Abstract
Background
We present our experience of ABO-incompatible renal transplant using immunoadsorption (IA) columns. We have compared efficacy of two commercially available columns.
Methods
This single-center prospective study was conducted at Army Hospital Research and Referral, Delhi. All consecutive ABO-incompatible renal transplants from January 2014 to February 2018 were analyzed. Of 30 patients who underwent transplantations, 28 underwent antibody depletion with immunoadsorption columns. Of them, 14 cases were in the “Glycosorb group,” while 14 in the “Adsopak group.”
Results
The donors in the Adsopak group were older than those in the Glycosorb group (p < 0.05). Both groups had spousal donors in majority. The cutoff for the antibody titer was 1:8. The median titer in the Adsopak group was 128 (range, 1:4 to 1:2048), while that in the Glycosorb group was 24 (range, 1:8 to 1:128). All patients in the Glycosorb group had baseline titers ≤1:128, while 13 patients in the Adsopak group had baseline titers ≤1:512. Nil titer was achievable with Glycosorb® (50%,7/14) but not with Adsopak® (P < 0.01). Around 4 sessions were required for the Glycosorb group, while around 8 sessions were required for the Adsopak group before transplantation (p < 0.001). The Glycosorb group was advantageous in terms of graft failure because no rejection was noticed in these patients in their follow-up period. Three patients in the Adsopak group developed rejection (two had mixed rejection, and one had antibody-mediated rejection). Four patients died of sepsis (three in the Glycosorb and one in the Adsopak group). Lower baseline serum creatinine level was achieved in the Glycosorb group.
Conclusions
Results of ABO-incompatible renal transplantation were satisfactory, and the use of immunoadsorption columns could effectively deplete antibody titers. Glycosorb columns were more efficient than Adsopak columns. Graft survival was better with Glycosorb. Posttransplant infections were a major cause of mortality.
Keywords: ABO-incompatible transplantation, Immunoadsorption columns, Adsopak, Glycosorb
Introduction
Renal transplantation is the best treatment form for patients with chronic kidney disease requiring renal replacement therapy.1 In developing countries such as India, transplant options are limited and ABO incompatibility remains an important limiting factor among the living donors. It leads to rejection of around 35% living kidney donors.2 Living donor renal transplantation is traditionally planned in accordance with the rules of blood group compatibility. Unintentional breach of the ABO barrier has led to immediate or irreparable graft losses.3, 4 Since the last two decades, ABO-incompatible (ABOi) transplantation protocols have evolved from splenectomy to specific antibody removal by immunoadsorption (IA) techniques. ABOi renal transplantation is still uncommon in developing countries because of high cost, lack of experience and infrastructure, and risk of antibody-mediated rejection. ABOi transplantation protocols differ the world over with respect to antibody removal techniques, accepted and target antibody titer, method of antibody detection, and immunosuppression maintenance.5 Most European preconditioning protocols are based on IA techniques using antigen-specific columns.6 Two types of antigen-specific IA columns are commercially available, Glycosorb®-ABO column which is a single-use column and ABO Adsopak® column which is reuseable. Both are low-molecular-weight carbohydrate columns with immobilized blood group A or B antigens linked to a sepharose matrix. These columns specifically deplete anti-A or anti-B antibodies, and their use together with anti-CD20 antibody administration has showed excellent results on 5-year follow-up.7
The ABOi living donor renal transplantation program was started at our center in July 2013 and began using IA columns for the antibody depletion technique in January 2014. We present our experience of ABOi renal transplant using IA columns. We also have compared the efficacy of the two commercially available columns in India.
Materials and methods
-
I.
Study design
This is a single-center prospective study
-
II.
Study place
The study was conducted in a tertiary care hospital in India.
-
III.
Study sample
All consecutive ABOi renal transplants from January 2014 to February 2018 were analyzed. A total of 30 patients underwent ABOi renal transplantations, of which 28 underwent antibody depletion with immunoadsorption columns.
-
IV.Inclusion criteria
-
1.Patients suffering from chronic kidney disease stage 5D with no live related donor of compatible blood group.
-
2.Patients aged at least 18 years and able to give consent regarding ABOi renal transplantation.
-
3.Patients proposing a live related donor to whom he/she has a baseline anti–blood group titer ≤1:2048.
-
4.Patients vaccinated against hepatitis B and pneumococcal disease at least 15 days before beginning desensitization.
-
1.
-
V.Exclusion criteria
-
1.Patients suffering from an unstable cardiovascular condition.
-
2.Patients with any form of active bacterial, viral, or other infection.
-
3.Patients with complement-dependent cytotoxicity (CDC) or flow crossmatch positivity with the donor.
-
4.Patients with a known or suspected hereditary complement deficiency.
-
5.Patients with a known hypersensitivity to the treatment drug or any of its excipients.
-
6.Patients with a history of illicit drug use or alcohol abuse in the previous year.
-
7.Patients with any medical condition that might interfere with the patient's participation in the study, pose an added risk for the patient, or confound the assessment of the patient (e.g., severe cardiovascular or pulmonary disease).
-
1.
-
VI.
Antibody titer measurement
Recipient serum IgG and IgM antibody titers were measured against donor ABO blood group antigens by the gel card test which was conducted by the column agglutination technique. The low-ionic-strength saline indirect antiglobulin test technique (Ortho Clinical Diagnostics, Johnson and Johnson, USA) was used. Procedures were performed according to the manufacturer's manual. Twenty-five microliters of serially diluted serum and 50 μL of the prepared group A or B 0.8% red blood cell suspension were added to the gel card microcolumns. Antibody titer was monitored twice daily in the first week after transplantation and once daily thereafter until discharge.
-
VII.
Immunization protocol
All recipients were subjected to pneumococcal 13-valent vaccination on preoperative day 120 and were vaccinated again with pneumococcal 23-valent vaccine on preoperative day 45.
-
VIII.
Immunological studies
All recipients and donors were subjected to human leukocyte antigen (HLA) matching on HLA-A, HLA-B, and HLA-DR locus. Crossmatching was performed by CDC and flow cytometry on preoperative day 17, before rituximab administration.
-
IX.
Preconditioning protocol
The detailed ABO desensitization protocol is depicted in Table 1.
-
A)
Antibody removal technique
Antigen-specific immunoadsorption for patients was started on preoperative day 7. For the purpose of immunoadsorption, the plasma was separated using membrane filters, using the Dialog+® hemodialysis machine (B Braun, Melsungen, Germany) at a rate of 60 ml/min; the plasma was then passed through the immunoadsorption column and then reinfused back into the patient. Initial 14 cases were treated with Glycosorb®-ABO columns (Glycorex Transplantation AB, Lund, Sweden), and later, the next 14 cases were treated with ABO Adsopak® columns (POCARD Ltd, Moscow, Russia). These two groups were labeled “Gycosorb group” and “Adsopak group,” respectively. The target IgG level titer was ≤1:8, and surgery was performed only if the titer was within the target range. During each IA, 1.5 to 2 plasma volumes per patient were processed, and on the day of hemodialysis, IA was performed after the dialysis session. For the Glycosorb group, the column was run up to 6 h for each session, and for the Adsopak group, it was run for 4 h and then regenerated, after which another session for 4 h was conducted (total 8 h per session).
Adsopak column regeneration procedure
The Adsopak column was regenerated in 4 steps over half an hour. In the first step, washing was carried out with 1 L of 0.9% NaCl at a rate of 150 ml/min. In the second step, antibody removal was carried out with “regeneration solution 1”, 0.5–1 L of solution containing HCl and glycine (pH 2–3), at a rate of 150 ml/min. In the third step, pH restoration was performed with “regeneration solution 2,” 0.5 L of solution containing phosphate buffer, at a rate of 150 ml/min. In the fourth step, column restoration was performed with a solution containing 2 ml of sodium nitrite and 0.5 L of phosphate buffer (total 502 ml) at a rate of 150 ml/min. At the end of this step, the column was stored in a fridge at 2–8 °C.
-
B)
Antibody synthesis blocking
Patients were administered intravenous rituximab on preoperative day 15 before transplantation. During the initial period, patients in the Glycosorb group were treated with rituximab, 500 mg twice, two weeks apart. Later, patients in the Adsopak group were administered a lesser dose of rituximab, 200 mg once. Seven days before transplantation, the CD19 assay was carried out and the need for repeat therapy was decided accordingly.
-
C)
Induction of immunosuppression
Intraoperatively, patients received intravenous methylprednisolone (500 mg) and intravenous basiliximab (Anti CD25) (20 mg) induction. Basiliximab was repeated on postoperative day 4. Intravenous methylprednisolone was repeated on postoperative day 1 and day 2 at a dose of 250 mg and 125 mg, respectively, and was overlapped with oral prednisolone 25 mg/day.
-
D)
Maintenance of immunosuppression
All patients were started on tacrolimus and mycophenolate mofetil (MMF) on preoperative day 7, which was continued throughout the posttransplant period. Tacrolimus was given at a dose of 0.1 mg/kg/day, divided into two doses, while MMF was given at a dose of 2000 mg/day, divided into two doses. Tacrolimus levels were monitored by liquid chromatography with tandem mass spectrometry (LC-MS/MS) method.
-
X.
Prophylactic therapy
Table 1.
ABO desensitization protocol.
| Protocol | Antibody synthesis blocking | Antibody removal technique | Immunosuppression |
|
|---|---|---|---|---|
| Induction | Maintenance | |||
| Technique | Anti-CD20 | Antigen-specific immunoadsorption column | Anti CD25 | Triple-drug immunosuppression |
| Day | Preoperative day 15 | Preoperative day 7 | Day 0, 4 | Dual immunosuppression from preoperative day 7 |
| Protocol | Inj rituximab | Glycosorb®-ABO; or ABO Adsopak® columns | Inj basiliximab 20 mg | Tacrolimus, mycophenolate mofetil, prednisolone |
| Monitoring | CD19 assay | Isoagglutinin IgG titers | Tacrolimus C0 levels | |
All patients received trimethoprim-sulfamethoxazole prophylaxis for 6 months for pneumocystis jiroveci infection and clotrimazole prophylaxis for fungal infection. Valganciclovir prophylaxis was given for 100 days to patients with D+/R-cytomegalovirus IgG serology.
-
XI.
Statistical analysis
The data so collected were entered in MS EXCEL. Results are expressed as mean ± standard deviation and median. Comparison of continuous and categorical variables was made using the student t-test and chi-square test, respectively. The level of significance was defined as p < 0.05. SPSS® (Statistical Package for the Social Sciences), version 22, Statistics for windows (IBM® Corp, Armonk, NY) was used for data analysis.
Results
Of a total of 30 patients, 28 underwent ABOi renal transplantation using IA columns. Of them, 14 cases were in the “Glycosorb group,” while 14 cases were placed in the “Adsopak group.” The remaining two cases underwent transplantation without using columns as the baseline titer was within the acceptable range as per protocol.
-
I.
Demographic profile
Table 2 depicts the descriptive statistics of these patients. The donors in the Adsopak group were older than those in the Glycosorb group, and this difference was statistically significant. There was sex mismatch in both groups, with most recipients being male and donors being female (statistically insignificant). Both groups had spousal donors in majority.
-
II.
IA data
Table 2.
Patient characteristics.
| Patient characteristics |
Glycosorb group (n = 14) |
Adsopak group (n = 14) |
P value |
|
Recipient data | |||
| Age in years (mean ± SD) | 35.5 ± 9.6 | 36.8 ± 9 | 0.71 |
| Sex (male:female) | 9:5 | 10:4 | 0.685 |
| Basic disease | |||
| Chronic glomerulonephritis | 04 | 12 | – |
| Chronic interstitial nephritis | 09 | 01 | – |
| ADPKD | 0 | 01 | – |
| Diabetic nephropathy | 01 | 0 | – |
| Blood group | |||
| A to O | 0 | 03 | – |
| B to O | 02 | 03 | – |
| AB to O | 0 | 0 | – |
| A to B | 01 | 03 | – |
| AB to B | 04 | 03 | – |
| B to A | 04 | 02 | – |
| AB to A | 03 | 0 | – |
| Time on dialysis, months (±SD) | 15.8 ± 18.3 | 12 ± 7.6 | 0.48 |
| Mode of dialysis | |||
| Hemodialysis | 13 | 13 | – |
| Peritoneal dialysis | 01 | 01 | – |
| PRA (≥ 5%) | 02 | 0 | – |
| Pretransplant blood transfusion |
03 |
03 |
– |
|
Donor data | |||
| Age in years (mean ± SD) | 39.5 ± 5.2 | 45.1 ± 8.4 | 0.04 |
| Sex (male:female) | 5:9 | 03:11 | 0.40 |
| Relation to patient | 0.40 | ||
| Spouse | 11 | 09 | |
| Parent | 03 | 05 | |
| Sibling | 0 | 0 | |
| HLA mismatch (0–6) | 3.5 ± 0.76 | 3.9 ± 1.73 | 0.43 |
SD, standard deviation; ADPKD, autosomal dominant polycystic kidney disease; HLA, human leukocyte antigen.
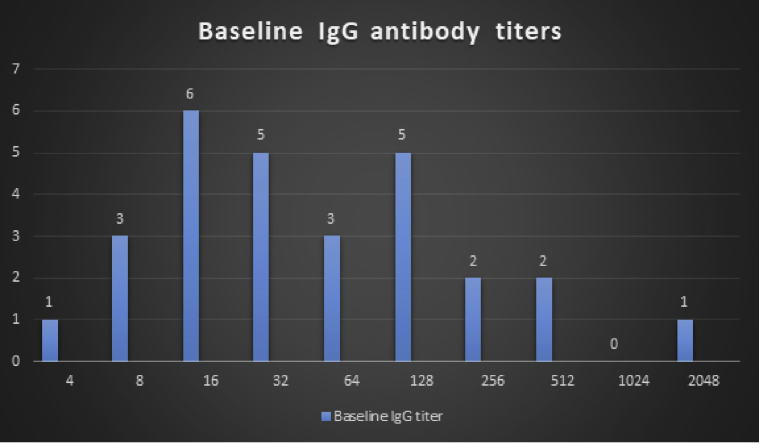
The IA data of two groups are compared in Table 3. The cutoff for antibody titer for transplantation was 1:8. The baseline IgG titer distribution of the patients is depicted in Fig. 1. The median titer in the Adsopak group was 128 (range, 1:4 to 1:2048), while that in the Glycosorb group was 24 (range, 1:8 to 1:128). The highest titer in the Glycosorb group was 1:128, while that in the Adsopak group was 1:2048. All patients in the Glycosorb group had baseline titers ≤1:128, while 13 patients in the Adsopak group had baseline titers ≤1:512. Nil titer was achievable with Glycosorb® (50%, 7/14), but no patient treated with Adsopak® could achieve a nil pretransplant titer (statistically significant).
Table 3.
Immunoadsorption data.
| Immunoadsorption data | Glycosorb group (n = 14) | Adsopak group (n = 14) | P value |
|---|---|---|---|
| Baseline antibody titer | |||
| Lowest | 1:8 | 1:4 | – |
| Highest | 1:128 | 1:2048 | – |
| Pretransplant antibody titer | |||
| Nil | 07 | 0 | 0.0023 |
| 1:2 | 03 | 02 | – |
| 1:4 | 04 | 06 | – |
| 1:8 | 0 | 06 | – |
| IA column quantity per patient | 1.86 ± 0.95 | 1.93 ± 1.2 | 0.865 |
| IA column sessions | |||
| Mean ± SD | 4.1 ± 1.56 | 8.43 ± 4.01 | 0.0009 |
| Median | 4.0 | 8.0 | – |
IA, immunoadsorption.
Fig. 1.
Baseline IgG ABO antibody titer distribution of the patients.
Although the Adsopak® columns were reuseable, they lost their efficacy after multiple reuse and additional columns had to be used for 6 patients. A mean of around 4 sessions were required in the Glycosorb group, while a mean of around 8 sessions were required in the Adsopak group before transplantation (statistically significant). In the Adsopak group, two patients required 13 sessions of IA and one of them had a baseline antibody titer of 1:2048. One patient in the Adsopak group required 16 IA sessions, although the baseline titer was 1:512.
-
III.
Recipient outcome
The recipient outcome of two groups is depicted in Table 4. Two cases did not require IA, and both cases achieved baseline serum creatinine <1 mg/dl and remained rejection free over a follow-up period of 2 years. As the Glycosorb group was inducted first in the study, followed by the Adsopak group, the mean follow-up period was higher in the Glycosorb group (statistically significant). The Glycosorb group was advantageous in terms of graft failure because no rejection was noticed in these patients in their follow-up period (statistically insignificant). Three patients in the Adsopak group developed rejection, of whom two had mixed rejection and one patient had antibody-mediated rejection. All rejection episodes were because of preformed anti HLA antibodies and had developed within one month of transplantation. One patient recovered graft function with antirejection therapy. Three patients died in the Glycosorb group, the cause of death being sepsis in all of them. One patient died in the Adsopak group because of sepsis. A lower baseline serum creatinine level was achieved in the Glycosorb group. No death occurred during the period of IA.
Table 4.
Recipient outcome.
| Recipient outcome | Glycosorb group (n = 14) | Adsopak group (n = 14) | P value |
|---|---|---|---|
| Follow-up, months (mean ± SD) | 30 ± 3.88 | 10.28 ± 1.45 | <0.001 |
| Baseline serum creatinine (mean, mg/dl) | 1.18 ± 0.39 | 1.86 ± 1.35 | 0.081 |
| Graft dysfunction | 0.066 | ||
| Antibody-mediated rejection | 0 | 01 | – |
| Acute cellular rejection | 0 | 0 | – |
| Mixed rejection | 0 | 02 | – |
| Recipient death | 03 | 01 | 0.280 |
SD, standard deviation.
Discussion
Introduction of IA columns in ABOi preconditioning protocols has led to considerably good graft and recipient outcomes. Although expensive, a major advantage of antigen-specific IA is efficient depletion of circulating antibodies without considerable losses of protective antibodies and other essential plasma components including coagulation factors.8 In 2005, Tyden et al.9 first published their report on a protocol without splenectomy, based on antigen-specific IA, rituximab, and a conventional triple-drug immunosuppressive protocol and proved that ABOi transplantations could be performed without splenectomy and standard immunosuppression with excellent results. In 2015, Schiesser et al.10 reported successful reuse of Glycosorb® columns, making the process more commercially sustainable. Data on the use of Adsopak® columns are scarce. In 2011, Moysyuk et al.11 had reported the first Russian experience of antigen-specific IA using reusable Adsopak® columns. The author could not find any further published data on their use and experiences. On vast review of literature, neither information on Adsopak® columns nor comparison of these two commercially available IA columns could be found. Most of studies on ABOi transplantation using IA columns had reported successful use of Glycosorb® columns. This study is the first of its kind to compare the outcomes of these two alternatives.
We used a modified Freiburg University Hospital protocol,12 and IV Ig was not used. The minimum pretransplant antibody titer was ≤1:8, compared with the original protocol, in which the cutoff was 1:4. However, this is a more restrictive strategy than used in other transplantation centers, accepting 1:32 or 1:16 as target titers.13 Because of limited experience with ABOi transplantation at our center, patients with antibody titer <1:8 after rituximab administration were also subjected to IA, although Masterson et al.14 had demonstrated that antibody depletion was not necessary in cases with low initial antibody titer.
The recipient profile was not statistically different in the two groups in our study. However, the donors in the Adsopak group were older, with greater female preponderance and a higher HLA mismatch. Although the median index titer levels were higher in the Adsopak group, there was significant difference in the efficiency of two IA alternatives. Glycosorb® was more efficient in terms of titer reduction and the number of sessions required. Overall, an average of 6 pretransplant IA sessions was required and posttransplant IA sessions were not required in most cases. Montgomery et al.15 reported that the number of sessions of plasma exchange ranged from 2 for a titer of 1:16 to 10 for a titer of 1:512. An average of 2–5 posttransplantation sessions was also required.15 Lipshutz et al.16 observed that the number of plasma exchange sessions depends on the initial isoagglutinin titers, ranging from 4 to 5 for a titer of 1:16 to 18 for a titer of 1:1024. The number of posttransplantation sessions ranged from 0 to 12. A study conducted in Japan showed that around 3 to 4 sessions of double filtration plasmapheresis were required before transplantation.17, 18 No posttransplant apheresis was carried out unless there was antibody-mediated rejection.18 On average, 4 sessions of antigen-specific IA were conducted by Tyden et al.,9 and around 7 sessions by Donauer et al.19 In our study, three cases in the Adsopak group required posttransplant plasmapheresis for antibody-mediated rejection as part of antirejection therapy.
Few successful ABOi renal transplantations have been performed with high initial antibody titers (>1:1024),13, 15, 16 and only few case reports of successful transplants with a titer of 1:2048 have been reported.20 In our study, one patient with titer 1:2048 was successfully transplanted and achieved good graft function. Only IgG titers were monitored in our study because IgG is more specific for antibody-mediated rejection than IgM.21
As the initial 14 transplantations were performed using Glycosorb® columns and the next 14 cases were subjected to IA using Adsopak® columns, the mean follow-up period was higher in the Glycosorb group. A lower baseline serum creatinine level was achieved using Glycosorb®, although recipients had lower index antibody titers in this group. Overall, death-censored graft survival rate was 92.85%, and it was higher in the Glycosorb group (100%, mean follow-up: 30 months) compared with the Adsopak group (85.71%, mean follow-up: 10 months). Overall, the patient survival rate was 85.71% which was higher in the Adsopak group (92.85%) than in the Glycosorb group (78.57%). Montgomery et al.15 in their study had found 1-year graft survival rate and patient survival rate of 98.3% and 96.3%, respectively. Lipshutz et al.16 reported a death-censored graft survival rate of 94.4% and patient survival rate of 100%. Morath et al.13 reported an 18-month graft survival rate of 100% and a patient survival rate of 94.4%. Fuchinoue et al.22 and Oettl et al.23 have described a 100% graft survival rate at 1 year. In another study, Wilpert et al.24 have reported a death-censored graft survival rate of 100% and patient survival rate of 98%. Schousboe et al.25 reported a 1-year graft survival rate of 97.2%. Morath et al.13 reported an 18-month graft survival rate of 100% and a patient survival rate of 94.4%. Barnett et al.26 reported a death-censored allograft survival rate of 98.4% and a patient survival rate of 94.5% at 1 year of follow-up. Jha et al.27 reported death-censored graft survival rate of 95% and patient survival arte of 90% in their study conducted in the same geographic location. Thus, graft survival rate of the Glycosorb group is comparable with that of the previous studies, but that of the Adsopak group is lower probably due to higher degree of HLA mismatch and higher initial antibody titers in this group. The patient survival rate of the Adsopak group was comparable with that of the previous studies, but it was lower in the Glycosorb group probably due to higher mean follow-up period of 30 months in this group. However, the studies conducted in this part of world show comparable data. In addition, being a developing country, poor hygiene and higher infection rate might have contributed to lower patient survival in the long run. All deaths in the study were due to fulminant sepsis.
Conclusion
We conclude that the results of ABOi transplantation at our center were satisfactory and that the use of IA columns could effectively deplete the antibody titers. We also conclude that higher titer reduction was achieved with a lower of sessions of IA by the Glycosorb® columns. In addition, the graft survival was better with Glycosorb® columns. Posttransplant infections and sepsis were major causes of mortality, which remains a major concern in the developing world.
Conflicts of interest
The authors have none to declare.
References
- 1.Wolfe R.A., Ashby V.B., Milford E.L. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.Segev D.L., Gentry S.E., Warren D.S., Reeb B., Montgomery R.A. Kidney paired donation and optimizing the use of live donor organs. J Am Med Assoc. 2005;293:1883–1890. doi: 10.1001/jama.293.15.1883. [DOI] [PubMed] [Google Scholar]
- 3.Starzl T.E., Marchioro T.L., Holmes J.H. Renal homografts in patients with major donor-recipient blood group incompatibilities. Surgery. 1964;55:195–200. [PMC free article] [PubMed] [Google Scholar]
- 4.Cook D.J., Graver B., Terasaki P.I. ABO incompatibility in cadaver donor kidney allografts. Transplant Proc. 1987;19:4549–4552. [PubMed] [Google Scholar]
- 5.Ray D.S., Thukral S. Outcome of ABO-Incompatible living donor renal transplantations: a single-center experience from eastern India. Transplant Proc. 2016;48(8):2622–2628. doi: 10.1016/j.transproceed.2016.06.048. [DOI] [PubMed] [Google Scholar]
- 6.Tyden G., Kumlien G., Fehrman I. Successful ABO-incompatible kidney transplantations without splenectomy using antigen-specific immunoadsorption and rituximab. Transplantation. 2003;76(4):730–731. doi: 10.1097/01.TP.0000078622.43689.D4. [DOI] [PubMed] [Google Scholar]
- 7.Van Agteren M., Weimar W., de Weerd A.E. The first fifty ABO blood group incompatible kidney transplantations: the Rotterdam experience. J Transplant. 2014;2014:913902. doi: 10.1155/2014/913902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valli P.V., Puga Yung G., Fehr T. Changes of circulating antibody levels induced by ABO antibody adsorption for ABO-incompatible kidney transplantation. Am J Transplant. 2009;9:1072. doi: 10.1111/j.1600-6143.2009.02579.x. [DOI] [PubMed] [Google Scholar]
- 9.Tyden G., Kumlien G., Genberg H. ABO incompatible kidney transplantations without splenectomy, using antigen-specific immunoadsorption and rituximab. Am J Transplant. 2005;5:145. doi: 10.1111/j.1600-6143.2004.00653.x. [DOI] [PubMed] [Google Scholar]
- 10.Schiesser M., Steinemann D.C., Hadaya K. The reuse of immunoadsorption columns in ABO-incompatible kidney transplantation is efficient: the Swiss experience. Transplantation. 2015;99:1030–1035. doi: 10.1097/TP.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 11.Moysyuk Y.G., Sushkov A.I., Pulkova N.V. The first Russian experience of abo-incompatible kidney transplantation with antigen-specific immunoadsorption. Vestnik Transplantologii i Iskusstvennyh Organov. 2011;13(4):6–18. [Google Scholar]
- 12.Zschiedrich S., Kramer-Zucker A., Janigen B. An update on ABO-incompatible kidney transplantation. Transpl Int. 2015;28(4):387–397. doi: 10.1111/tri.12485. [DOI] [PubMed] [Google Scholar]
- 13.Morath C., Becker L.E., Leo A. ABO incompatible kidney transplantation enabled by non-antigen specific immune-adsorption. Transplantation. 2012;93(8):827–834. doi: 10.1097/TP.0b013e31824836ae. [DOI] [PubMed] [Google Scholar]
- 14.Masterson R., Hughes P., Walker R.G. ABO incompatible renal transplantation without antibody removal using conventional immunosuppression alone. Am J Transplant. 2014;14(12):2807–2813. doi: 10.1111/ajt.12920. [DOI] [PubMed] [Google Scholar]
- 15.Montgomery R.A., Locke J.E., King K.E. ABO incompatible renal transplantation: a paradigm ready for broad implementation. Transplantation. 2009;87:1246–1255. doi: 10.1097/TP.0b013e31819f2024. [DOI] [PubMed] [Google Scholar]
- 16.Lipshutz G.S., McGuire S., Zhu Q. ABO blood type-incompatible kidney transplantation and access to organs. Arch Surg. 2011;146:453–458. doi: 10.1001/archsurg.2011.40. [DOI] [PubMed] [Google Scholar]
- 17.Ishida H., Koyama I., Sawada T. Anti-AB titer changes in patients with ABO incompatibility after living related kidney transplantations: survey of 101 cases to determine whether splenectomies are necessary for successful transplantation. Transplantation. 2000;70:681–685. doi: 10.1097/00007890-200008270-00024. [DOI] [PubMed] [Google Scholar]
- 18.Toki D., Ishida H., Horita S. Blood group O recipients associated with early graft deterioration in living ABO-incompatible kidney transplantation. Transplantation. 2009;88:1186–1193. doi: 10.1097/TP.0b013e3181ba07ec. [DOI] [PubMed] [Google Scholar]
- 19.Donauer J., Wilpert J., Geyer M. ABO-incompatible kidney transplantation using antigen-specific immunoadsorption and rituximab: a single center experience. Xenotransplantation. 2006;13:108–110. doi: 10.1111/j.1399-3089.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- 20.Koshino K., Okamoto M., Sakai K. The excellent outcomes of ABO-incompatible kidney transplantation with high titer (>x2048) using anti-CD20 and anti-CD25 antibody without splenectomy: two case reports. Transplant Proc. 2011;43:2379–2382. doi: 10.1016/j.transproceed.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 21.Shimmura H., Tanabe K., Ishikawa N. TomaRole of anti-A/B antibody titers in results of ABO-incompatible kidney transplantation. Transplantation. 2000;70:1331–1335. doi: 10.1097/00007890-200011150-00011. [DOI] [PubMed] [Google Scholar]
- 22.Fuchinoue S., Ishii Y., Sawada T. The 5-year outcome of ABO-incompatible kidney transplantation with rituximab induction. Transplantation. 2011;91:853–857. doi: 10.1097/TP.0b013e31820f08e8. [DOI] [PubMed] [Google Scholar]
- 23.Oettl T., Halter J., Bachmann A. ABO blood group-incompatible living donor kidney transplantation: a prospective, single-centre analysis including serial protocol biopsies. Nephrol Dial Transplant. 2009;24:298–303. doi: 10.1093/ndt/gfn478. [DOI] [PubMed] [Google Scholar]
- 24.Wilpert J., Fischer K.G., Pisarski P. Long-term outcome of ABO-incompatible living donor kidney transplantation based on antigen-specific desensitization. An observational comparative analysis. Nephrol Dial Transplant. 2010;25:3778–3786. doi: 10.1093/ndt/gfq229. [DOI] [PubMed] [Google Scholar]
- 25.Schousboe K., Titlestad K., Baudier F. BistrupABO-incompatible kidney transplantation. Dan Med Bull. 2010;57:A4197. [PubMed] [Google Scholar]
- 26.Barnett N., Manook M., Nagendran M. Tailored desensitization strategies in ABO blood group antibody incompatible renal transplantation. Transpl Int. 2014;27:187–196. doi: 10.1111/tri.12234. [DOI] [PubMed] [Google Scholar]
- 27.Jha P.K., Bansal S.B., Sethi S.K. ABO-incompatible renal transplantation in developing world- crossing the immunological (and mental) barrier. Indian J Nephrol. 2016;26(2):113–118. doi: 10.4103/0971-4065.159557. [DOI] [PMC free article] [PubMed] [Google Scholar]