodocyclaceae, and Pseudomonas were the main contributors in the assimilation of acetate to conduct extracellular electron transfer in BES. Kimura and Okabe (2013) also confirmed the predominance of Geobacter sp. as acetoclastic exoelectrogens in BES. The direct interspecies electron transfer (DIET) may be associated with the processes of Geobacter and other EAMs in utilizing acetate to conducted electricity (Cheng and Call 2016). Considering the existence of Pseudomonas in biofilm and suspension, phenazines produced by Pseudomonas can also transfer the electrons from the oxidation of acetate to the anode (Pham et al., 2008; Rabaey et al., 2005), as revealed by the cyclic voltammetry result (Figure S11). This indirect electron transfer mechanism can promote redox interactions for the microbial community and increase the performance of anode biofilms (Cheng and Call 2016). It can be suggested that acetate, rather than BPS, served as an electron donor for electricity generation by EAMs during BPS degradation in this study.
To date, this is the first study investigating the network among microbes with different functions in the bioelectrochemical degradation of pollutants, which can provide information on what microbes are physically and/or functionally associated in a microbial community (Zhou et al., 2011). EAMs cannot directly degrade contaminants but utilize their metabolites (Borole et al., 2011; Cheng and Call 2016), which refers to the metabolites mediated interspecies electron transfer (MIET). Considering acetate and other low-molecular byproducts can also be transformed from BPS (Figure 3), the removal of these simple organic products such as acetate by EAMs and other synergistic microbes theoretically reduces potential feedback inhibition effects (Kiely et al., 2011), thereby accelerate the biodegradation of BPS. These presumptions can also explain the intense microbial networks between EAMs and the non-EAMs (including BPS assimilators) that observed in biofilms. Therefore, this MIET-based syntrophic interaction and the DIET-based electrochemical interaction collectively established the redox equilibrium in bioelectrochemically enhanced degradation of BPS.
Moreover, SIAM was used to identify hydrolysis and oxidation processes as the initial and key transformation pathways for bioelectrochemical degradation; followed by the hydroxylation of one or two phenolic rings, aromatic ring cleavage, or alkylation in the formation of highly (but not fully) isotopic products. These isotopic-validated pathways were similar to those in aerobic degradation of BPS in sludge and sediment (Choi and Lee 2017; Huang et al., 2019; Kovačič et al., 2021; Ogata et al., 2013), including hydrolysis, methylation, cleavage and the coupling of smaller bisphenol moieties. A recent study on other BP analogs degradation by bacteria also reported for the common biodegradation pathways (Noszczyńska and Piotrowska-Seget 2018). Previous studies also confirmed the possibility of dihydroxybenzene transformation to benzoquinone, diene acid, benzoic acid, and aromatic ring-cleavage products by EAMs (Milligan and Häggblom 1998; Su et al., 2019; Uchimiya and Stone 2006; Zhou et al., 2020). Hence, we can propose that the driving reaction with anode as electron acceptor can produce degradation pathways similar to those under aerobic reaction.
In the conventionally anaerobic condition, the biodegradation of organic compounds was highly dependent upon the presence of electron acceptors or electron donors (Berry et al., 1987). Previous studies indicated that the degradation pathways of recalcitrant substances in BES may be directly dependent on degradation of the anode or cathode, which has been illustrated to be used as a sole electron acceptor or donor in BES (Huang et al., 2011; Logan 2009; Strycharz et al., 2010). Sun et al. (2019) proposed that the deoxidation of sulfone (lost oxygen atoms) and the following C-S bond cleavage were the two common transformation pathways for BPS under anaerobic conditions and in BES cathode, which was quite different from the present study. It can be explained by the cathodic degradation of BPS in their study caused by the application of negative anodic potential. The influence of the applied potential on the degradation pathways of recalcitrant substances has also been investigated by several studies (Feng et al., 2017; Strycharz et al. 2008, 2010). Therefore, we speculate that the BPS degradation pathways in BES were stable under different positive potentials in the present study (from 0.2V to 0.6V).
SIP techniques offer an excellent opportunity to identify new unexpected degradation pathways and reveal hidden metabolic mechanisms. This work illustrates the potential of using SIP techniques to study the bioelectrochemical degradation mechanisms of pollutants, which strengthens the promise of integrating two stable isotopic approaches in BES platforms. The proposed unique microbial cooperation mechanism in electroactive biofilm provides new insights into the enhanced degradation mechanisms in BES for several other structure-similar pollutants. This research can also beneficially inform the design of EAB structures and the development of specific engineered assimilators for BESs in future wastewater treatment applications.
Limitations of the study
However, this study only tracked the short-term process from BPS to primary degradation products in BES using stable isotope. Future work covering long-term investigation on the stable isotope dynamics during entire contaminant assimilation process (from BPS to inorganic carbon) is needed to disclose more detailed and reliable fundamental information related to metabolic and redox interactions in BESs for pollutants remediation.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Prof. Yong Yuan (yuanyong@soil.gd.cn).
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate datasets/code.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This study was supported jointly by the National Natural Science Foundation of China (Nos. 41877045, 41907339 and 21876032) and China Postdoctoral Research Funds (No. 2018M640765).
Author contributions
Conceptualization: Rui Hou and Yong Yuan; Methodology and Investigation: Rui Hou, Lin Gan, and Jibing Li: Writing – Original Draft: Rui Hou; Methodology: Yi Wang and Jibing Li; Writing – Review & Editing: Yong Yuan and Shungui Zhou; Funding Acquisition: Yong Yuan; Resources: Yong Yuan and Jibing Li; Supervision: Yong Yuan and Shungui Zhou.
Declaration of interests
The authors declare no competing interests.
Published: January 22, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.102014.
Supplemental information
References
- Badia-Fabregat M., Rosell M., Caminal G., Vicent T., Marco-Urrea E. Use of stable isotope probing to assess the fate of emerging contaminants degraded by white-rot fungus. Chemosphere. 2014;103:336–342. doi: 10.1016/j.chemosphere.2013.12.029. [DOI] [PubMed] [Google Scholar]
- Baran R., Bowen B.P., Bouskill N.J., Brodie E.L., Yannone S.M., Northen T.R. Metabolite identification in Synechococcus sp. PCC 7002 using untargeted stable isotope assisted metabolite profiling. Anal. Chem. 2010;82:9034–9042. doi: 10.1021/ac1020112. [DOI] [PubMed] [Google Scholar]
- Bernard L., Mougel C., Maron P.A., Nowak V., Lévêque J., Henault C., Haichar F.E.Z., Berge O., Marol C., Balesdent J. Dynamics and identification of soil microbial populations actively assimilating carbon from 13C-labelled wheat residue as estimated by DNA- and RNA-SIP techniques. Environ. Microbiol. 2007;9:752–764. doi: 10.1111/j.1462-2920.2006.01197.x. [DOI] [PubMed] [Google Scholar]
- Berry D.F., Francis A.J., Bollag J.M. Microbial metabolism of homocyclic and heterocyclic aromatic compounds under anaerobic conditions. Microbiol. Rev. 1987;51:43–59. doi: 10.1128/mr.51.1.43-59.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhute S.S., Escobedo B., Haider M., Mekonen Y., Ferrer D., Hillyard S.D., Friel A.D., van Breukelen F., Hedlund B.P. The gut microbiome and its potential role in paradoxical anaerobism in pupfishes of the Mojave Desert. Anim. Microbiome. 2020;2:20. doi: 10.1186/s42523-020-00037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borole A.P., Reguera G., Ringeisen B., Wang Z.-W., Feng Y., Kim B.H. Electroactive biofilms: current status and future research needs. Energy Environ. Sci. 2011;4:4813–4834. [Google Scholar]
- Bueschl C., Kluger B., Neumann N.K.N., Doppler M., Maschietto V., Thallinger G.G., Meng-Reiterer J., Krska R., Schuhmacher R. MetExtract II: a software suite for stable isotope-assisted untargeted metabolomics. Anal. Chem. 2017;89:9518–9526. doi: 10.1021/acs.analchem.7b02518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call D.F., Wagner R.C., Logan B.E. Hydrogen production by <em>Geobacter</em> species and a mixed consortium in a microbial electrolysis cell. Appl. Environ. Microbiol. 2009;75:7579. doi: 10.1128/AEM.01760-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M., Feng Y., Wang N., Li Y., Li N., Liu J., He W. Electrochemical regulation on the metabolism of anode biofilms under persistent exogenous bacteria interference. Electrochim. Acta. 2020;340:135922. [Google Scholar]
- Cao Z., Zhang M., Zhang J., Zhang H. Impact of continuous and intermittent supply of electric assistance on high-strength 2,4-dichlorophenol (2,4-DCP) degradation in electro-microbial system. Bioresour. Technol. 2016;212:138–143. doi: 10.1016/j.biortech.2016.03.165. [DOI] [PubMed] [Google Scholar]
- Capellades J., Navarro M., Samino S., Garcia-Ramirez M., Hernandez C., Simo R., Vinaixa M., Yanes O. geoRge: a computational tool to detect the presence of stable isotope labeling in LC/MS-based untargeted metabolomics. Anal. Chem. 2016;88:621–628. doi: 10.1021/acs.analchem.5b03628. [DOI] [PubMed] [Google Scholar]
- Chae K.-J., Choi M.-J., Lee J.-W., Kim K.-Y., Kim I.S. Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresour. Technol. 2009;100:3518–3525. doi: 10.1016/j.biortech.2009.02.065. [DOI] [PubMed] [Google Scholar]
- Chen D., Kannan K., Tan H., Zheng Z., Feng Y.-L., Wu Y., Widelka M. Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity—a review. Environ. Sci. Technol. 2016;50:5438–5453. doi: 10.1021/acs.est.5b05387. [DOI] [PubMed] [Google Scholar]
- Cheng Q., Call D.F. Hardwiring microbes via direct interspecies electron transfer: mechanisms and applications. Environ. Sci. Process. Impacts. 2016;18:968–980. doi: 10.1039/c6em00219f. [DOI] [PubMed] [Google Scholar]
- Choi Y.J., Lee L.S. Aerobic soil biodegradation of bisphenol (BPA) alternatives bisphenol S and bisphenol AF compared to BPA. Environ. Sci. Technol. 2017;51:13698–13704. doi: 10.1021/acs.est.7b03889. [DOI] [PubMed] [Google Scholar]
- Comte S., Guibaud G., Baudu M. Relations between extraction protocols for activated sludge extracellular polymeric substances (EPS) and EPS complexation properties: Part I. Comparison of the efficiency of eight EPS extraction methods. Enzyme Microb. Technol. 2006;38:237–245. [Google Scholar]
- Creek D.J., Chokkathukalam A., Jankevics A., Burgess K.E.V., Breitling R., Barrett M.P. Stable isotope-assisted metabolomics for network-wide metabolic pathway elucidation. Anal. Chem. 2012;84:8442–8447. doi: 10.1021/ac3018795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creek D.J. Stable isotope labeled metabolomics improves identification of novel metabolites and pathways. Bioanalysis. 2013;5:1807–1810. doi: 10.4155/bio.13.131. [DOI] [PubMed] [Google Scholar]
- Daghio M., Espinoza Tofalos A., Leoni B., Cristiani P., Papacchini M., Jalilnejad E., Bestetti G., Franzetti A. Bioelectrochemical BTEX removal at different voltages: assessment of the degradation and characterization of the microbial communities. J. Hazard. Mater. 2018;341:120–127. doi: 10.1016/j.jhazmat.2017.07.054. [DOI] [PubMed] [Google Scholar]
- Dumont M.G., Murrell J.C. Stable isotope probing — linking microbial identity to function. Nat. Rev. Microbiol. 2005;3:499–504. doi: 10.1038/nrmicro1162. [DOI] [PubMed] [Google Scholar]
- Fan M., Zhou N., Li P., Chen L., Chen Y., Shen S., Zhu S. Anaerobic co-metabolic biodegradation of tetrabromobisphenol A using a bioelectrochemical system. J. Hazard. Mater. 2017;321:791–800. doi: 10.1016/j.jhazmat.2016.09.068. [DOI] [PubMed] [Google Scholar]
- Feng H., Wang Y., Zhang X., Shen D., Li N., Chen W., Huang B., Liang Y., Zhou Y. Degradation of p-fluoronitrobenzene in biological and bioelectrochemical systems: differences in kinetics, pathways, and microbial community evolutions. Chem. Eng. J. 2017;314:232–239. [Google Scholar]
- Fernando E.Y., Keshavarz T., Kyazze G. The use of bioelectrochemical systems in environmental remediation of xenobiotics: a review. J. Chem. Technol. Biotechnol. 2019;94:2070–2080. [Google Scholar]
- Ha P.T., Lee T.K., Rittmann B.E., Park J., Chang I.S. Treatment of alcohol distillery wastewater using a bacteroidetes-dominant thermophilic microbial fuel cell. Environ. Sci. Technol. 2012;46:3022–3030. doi: 10.1021/es203861v. [DOI] [PubMed] [Google Scholar]
- Hao Y.T., Wu S.G., Jakovlić I., Zou H., Li W.X., Wang G.T. Impacts of diet on hindgut microbiota and short-chain fatty acids in grass carp (Ctenopharyngodon idellus) Aquac. Res. 2017;48:5595–5605. [Google Scholar]
- Hasany M., Mardanpour M.M., Yaghmaei S. Biocatalysts in microbial electrolysis cells: a review. Int. J. Hydrogen Energy. 2016;41:1477–1493. [Google Scholar]
- Hou R., Luo X., Liu C., Zhou L., Wen J., Yuan Y. Enhanced degradation of triphenyl phosphate (TPHP) in bioelectrochemical systems: kinetics, pathway and degradation mechanisms. Environ. Pollut. 2019;254:113040. doi: 10.1016/j.envpol.2019.113040. [DOI] [PubMed] [Google Scholar]
- Huang L., Cheng S., Chen G. Bioelectrochemical systems for efficient recalcitrant wastes treatment. J. Chem. Technol. Biotechnol. 2011;86:481–491. [Google Scholar]
- Huang W.-C., Jia X., Li J., Li M. Dynamics of microbial community in the bioreactor for bisphenol S removal. Sci. Total Environ. 2019;662:15–21. doi: 10.1016/j.scitotenv.2019.01.173. [DOI] [PubMed] [Google Scholar]
- Ji J., Kulshreshtha S., Kakade A., Majeed S., Li X., Liu P. Bioaugmentation of membrane bioreactor with Aeromonas hydrophila LZ-MG14 for enhanced malachite green and hexavalent chromium removal in textile wastewater. Int. Biodeterior. Biodegradation. 2020;150:104939. [Google Scholar]
- Jia J., Tang Y., Liu B., Wu D., Ren N., Xing D. Electricity generation from food wastes and microbial community structure in microbial fuel cells. Bioresour. Technol. 2013;144:94–99. doi: 10.1016/j.biortech.2013.06.072. [DOI] [PubMed] [Google Scholar]
- Jones M.D., Crandell D.W., Singleton D.R., Aitken M.D. Stable-isotope probing of the polycyclic aromatic hydrocarbon-degrading bacterial guild in a contaminated soil. Environ. Microbiol. 2011;13:2623–2632. doi: 10.1111/j.1462-2920.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kästner M., Streibich S., Beyrer M., Richnow H.H., Fritsche W. formation of bound residues during microbial degradation of [14C]anthracene in soil. Appl. Environ. Microbiol. 1999;65:1834–1842. doi: 10.1128/aem.65.5.1834-1842.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang F., Alvarez P.J., Zhu D. Microbial extracellular polymeric substances reduce Ag+ to silver nanoparticles and antagonize bactericidal activity. Environ. Sci. Technol. 2014;48:316–322. doi: 10.1021/es403796x. [DOI] [PubMed] [Google Scholar]
- Kiely P.D., Regan J.M., Logan B.E. The electric picnic: synergistic requirements for exoelectrogenic microbial communities. Curr. Opin. Biotechnol. 2011;22:378–385. doi: 10.1016/j.copbio.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Kimura Z.-i., Okabe S. Acetate oxidation by syntrophic association between Geobacter sulfurreducens and a hydrogen-utilizing exoelectrogen. ISME J. 2013;7:1472–1482. doi: 10.1038/ismej.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovačič A., Gys C., Kosjek T., Covaci A., Heath E. Photochemical degradation of BPF, BPS and BPZ in aqueous solution: identification of transformation products and degradation kinetics. Sci. Total Environ. 2019;664:595–604. doi: 10.1016/j.scitotenv.2019.02.064. [DOI] [PubMed] [Google Scholar]
- Kovačič A., Gys C., Gulin M.R., Gornik T., Kosjek T., Heath D., Covaci A., Heath E. Kinetics and biotransformation products of bisphenol F and S during aerobic degradation with activated sludge. J. Hazard. Mater. 2021;404:124079. doi: 10.1016/j.jhazmat.2020.124079. [DOI] [PubMed] [Google Scholar]
- Lee D.G., Cho K.-C., Chu K.-H. Identification of triclosan-degrading bacteria in a triclosan enrichment culture using stable isotope probing. Biodegradation. 2014;25:55–65. doi: 10.1007/s10532-013-9640-7. [DOI] [PubMed] [Google Scholar]
- Li D.B., Huang Y.X., Li J., Li L.L., Tian L.J., Yu H.Q. Electrochemical activities of Geobacter biofilms growing on electrodes with various potentials. Electrochim. Acta. 2017;225:452–457. [Google Scholar]
- Li H., Zhang S., Yang X.-L., Yang Y.-L., Xu H., Li X.-N., Song H.-L. Enhanced degradation of bisphenol A and ibuprofen by an up-flow microbial fuel cell-coupled constructed wetland and analysis of bacterial community structure. Chemosphere. 2019;217:599–608. doi: 10.1016/j.chemosphere.2018.11.022. [DOI] [PubMed] [Google Scholar]
- Li J., Luo C., Song M., Dai Q., Jiang L., Zhang D., Zhang G. Biodegradation of phenanthrene in polycyclic aromatic hydrocarbon-contaminated wastewater revealed by coupling cultivation-dependent and -independent approaches. Environ. Sci. Technol. 2017;51:3391–3401. doi: 10.1021/acs.est.6b04366. [DOI] [PubMed] [Google Scholar]
- Logan B.E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 2009;7:375–381. doi: 10.1038/nrmicro2113. [DOI] [PubMed] [Google Scholar]
- Logan B.E., Rossi R., Ragab A.a., Saikaly P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 2019;17:307–319. doi: 10.1038/s41579-019-0173-x. [DOI] [PubMed] [Google Scholar]
- Lovley D.R. Live wires: direct extracellular electron exchange for bioenergy and the bioremediation of energy-related contamination. Energy Environ. Sci. 2011;4:4896–4906. [Google Scholar]
- Lu L., Huggins T., Jin S., Zuo Y., Ren Z.J. Microbial metabolism and community structure in response to bioelectrochemically enhanced remediation of petroleum hydrocarbon-contaminated soil. Environ. Sci. Technol. 2014;48:4021–4029. doi: 10.1021/es4057906. [DOI] [PubMed] [Google Scholar]
- Luo C., Hou R., Chen G., Liu C., Zhou L., Yuan Y. UVC-assisted electrochemical degradation of novel bisphenol analogues with boron-doped diamond electrodes: kinetics, pathways and eco-toxicity removal. Sci. Total Environ. 2019;711:134539. doi: 10.1016/j.scitotenv.2019.134539. [DOI] [PubMed] [Google Scholar]
- Luo H., Liu G., Zhang R., Jin S. Phenol degradation in microbial fuel cells. Chem. Eng. J. 2009;147:259–264. [Google Scholar]
- Milligan P.W., Häggblom M.M. Biodegradation of resorcinol and catechol by denitrifying enrichment cultures. Environ. Toxicol. Chem. 1998;17:1456–1461. [Google Scholar]
- Miran W., Jang J., Nawaz M., Shahzad A., Lee D.S. Biodegradation of the sulfonamide antibiotic sulfamethoxazole by sulfamethoxazole acclimatized cultures in microbial fuel cells. Sci. Total Environ. 2018;627:1058–1065. doi: 10.1016/j.scitotenv.2018.01.326. [DOI] [PubMed] [Google Scholar]
- Motteran F., Braga J.K., Silva E.L., Varesche M.B.A. Influence of sucrose on the diversity of bacteria involved in nonionic surfactant degradation in fluidized bed reactor. Water Air Soil Pollut. 2016;228:21. [Google Scholar]
- Motteran F., Okada D.Y., Delforno T.P., Varesche M.B.A. Influence of cosubstrates for linear anionic sulfonated alkylbenzene degradation and methane production in anaerobic batch reactors. Process Saf. Environ. Prot. 2020;139:60–68. [Google Scholar]
- Ng I.S., Hsueh C.-C., Chen B.-Y. Electron transport phenomena of electroactive bacteria in microbial fuel cells: a review of Proteus hauseri. Bioresourc. Bioprocess. 2017;4:53. [Google Scholar]
- Noszczyńska M., Piotrowska-Seget Z. Bisphenols: application, occurrence, safety, and biodegradation mediated by bacterial communities in wastewater treatment plants and rivers. Chemosphere. 2018;201:214–223. doi: 10.1016/j.chemosphere.2018.02.179. [DOI] [PubMed] [Google Scholar]
- Ogata Y., Goda S., Toyama T., Sei K., Ike M. The 4-tert-Butylphenol-Utilizing bacterium sphingobium fuliginis OMI can degrade bisphenols via phenolic ring hydroxylation and meta-cleavage pathway. Environ. Sci. Technol. 2013;47:1017–1023. doi: 10.1021/es303726h. [DOI] [PubMed] [Google Scholar]
- Pellis A., Cantone S., Ebert C., Gardossi L. Evolving biocatalysis to meet bioeconomy challenges and opportunities. New Biotechnol. 2018;40:154–169. doi: 10.1016/j.nbt.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Pham H., Boon N., Marzorati M., Verstraete W. Enhanced removal of 1,2-dichloroethane by anodophilic microbial consortia. Water Res. 2009;43:2936–2946. doi: 10.1016/j.watres.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Pham T.H., Boon N., De Maeyer K., Höfte M., Rabaey K., Verstraete W. Use of Pseudomonas species producing phenazine-based metabolites in the anodes of microbial fuel cells to improve electricity generation. Appl. Microbiol. Biotechnol. 2008;80:985–993. doi: 10.1007/s00253-008-1619-7. [DOI] [PubMed] [Google Scholar]
- Rabaey K., Boon N., Höfte M., Verstraete W. Microbial phenazine production enhances electron transfer in biofuel cells. Environ. Sci. Technol. 2005;39:3401–3408. doi: 10.1021/es048563o. [DOI] [PubMed] [Google Scholar]
- Rabaey K., Rozendal R.A. Microbial electrosynthesis — revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010;8:706–716. doi: 10.1038/nrmicro2422. [DOI] [PubMed] [Google Scholar]
- Rismani-Yazdi H., Carver S.M., Christy A.D., Yu Z., Bibby K., Peccia J., Tuovinen O.H. Suppression of methanogenesis in cellulose-fed microbial fuel cells in relation to performance, metabolite formation, and microbial population. Bioresour. Technol. 2013;129:281–288. doi: 10.1016/j.biortech.2012.10.137. [DOI] [PubMed] [Google Scholar]
- Ruth R.J., Louise B.A. Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ. Health Perspect. 2015;123:643–650. doi: 10.1289/ehp.1408989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saratale G.D., Saratale R.G., Shahid M.K., Zhen G., Kumar G., Shin H.-S., Choi Y.-G., Kim S.-H. A comprehensive overview on electro-active biofilms, role of exo-electrogens and their microbial niches in microbial fuel cells (MFCs) Chemosphere. 2017;178:534–547. doi: 10.1016/j.chemosphere.2017.03.066. [DOI] [PubMed] [Google Scholar]
- Sathyamoorthy S., Hoar C., Chandran K. Identification of bisphenol A-assimilating microorganisms in mixed microbial communities using 13C-DNA stable isotope probing. Environ. Sci. Technol. 2018;52:9128–9135. doi: 10.1021/acs.est.8b01976. [DOI] [PubMed] [Google Scholar]
- Schymanski E.L., Jeon J., Gulde R., Fenner K., Ruff M., Singer H.P., Hollender J. Identifying small molecules via high resolution mass spectrometry: communicating confidence. Environ. Sci. Technol. 2014;48:2097–2098. doi: 10.1021/es5002105. [DOI] [PubMed] [Google Scholar]
- Shehab N.A., Ortiz-Medina J.F., Katuri K.P., Hari A.R., Amy G., Logan B.E., Saikaly P.E. Enrichment of extremophilic exoelectrogens in microbial electrolysis cells using Red Sea brine pools as inocula. Bioresour. Technol. 2017;239:82–86. doi: 10.1016/j.biortech.2017.04.122. [DOI] [PubMed] [Google Scholar]
- Sheng G.-P., Yu H.-Q., Li X.-Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: a review. Biotechnol. Adv. 2010;28:882–894. doi: 10.1016/j.biotechadv.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Song L., Wang Y., Tang W., Lei Y. Bacterial community diversity in municipal waste landfill sites. Appl. Microbiol. Biotechnol. 2015;99:7745–7756. doi: 10.1007/s00253-015-6633-y. [DOI] [PubMed] [Google Scholar]
- Song M., Jiang L., Zhang D., Luo C., Wang Y., Yu Z., Yin H., Zhang G. Bacteria capable of degrading anthracene, phenanthrene, and fluoranthene as revealed by DNA based stable-isotope probing in a forest soil. J. Hazard. Mater. 2016;308:50–57. doi: 10.1016/j.jhazmat.2016.01.009. [DOI] [PubMed] [Google Scholar]
- Song Y., Xiao L., Jayamani I., He Z., Cupples A.M. A novel method to characterize bacterial communities affected by carbon source and electricity generation in microbial fuel cells using stable isotope probing and Illumina sequencing. J. Microbiol. Methods. 2015;108:4–11. doi: 10.1016/j.mimet.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Srikanth S., Kumar M., Singh D., Singh M.P., Puri S.K., Ramakumar S.S.V. Long-term operation of electro-biocatalytic reactor for carbon dioxide transformation into organic molecules. Bioresour. Technol. 2018;265:66–74. doi: 10.1016/j.biortech.2017.12.075. [DOI] [PubMed] [Google Scholar]
- Strycharz S.M., Woodard T.L., Johnson J.P., Nevin K.P., Sanford R.A., Löffler F.E., Lovley D.R. Graphite electrode as a sole electron donor for reductive dechlorination of tetrachlorethene by <em>Geobacter lovleyi</em>. Appl. Environ. Microbiol. 2008;74:5943. doi: 10.1128/AEM.00961-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strycharz S.M., Gannon S.M., Boles A.R., Franks A.E., Nevin K.P., Lovley D.R. Reductive dechlorination of 2-chlorophenol by Anaeromyxobacter dehalogenans with an electrode serving as the electron donor. Environ. Microbiol. Rep. 2010;2:289–294. doi: 10.1111/j.1758-2229.2009.00118.x. [DOI] [PubMed] [Google Scholar]
- Su C., Lu Y., Deng Q., Chen S., Pang G., Chen W., Chen M., Huang Z. Performance of a novel ABR-bioelectricity-Fenton coupling reactor for treating traditional Chinese medicine wastewater containing catechol. Ecotoxicol. Environ. Saf. 2019;177:39–46. doi: 10.1016/j.ecoenv.2019.03.112. [DOI] [PubMed] [Google Scholar]
- Sun J., Xu Y., Jiang L., Fan M., Xu H., Chen Y., Shen S. Highly efficient transformation of bisphenol S by anaerobic cometabolism in the bioelectrochemical system. J. Environ. Eng. 2019;145:04019079. [Google Scholar]
- Tian X., Song Y., Shen Z., Zhou Y., Wang K., Jin X., Han Z., Liu T. A comprehensive review on toxic petrochemical wastewater pretreatment and advanced treatment. J. Clean. Prod. 2020;245:118692. [Google Scholar]
- Tian Z., Vila J., Yu M., Bodnar W., Aitken M.D. Tracing the biotransformation of polycyclic aromatic hydrocarbons in contaminated soil using stable isotope-assisted metabolomics. Environ. Sci. Technol. Lett. 2018;5:103–109. doi: 10.1021/acs.estlett.7b00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimiya M., Stone A.T. Aqueous oxidation of substituted dihydroxybenzenes by substituted benzoquinones. Environ. Sci. Technol. 2006;40:3515–3521. doi: 10.1021/es052578k. [DOI] [PubMed] [Google Scholar]
- Wang L., Liu Y., Ma J., Zhao F. Rapid degradation of sulphamethoxazole and the further transformation of 3-amino-5-methylisoxazole in a microbial fuel cell. Water Res. 2016;88:322–328. doi: 10.1016/j.watres.2015.10.030. [DOI] [PubMed] [Google Scholar]
- Wang Q., Lu X., Cao Y., Ma J., Jiang J., Bai X., Hu T. Degradation of Bisphenol S by heat activated persulfate: kinetics study, transformation pathways and influences of co-existing chemicals. Chem. Eng. J. 2017;328:236–245. [Google Scholar]
- Wei L., Li J., Xue M., Wang S., Li Q., Qin K., Jiang J., Ding J., Zhao Q. Adsorption behaviors of Cu2+, Zn2+ and Cd2+ onto proteins, humic acid, and polysaccharides extracted from sludge EPS: sorption properties and mechanisms. Bioresour. Technol. 2019;291:121868. doi: 10.1016/j.biortech.2019.121868. [DOI] [PubMed] [Google Scholar]
- Xu H., Tong N., Huang S., Zhou S., Li S., Li J., Zhang Y. Degradation of 2,4,6-trichlorophenol and determination of bacterial community structure by micro-electrical stimulation with or without external organic carbon source. Bioresour. Technol. 2018;263:266–272. doi: 10.1016/j.biortech.2018.05.015. [DOI] [PubMed] [Google Scholar]
- Yan W., Xiao Y., Yan W., Ding R., Wang S., Zhao F. The effect of bioelectrochemical systems on antibiotics removal and antibiotic resistance genes: a review. Chem. Eng. J. 2019;358:1421–1437. [Google Scholar]
- Yan Z., He Y., Cai H., Van Nostrand J.D., He Z., Zhou J., Krumholz L.R., Jiang H.-L. Interconnection of key microbial functional genes for enhanced benzo[a]pyrene biodegradation in sediments by microbial electrochemistry. Environ. Sci. Technol. 2017;51:8519–8529. doi: 10.1021/acs.est.7b00209. [DOI] [PubMed] [Google Scholar]
- Yang G., Huang L., Yu Z., Liu X., Chen S., Zeng J., Zhou S., Zhuang L. Anode potentials regulate Geobacter biofilms: new insights from the composition and spatial structure of extracellular polymeric substances. Water Res. 2019;159:294–301. doi: 10.1016/j.watres.2019.05.027. [DOI] [PubMed] [Google Scholar]
- Yang T.H., Coppi M.V., Lovley D.R., Sun J. Metabolic response of Geobacter sulfurreducens towards electron donor/acceptor variation. Microb. Cell Fact. 2010;9:90. doi: 10.1186/1475-2859-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Xu M., Guo J., Sun G. Bacterial extracellular electron transfer in bioelectrochemical systems. Process Biochem. 2012;47:1707–1714. [Google Scholar]
- Zakaria B.S., Dhar B.R. Changes in syntrophic microbial communities, EPS matrix, and gene-expression patterns in biofilm anode in response to silver nanoparticles exposure. Sci. Total Environ. 2020;734:139395. doi: 10.1016/j.scitotenv.2020.139395. [DOI] [PubMed] [Google Scholar]
- Zemb O., Lee M., Gutierrez-Zamora M.L., Hamelin J., Coupland K., Hazrin-Chong N.H., Taleb I., Manefield M. Improvement of RNA-SIP by pyrosequencing to identify putative 4-n-nonylphenol degraders in activated sludge. Water Res. 2012;46:601–610. doi: 10.1016/j.watres.2011.10.047. [DOI] [PubMed] [Google Scholar]
- Zeng X., Borole A.P., Pavlostathis S.G. Biotransformation of furanic and phenolic compounds with hydrogen gas production in a microbial electrolysis cell. Environ. Sci. Technol. 2015;49:13667–13675. doi: 10.1021/acs.est.5b02313. [DOI] [PubMed] [Google Scholar]
- Zhan Y., Liu W., Bao Y., Zhang J., Petropoulos E., Li Z., Lin X., Feng Y. Fertilization shapes a well-organized community of bacterial decomposers for accelerated paddy straw degradation. Sci. Rep. 2018;8:7981. doi: 10.1038/s41598-018-26375-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Feng H., Shan D., Shentu J., Wang M., Yin J., Shen D., Huang B., Ding Y. The effect of electricity on 2–fluoroaniline removal in a bioelectrochemically assisted microbial system (BEAMS) Electrochim. Acta. 2014;135:439–446. [Google Scholar]
- Zheng Y., Xiao Y., Yang Z.-H., Wu S., Xu H.-J., Liang F.-Y., Zhao F. The bacterial communities of bioelectrochemical systems associated with the sulfate removal under different pHs. Process Biochem. 2014;49:1345–1351. [Google Scholar]
- Zhou J., Deng Y., Luo F., He Z., Yang Y. Phylogenetic molecular ecological network of soil microbial communities in response to elevated CO<sub>2</sub>. mBio. 2011;2 doi: 10.1128/mBio.00122-11. e00122–e00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Zou Q., Fan M., Xu Y., Chen Y. Highly efficient anaerobic co-degradation of complex persistent polycyclic aromatic hydrocarbons by a bioelectrochemical system. J. Hazard. Mater. 2020;381:120945. doi: 10.1016/j.jhazmat.2019.120945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate datasets/code.