Abstract
Among patients with axial spondyloarthritis (axSpA), non-radiographic axial spondyloarthritis (nr-axSpA) is distinguished from ankylosing spondylitis (AS) by a lack of obvious radiographic changes in the sacroiliac joint. Tumor necrosis factor inhibitor (TNFi) has been used as a highly effective treatment in patients with AS and has shown good efficacy and safety in clinical trials in patients with nr-axSpA. As the pathophysiological mechanism for axSpA has started to become better recognized, various drugs other than TNFi, all of which are related to the interleukin-17 (IL-17) axis, are being evaluated in patients with axSpA. IL-17 inhibitors, such as secukinumab and ixekizumab, are effective drugs for patients with AS. A recent clinical trial reported that ixekizumab, a monoclonal antibody against IL-17A, was also effective in patients with nr-axSpA. In a COAST-X study, ixekizumab was superior to a placebo for improving signs and symptoms in patients with nr-axSpA at weeks 16 and 52. The adverse events were no different from those found in previous ixekizumab studies, and no new safety signals were identified. However, when considering several IL-17 inhibitors, it is necessary to obtain sufficient data to identify the exacerbation of extra-articular manifestation. In terms of effectiveness and safety, ixekizumab may be an appropriate alternative to TNFi in nr-axSpA patients.
Keywords: biologics, IL-16, ixekizumab, spondyloarthritis, treatment
Introduction
Axial spondyloarthritis (axSpA) is a chronic inflammatory disease that mainly affects the spine and sacroiliac joints. The occurrence of axSpA is closely related to the presence of human leukocyte antigen B27 (HLA-B27); it also has various extra-articular manifestations, such as uveitis, inflammatory bowel disease (IBD), and psoriasis. Depending on the presence of definitive radiographic sacroiliitis, axSpA is divided into non-radiographic axial spondyloarthritis (nr-axSpA) and radiographic axial spondyloarthritis, also known as ankylosing spondylitis (AS).1–3
To date, the 1984 modified New York classification criteria for AS have been used for diagnostic purposes in clinical practice.4 However, it was difficult to identify and treat patients with axSpA in the early stages because the condition could be diagnosed as AS if bilateral grade 2 or unilateral grade 3 sacroiliitis was present. Therefore, to avoid incorrect diagnosis, the Assessment of Spondyloarthritis international Society (ASAS)5,6 proposed the identification of patients with “atypical disease” that shared the clinical manifestation of AS.1 Nr-axSpA is clinically a relevant subgroup of axSpA. The diagnosis of nr-axSpA was introduced to identify patients with axSpA who had no structural changes in the sacroiliac joints.3 Therefore, the concept of nr-axSpA allows patients with axSpA to be diagnosed even without definite sacroiliitis on radiographs and treated with biologics, such as tumor necrosis factor inhibitor (TNFi) and interleukin-17 (IL-17) inhibitor.
Various clinical trials in patients with nr-axSpA have found that TNFi improves symptoms and reduces the burden of illness.7 In addition, IL-17 inhibitors have been introduced among the new therapeutic options for AS. IL-17 is a cytokine that plays an important role in the pathophysiology of axSpA. Secukinumab, a monoclonal antibody against IL-17A, is the first IL-17 inhibitor to be approved as a treatment for AS. Furthermore, no radiographic progression [a change of <2 in the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS) from baseline] was observed in 79% of patients treated with secukinumab, which was very encouraging.8 Ixekizumab, another monoclonal antibody against IL-17A, is also approved for the treatment of AS. Three phase III studies (COAST-V,9 COAST-W,10 and COAST-X11) have assessed the efficacy of ixekizumab in axSpA. The COAST-X study assessed the efficacy of ixekizumab in TNFi-naïve patients with nr-axSpA and found a significant improvement in the ASAS 40 response compared with the placebo response.11
In this article, we review the pathogenesis of IL-17 and clinical studies for patients with nr-axSpA. To clarify the options in nr-axSpA treatment, we discuss the effectiveness, safety, and benefits of ixekizumab with reference to clinical studies on IL-17 inhibitors and TNFi in the treatment of nr-axSpA and AS.
The role of IL-17 in the pathogenesis of SpA
There are various diseases in the subgroup of spondyloarthritis (SpA), including AS, nr-axSpA, psoriatic arthritis, reactive arthritis, enteropathic arthritis, and undifferentiated SpA. As they share a similar pathogenesis, these various types of SpA show similar articular and extra-articular manifestations and share classification criteria and treatment principles.12 Recent studies on the genetics of patients with SpA and arthritis animal models have found that the IL-23-IL17 axis is involved in the pathogenesis of SpA.12
The presence of HLA-B27 is an important genetic risk in patients with SpA. Variants in the HLA-B27 region are associated with the direct presentation of an arthritogenic peptide to cytotoxic CD8+ T cells.13 In addition, variants in this locus promote HLA-B27 homodimerization instead of heterodimerization; HLA-B27 protein misfolding can also occur, activating the unfolded protein response and increasing IL-23 production.14,15 HLA-B27 homodimers bind with increased affinity to the killer cell immunoglobulin-like receptor 3DL2 (KIR3DL2), which is expressed on IL-17+CD4+ T cells from the blood and synovial fluid of patients with AS.16 These results suggest that there is an association between major histocompatibility complex class I and the IL-23–IL-17 axis. In addition, the IL12B, IL23A, and IL23R loci are also related to the IL-23–IL-17 axis.12
The IL-17 family consists of IL-17A, IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F. IL-17A is the most well-known IL-17 subtype that is associated with several inflammatory diseases.12 IL-17A results in the transcription of pro-inflammatory genes, leading to the secretion of a range of pro-inflammatory cytokines and immune cell-attracting chemokines in target cells such as fibroblasts, epithelial cells, and synoviocytes.17,18 In addition, IL-17A increases levels of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor, both of which are associated with granulopoiesis in stromal cells, macrophages, and T cells.19 In inflammatory arthritis, IL-17A induces the production of matrix metalloproteinases and the receptor activator of nuclear factor-κB ligand and promotes angiogenesis, all of which contribute to joint and bone destruction.20–22 The function of IL-17F is similar to that of IL-17A, but it has a lower potency.23 Both IL-17A and IL-17F can be secreted as homodimers as well as an IL-17A–IL-17F heterodimer.24
In patients with SpA, IL-17 has been identified directly in the blood and synovial fluid. IL-17 and Il-23 levels have been found to be considerably higher in the serum of patients with SpA such as AS, reactive arthritis, and undifferentiated SpA when compared with the serum of healthy subjects.25,26 In patients with AS who had manifestations of peripheral arthritis, it was observed that the cytokine profile was different between AS and rheumatoid arthritis (RA); moreover, the IL-17 cytokine level was high in patients refractory to both conventional disease-modifying antirheumatic drugs and biologics.27 Additionally, the serum level of IL-17 was found to be directly correlated with the measures of disease activity such as the Bath Ankylosing Spondylitis Disease Activity Index.28 This evidence indicates that the IL-17 pathway plays an important role in the pathogenesis of SpA.
Biologics targeting IL-17 in SpA
Secukinumab is a fully recombinant human immunoglobulin G (IgG)1 kappa monoclonal antibody that directly inhibits IL-17A. Secukinumab was first approved for the treatment of AS by the European Medical Agency (EMA) in March 2015, and by the Food and Drug Administration (FDA) in January 2016.29 The FDA and EMA approved secukinumab for the treatment of nr-axSpA in June and April 2020, respectively.
Ixekizumab is an IgG4 monoclonal antibody that has an affinity to the homodimer IL-17A and the heterodimer IL-17A/F. Ixekizumab was also approved by the EMA and FDA in August 2019 for the treatment of AS.30 Recently, the COAST-X phase III study11 showed ixekizumab to have efficacy in treating patients with nr-axSpA. Ixekizumab was approved for the treatment of nr-axSpA by the FDA and the EMA in June 2020.
Brodalumab is a human monoclonal antibody that binds with high affinity to human IL-17 receptor A and can be a significantly efficacious therapeutic alternative for psoriasis and psoriatic arthritis.31,32 Although adverse psychiatric events were observed in patients with psoriasis, a clinical study is underway with AS.33,34 The trial has presented meaningful results and shows that brodalumab still has potential for the treatment of axSpA.
Bimekizumab is a humanized monoclonal antibody that selectively neutralizes IL-17A and IL-17F. It is being studied as a treatment for psoriasis, psoriatic arthritis, and AS.35–38 A 48-week phase IIb study was recently published on patients with active AS. A significant dose response compared with that of the placebo was observed, and a phase III study is being conducted in patients with AS and nr-axSpA.38
Current evidence-based treatment for patients with nr-axSpA
Using the ASAS classification criteria, axSpA has been divided into AS and nr-axSpA. This distinction depends on whether a definitive structural change to the sacroiliac joint is seen on a plain radiograph.3 Usually, if a radiographic change is observed, AS is diagnosed.4 However, patients with early disease cannot be classified according to the 1984 modified New York criteria for AS. Therefore, it is essential to differentiate between AS and nr-axSpA. Due to the classification of the disease spectrum, obtaining approval to use TNFi for treating AS could enable additional labeling for nr-axSpA; TNFi can then be used to treat all patients who manifest severe clinical features of AS.1,39 In a 10-year follow-up study of cases of undifferentiated SpA with radiographs, 35.7% showed the development of AS, and HLA-B27 and buttock pain were meaningful predictors of progression.40 In a recent retrospective study, 26% of nr-axSpA patients progressed to AS during the 15-year follow-up period.41 Not all patients with nr-axSpA developed radiographic sacroiliitis, which suggested that nr-axSpA may not simply be an initial stage of AS. This evidence motivates the active treatment of patients with nr-axSpA.
In the 2016 update of the ASAS-EULAR management recommendation for axSpA, a single set of management recommendations was provided for patients with the whole spectrum of axSpA disease including AS and nr-axSpA.42 Recently, the Spondylitis Association of America and the Spondyloarthritis Research and Treatment Network released updated recommendations for the treatment of axSpA, which comprises AS and nr-axSpA.43 As the research on nr-axSpA did not show sufficient clinical evidence, clinical drug studies conducted in patients with AS were referenced. Similar to the treatment for AS, non-steroidal anti-inflammatory drugs (NSAIDs) and physical therapy are mainstays of treatment for patients with nr-axSpA. For patients who do not show an adequate response to NSAIDs, clinical trials have shown that various TNFi treatments have been effective in the treatment of nr-axSpA.39,44–47 The known predictors for a good response to TNFi were male sex, a low Bath Ankylosing Spondylitis Functional Index, high C-reactive protein (CRP) levels, short disease duration, HLA-B27 positivity, and magnetic resonance imaging (MRI) changes.2,48,49
The effect of TNFi treatment on structural damage in patients with nr-axSpA was investigated. In a small sample study, treatment with adalimumab in patients with nr-axSpA showed no significant change in the mSASSS after about 2 years of follow-up.50 In the RAPID-axSpA study of patients with AS and nr-axSpA, a 4-year radiographic change was evaluated in patients treated with certolizumab pegol.51 The mean mSASSS changes were 0.06 from baseline to week 204; –0.01 from baseline to week 96; and 0.07 from week 96 to week 204. Overall, 4.5% of patients with nr-axSpA fulfilled the modified New York criteria at week 204. It is impossible to compare with controls, but it may be helpful in significantly reducing the radiographic progression in patients with nr-axSpA. However, we are aware that not all patients with nr-axSpA will develop radiographic sacroiliitis.
In the recommendations of biologics for patients with axSpA, TNFi is recommended over secukinumab or ixekizumab as the first biologic to be used, and secukinumab or ixekizumab is recommended over the use of a second TNFi in patients with a primary nonresponse to the first TNFi. Treatment with secukinumab or ixekizumab was strongly recommended over no treatment with either in patients with AS, while the use of these medications was conditionally recommended in nr-axSpA because clinical trials in nr-axSpA have not been reported.43
Clinical trials of ixekizumab in patients with nr-axSpA
Efficacy
Deodhar et al. reported the efficacy and safety of ixekizumab in patients with nr-axSpA.11 COAST-X was a multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase III trial. In particular, the institutions participating in the study were distributed among various regions.
Patients with nr-axSpA were defined as adults (aged ⩾18 years) with active axSpA without definite radiographic sacroiliitis, objective signs of inflammation (via MRI or CRP level), and an inadequate response or intolerance to NSAIDs. Patients were randomly assigned (1:1:1) to receive subcutaneous 80 mg ixekizumab every 4 weeks (Q4W), every 2 weeks (Q2W), or a placebo. The primary endpoints were the ASAS40 response at weeks 16 and 52.
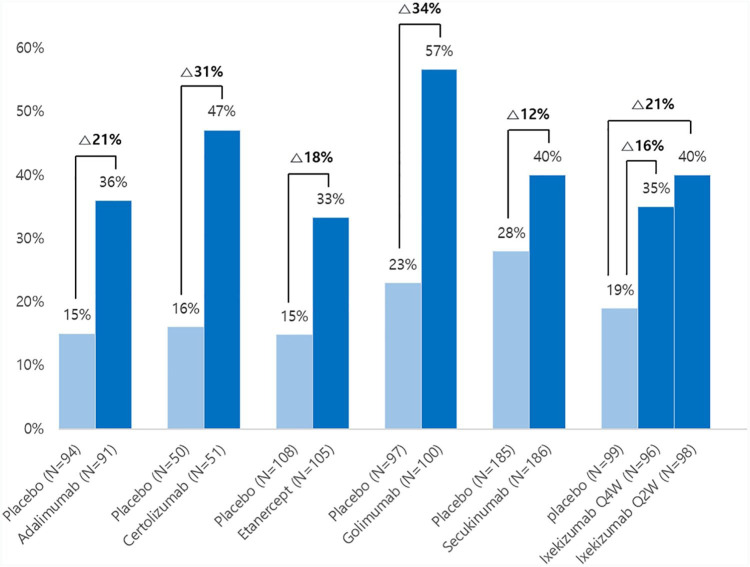
In the evaluation of the primary endpoint, the ASAS40 response at 16 and 52 weeks showed a statistically significant difference in the ixekizumab Q4W and Q2W treatment groups compared with the placebo group (Figure 1). Overall, 290 (96%) of 303 patients completed the first 16 weeks, and 265 (87%) completed the whole 52 weeks. Among them, 34 (35%) of 96 patients in the ixekizumab Q4W group (p = 0·0094 versus placebo), 41 (40%) of 102 patients in the ixekizumab Q2W group (p = 0·0016 versus placebo) and 20 (19%) of 105 patients in the placebo group achieved ASAS40 at week 16. In total, 29 (30%) of 96 patients in the ixekizumab Q4W group (p = 0·0045 versus placebo), 32 (31%) of 102 patients in the ixekizumab Q2W group (p = 0·0037 versus placebo), and 14 (13%) of 105 patients in the placebo group achieved ASAS40 at week 52. These results were similar to the ASAS40 response in the PREVENT study, which was a randomized controlled trial evaluating the efficacy and safety of secukinumab 150 mg in anti-TNF-naïve patients with nr-axSpA (Figure 1).52 Notably, a higher ASAS40 response rate was seen in ixekizumab treatment groups at week 1 [13 (14%) of 96, p = 0·0077, for ixekizumab Q4W and 12 (12%) of 102, p = 0·0123, for ixekizumab Q2W versus one (1%) of 105 for placebo]. In addition, they showed a greater reduction in the sacroiliac joint and Spondyloarthritis Research Consortium of Canada (SPARCC) scores than those of the placebo group. The least square mean change (SE) was –0.31 (0.54) in the placebo group, –3.38 (0.55) in the Q4W group, and –4.52 (0.53) in the Q2W group at week 16 and –1.92 (0.87) in the placebo group, –4.40 (0.73) in the Q4W group, and –6.16 (0.71) in the Q2W group at week 52.
Figure 1.
The findings of the Assessment of Spondyloarthrtis International Society-40 in clinical trials for the treatment of adalimumab,47 certolizumab,45 and etanercept44 at 12 weeks and that of golimumab,46 secukinumab,53 and ixekizumab11 at 16 weeks in patients with nr-axSpA. The certolizumab group had 400 mg certolizumab administered every 4 weeks, and the secukinumab group was a loading dose group.
In the post hoc analysis of COAST-X based on HLA-B27 status and disease duration, there was a significant ASAS40 response rate at 16 weeks in the ixekizumab Q4W and Q2W groups compared with that in the placebo group for patients with positive HLA-B27 (38% and 44% versus 22%, respectively) and in patients with a disease duration less than 5 years (both 42% versus 18%, respectively).54 However, if more patients were recruited, patients with a negative HLA-B27 or a disease duration of 5 years or more may also show a significant ASAS40 response in the ixekizumab group. In the evaluation of enthesitis, the ixekizumab treatment group showed improvement in the SPARCC enthesitis score in patients with nr-axSpA. In addition, among patients who achieved complete resolution of enthesitis, the ixekizumab Q4W and Q2W groups showed improvement of ASAS40 compared with that in the placebo group (52.2% and 57.7% versus 31.3%, respectively).55
In the subgroups stratified by baseline CRP and sacroiliac joint MRI status (CRP >5 mg/L and SPARCC score ⩾2; CRP ⩽5 mg/L and SPARCC score ⩾2; CRP >5 mg/L and SPARCC score <2), ASAS40 and ASDAS <2.1, BASDAI50, and Short Form-36 physical component scores were greater in the ixekizumab treatment group than in the placebo group.56,57 In addition, fatigue, spinal pain, spinal pain at night, and stiffness were improved at 16 and 52 weeks.58
Safety
The biologics, including TNFi and IL-17 inhibitors, affect host immunity and therefore cannot be free from all safety issues in patients with axSpA. The frequency of the treatment-emergent adverse events (TEAEs) was similar in the three groups [60 (57%) in the placebo group, 63 (66%) in the ixekizumab Q4W group, and 79 (77%) in the ixekizumab Q2W group]. Moreover, the frequency of serious adverse events and discontinuation were also similar among the three groups. The common TEAEs were nasopharyngitis that occurred in eight (8%) patients in the placebo group, 18 (19%) in the ixekizumab Q4W group, and 16 (16%) in the ixekizumab Q2W group. Injection-site reaction occurred in four (4%) patients in the placebo group, 11 (11%) in the ixekizumab Q4W group, and 17 (17%) in the ixekizumab Q2W group. The incidence of headache, upper respiratory tract infection, and hypertension was similar among the three groups. When considering that IL-17 is associated with increased granulopoiesis,19 ixekizumab may induce neutropenia. However, only one (1%) patient had grade 4 neutropenia in the placebo group. In the COAST-W study, one patient had grade 4 neutropenia in the ixekizumab Q4W group. In the COAST-V study, there was no grade 3 or 4 neutropenia in the ixekizumab group. The occurrence of infection was not significantly different either among the three groups. Herpes zoster occurred in one patient (1%) in the placebo and two patients (2%) in the ixekizumab Q4W, but none in the ixekizumab Q2W groups. Importantly, reactivation of tuberculosis did not occur in any patient in the three groups. Tuberculosis reactivation did not occur in the patients in the COAST-V and COAST-W studies either. In a systematic review of tuberculosis reactivation in treatment with IL-17 inhibitors for psoriasis, there were no cases of tuberculosis reactivation.59
Anterior uveitis and inflammatory bowel disease
Although IL-17 has an important role in the pathogenesis of uveitis, clinical trials of secukinumab treatment for non-infectious uveitis have been withdrawn because they did not meet the primary end points.60 In previous clinical trials of patients with AS, it was questionable whether secukinumab was effective for treating uveitis.61,62 In a 2019 treatment recommendation, secukinumab and ixekizumab are not recommended in patients with IBD or recurrent uveitis, because TNFi monoclonal antibodies are better options.43 Recently, in a large population of patients with AS treated with secukinumab from three phase III MEASURE trials that were conducted over 4 years, secukinumab did not increase the risk of uveitis (the exposure-adjusted incidence rate of uveitis was 1.4 per 100 patient-years).63 Regarding treatment with ixekizumab, one patient with a history of anterior uveitis in the COAST-V study and five patients (three in the Q2W and two in the Q4W group) in the COAST-W study had anterior uveitis. Since the COAST-W study was conducted on patients who did not respond to TNFi, the incidence of uveitis would have been higher than that seen in the COAST-V or COAST-X study. As a result, in the COAST-V and COAST-X studies, uveitis did not develop significantly compared with that in the placebo group. Long-term real-world studies are needed to assess the relationship between treatment with IL-17 inhibitors and flare-ups of uveitis.
In IBD, the control of the IL-23–IL-17 axis may have a paradoxical effect in the intestine. Anti-IL-23 antibody reduces T helper 17 autoimmunity, thereby improving colitis, whereas IL-17 inhibitors impair intestinal wall integrity and exacerbate the disease.64 In clinical trials of IBD, secukinumab had no effect on moderate-to-severe Crohn’s disease.65 In addition, secukinumab does not appear to be related to IBD exacerbation in a pooled secukinumab safety analysis with psoriasis, psoriatic arthritis, and AS treated with secukinumab (21 clinical trials).66 Therefore, caution should be exercised when prescribing secukinumab to patients with IBD or a personal history suggesting IBD. In the COAST-V study for ixekizumab, one patient had Crohn’s disease in the ixekizumab Q2W group. In the COAST-W study, one patient in the placebo group and three in the ixekizumab Q4W group had IBD events. In the COAST-X study, two patients had IBD events; a flare of ulcerative colitis in a patient from the placebo group who had pre-existing ulcerative colitis and a case of Crohn’s disease in a patient with pre-existing diarrhea in the ixekizumab Q4W group. There are still insufficient data to conclude a relationship between IL-17 inhibitors and IBD exacerbation.
Depression
Depression-related events occurred in four patients (4%) in the ixekizumab Q2W group only. Among them, a serious event of major depression occurred in a patient with pre-existing anxiety. The other three cases were non-serious: one patient had worsening pre-existing depression, one had recurrent depression on the day of the injection, and one had an adverse event of altered mood that resolved after 3 days without treatment. All four remained on the ixekizumab treatment until the study was completed. In the COAST-V study, none of the patients had complications such as depression.9 In the COAST-W study, five patients had a depressive episode in the placebo group (5.8%), one in the Q2W (1%) group, and none in the Q4W group.67 With respect to the long-term safety of secukinumab, no cases of suicide-related adverse events were reported in the AS studies on secukinumab treatment, but there were several cases in psoriasis and psoriatic arthritis.68 Long-term studies are needed, but ixekizumab is unlikely to be associated with depression in patients with r-axSpA or AS.
Ixekizumab can be an alternative to TNFi
Patients with nr-axSpA have a substantial burden of illness, with self-reported disease activity and functional impairments comparable with those in patients with AS, although they have lack of radiographic changes.7 In recent treatment trials, biologics have been effective in reducing the disease activity of nr-axSpA as well as improving the quality of life.44,45,47,50 As with TNF inhibitors, patients with nr-axSpA had a good response to ixekizumab. In addition, there was a significant reduction in the change in MRI findings of the sacroiliac joint. Although there are not enough data associated with IL-17 treatment in patients with nr-axSpA, referring to the data associated with ixekizumab in the COAST-W study and secukinumab in the MEASURE 2 study on patients with AS may be an appropriate alternative in patients who do not respond to TNFi.
One of the things we noticed in the studies on ixekizumab is the safety against tuberculosis reactivation. Management of latent tuberculosis infection must precede TNFi treatment to prevent tuberculosis reactivation. However, there are still patients with reactivation at the early stage of TNFi treatment; the situation is very serious in a few patients. IL-17 inhibitors may be preferred over TNFi in patients with a high risk of tuberculosis reactivation or in a region with a high prevalence of tuberculosis.69–72 In addition, it is necessary to study further whether an IL-17 inhibitor may be an alternative treatment for patients who presented with tuberculosis reactivation during TNFi therapy.73,74
The evidence that IL-17 inhibitors are effective for extra-articular manifestation such as psoriasis may also be a reason for treatment with ixekizumab.75,76 However, more research is needed in extra-articular manifestation, such as uveitis, IBD, enthesitis, and peripheral arthritis.
Finding the best biologics for patients with nr-axSpA
Various biologics and targeted synthetic disease-modifying antirheumatic drugs are used in RA treatment. In patients with RA, when primary drugs are ineffective, patients can be switched to drugs with a different mechanism of action.77–79 Moreover, several studies have attempted to identify biomarkers in patients with RA that could predict treatment outcomes and therefore could guide physicians in their decision to prescribe the most suitable biologics.80 In addition to TNFi, biologics that have been used for a long time in AS and various IL-17 inhibitors have been released as new therapeutic drugs. As in RA, axSpA requires research into new and diverse treatment strategies. An attempt to find a biomarker that can predict the therapeutic response of biologics in axSpA can also improve symptoms and prognosis and can reduce medical costs.81 Although the response rates of TNFi and IL-17 inhibitors are similar in the clinical trials of patients with nr-axSpA, there will be some patients who respond better to IL-17 inhibitors than to TNFi. Therefore, in nr-axSpA, it is necessary to divide the group of patients responding to TNFi or IL-17 inhibitors, to distinguish potential responders, and to derive an optimal strategy of switching to TNFi or an IL-17 inhibitor.82 Research on the selection of optimal biologics for individual patients should be actively conducted in AS and nr-axSpA, as various studies are attempted to select the most effective drug for each individual among various classes of drugs in RA.
Conclusion
IL-17 is a cytokine that is closely related to the pathogenesis of axSpA including nr-axSpA. Ixekizumab is an effective drug for early improvement of symptoms and inflammation and for preventing disability by inhibiting the IL-17 cytokine in patients with nr-axSpA and is as effective as TNFi. Ixekizumab may have an advantage over TNFi for tuberculosis reactivation, but more data are needed to address the concerns regarding uveitis and IBD. Therefore, ixekizumab is suitable as an effective treatment in patients with axSpA and nr-axSpA.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Bon San Koo  https://orcid.org/0000-0002-4212-2634
https://orcid.org/0000-0002-4212-2634
Tae-Hwan Kim  https://orcid.org/0000-0002-3542-2276
https://orcid.org/0000-0002-3542-2276
Contributor Information
Bon San Koo, Department of Internal Medicine, Inje University Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea.
Tae-Hwan Kim, Department of Rheumatology, Hanyang University Hospital for Rheumatic Diseases, 222-1, Wangsimni-ro, Seongdong-gu, Seoul, 04763, Korea.
References
- 1. Deodhar A, Strand V, Kay J, et al. The term non-radiographic axial spondyloarthritis is much more important to classify than to diagnose patients with axial spondyloarthritis. Ann Rheum Dis 2016; 75: 791–794. [DOI] [PubMed] [Google Scholar]
- 2. Ghosh N, Ruderman EM. Nonradiographic axial spondyloarthritis: clinical and therapeutic relevance. Arthritis Res Ther 2017; 19: 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sieper J, van der Heijde D. Review: Nonradiographic axial spondyloarthritis: new definition of an old disease? Arthritis Rheum 2013; 65: 543–551. [DOI] [PubMed] [Google Scholar]
- 4. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984; 27: 361–368. [DOI] [PubMed] [Google Scholar]
- 5. Rudwaleit M, Landewe R, van der Heijde D, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 2009; 68: 770–776. [DOI] [PubMed] [Google Scholar]
- 6. Rudwaleit M, van der Heijde D, Landewe R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009; 68: 777–783. [DOI] [PubMed] [Google Scholar]
- 7. Boonen A, Sieper J, van der Heijde D, et al. The burden of non-radiographic axial spondyloarthritis. Semin Arthritis Rheum 2015; 44: 556–562. [DOI] [PubMed] [Google Scholar]
- 8. Braun J, Baraliakos X, Deodhar A, et al. Secukinumab shows sustained efficacy and low structural progression in ankylosing spondylitis: 4-year results from the MEASURE 1 study. Rheumatology (Oxford) 2019; 58: 859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet 2018; 392: 2441–2451. [DOI] [PubMed] [Google Scholar]
- 10. Deodhar A, Poddubnyy D, Pacheco-Tena C, et al. Efficacy and safety of ixekizumab in the treatment of radiographic axial spondyloarthritis: sixteen-week results from a phase III randomized, double-blind, placebo-controlled trial in patients with prior inadequate response to or intolerance of tumor necrosis factor inhibitors. Arthritis Rheumatol 2019; 71: 599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deodhar A, van der Heijde D, Gensler LS, et al. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet 2020; 395: 53–64. [DOI] [PubMed] [Google Scholar]
- 12. Taams LS, Steel KJA, Srenathan U, et al. IL-17 in the immunopathogenesis of spondyloarthritis. Nat Rev Rheumatol 2018; 14: 453–466. [DOI] [PubMed] [Google Scholar]
- 13. Fiorillo MT, Maragno M, Butler R, et al. CD8+ T-cell autoreactivity to an HLA-B27-restricted self-epitope correlates with ankylosing spondylitis. J Clin Invest 2000; 106: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allen RL, O’Callaghan CA, McMichael AJ, et al. Cutting edge: HLA-B27 can form a novel beta 2-microglobulin-free heavy chain homodimer structure. J Immunol 1999; 162: 5045–5048. [PubMed] [Google Scholar]
- 15. DeLay ML, Turner MJ, Klenk EI, et al. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum 2009; 60: 2633–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bowness P, Ridley A, Shaw J, et al. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J Immunol 2011; 186: 2672–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shahrara S, Pickens SR, Mandelin AM, 2nd, et al. IL-17-mediated monocyte migration occurs partially through CC chemokine ligand 2/monocyte chemoattractant protein-1 induction. J Immunol 2010; 184: 4479–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yao Z, Painter SL, Fanslow WC, et al. Human IL-17: a novel cytokine derived from T cells. J Immunol 1995; 155: 5483–5486. [PubMed] [Google Scholar]
- 19. Schwarzenberger P, La Russa V, Miller A, et al. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J Immunol 1998; 161: 6383–6389. [PubMed] [Google Scholar]
- 20. Chabaud M, Garnero P, Dayer JM, et al. Contribution of interleukin 17 to synovium matrix destruction in rheumatoid arthritis. Cytokine 2000; 12: 1092–1099. [DOI] [PubMed] [Google Scholar]
- 21. Jo S, Wang SE, Lee YL, et al. IL-17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Res Ther 2018; 20: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest 1999; 103: 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res 2007; 17: 435–440. [DOI] [PubMed] [Google Scholar]
- 24. Wright JF, Bennett F, Li B, et al. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J Immunol 2008; 181: 2799–2805. [DOI] [PubMed] [Google Scholar]
- 25. Romero-Sanchez C, Jaimes DA, Londono J, et al. Association between Th-17 cytokine profile and clinical features in patients with spondyloarthritis. Clin Exp Rheumatol 2011; 29: 828–834. [PubMed] [Google Scholar]
- 26. Wendling D, Cedoz JP, Racadot E, et al. Serum IL-17, BMP-7, and bone turnover markers in patients with ankylosing spondylitis. Joint Bone Spine 2007; 74: 304–305. [DOI] [PubMed] [Google Scholar]
- 27. Koo BS, Jo S, Kwon E, et al. Effect of biologics in the level of cytokines in the synovial fluid of patients with ankylosing spondylitis. Korean J Intern Med 2020; 35: 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen WS, Chang YS, Lin KC, et al. Association of serum interleukin-17 and interleukin-23 levels with disease activity in Chinese patients with ankylosing spondylitis. J Chin Med Assoc 2012; 75: 303–308. [DOI] [PubMed] [Google Scholar]
- 29. Blair HA. Secukinumab: a review in ankylosing spondylitis. Drugs 2019; 79: 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Benucci M, Damiani A, Li Gobbi F, et al. Therapeutic potential of ixekizumab in the treatment of ankylosing spondylitis: a review on the emerging clinical data. Ther Clin Risk Manag 2020; 16: 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med 2015; 373: 1318–1328. [DOI] [PubMed] [Google Scholar]
- 32. Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med 2014; 370: 2295–2306. [DOI] [PubMed] [Google Scholar]
- 33. Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol 2018; 78: 81–89e85. [DOI] [PubMed] [Google Scholar]
- 34. Wei JC-C, Kim T-H, Kishimoto M, et al. Efficacy and safety of brodalumab, an anti-interleukin-17 receptor a monoclonal antibody, in patients with axial spondyloarthritis: a 16 week results of a phase 3, multicenter, randomized, double-blind, placebo-controlled study. Ann Rheum Dis 2019; 78: 195. [Google Scholar]
- 35. Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis 2018; 77: 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol 2017; 83: 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Papp KA, Merola JF, Gottlieb AB, et al. Dual neutralization of both interleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. J Am Acad Dermatol 2018; 79: 277–286.e210. [DOI] [PubMed] [Google Scholar]
- 38. van der Heijde D, Gensler LS, Deodhar A, et al. Dual neutralisation of interleukin-17A and interleukin-17F with bimekizumab in patients with active ankylosing spondylitis: results from a 48-week phase IIb, randomised, double-blind, placebo-controlled, dose-ranging study. Ann Rheum Dis 2020; 79: 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barkham N, Keen HI, Coates LC, et al. Clinical and imaging efficacy of infliximab in HLA-B27-positive patients with magnetic resonance imaging-determined early sacroiliitis. Arthritis Rheum 2009; 60: 946–954. [DOI] [PubMed] [Google Scholar]
- 40. Sampaio-Barros PD, Bortoluzzo AB, Conde RA, et al. Undifferentiated spondyloarthritis: a longterm followup. J Rheumatol 2010; 37: 1195–1199. [DOI] [PubMed] [Google Scholar]
- 41. Wang R, Gabriel SE, Ward MM. Progression of nonradiographic axial spondyloarthritis to ankylosing spondylitis: a population-based cohort study. Arthritis Rheumatol 2016; 68: 1415–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017; 76: 978–991. [DOI] [PubMed] [Google Scholar]
- 43. Ward MM, Deodhar A, Gensler LS, et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2019; 71: 1599–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dougados M, van der Heijde D, Sieper J, et al. Symptomatic efficacy of etanercept and its effects on objective signs of inflammation in early nonradiographic axial spondyloarthritis: a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol 2014; 66: 2091–2102. [DOI] [PubMed] [Google Scholar]
- 45. Landewe R, Braun J, Deodhar A, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann Rheum Dis 2014; 73: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sieper J, van der Heijde D, Dougados M, et al. A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2015; 67: 2702–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sieper J, van der Heijde D, Dougados M, et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann Rheum Dis 2013; 72: 815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Flouri ID, Markatseli TE, Boki KA, et al. Comparative analysis and predictors of 10-year tumor necrosis factor inhibitors drug survival in patients with spondyloarthritis: first-year response predicts longterm drug persistence. J Rheumatol 2018; 45: 785–794. [DOI] [PubMed] [Google Scholar]
- 49. Maneiro JR, Souto A, Salgado E, et al. Predictors of response to TNF antagonists in patients with ankylosing spondylitis and psoriatic arthritis: systematic review and meta-analysis. RMD Open 2015; 1: e000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cantarini L, Fabbroni M, Talarico R, et al. Effectiveness of adalimumab in non-radiographic axial spondyloarthritis: evaluation of clinical and magnetic resonance imaging outcomes in a monocentric cohort. Medicine (Baltimore) 2015; 94: e1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van der Heijde D, Baraliakos X, Hermann KA, et al. Limited radiographic progression and sustained reductions in MRI inflammation in patients with axial spondyloarthritis: 4-year imaging outcomes from the RAPID-axSpA phase III randomised trial. Ann Rheum Dis 2018; 77: 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Deodhar A, Blanco R, Dokoupilova E, et al. Secukinumab improves signs and symptoms of non-radiographic axial spondyloarthritis: primary results of a randomized controlled phase III study. Arthritis Rheumatol. Epub ahead of print 7 August 2020. DOI: 10.1002/art.41477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Braun J, Blanco R, Dokoupilova E, et al. OP0106 secukinumab 150 mg significantly improved signs and symptoms of non-radiographic axial spondyloarthritis: 52-week results from the phase iii prevent study. Ann Rheum Dis 2020; 79: 69.31229952 [Google Scholar]
- 54. Navarro-Compán V, Maldonado-Cocco J, Rahman P, et al. Response to treatment with ixekizumab in patients with active non-radiographic axial spondyloarthritis based on HLA-B27 status and disease duration. Arthritis Rheumatol 2020; 72 (Suppl. 10): abstract 0361. [Google Scholar]
- 55. Schett G, Van den Bosch F, Baraliakos X, et al. Ixekizumab improves signs and symptoms of patients with radiographic and non-radiographic axial spondyloarthritis and extra-articular manifestation of enthesitis through 16 weeks. Arthritis Rheumatol 2020; 72 (Suppl. 10): abstract 0880. [Google Scholar]
- 56. Maksymowych WP, Marzo-Ortega H, Ø’stergaard M, et al. Efficacy of ixekizumab on disease activity and quality of life in patients with active nonradiographic axial spondyloarthritis and objective signs of inflammation, stratified by baseline CRP/Sacroiliac joint MRI status [abstract]. Arthritis Rheumatol 2020; 72 (Suppl. 10). [Google Scholar]
- 57. Walsh J, Magrey M, Kiltz U, et al. Ixekizumab significantly improves self-reported overall health as measured by short-form-36 in patients with active non-radiographic axial spondyloarthritis: 16- and 52-week results of a phase 3 randomized trial (COAST-X). Arthritis Rheumatol 2020; 71(Suppl. 10): abstract 1545. [Google Scholar]
- 58. Mease P, Deodhar A, Rahman P, et al. Ixekizumab treatment improves fatigue, spinal pain, stiffness, and sleep in patients with nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2020; 72(Suppl. 10): abstract 0890. [Google Scholar]
- 59. Fowler E, Ghamrawi RI, Ghiam N, et al. Risk of tuberculosis reactivation during interleukin-17 inhibitor therapy for psoriasis: a systematic review. J Eur Acad Dermatol Venereol 2020; 34: 1449–1456. [DOI] [PubMed] [Google Scholar]
- 60. Dick AD, Tugal-Tutkun I, Foster S, et al. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology 2013; 120: 777–787. [DOI] [PubMed] [Google Scholar]
- 61. Baeten D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med 2015; 373: 2534–2548. [DOI] [PubMed] [Google Scholar]
- 62. Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab and sustained improvement in signs and symptoms of patients with active ankylosing spondylitis through two years: results from a phase III study. Arthritis Care Res (Hoboken) 2017; 69: 1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Deodhar AA, Miceli-Richard C, Baraliakos X, et al. Incidence of uveitis in secukinumab-treated patients with ankylosing spondylitis: pooled data analysis from three phase 3 studies. ACR Open Rheumatol 2020; 2: 294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fauny M, Moulin D, D’Amico F, et al. Paradoxical gastrointestinal effects of interleukin-17 blockers. Ann Rheum Dis 2020; 79: 1132–1138. [DOI] [PubMed] [Google Scholar]
- 65. Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012; 61: 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schreiber S, Colombel JF, Feagan BG, et al. Incidence rates of inflammatory bowel disease in patients with psoriasis, psoriatic arthritis and ankylosing spondylitis treated with secukinumab: a retrospective analysis of pooled data from 21 clinical trials. Ann Rheum Dis 2019; 78: 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dougados M, Wei JC, Landewe R, et al. Efficacy and safety of ixekizumab through 52 weeks in two phase 3, randomised, controlled clinical trials in patients with active radiographic axial spondyloarthritis (COAST-V and COAST-W). Ann Rheum Dis 2020; 79: 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Deodhar A, Mease PJ, McInnes IB, et al. Long-term safety of secukinumab in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: integrated pooled clinical trial and post-marketing surveillance data. Arthritis Res Ther 2019; 21: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cantini F, Nannini C, Niccoli L, et al. Risk of tuberculosis reactivation in patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis receiving non-anti-tnf-targeted biologics. Mediators Inflamm 2017; 2017: 8909834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cantini F, Niccoli L, Goletti D. Tuberculosis risk in patients treated with non-anti-tumor necrosis factor-alpha (TNF-alpha) targeted biologics and recently licensed TNF-alpha inhibitors: data from clinical trials and national registries. J Rheumatol Suppl 2014; 91: 56–64. [DOI] [PubMed] [Google Scholar]
- 71. Jung SM, Ju JH, Park MS, et al. Risk of tuberculosis in patients treated with anti-tumor necrosis factor therapy: a nationwide study in South Korea, a country with an intermediate tuberculosis burden. Int J Rheum Dis 2015; 18: 323–330. [DOI] [PubMed] [Google Scholar]
- 72. Navarra SV, Tang B, Lu L, et al. Risk of tuberculosis with anti-tumor necrosis factor-alpha therapy: substantially higher number of patients at risk in Asia. Int J Rheum Dis 2014; 17: 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kim HW, Kwon SR, Jung KH, et al. Safety of resuming tumor necrosis factor inhibitors in ankylosing spondylitis patients concomitant with the treatment of active tuberculosis: a retrospective nationwide registry of the Korean Society of Spondyloarthritis Research. PLoS One 2016; 11: e0153816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kim YJ, Kim YG, Shim TS, et al. Safety of resuming tumour necrosis factor inhibitors in patients who developed tuberculosis as a complication of previous TNF inhibitors. Rheumatology (Oxford) 2014; 53: 1477–1481. [DOI] [PubMed] [Google Scholar]
- 75. Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 2015; 386: 541–551. [DOI] [PubMed] [Google Scholar]
- 76. Ruyssen-Witrand A, Perry R, Watkins C, et al. Efficacy and safety of biologics in psoriatic arthritis: a systematic literature review and network meta-analysis. RMD Open 2020; 6: e001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Park EJ, Kim H, Jung SM, et al. The use of biological disease-modifying antirheumatic drugs for inflammatory arthritis in Korea: results of a Korean expert consensus. J Rheum Dis 2020; 27: 4–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016; 68: 1–26. [DOI] [PubMed] [Google Scholar]
- 79. Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020; 79: 685–699. [DOI] [PubMed] [Google Scholar]
- 80. Cuppen BV, Welsing PM, Sprengers JJ, et al. Personalized biological treatment for rheumatoid arthritis: a systematic review with a focus on clinical applicability. Rheumatology (Oxford) 2016; 55: 826–839. [DOI] [PubMed] [Google Scholar]
- 81. Sullivan SD, Alfonso-Cristancho R, Carlson J, et al. Economic consequences of sequencing biologics in rheumatoid arthritis: a systematic review. J Med Econ 2013; 16: 391–396. [DOI] [PubMed] [Google Scholar]
- 82. Poddubnyy D, Sieper J. What is the best treatment target in axial spondyloarthritis: tumour necrosis factor alpha, interleukin 17, or both? Rheumatology (Oxford) 2018; 57: 1145–1150. [DOI] [PubMed] [Google Scholar]