Abstract
Purpose
To evaluate the association between baseline ocular variables and the widening of the anterior chamber angle by laser peripheral iridotomy (LPI) in primary angle closure suspects (PACS) using a new Fourier-domain swept-source anterior segment optical coherence tomography (FD-ASOCT).
Method
Sixty-six PACS eyes of 41 individuals were enrolled in this prospective interventional case series. An FD-ASOCT (Casia SS-1000 OCT; Tomey, Nagoya, Japan) was used to measure biometric baseline variables and at 1 month after the LPI. Paired t test was used to compare the difference between pre-and post-LPI measurements. Multivariate regression analysis was used to test for an association between baseline iris thickness and volume, anterior chamber depth and volume, and lens vault with a widening of the angle after an LPI. Changes in trabecular iris space area and angle opening distance after the LPI were main outcome measures.
Results
The mean age of participants was 58.6 ± 8.7 years, 68.2% of whom were female. The angle opening distance, recess area, and trabecular iris surface area at 500 μm increased by 48 to 73% (all P < 0.001). Lens vault and iris volume did not change. A low anterior chamber volume and low iris volume were associated with angle greater deepening by LPI.
Conclusion
Eyes with a shallow anterior chamber and thinner irises are more likely to experience angle opening from an LPI.
Keywords: Primary angle closure suspect; Laser peripheral iridotomy, swept-source anterior segment OCT; Anterior chamber volume, iris volume, CASIA OCT
Introduction
Primary angle closure glaucoma (PACG) is a major cause of vision loss around the world and in Asia in particular [1]. PACG has the highest prevalence in East Asia (1.1%) [2] compared to only 0.4% in Iran [3], which is more similar to Caucasian populations [2]. The anatomical features considered to put an eye at risk of angle closure are a crowded anterior segment and narrow anterior chamber angle which are often associated with a small corneal diameter, a short axial length, a shallow anterior chamber, a thick lens and iris, and lens vault [2, 4-11]. Laser peripheral iridotomy (LPI) is a relatively safe procedure that helps reverse appositional angle closure, thereby relieving pupillary block, widening the angle, and preventing optic nerve damage from increased intraocular pressure [12-16]. However, 60% of patients treated with an LPI continue to have some degree of angle closure [15, 17-19]. Because the indication for an LPI is quite subjective and the number of primary angle closure suspects (PACS) patients is so large, treating everybody would pose serious challenges and not be cost-effective. Moreover, despite being a relatively low-risk procedure, the risk of cataract development and endothelial cell loss has not been evaluated in long-term prospective studies [20-22]. The value of iris thickness, anterior chamber depth, iris curve, and lens vault to predict a successful LPI has previously been found to be relatively low [13, 23, 24].
In the present study, we used a new Fourier-domain swept-source anterior segment optical coherence tomography (FD-ASOCT; Casia SS-1000 OCT; Tomey, Nagoya, Japan) with a high scan speed (30,000 A-scans per second) that allows us to image the angle by 128 cross-sections in less than 3 s. We hypothesized that this FD-ASOCT would allow for a more accurate assessment of anterior chamber baseline variables that predict widening of the anterior chamber angles following an LPI.
Methods
Study design
This study was approved by the ethics committee of Shahid Beheshti University of Medical Sciences and carried out in accordance with tenets of the Declaration of Helsinki. A total of 72 eyes of 43 consecutive Iranian individuals with PACS were included from the general ophthalmology clinic between May 2015 and December 2015. After a thorough discussion of the benefits, shortcomings, and potential complications of an LPI, a written informed consent was obtained from all. Inclusion criteria were angle closure on gonioscopic exam by Shaffer gonioscopic criteria with a scleral spur visibility score (SSVS) of at least 1 in each angle (described below) and age above 18 years. Exclusion criteria were peripheral synechiae, an intraocular pressure above 21 mmHg, a corneal opacity that would interfere with the image acquisition, signs of glaucomatous optic neuropathy, and any systemic condition or use of anticoagulants that could increase the risk of bleeding. Diagnosis of PACS, PAC, and PACG was based on the criteria established by the International Society of Geographic and Epidemiologic Ophthalmology (ISGEO) [25]. PACS was defined as an eye in which the posterior trabecular meshwork was not seen over 270 ° or more of the angle circumference on gonioscopy. Primary angle closure (PAC) was defined using PACS criteria plus PAS reaching the scleral spur or beyond; evidence of prior acute intraocular pressure (IOP) elevation, including iris atrophy, glaukomflecken, and a dilated nonreactive pupil; or elevated IOP without signs of glaucomatous damage. PACG was defined by PAC and glaucomatous optic neuropathy or visual field defects.
At baseline, all patients underwent a comprehensive ophthalmic examination by an experienced glaucoma specialist including determination of best corrected visual acuity (BCVA), slit-lamp examination (Haag-Streit, Bern, Switzerland), Goldmann applanation tonometry (model AT900, Haag-Streit), gonioscopy with a Zeiss-style 4-mirror lens (model OPDSG, Ocular Instruments, Inc., Bellevue, WA), fundus examination, and perimetry (Humphrey visual field analyzer; model 750; Carl Zeiss Meditec, Dublin, CA, USA).
Gonioscopy was performed by a trained ophthalmologist (HE) at × 16 magnification in a darkroom setting using the Shaffer gonioscopic classification as described in the following: an angle between the iris and the trabecular meshwork surface of 35 to 45° was graded 4, between 20 and 35° as grade 3, between 10 to 20° as grade 2, and less than 10° was classified as grade 1. Grade 0 was assigned if angle structures were not observed [26]. IOP was measured twice by the same ophthalmologist between 9 and 11 AM. The average of 2 readings was recorded as patient’s IOP. If two IOPs were different by more than 2 mmHg, a third measurement was taken, and the median was recorded.
Swept-source anterior segment optical coherence tomography
The anterior chamber angle scan protocol of the Casia SS-1000 OCT (FD-ASOCT) was used to obtain a volume scan consisting of 128 radial scans, each 16 mm in length and 6 mm in depth. Measurements were done in the dark (0.3 lx) while the individual was asked to fixate on an internal target for the 2.4 s required to complete data acquisition. Images with motion or lid artifacts were excluded. After the scleral spur was manually determined, the trabecular iris space area (TISA), the angle opening distance (AOD), and trabecular iris angle (TIA) were measured by the instrument’s software in the nasal and temporal quadrant. The scleral spur was identified in the FD-ASOCT image as a protrusion of sclera into the anterior chamber. When the scleral spur was clearly visible, a visibility score (SSVS) of 2 was denoted [27]. In cases of no detectable scleral spur, SSVS was graded 0, while moderate clarity of the scleral spur was graded as 1 [27]. A glaucoma specialist experienced with this method (MP) determined the scleral spur location for each image.
By definition, the AOD is the perpendicular distance between a point 500 μm (AOD 500) or 750 μm (AOD 750) anterior to the scleral spur and the opposing iris. TISA is a trapezoidal area (TISA 500 or 750) bounded by the AOD 500 or 750, the anterior iris surface, the inner corneoscleral wall, and the perpendicular distance between the scleral spur and the opposing iris. Angle recess area (in mm2) is defined as the triangular area (ARA 500 or 750) bounded by the AOD 500 or 750, the anterior iris surface, and the inner corneoscleral wall [28]. Iris thickness was measured at 2000 μm from the scleral spur (IT2000). Since the peripheral iridotomies were created closer to the superior and temporal angles, only the nasal angles were analyzed. The iris volume, anterior chamber depth, and anterior chamber volume were also measured. These variables have shown to have low measurement variabilities [29].
The anterior chamber depth was determined as axial distance from the corneal endothelium to the anterior lens surface. Lens vault (LV) was defined as the perpendicular distance from the anterior lens surface to the horizontal line connecting the two scleral spurs.
Laser peripheral iridotomy technique
All LPIs were performed by the same ophthalmologist (NA). Pupillary constriction was achieved with 2% pilocarpine, and topical tetracaine was applied to the eye for anesthesia. An Abraham iridotomy lens was used with hypromellose 2.5% ophthalmic lubricant solution (Goniovisc; HUB pharmaceuticals, Rancho Cucamonga, CA) as a coupling agent. A single 4 mJ shot was applied with a neodymium-doped yttrium aluminum garnet (YAG) laser operating at 1064 nm (Optimis Fusion, Quantel Medical, Paris, France) and was used initially to gauge the tissue response to achieve patency and enlargement of the iridotomy. An average of 15 shots was needed. The surgeon confirmed the patency of the LPI by transillumination. A drop of apraclonidine and betamethasone was applied before discharging the patient. All LPIs were created in the superotemporal location between the 10–11 and 1–2 o’clock positions on the right and left eyes, respectively. All patients were started on betamethasone four times a day for 4 days and reexamined 2 and 4 weeks after the procedure. At the follow-up visit at 4 weeks, FD-ASOCT imaging was repeated and performed by the same operator, at the same time of the day and with the same protocol.
Statistical analysis
All data was computed as mean, standard deviation, median, and range. Differences in preoperative and postoperative measurements for AOD 500, AOD 750, TISA 500, TISA 750, ARA 500, ARA 750, TIA 500, TIA 750, LV, AC volume, iris thickness, and iris volume were compared by paired Student’s t tests. Univariate and multivariate linear regression models, the latter adjusted for age, sex, refractive error, and axial length, were used to test for an association between the baseline measurements of the iris thickness, iris volume, LV, and anterior chamber volume (ACV) and the changes in AOD 500, AOD 750, TISA 500, and TISA 750. The significance was set at P < 0.05 for this study. Among all the variables included in our study, the nasal AOD 500 produced the maximum sample size, which we used to compute the proper sample size. To have a power of 95% to detect a difference as large as 0.10 before and after LPI when the standard deviation of this change was calculated to be 0.15 based on a pilot study, a total sample size of 64 eyes would be needed. All statistical analysis was performed by SPSS software (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0; Armonk, NY: IBM Corporation).
Results
Seventy-two eyes of 43 consecutive patients with PACS were enrolled in this study. Six eyes were excluded due to poor visualization of the scleral spur in FD-ASOCT images. At the time of the imaging, none of the individuals were using pilocarpine or other systemic agents that could constrict or dilate the pupil. Data from 66 eyes of 41 patients (28 female, 13 male) was used for the final analysis. The mean age was 58.6 ± 8.7 years. Mean IOP at the time of presentation was 13 ± 2.7 mmHg and remained unchanged at 1 month (13.3 ± 2.1 mmHg) after the LPI (P = 0.421). Mean central corneal thickness was 538 ± 29 μm (Table 1). The mean pre-LPI gonioscopic angle width (Shaffer grade) was 0.72 ± 0.41, which increased significantly to 1.83 ± 0.71 1 month after the LPI (P < 0.001).
Table 1.
Baseline demographics and ocular examination
| Male | 13 (31.7%) |
|---|---|
| Female | 28 (68.2%) |
| Age (years) | 58.6 ± 8.7 |
| CCT (μm) | 538 ± 29 |
| Angle opening (mm) | 0.7 ± 0.4 |
| IOP (mmHg) | 13 ± 2.7 |
| Lens thickness (mm) | 4.18 ± 0.81 |
| Axial length (mm) | 22.5 ± 1.2 |
| SE refraction (diopter) | 1.27 ± 1.21 |
CCT, central corneal thickness; IOP, intraocular pressure; SE, spherical equivalent refraction
AOD 500 (0.35 ± 0.14 vs. 0.46 ± 0.15 mm, P < 0.001), TISA 500 (0.17 ± 0.12 vs. 0.18 ± 0.06 mm, P < 0.001), ARA 500 (0.22 ± 0.09 vs.0.27 ± 0.1 mm2, P < 0.001), ACD (2.37 ± 0.29 vs. 2.45 ± 0.37, P = 0.001), and AC width (106.9 ± 26.4 vs. 119.8 ± 25.6, P< 0.001) increased after LPI (Fig. 1 and Supplementary Table 1). However, there was no significant change in LV (0.27 ± 0.18 vs.0.27 ± 0.19, P + 0.761) and iris volume (28.4 ± 4.8 vs. 28.6 ± 5, P = 0.755) (Fig. 1). Baseline iris thickness measured 2 mm from the scleral spur was 459 ± 34 μm that changed insignificantly to 458 ± 35 μm 1-month post-LPI (P = 0.071).
Fig. 1.
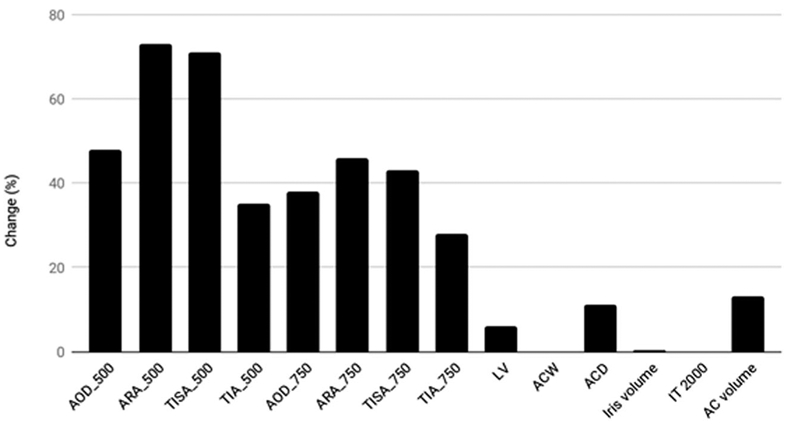
Changes in mean anterior segment variables. Only LV, ACW, iris volume, and IT2000 did not change significantly (P > 05). AOD angle opening distance at 500 and 750 μm; ARA angle recess area at 500 and 750 μm. TISA, trabecular iris surface area; TIA, trabecular iris angle; ACD, anterior chamber depth; ACV, anterior chamber volume; ACW, anterior chamber width; LV, lens vault; IT2000 iris thickness 2000 μm from the scleral spur
The multivariate linear regression model found a significant association between lower baseline anterior chamber volume and AOD 500, AOD 750, TISA 500, and TISA 750 opening at 1-month post-LPI. (respective P values: 0.036, 0.048, 0.020, and 0.041, Table 2, Fig. 2). The baseline iris volume was associated with a significant angle opening at AOD 500, TISA 500, and TISA 750 1 month after the LPI (Ps = 0.022, 0.001, and 0.041, respectively; Table 2 and Fig. 3). For each 1 μl of lower preoperative iris volume, TISA 500 increased by an average of 0.01 mm2.
Table 2.
The unadjusted univariate and adjusted multivariate linear regression for factors associated with increased anterior opening distance
| Unadjusted* |
Adjusted** |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Regression coefficient | 95% CI | P | Regression coefficient | 95% CI | P | ||||
| Lower | Upper | Lower | Upper | ||||||
| ΔAOD_500 | Age | 0 | 0 | 0.01 | 0.375 | 0 | 0 | 0.01 | 0.249 |
| Gender | 0.05 | −0.06 | 0.16 | 0.346 | 0.06 | −0.04 | 0.16 | 0.257 | |
| LV | −0.09 | −0.51 | 0.34 | 0.681 | 0.17 | −0.06 | 0.4 | 0.147 | |
| cACD | −0.16 | −0.72 | 0.4 | 0.562 | −0.13 | −0.28 | 0.01 | 0.072 | |
| Iris Volume | −0.01 | −0.02 | 0 | 0.141 | −0.01 | −0.02 | 0 | 0.022 | |
| IT2000 | 0.04 | −0.04 | 0.12 | 0.35 | 0.001 | < 0.001 | 0.002 | 0.232 | |
| ACV | −0.04 | −0.08 | 0.01 | 0.03 | −0.01 | −0.01 | 0 | 0.036 | |
| ΔTISA_500 | Age | 0 | 0 | 0.01 | 0.366 | 0 | 0 | 0.01 | 0.193 |
| Gender | −0.07 | −0.15 | 0.01 | 0.081 | −0.06 | −0.15 | 0.03 | 0.19 | |
| LV | −0.12 | −0.44 | 0.21 | 0.466 | 0.08 | −0.13 | 0.3 | 0.442 | |
| cACD | −0.05 | −0.47 | 0.38 | 0.827 | −0.12 | −0.25 | 0.01 | 0.06 | |
| Iris Volume | −0.01 | −0.02 | 0 | 0.007 | −0.01 | −0.02 | −0.01 | 0.001 | |
| IT2000 | 0.01 | −0.05 | −0.05 | 0.756 | < 0.001 | < 0.001 | 0.001 | 0.363 | |
| ACV | 0 | −0.04 | 0.03 | 0.83 | −0.01 | −0.01 | 0 | 0.4 | |
| ΔAOD_750 | Age | 0 | −0.01 | 0.01 | 0.863 | 0 | −0.01 | 0.01 | 0.622 |
| Gender | 0.08 | −0.08 | 0.23 | 0.304 | 0.1 | −0.04 | 0.24 | 0.161 | |
| LV | 0.07 | −0.55 | 0.68 | 0.826 | 0.28 | −0.04 | 0.59 | 0.084 | |
| cACD | −0.16 | −0.96 | 0.65 | 0.694 | −0.17 | −0.37 | 0.02 | 0.083 | |
| Iris Volume | −0.01 | −0.02 | 0.01 | 0.336 | −0.01 | −0.02 | 0 | 0.103 | |
| IT2000 | −0.01 | −0.04 | 0.01 | 0.206 | 0.001 | < 0.001 | 0.002 | 0.245 | |
| ACV | −0.06 | −0.32 | 0.2 | 0.653 | −0.07 | −0.14 | 0.01 | 0.048 | |
| ΔTISA_750 | Age | 0 | 0 | 0.01 | 0.315 | 0 | 0 | 0 | 0.291 |
| Gender | 0.04 | −0.02 | 0.11 | 0.188 | 0.05 | −0.01 | 0.11 | 0.125 | |
| LV | −0.05 | −0.32 | 0.23 | 0.728 | 0.13 | −0.01 | 0.28 | 0.074 | |
| cACD | −0.18 | −0.54 | 0.17 | 0.305 | −0.09 | −0.18 | 0 | 0.06 | |
| Iris Volume | 0 | −0.01 | 0 | 0.258 | −0.01 | −0.01 | 0 | 0.04 | |
| IT2000 | 0.03 | −0.03 | 0.08 | 0.325 | < 0.001 | < 0.001 | 0.001 | 0.173 | |
| ACV | −0.31 | −0.82 | 0.19 | 0.215 | −0.42 | −0.83 | 0 | 0.041 | |
AOD, angle opening distance; LV, lens vault; cACD, central anterior chamber depth; IT, iris thickness; ACV, anterior chamber volume; TISA, trabecular iris space area
Based on multivariate general linear model
Simultaneous effect of all explanatory variables. Based on multivariate analysis of covariance (MANCOVA)
Fig. 2.
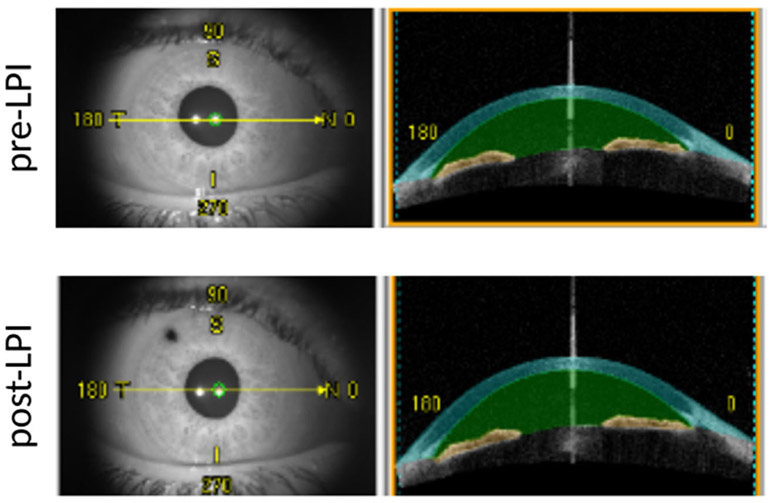
Anterior chamber measurements. Sixty-four anterior segment cross-sectional images of 128 angle meridians were analyzed. Instrument software automatically detected anterior and posterior surface of the iris (yellow color) and calculated iris volume. Anterior chamber volume increased from 111.84 mm3 at the baseline (upper row) to 121.34mm3 post-LPI (lower row)
Fig. 3.
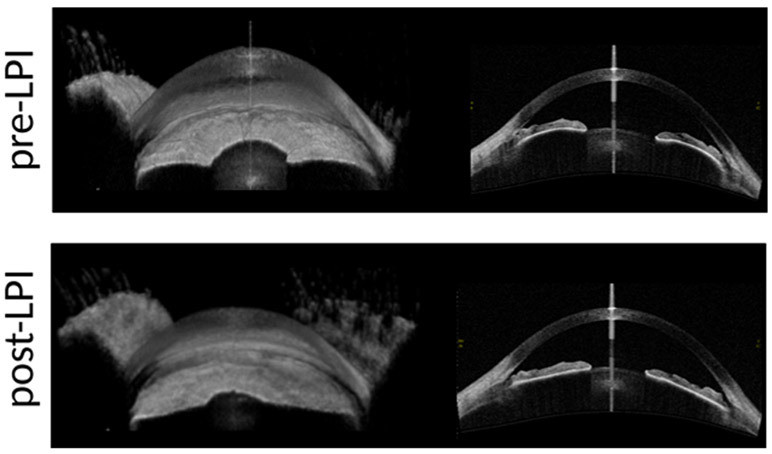
Iris volume was calculated by summing up pixel volumes derived from each B-scan image. Before the LPI, iris had high curvature as evident by 3D-image of the iris (upper left) and cross-sectional image of the iris (lower left). Iris resumes a flatter configuration after the LPI (3D-upper right and cross-sectional lower right images)
Discussion
Our study evaluated the association between anterior chamber baseline parameters and LPI responses in PACS eyes. We observed significant widening of the angle after LPI evidenced by gonioscopic exam and increase in ACV, ACD, AOD 250, AOD 500, AOD 750, TISA 500, TISA 750, ARA 750, and TIA. Our findings are comparable to other imaging studies that used anterior segment OCT to evaluate the changes [30, 13, 24, 31, 32]. In contrast to the ASOCT used in those studies, which generated 12 cross-sectional images, the FD-ASOCT used here can rapidly obtain 128 radial scans to compute the iris and anterior chamber volumes. The novel finding of our study is the association between a lower baseline iris volume and an increased angle widening after an LPI as best seen in TISA 500. A low baseline anterior chamber volume was also associated with a more significant increase in AOD, ARA, TISA, and TIA 1-month post-LPI, but a clinically useful prediction formula could not be derived.
A possible explanation for an inverse relationship between a thinner iris and an improved angle deepening after an LPI is that a thinner iris might be more prone to bulging forward during a relative pupillary block [33]. Two ASOCT studies have examined the relationship between pre-LPI iris thickness and the amount of angle opening after the LPI. How et al. showed a positive association [23], but Lee et al. found a negative relationship [24]. Both studies used an ASOCT that uses a single A-scan at 2 mm from the scleral spur. This method is influenced by the regional differences of the iris and may not be an accurate representation of the iris volume. Our use of an FD-ASOCT allowed us to measure the entire volume of the iris instead of its thickness at a single point. Thinner irises were found to have less collagen type 1 resulting in altered biomechanical behavior [34].
The mean ACV in our study increased from 106.9 ± 26.4 to 119.8 ± 25.6 ml after LPI, consistent with previous reports, despite different methods [35, 36]. Interestingly, we found that the central anterior chamber depth (ACD) increased by 50 μm, not enough to explain the observed increase in ACV, which has to be the result of a flattened iris. It has been suggested that aqueous accumulates in the vitreous which pushes the lens anteriorly during a pupillary block [15]. When the LPI resolves the pupillary block, the aqueous accumulation is resolved, and the lens can shift posteriorly.
AOD was a similar variable that was significantly impacted by the iris and anterior chamber volume but to a lesser extent. AOD treats the iris surface as a straight line [37] and is a less accurate way of measuring angle changes compared to TISA and AOD [38].
This study had several limitations. We included only eyes suspected, but not established, to have primary angle closure because the decision to treat is evident in the latter. This makes it difficult to estimate how eyes with confirmed angle closure would respond. Because only Iranian patients were included in this study, the results are based on a 67–80% ethnically white (Indo-European/Caucasian) population limiting its generalization to other ethnicities. Only one experienced glaucoma; specialist determined the diagnosis and indication to reduce variability. The relatively small sample size, focus on data from the nasal quadrant and lack of automatic identification of the scleral spur, also have to be considered.
In summary, this study showed that a lower iris and anterior chamber volume measured by swept-source anterior segment OCT are associated with a larger angle opening after LPI in PACS eyes. These findings will aid clinicians in deciding whether an LPI should be attempted or a primary lens extraction might be indicated.
Supplementary Material
Acknowledgments
Funding We acknowledge support from the Initiative to Cure Glaucoma of the Eye and Ear Foundation of Pittsburgh; NIH CORE Grant P30 EY08098 to the Department of Ophthalmology, and from an unrestricted grant from Research to Prevent Blindness, NY, NY.
Footnotes
Conflict of interest NAL has received honoraria for Trabectome wet labs and lectures from Neomedix Corp.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent Written consent form was received from all participants in this study.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00417-018-4092-8) contains supplementary material, which is available to authorized users.
References
- 1.Sun X, Dai Y, Chen Y et al. (2017) Primary angle closure glaucoma: what we know and what we don’t know. Prog Retin Eye Res 57:26–45 [DOI] [PubMed] [Google Scholar]
- 2.Tham Y-C, Li X, Wong TY et al. (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121:2081–2090 [DOI] [PubMed] [Google Scholar]
- 3.Pakravan M, Yazdani S, Javadi M-A et al. (2013) A population-based survey of the prevalence and types of glaucoma in Central Iran: the Yazd eye study. Ophthalmology 120:1977–1984 [DOI] [PubMed] [Google Scholar]
- 4.Lowe RF (1970) Aetiology of the anatomical basis for primary angle-closure glaucoma. Biometrical comparisons between normal eyes and eyes with primary angle-closure glaucoma. Br J Ophthalmol 54:161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee DA, Brubaker RF, Ilstrup DM (1984) Anterior chamber dimensions in patients with narrow angles and angle-closure glaucoma. Arch Ophthalmol 102:46–50 [DOI] [PubMed] [Google Scholar]
- 6.Marchini G, Pagliarusco A, Toscano A et al. (1998) Ultrasound biomicroscopic and conventional ultrasonographic study of ocular dimensions in primary angle-closure glaucoma. Ophthalmology 105:2091–2098 [DOI] [PubMed] [Google Scholar]
- 7.Tarongoy P, Ho CL, Walton DS (2009) Angle-closure glaucoma: the role of the lens in the pathogenesis, prevention, and treatment. Surv Ophthalmol 54:211–225 [DOI] [PubMed] [Google Scholar]
- 8.Wang N, Ouyang J, Zhou W et al. (2000) [Multiple patterns of angle closure mechanisms in primary angle closure glaucoma in Chinese]. Zhonghua Yan Ke Za Zhi 36:46–51 5, 6 [PubMed] [Google Scholar]
- 9.Congdon NG, Youlin Q, Quigley H et al. (1997) Biometry and primary angle-closure glaucoma among Chinese, white, and black populations. Ophthalmology 104:1489–1495 [DOI] [PubMed] [Google Scholar]
- 10.Nongpiur ME, He M, Amerasinghe N et al. (2011) Lens vault, thickness, and position in Chinese subjects with angle closure. Ophthalmology 118:474–479 [DOI] [PubMed] [Google Scholar]
- 11.Thomas R, George R, Parikh R et al. (2003) Five year risk of progression of primary angle closure suspects to primary angle closure: a population based study Br J Ophthalmol 87:450–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz LW, Rodrigues MM, Spaeth GL et al. (1978) Argon laser iridotomy in the treatment of patients with primary angle-closure or pupillary block glaucoma: a clinicopathologic study. Ophthalmology 85:294–309 [DOI] [PubMed] [Google Scholar]
- 13.Lei K, Wang N, Wang L, Wang B (2007) Morphological changes of the anterior segment after laser peripheral iridotomy in primary angle closure. Eye 23:345–350 [DOI] [PubMed] [Google Scholar]
- 14.McGalliard JN, Wishart PK (1990) The effect of Nd:YAG iridotomy on intraocular pressure in hypertensive eyes with shallow anterior chambers. Eye 4(Pt 6):823–829 [DOI] [PubMed] [Google Scholar]
- 15.Dada T, Mohan S, Sihota R et al. (2007) Comparison of ultrasound biomicroscopic parameters after laser iridotomy in eyes with primary angle closure and primary angle closure glaucoma. Eye 21:956–961 [DOI] [PubMed] [Google Scholar]
- 16.He M, Friedman DS, Ge J et al. (2007) Laser peripheral iridotomy in eyes with narrow drainage angles: ultrasound biomicroscopy outcomes. The Liwan Eye Study. Ophthalmology 114:1513–1519 [DOI] [PubMed] [Google Scholar]
- 17.Yao B-Q, Wu L-L, Zhang C, Wang X (2009) Ultrasound biomicroscopic features associated with angle closure in fellow eyes of acute primary angle closure after laser iridotomy. Ophthalmology 116:444–448.e2 [DOI] [PubMed] [Google Scholar]
- 18.Gazzard G, Friedman DS, Devereux JG et al. (2003) A prospective ultrasound biomicroscopy evaluation of changes in anterior segment morphology after laser iridotomy in Asian eyes. Ophthalmology 110:630–638 [DOI] [PubMed] [Google Scholar]
- 19.Lee KS, Sung KR, Kang SY et al. (2011) Residual anterior chamber angle closure in narrow-angle eyes following laser peripheral iridotomy: anterior segment optical coherence tomography quantitative study. Jpn J Ophthalmol 55:213–219 [DOI] [PubMed] [Google Scholar]
- 20.Quigley HA (1981) Long-term follow-up of laser iridotomy. Ophthalmology 88:218–224 [DOI] [PubMed] [Google Scholar]
- 21.Pollack IP (1981) Laser iridotomy in the treatment of angle-closure glaucoma. Ann Ophthalmol 13:549–550 [PubMed] [Google Scholar]
- 22.Friedman DS (2001) Who needs an iridotomy? Br J Ophthalmol 85:1019–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.How AC, Baskaran M, Kumar RS et al. (2012) Changes in anterior segment morphology after laser peripheral iridotomy: an anterior segment optical coherence tomography study. Ophthalmology 119: 1383–1387 [DOI] [PubMed] [Google Scholar]
- 24.Lee RY, Kasuga T, Cui QN et al. (2014) Association between baseline iris thickness and prophylactic laser peripheral iridotomy outcomes in primary angle-closure suspects. Ophthalmology 121:1194–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ (2002) The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol 86:238–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaffer RN (1960) Primary glaucomas. Gonioscopy, ophthalmoscopy and perimetry. Trans Am Acad Ophthalmol Otolaryngol 64:112–127 [PubMed] [Google Scholar]
- 27.Liu S, Li H, Dorairaj S et al. (2010) Assessment of scleral spur visibility with anterior segment optical coherence tomography. J Glaucoma 19:132–135 [DOI] [PubMed] [Google Scholar]
- 28.Mak H, Xu G, Leung CK-S (2013) Imaging the iris with swept-source optical coherence tomography: relationship between iris volume and primary angle closure. Ophthalmology 120:2517–2524 [DOI] [PubMed] [Google Scholar]
- 29.Liu S, Yu M, Ye C et al. (2011) Anterior chamber angle imaging with swept-source optical coherence tomography: an investigation on variability of angle measurement. Invest Ophthalmol Vis Sci 52:8598–8603 [DOI] [PubMed] [Google Scholar]
- 30.Lee RY, Kasuga T, Cui QN et al. (2013) Association between baseline angle width and induced angle opening following prophylactic laser peripheral iridotomy. Invest Ophthalmol Vis Sci 54:3763–3770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoong Leong JC, O’Connor J, Soon Ang G, Wells AP (2014) Anterior segment optical coherence tomography changes to the anterior chamber angle in the short-term following laser peripheral iridoplasty. J Curr Glaucoma Pract 8:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ang BCH, Nongpiur ME, Aung T et al. (2016) Changes in Japanese eyes after laser peripheral iridotomy: an anterior segment optical coherence tomography study. Clin Exp Ophthalmol 44:159–165 [DOI] [PubMed] [Google Scholar]
- 33.Ng WT, Morgan W (2012) Mechanisms and treatment of primary angle closure: a review. Clin Exp Ophthalmol 40:e218–e228 [DOI] [PubMed] [Google Scholar]
- 34.He M, Lu Y, Liu X et al. (2008) Histologic changes of the iris in the development of angle closure in Chinese eyes. J Glaucoma 17:386–392 [DOI] [PubMed] [Google Scholar]
- 35.Oka N, Otori Y, Okada M et al. (2006) Clinical study of anterior ocular segment topography in angle-closure glaucoma using the three-dimensional anterior segment analyzer Pentacam. Nippon Ganka Gakkai Zasshi 110(5):398–403 [PubMed] [Google Scholar]
- 36.Coakes RL, Lloyd-Jones D, Hitchings RA (1979) Anterior chamber volume. Its measurement and clinical application. Trans Ophthalmol Soc U K 99:78–81 [PubMed] [Google Scholar]
- 37.Dorairaj S, Liebmann JM, Ritch R (2007) Quantitative evaluation of anterior segment parameters in the era of imaging. Trans Am Ophthalmol Soc 105:99–108 discussion 108–10 [PMC free article] [PubMed] [Google Scholar]
- 38.Radhakrishnan S, Goldsmith J, Huang D et al. (2005) Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Arch Ophthalmol 123:1053–1059 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.